Bluegill Population Demographics as Related to Abiotic and Biotic Factors in Florida Lakes
Abstract
1. Introduction
2. Materials and Methods
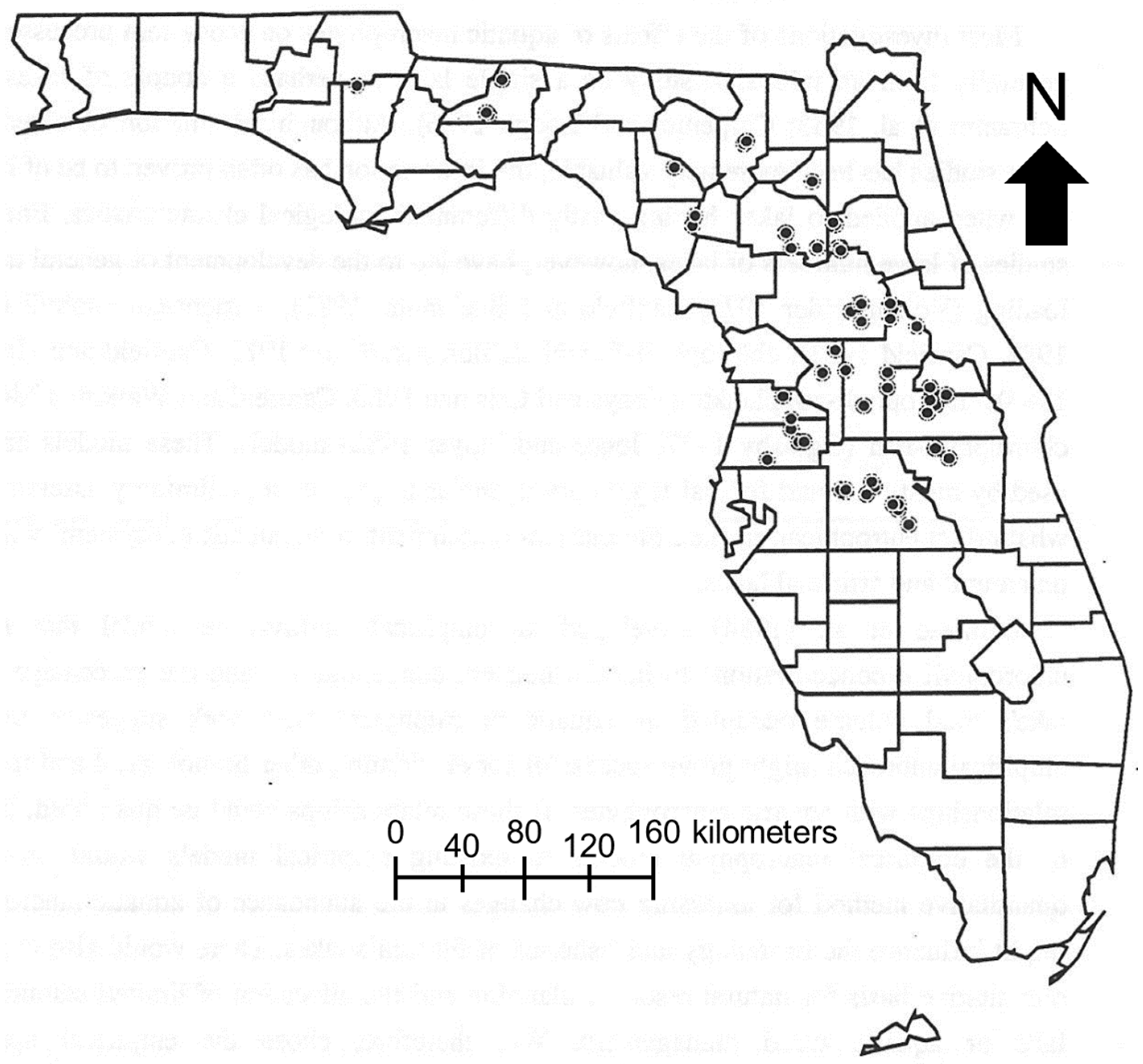
2.1. Study Area
2.2. Data Collection
2.3. Statistical Analysis
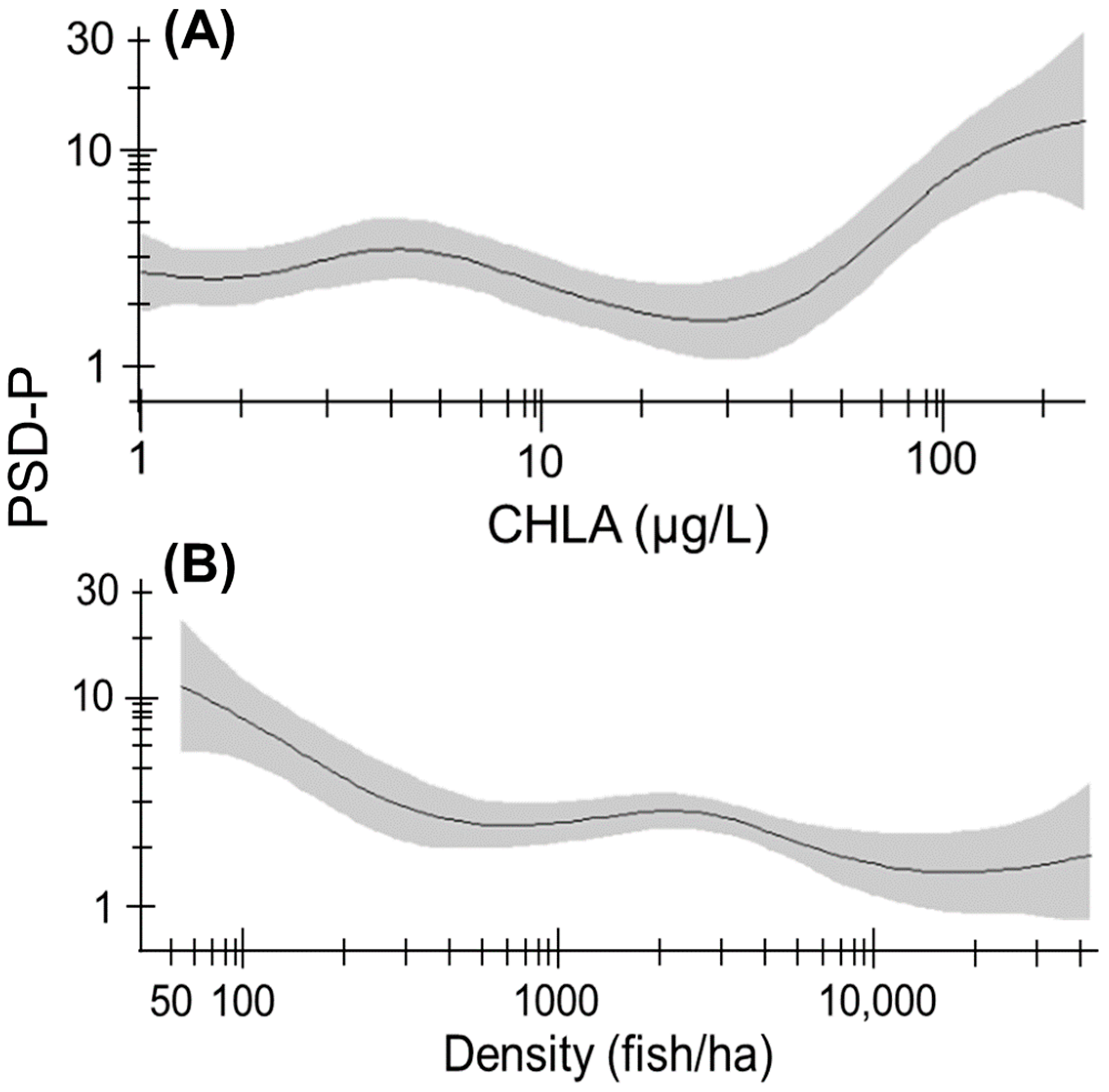
3. Results
3.1. Abiotic and Biotic Factors
3.2. Density
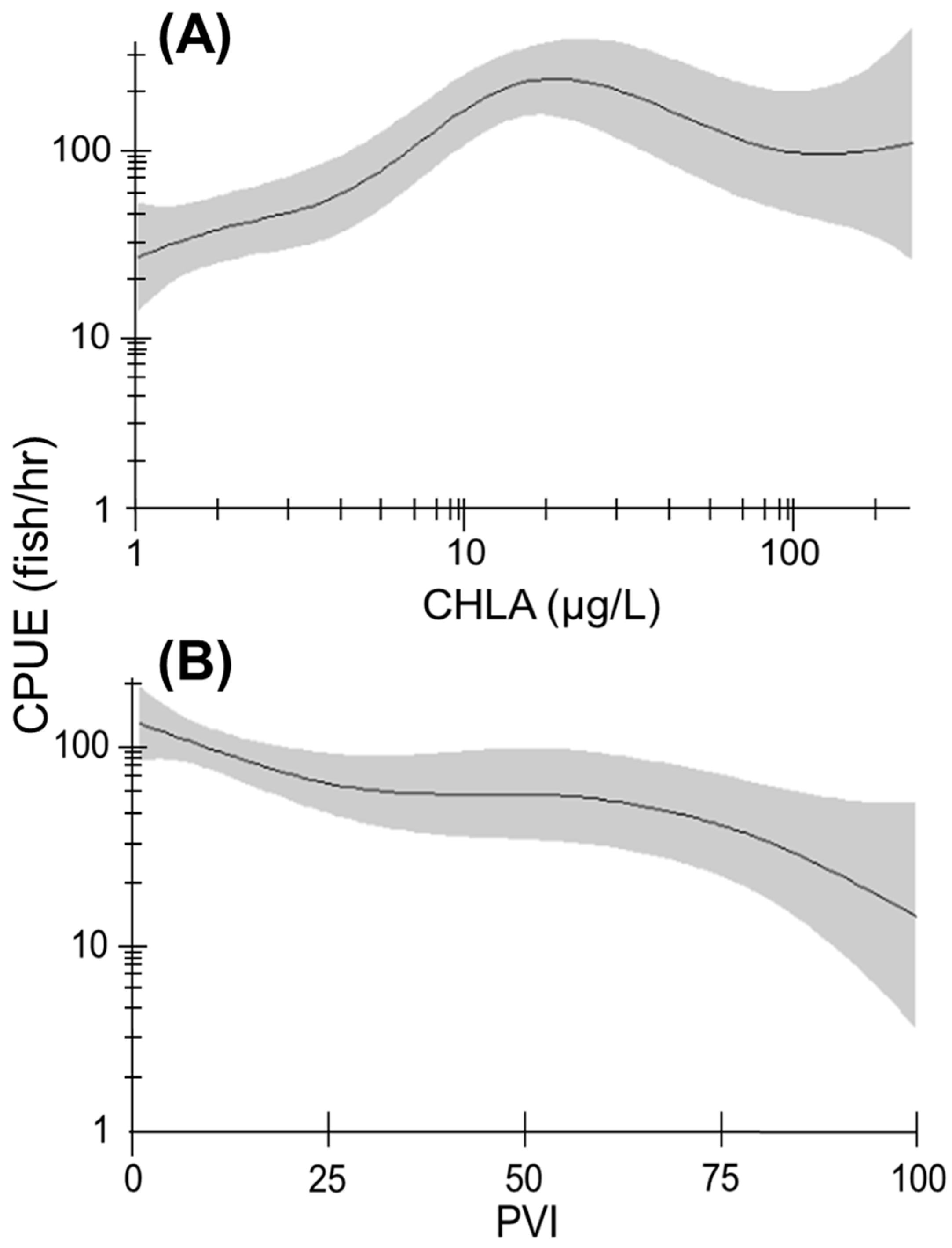
3.3. Relative Abundance
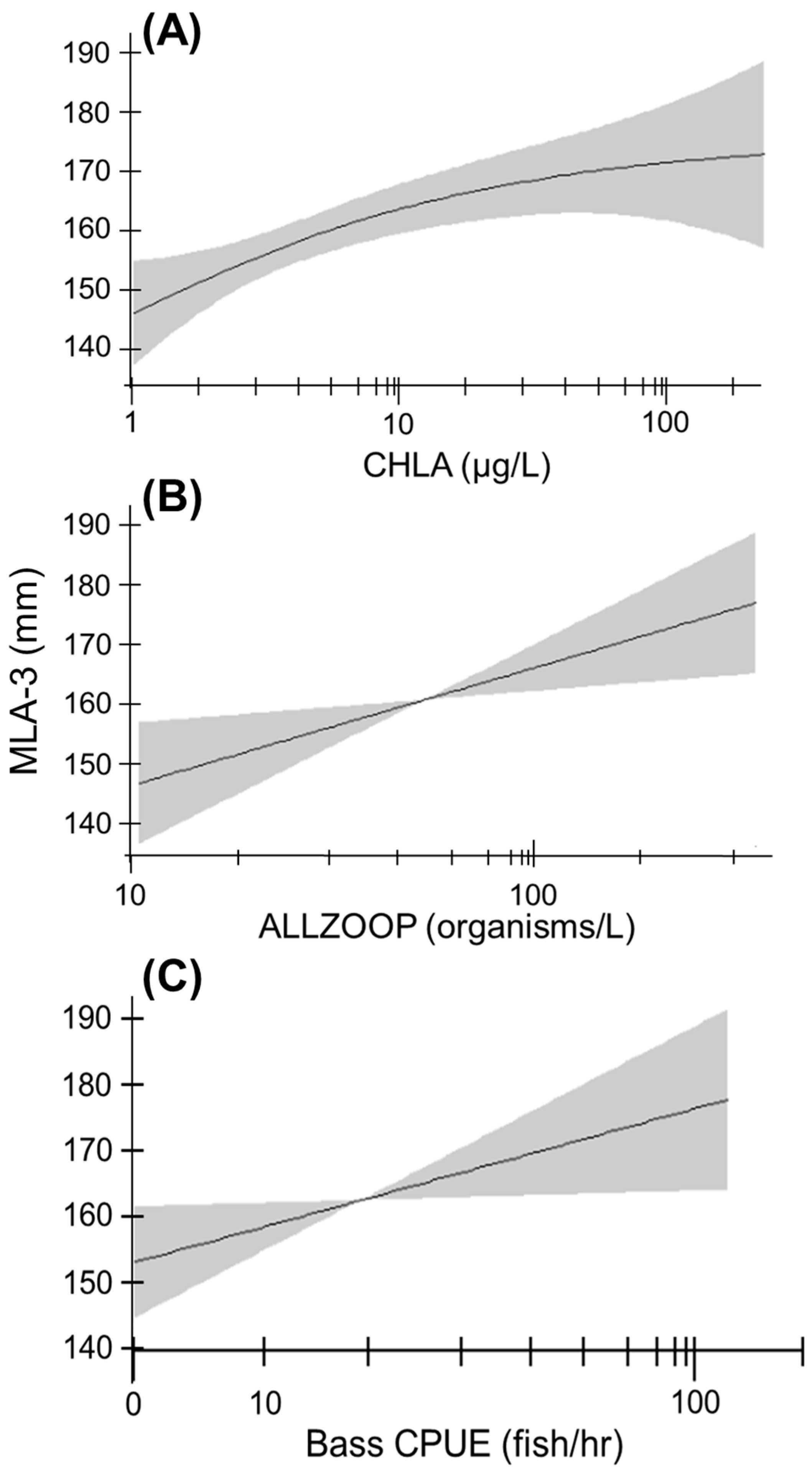
3.4. Growth
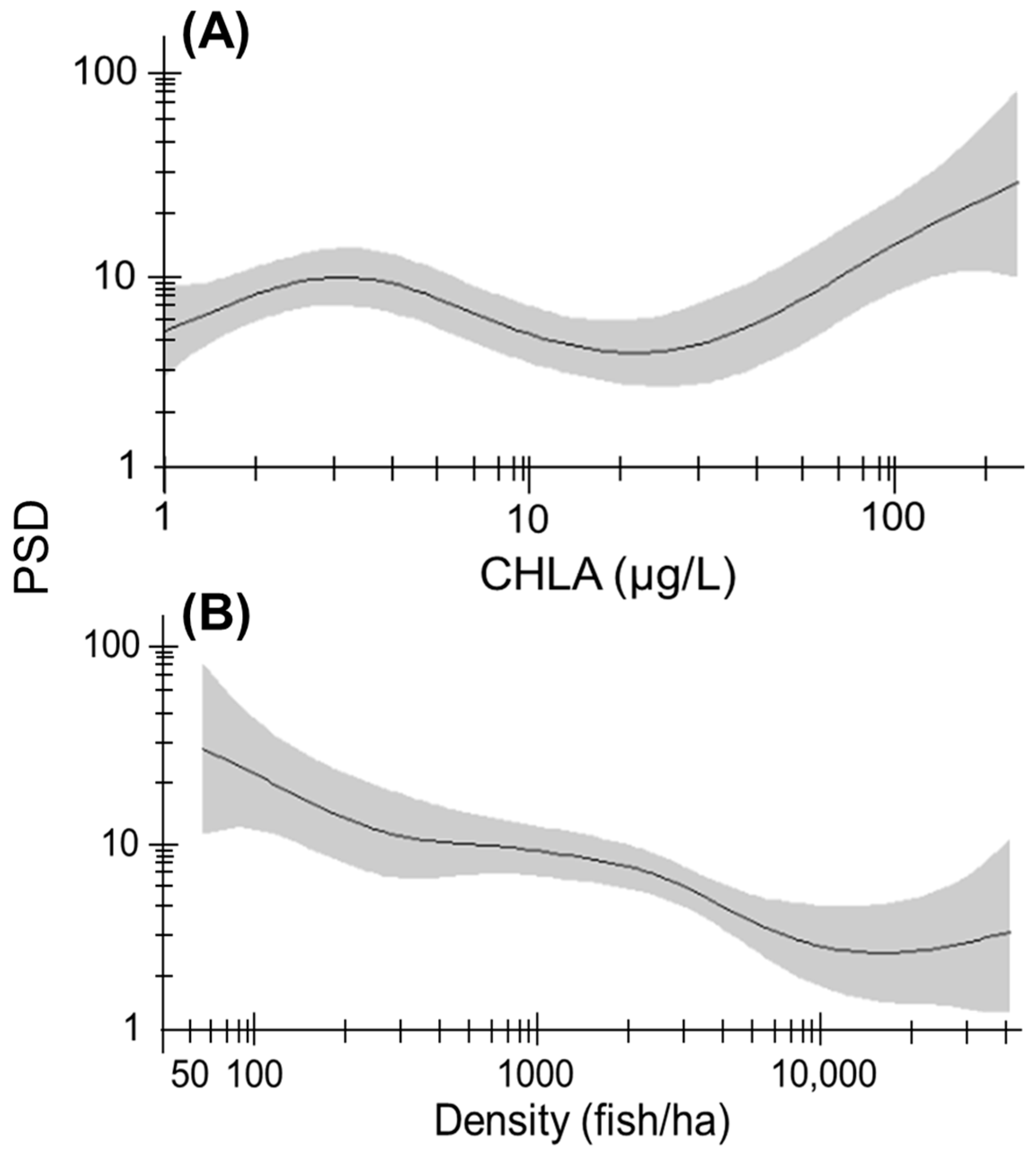
3.5. Size Structure
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lyons, J.; Rypel, A.L.; Hansen, J.F.; Rowe, D.C. Fillet weight and fillet yield: New metrics for the management of panfish and other consumption-oriented recreational fisheries. N. Am. J. Fish. Manag. 2017, 37, 550–557. [Google Scholar] [CrossRef]
- USDOI (United States Department of the Interior). National Survey of Fishing, Hunting, & Wildlife-Associated Recreation (FHWAR): 2016; United States Department of the Interior, United States Fish and Wildlife Service, United States Department of Commerce, United States Census Bureau: Washington, DC, USA, 2018. [Google Scholar]
- Caldwell, D.K.; Odum, H.T.; Hellier, T.R., Jr.; Berry, F.H. Populations of Spotted Sunfish and Florida Largemouth Bass in a constant-temperature spring. Trans. Am. Fish. Soc. 1957, 85, 120–134. [Google Scholar] [CrossRef]
- Sammons, S.M.; Maceina, M.J. Evaluating the potential effectiveness of harvest restrictions on riverine sunfish populations in Georgia, USA. Fish. Manag. Ecol. 2008, 15, 167–178. [Google Scholar] [CrossRef]
- Willis, D.J.; Watson, D.; Hoyer, M.; Canfield, D.E., Jr. Factors related to Warmouth Lepomis gulosus biomass and density in Florida lakes. Fla. Sci. 2009, 72, 218–226. [Google Scholar]
