Effects of Multiple Freeze-Thaw Cycles on Protein and Lipid Oxidation, Microstructure and Quality Characteristics of Rainbow Trout (Oncorhynchus mykiss)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Color Texture and Shear Force Analysis
2.3. pH and WHC (Thawing Loss, Cooking Loss) Analysis
2.4. Lipid Oxidation
2.5. Preparation of Myofibrillar Protein (MP)
2.6. Determination of Carbonyl Content
2.7. Determination of Total Sulfhydryl (SH) Content
2.8. Muscle Fiber Microstructure
2.9. Approximate Nutrition Component and Amino Acid Composition Analysis
2.10. Statistical Analysis
3. Results and Discussion
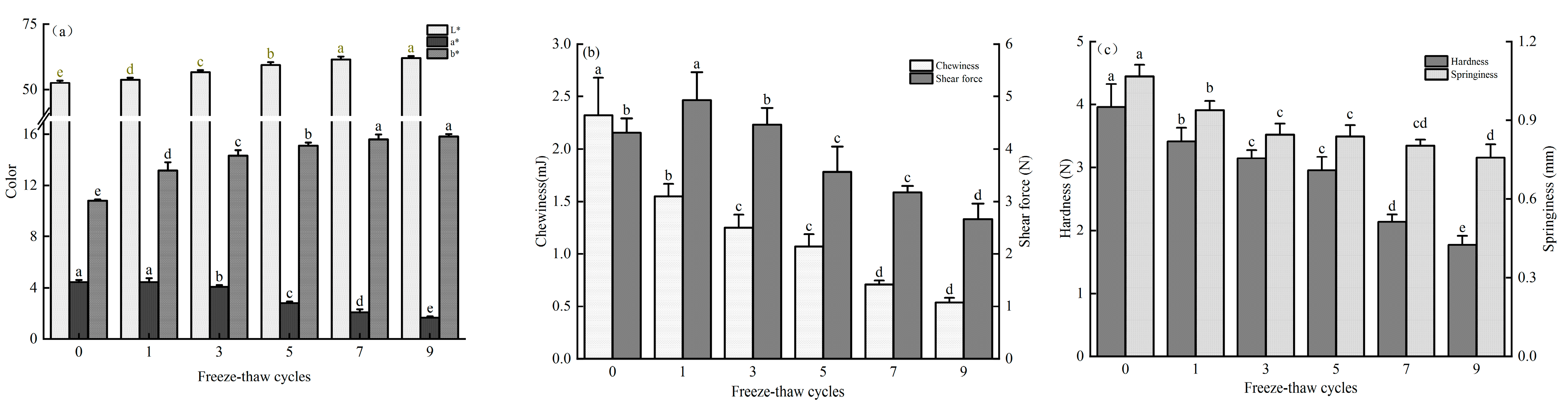
3.1. Effect of Freeze-Thaw Cycles on Color, Shear Force, and Texture
3.2. Effect of Freeze-Thaw Cycles on pH, Thawing Loss, and Cooking Loss
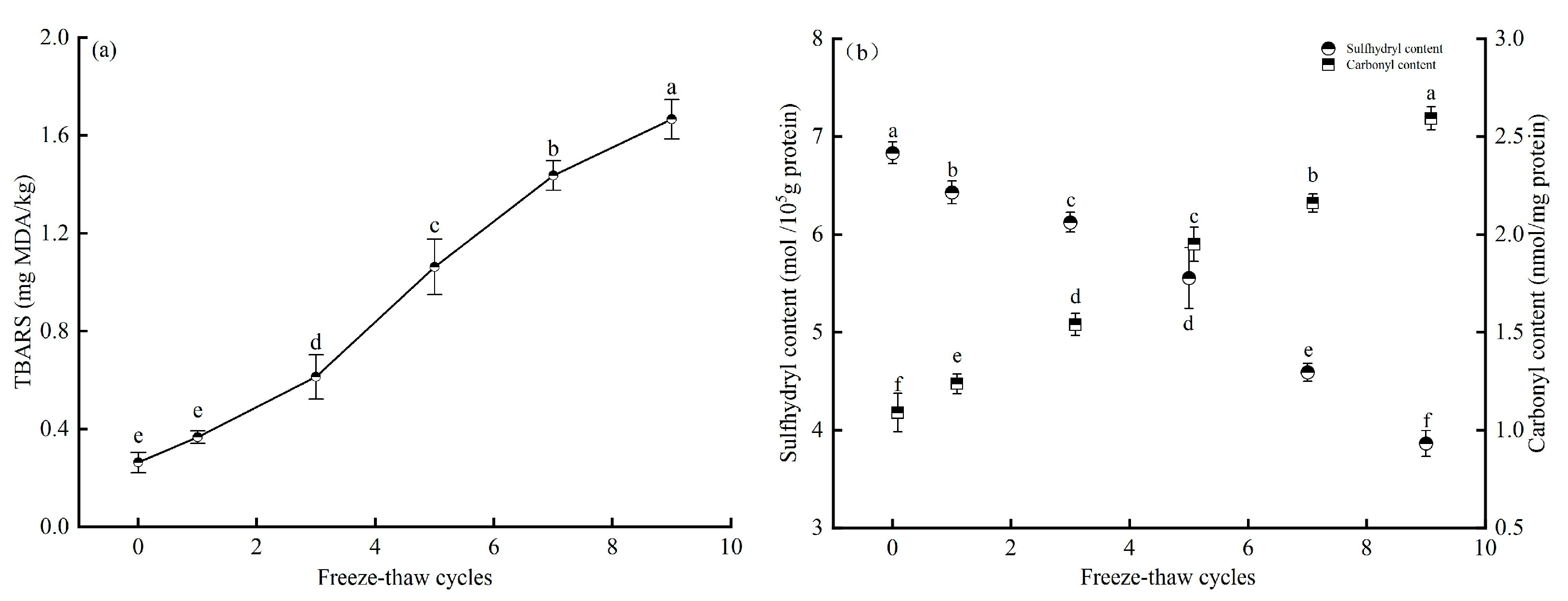
3.3. Effect of Freeze-Thaw Cycles on Lipid and Protein Oxidation
3.4. Effect of Freeze-Thaw Cycles on Tissue Histology