- Colle, D.E.; Shireman, J.; Haller, W.; Joyce, J.; Canfield, D.E. Influence of hydrilla on harvestable sport-fish populations, angler use, and angler expenditures at Orange Lake, Florida. N. Am. J. Fish. Manag. 1987, 7, 410–417. [Google Scholar] [CrossRef]
- Crawford, S.; Allen, M.S. Fishing and natural mortality of Bluegills and Redear Sunfish at Lake Panasoffkee, Florida: Implications for size limits. N. Am. J. Fish. Manag. 2006, 26, 42–51. [Google Scholar] [CrossRef]
- Sammons, S.M.; Partridge, D.; Maceina, M.J. Differences in population metrics between Bluegill and Redear Sunfish: Implications for the effectiveness of harvest restrictions. N. Am. J. Fish. Manag. 2006, 26, 777–787. [Google Scholar] [CrossRef]
- Hanson, C.W.; Sauls, B. Status of recreational saltwater fishing in Florida: Characterization of license sales, participation, and fishing effort. In American Fisheries Society Symposium; American Fisheries Society: Bethesda, MD, USA, 2011; Volume 75, pp. 355–365. [Google Scholar]
- Froese, R.; Pauly, D. FishBase: Bluegill, Lepomis macrochirus Rafinesque, 1819. 2022. Available online: https://www.fishbase.se/Summary/SpeciesSummary.php?ID=3375&AT=bluegill (accessed on 2 February 2023).
- Edison, T.W.; Wahl, D.; Diana, M.; Philipp, D.; Austen, D.J. Angler opinion of potential Bluegill regulations on Illinois lakes: Effects of angler demographics and Bluegill population size structure. N. Am. J. Fish. Manag. 2006, 26, 800–811. [Google Scholar] [CrossRef]
- Tingley, R.W., III; Hansen, J.; Isermann, D.; Fulton, D.; Musch, A.; Paukert, C.P. Characterizing angler preferences for Largemouth Bass, Bluegill, and Walleye fisheries in Wisconsin. N. Am. J. Fish. Manag. 2019, 39, 676–692. [Google Scholar] [CrossRef]
- Winkelman, D.L.; Sager, C. Managing hybrid Bluegill fisheries: Estimating and predicting the effects of young anglers. In American Fisheries Society Symposium 44:207–214; American Fisheries Society: Bethesda, MD, USA, 2004. [Google Scholar]
- Coble, D.W. Effects of angling on Bluegill populations: Management implications. N. Am. J. Fish. Manag. 1988, 8, 277–283. [Google Scholar] [CrossRef]
- Reed, J.R.; Parsons, B.G. Angler opinions of Bluegill management and related hypothetical effects on Bluegill fisheries in four Minnesota lakes. N. Am. J. Fish. Manag. 1999, 19, 515–519. [Google Scholar] [CrossRef]
- Jacobson, P.C. Experimental analysis of a reduced daily Bluegill limit in Minnesota. N. Am. J. Fish. Manag. 2005, 25, 203–210. [Google Scholar] [CrossRef]
- Rypel, A.L.; Lyons, J.; Griffin, J.; Simonson, T.D. Seventy-year retrospective on size-structure changes in the recreational fisheries of Wisconsin. Fisheries 2016, 41, 230–243. [Google Scholar] [CrossRef]
- Snow, H.E.; Staggs, M.D. Factors Related to Fish Growth in Northwestern Wisconsin Lakes; Wisconsin Department of Natural Resources, Bureau of Fish Management Report 162; The Wisconsin Department of Natural Resources: Madison, WI, USA, 1994. [Google Scholar]
- Jackson, D.C.; Brown-Peterson, N.J. Habitat, accessibility, and watershed variables as they relate to Largemouth Bass and Bluegill in Mississippi’s National Forest impoundments. J. Southeast. Assoc. Fish Wildl. Agencies 1995, 49, 26–36. [Google Scholar]
- Trebitz, A.S.; Carpenter, S.; Cunningham, P.; Johnson, B.; Lillie, R.; Marshall, D.; Martin, T.; Narf, R.; Pellett, T.; Stewart, S.; et al. A model of Bluegill-Largemouth Bass interactions in relation to aquatic vegetation and its management. Ecol. Model. 1997, 94, 139–156. [Google Scholar] [CrossRef]
- Tomcko, C.M.; Pierce, R.B. The relationship of Bluegill growth, lake morphometry, and water quality in Minnesota. Trans. Am. Fish. Soc. 2001, 130, 317–321. [Google Scholar] [CrossRef]
- Aday, D.D.; Shoup, D.; Veviackas, J.; Kline, J.; Wahl, D.H. Prey community responses to Bluegill and Gizzard Shad foraging: Implications for growth of juvenile Largemouth Bass. Trans. Am. Fish. Soc. 2005, 134, 1091–1102. [Google Scholar] [CrossRef]
- Michaletz, P.H.; Obrecht, D.; Jones, J.R. Influence of environmental variables and species interactions on sport fish communities in small Missouri impoundments. N. Am. J. Fish. Manag. 2012, 32, 1146–1159. [Google Scholar] [CrossRef]
- Michaletz, P.H. Interrelationships among Bluegill demographics, predator and competitor abundances and impoundment characteristics. J. Freshw. Ecol. 2020, 35, 491–506. [Google Scholar] [CrossRef]
- Jones, J.R.; Hoyer, M.V. Sportfish harvest predicted by summer chlorophyll-α concentration in Midwestern lakes and reservoirs. Trans. Am. Fish. Soc. 1982, 111, 176–179. [Google Scholar] [CrossRef]
- Diana, M.J.; Stein, J.; Oplinger, R.; Aday, D.; Hoxmeier, J.; Claussen, J.; Philipp, D.; Wahl, D.H. Quality Management of Bluegill: Factors Affecting Population Size Structure; Final Report to the Division of Fisheries, Illinois Department of Natural Resources, Federal Aid Project F-128-R; Illinois Natural History Survey, Center for Aquatic Ecology: Springfield, IL, USA, 2007. [Google Scholar]
- Knuth, D.S. Effects of Abiotic and Biotic Factors on Bluegill Reproduction and Growth in Seven Unexploited Surface Coal Mine Lakes. Master’s Thesis, Southern Illinois University, Carbondale, IL, USA, 2007. [Google Scholar]
- Gerking, S.D. Production and food utilization in a population of Bluegill sunfish. Ecol. Monogr. 1962, 32, 31–78. [Google Scholar] [CrossRef]
- Mittelbach, G.G. Competition among refuging sunfishes and effects of fish density on littoral zone invertebrates. Ecology 1988, 69, 614–623. [Google Scholar] [CrossRef]
- Schneider, J.C. Dynamics of quality Bluegill populations in two Michigan lakes with dense vegetation. N. Am. J. Fish. Manag. 1999, 19, 97–109. [Google Scholar] [CrossRef]
- Paukert, C.P.; Willis, D.; Klammer, J.A. Effects of predation and environment on quality of Yellow Perch and Bluegill populations in Nebraska Sandhill lakes. N. Am. J. Fish. Manag. 2002, 22, 86–95. [Google Scholar] [CrossRef]
- Cross, T.K.; McInerny, M.C. Spatial habitat dynamics affecting Bluegill abundance in Minnesota bass–panfish lakes. N. Am. J. Fish. Manag. 2005, 25, 1051–1066. [Google Scholar] [CrossRef]
- Hoxmeier, R.J.H.; Aday, D.; Wahl, D.H. Examining interpopulation variation in Bluegill growth rates and size structure: Effects of harvest, maturation, and environmental variables. Trans. Am. Fish. Soc. 2009, 138, 423–432. [Google Scholar] [CrossRef]
- Bevil, M.C.; Weber, M.J. Relative effects of density dependence versus environmental factors on Bluegill populations. N. Am. J. Fish. 2018, 38, 140–151. [Google Scholar] [CrossRef]
- FWC (Florida Fish and Wildlife Conservation Commission). The Economic Impacts of Saltwater Fishing in Florida; FWC: Tallahassee, FL, USA, 2022; Available online: https://myfwc.com/conservation/value/saltwater-fishing/#:~:text=4%20million%20Florida%20anglers%3A%20FWC,from%20the%202011%20National%20Survey (accessed on 2 February 2023).
- FWC (Florida Fish and Wildlife Conservation Commission). Manager Report (2020–2021), Fish and Wildlife Research Institute; FWC Freshwater Fisheries Research: Tallahassee, FL, USA, 2022. [Google Scholar]
- Schramm, H.L.; Jirka, K.J. Epiphytic macroinvertebrates as a food resource for Bluegills in Florida lakes. Trans. Am. Fish. Soc. 1989, 118, 416–426. [Google Scholar] [CrossRef]
- Canfield, D.E., Jr.; Hoyer, M.V. Aquatic Macrophytes and Their Relation to the Limnology of Florida Lakes; Bureau of Aquatic Plant Management, Florida Department of Natural Resources, Final Report: Tallahassee, FL, USA, 1992; Available online: https://lakewatch.ifas.ufl.edu/research/past-project-reports/ (accessed on 2 February 2023).
- Werner, E.E.; Hall, D.J. Niche shifts in sunfishes: Experimental evidence and significance. Science 1976, 191, 404–406. [Google Scholar] [CrossRef]
- Clugston, J.P. Centrarchid spawning in the Florida Everglades. Q. J. Fla. Acad. Sci. 1966, 29, 137–143. [Google Scholar]
- Hoyer, M.V.; Canfield, D.E., Jr. Largemouth Bass abundance and aquatic vegetation in Florida lakes: An empirical analysis. J. Aquat. Plant Manag. 1996, 34, 23–32. [Google Scholar]
- Parson, T.R.; Strickland, J.D. Discussion of spectrophotometric determination of marine-plant pigments, with revised equations of ascertaining chlorophylls and carotenoids. J. Mar. Res. 1963, 21, 155–163. [Google Scholar]
- Yentsch, C.S.; Menzel, D.W. A method for the determination of phytoplankton chlorophyll and phaeophytin by fluorescence. Deep. Sea Res. 1963, 10, 221–231. [Google Scholar] [CrossRef]
- Canfield, D.E., Jr.; Langeland, K.; Maceina, M.; Haller, W.; Shireman, J.; Jones, J.R. Trophic state classification of lakes with aquatic macrophytes. Can. J. Fish. Aquat. Sci. 1983, 40, 1713–1718. [Google Scholar] [CrossRef]
- Maceina, M.J.; Shireman, J.V. The use of a recording fathometer for the determination of distribution and biomass of hydrilla. J. Aquat. Plant Manag. 1980, 18, 34–39. [Google Scholar]
- Allen, M.S.; Hoyer, M.; Canfield, D.E., Jr. Factors related to Black Crappie occurrence, density, and growth in Florida lakes. N. Am. J. Fish. Manag. 1998, 18, 864–871. [Google Scholar] [CrossRef]
- Shireman, J.V.; Colle, D.; Durant, D.F. Efficiency of rotenone sampling with large and small block nets in vegetated and unvegetated habitats. Trans. Am. Fish. Soc. 1981, 110, 77–80. [Google Scholar] [CrossRef]
- Schramm, H.L., Jr.; Doerzbacher, J.F. Use of otoliths to age Black Crappie from Florida. J. Southeast. Assoc. Fish Wildl. Agencies 1982, 36, 95–105. [Google Scholar]
- Venables, W.N.; Dichmont, C.M. GLMs, GAMs and GLMMs: An overview of theory for applications in fisheries research. Fish. Res. 2004, 70, 319–337. [Google Scholar] [CrossRef]
- Agha, M.; Losee, J.; Litz, M.; Smith, C.; Schaffler, J.; Patton, W.; Dufault, A.; Madel, G.M. Temporal patterns and ecosystem correlates of Chum Salmon Oncorhynchus keta migration phenology in the Pacific Northwest. Can. J. Fish. Aquat. Sci. 2021, 78, 1565–1575. [Google Scholar] [CrossRef]
- Gabelhouse, D.W., Jr. A length-categorization system to assess fish stocks. N. Am. J. Fish. Manag. 1984, 4, 273–285. [Google Scholar] [CrossRef]
- Allen, M.S.; Hoyer, M.; Canfield, D.E., Jr. Factors related to Gizzard Shad and Threadfin Shad occurrence and abundance in Florida lakes. J. Fish Biol. 2000, 57, 291–302. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1984. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference; Springer Science: New York, NY, USA, 2002. [Google Scholar]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017. [Google Scholar]
- Hyndman, R.J.; Athanasopoulos, G. Forecasting: Principles and Practice, 2nd ed.; OTexts: Melbourne, Australia, 2018. [Google Scholar]
- Florida Atlas of Lakes. Florida Atlas of Lakes; University of South Florida Water Institute: Tampa, FL, USA, 2022; Available online: https://wateratlas.usf.edu/atlasoflakes/florida/ (accessed on 2 February 2023).
- Florida LAKEWATCH. Florida LAKEWATCH Reports; University of Florida: Gainesville, FL, USA, 2022; Available online: https://lakewatch.ifas.ufl.edu/datareports/?view=meta (accessed on 2 February 2023).
- R Development Core Team. R: A Language And Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Forsberg, C.; Ryding, S. Eutrophication parameters and trophic state indices in 30 Swedish waste-receiving lakes. Arch. Hydrobiol. 1980, 89, 189–207. [Google Scholar]
- Oglesby, R.T. Relationships of fish yield to lake phytoplankton standing crop, production, and morphoedaphic factors. J. Fish. Res. Board Can. 1977, 34, 2271–2279. [Google Scholar] [CrossRef]
- Schramm, H.L.; Willis, D. Assessment and harvest of Largemouth Bass–Bluegill ponds. In Small Impoundment Management in North America; Neal, J.W., Willis, D.W., Eds.; American Fisheries Society: Bethesda, MD, USA, 2012; pp. 181–213. [Google Scholar]
- Mittelbach, G.G. Foraging efficiency and body size: A study of optimal diet and habitat use by Bluegills. Ecology 1981, 62, 1370–1386. [Google Scholar] [CrossRef]
- Werner, E.E.; Hall, D.J. Ontogenetic habitat shifts in Bluegill: The foraging rate–predation risk trade-off. Ecology 1988, 69, 1352–1366. [Google Scholar] [CrossRef]
- Mittelbach, G.G.; Osenberg, C.W. Stage-structured interactions in Bluegill: Consequences of adult resource variation. Ecology 1993, 74, 2381–2394. [Google Scholar] [CrossRef]
- Crowder, L.B.; Cooper, W.E. Structural complexity and fish-prey interactions in ponds: A point of view. In Response of Fish to Habitat Structure in Standing Water; Johnson, D.L., Stein, R.A., Eds.; American Fisheries Society, North Central Division, Special Publication 6: Bethesda, MD, USA, 1979; pp. 2–10. [Google Scholar]
- Savino, J.F.; Stein, R.A. Predator-prey interactions between Largemouth Bass and Bluegills as influenced by simulated submersed vegetation. Trans. Am. Fish. Soc. 1982, 111, 255–266. [Google Scholar] [CrossRef]
- Pothoven, S.A.; Vondracek, B.; Pereira, D.L. Effects of vegetation removal on Bluegill and Largemouth Bass in two Minnesota lakes. N. Am. J. Fish. Manag. 1999, 19, 748–757. [Google Scholar] [CrossRef]
- Gotceitas, V.; Colgan, P. Selection between densities of artificial vegetation by young Bluegills avoiding predation. Trans. Am. Fish. Soc. 1987, 116, 40–49. [Google Scholar] [CrossRef]
- Lillie, R.A.; Budd, J. Habitat architecture of Myriophyllum spicatum L. as an index to habitat quality for fish and macroinvertebrates. J. Freshw. Ecol. 1992, 7, 113–125. [Google Scholar] [CrossRef]
- Collingsworth, P.D.; Kohler, C.C. Abundance and habitat use of juvenile sunfish among different macrophyte stands. Lake Reserv. Manag. 2010, 26, 35–42. [Google Scholar] [CrossRef]
- Scarnecchia, D.L. A reappraisal of gars and bowfins in fishery management. Fisheries 1992, 17, 6–12. [Google Scholar] [CrossRef]
- Bransky, J.W.; Dorn, N.J. Prey use of wetland benthivorous sunfishes: Ontogenetic, interspecific and seasonal variation. Environ. Biol. Fishes 2013, 96, 1329–1340. [Google Scholar] [CrossRef]
- Porter, N.J.; Bonvechio, T.; McCormick, J.; Quist, M.C. Population dynamics of Bowfin in a South Georgia reservoir: Latitudinal comparisons of population structure, growth, and mortality. J. Southeast. Assoc. Fish Wildl. Agencies 2014, 2014, 103–109. [Google Scholar]
- Hinch, S.G.; Collins, N.C. Relationships of littoral fish abundance to water chemistry and macrophyte variables in central Ontario lakes. Can. J. Fish. Aquat. Sci. 1993, 50, 1870–1878. [Google Scholar] [CrossRef]
- Haley, N.V., III; Wright, R.; DeVries, D.; Allen, M. Privately owned small impoundments in central Alabama: A survey and evaluation of management techniques for Largemouth Bass and Bluegill. N. Am. J. Fish. Manag. 2012, 32, 1180–1190. [Google Scholar] [CrossRef]
- Michaletz, P.H.; Doisy, K.; Rabeni, C.F. Influences of productivity, vegetation, and fish on macroinvertebrate abundance and size in Midwestern USA impoundments. Hydrobiologia 2005, 543, 147–157. [Google Scholar] [CrossRef]
- Bayley, P.B.; Austen, D.J. Capture efficiency of a boat electrofisher. Trans. Am. Fish. Soc. 2002, 131, 435–451. [Google Scholar] [CrossRef]
- Hangsleben, M.A.; Allen, M.; Gwinn, D.C. Evaluation of electrofishing catch per unit effort for indexing fish abundance in Florida lakes. Trans. Am. Fish. Soc. 2013, 142, 247–256. [Google Scholar] [CrossRef]
- Tomcko, C.M.; Pierce, R.B. Bluegill recruitment, growth, population size structure, and associated factors in Minnesota Lakes. N. Am. J. Fish. Manag. 2005, 25, 171–179. [Google Scholar] [CrossRef]
- Lemly, A.D.; Dimmick, J.F. Growth of young-of-the-year and yearling centrarchids in relation to zooplankton in the littoral zone of lakes. Copeia 1982, 1982, 305–321. [Google Scholar] [CrossRef]
- Theiling, C.H. The Relationships between Several Limnological Factors and Bluegill Growth in Michigan Lakes; Michigan Department of Natural Resources, Fisheries Research Report 1970; The Michigan Department of Natural Resources: Ann Arbor, MI, USA, 1990. [Google Scholar]
- Fletcher, C.M.; Collins, S.; Nannini, M.; Wahl, D.H. Competition during early ontogeny: Effects of native and invasive planktivores on the growth, survival, and habitat use of Bluegill. Freshw. Biol. 2019, 64, 697–707. [Google Scholar] [CrossRef]
- Anderson, R.O. Management of small warm water impoundments. Fisheries 1976, 5–7, 26–28. [Google Scholar]
- Guy, C.S.; Willis, D.W. Structural relationships of Largemouth Bass and Bluegill populations in South Dakota ponds. N. Am. J. Fish. Manag. 1990, 10, 338–343. [Google Scholar] [CrossRef]
- Otis, K.J.; Piette, R.; Keppler, J.; Rasmussen, P.W. A Largemouth Bass closed fishery to control an overabundant Bluegill population in a Wisconsin lake. J. Freshw. Ecol. 1998, 13, 391–403. [Google Scholar] [CrossRef]
- Novinger, G.D.; Legler, R.E. Bluegill population structure and dynamics. In New Approaches to the Management of Small Impoundments; Novinger, G.D., Dillard, J.G., Eds.; American Fisheries Society, North Central Division, Special Publication 5: Bethesda, MD, USA, 1978; pp. 37–49. [Google Scholar]
- Wiener, J.G.; Hanneman, W.R. Growth and condition of Bluegills in Wisconsin lakes: Effects of population density and lake pH. Trans. Am. Fish. Soc. 1982, 111, 761–767. [Google Scholar] [CrossRef]
- Tomcko, C.M. Bluegill Growth Rates in Minnesota; Minnesota Department of Natural Resources, Section of Fisheries Investigational Report Number 458; The Minnesota Department of Natural Resources: St. Paul, MI, USA, 1997. [Google Scholar]
- Schultz, R.D.; Jackson, Z.; Quist, M.C. Relating impoundment morphometry and water quality to Black Crappie, Bluegill, and Largemouth Bass populations in Iowa. In American Fisheries Society Symposium 62:479–491; American Fisheries Society: Bethesda, MD, USA, 2008. [Google Scholar]
- Nease, P.A. Nearshore Habitat and Land-Use Effects on Trophic Interactions and Growth of Largemouth Bass and Bluegill in Indiana’s Glacial Lakes. Master’s Thesis, Southern Illinois University, Carbondale, IL, USA, 2019. [Google Scholar]
- Peterson, N.R.; VanDeHey, J.; Willis, D.W. Size and age at maturity of Bluegill Lepomis macrochirus in southeastern South Dakota impoundments. J. Freshw. Ecol. 2010, 25, 303–312. [Google Scholar] [CrossRef]
- Sass, G.G.; Carpenter, S.; Gaeta, J.; Kitchell, J.; Ahrenstorff, T.D. Whole-lake addition of coarse woody habitat: Response of fish populations. Aquat. Sci. 2012, 74, 255–266. [Google Scholar] [CrossRef]
- Huber, W.C.; Brezonik, P.; Heaney, J.; Dickinson, R.; Preston, S.; Dwornik, D.; DeMaio, M.A. A classification of Florida lakes. Final report prepared for the Florida Department of Environmental Regulation. In Report ENV-05-82-1; Department of Environmental Engineering Sciences: Gainesville, FL, USA, 1982. [Google Scholar]
- Canfield, D.E., Jr. Prediction of chlorophyll a concentrations in Florida lakes: The importance of phosphorus and nitrogen. Water Resour. Bull. 1983, 19, 255–262. [Google Scholar] [CrossRef]
- Modde, T.; Scalet, C.G. Latitudinal growth effects on predator-prey interactions between Largemouth Bass and Bluegills in ponds. N. Am. J. Fish. Manag. 1985, 5, 227–232. [Google Scholar] [CrossRef]
- Drake, M.T.; Claussen, J.; Philipp, D.; Pereira, D.L. A comparison of Bluegill reproductive strategies and growth among lakes with different fishing intensities. N. Am. J. Fish. Manag. 1997, 17, 496–507. [Google Scholar] [CrossRef]
- Hankinson, T.L. Ecological notes on the fishes of Walnut Lake, Michigan. Trans. Am. Fish. Soc. 1911, 40, 195–206. [Google Scholar] [CrossRef]
- Ricker, W.E. Natural mortality among Indiana Bluegill sunfish. Ecology 1945, 26, 111–121. [Google Scholar] [CrossRef]
- Sammons, S.M.; Maceina, M.J. Variation in growth and survival of Bluegills and Redbreast Sunfish in Georgia rivers. N. Am. J. Fish. Manag. 2009, 29, 101–108. [Google Scholar] [CrossRef]
- Dequine, J.F. Management of Florida’s fresh-water fisheries. Trans. Am. Fish. Soc. 1950, 78, 38–41. [Google Scholar] [CrossRef]
- Belk, M.C.; Hales, L.S., Jr. Predation-induced differences in growth and reproduction of Bluegills (Lepomis macrochirus). Copeia 1993, 1993, 1034–1044. [Google Scholar] [CrossRef]

| Factor | Mean | Median | N | SE | Range | CV |
|---|---|---|---|---|---|---|
| Surface area (ha) | 406.0 | 55.0 | 60 | 226.0 | 2–12,412 | 4.31 |
| Chlorophyll-a (μg/L) | 28.1 | 9.5 | 60 | 6.0 | 1–241 | 1.66 |
| Adjusted chlorophyll-a (μg/L) | 50.0 | 21.0 | 60 | 8.9 | 1–350 | 1.37 |
| Zooplankton density (organisms/L) | 66.8 | 56.3 | 60 | 7.2 | 11–314 | 0.82 |
| PVI | 23.6 | 8.5 | 60 | 4.1 | 0.3–100 | 1.36 |
| Bluegill density (fish/ha) | 3618.3 | 1773.1 | 60 | 845.1 | 54–40,215 | 1.78 |
| Bluegill CPUE (fish/h) | 191.3 | 78.9 | 60 | 54.3 | 0–3062 | 2.20 |
| Bluegill length at age 1 (mm) | 64.2 | 61.0 | 55 | 2.4 | 38–110 | 0.28 |
| Bluegill length at age 2 (mm) | 120.9 | 118.0 | 55 | 2.8 | 86–175 | 0.17 |
| Bluegill length at age 3 (mm) | 159.3 | 156.5 | 54 | 3.1 | 111–212 | 0.14 |
| Bluegill PSD | 39.0 | 31.3 | 60 | 4.1 | 0–100 | 0.53 |
| Bluegill PSD-P | 18.0 | 8.7 | 60 | 3.0 | 0–93 | 0.38 |
| Florida Bass CPUE (SL fish/h) | 21.5 | 16.1 | 60 | 2.8 | 0–126 | 0.97 |
| Florida Bass PSD | 42.0 | 44.0 | 60 | 3.6 | 0–100 | 0.67 |
| Florida Bass PSD-P | 17.0 | 11.0 | 60 | 2.3 | 0–61 | 1.01 |
| Variable | SA | CHLA | ACHLA | ALLZOOP | PVI | Bluegill Density | Bass CPUE | Bass PSD |
|---|---|---|---|---|---|---|---|---|
| SA | - | - | - | - | - | - | - | - |
| CHLA | 0.45 | - | - | - | - | - | - | - |
| ACHLA | 0.34 | 0.72 | - | - | - | - | - | - |
| ALLZOOP | 0.33 | 0.31 | 0.36 | - | - | - | - | - |
| PVI | 0.02 | −0.34 | 0.25 | 0.25 | - | - | - | - |
| Bluegill density | 0.01 | 0.25 | 0.27 | 0.21 | −0.05 | - | - | - |
| Bass CPUE | 0.23 | 0.30 | 0.08 | <0.01 | −0.23 | 0.28 | - | - |
| Bass PSD | 0.07 | 0.22 | 0.29 | 0.13 | −0.04 | 0.01 | −0.29 | - |
| Bass PSD-P | 0.09 | 0.28 | −0.05 | −0.15 | −0.55 | 0.13 | 0.12 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlson, A.K.; Hoyer, M.V. Bluegill Population Demographics as Related to Abiotic and Biotic Factors in Florida Lakes. Fishes 2023, 8, 100. https://doi.org/10.3390/fishes8020100
Carlson AK, Hoyer MV. Bluegill Population Demographics as Related to Abiotic and Biotic Factors in Florida Lakes. Fishes. 2023; 8(2):100. https://doi.org/10.3390/fishes8020100
Chicago/Turabian StyleCarlson, Andrew K., and Mark V. Hoyer. 2023. "Bluegill Population Demographics as Related to Abiotic and Biotic Factors in Florida Lakes" Fishes 8, no. 2: 100. https://doi.org/10.3390/fishes8020100
APA StyleCarlson, A. K., & Hoyer, M. V. (2023). Bluegill Population Demographics as Related to Abiotic and Biotic Factors in Florida Lakes. Fishes, 8(2), 100. https://doi.org/10.3390/fishes8020100






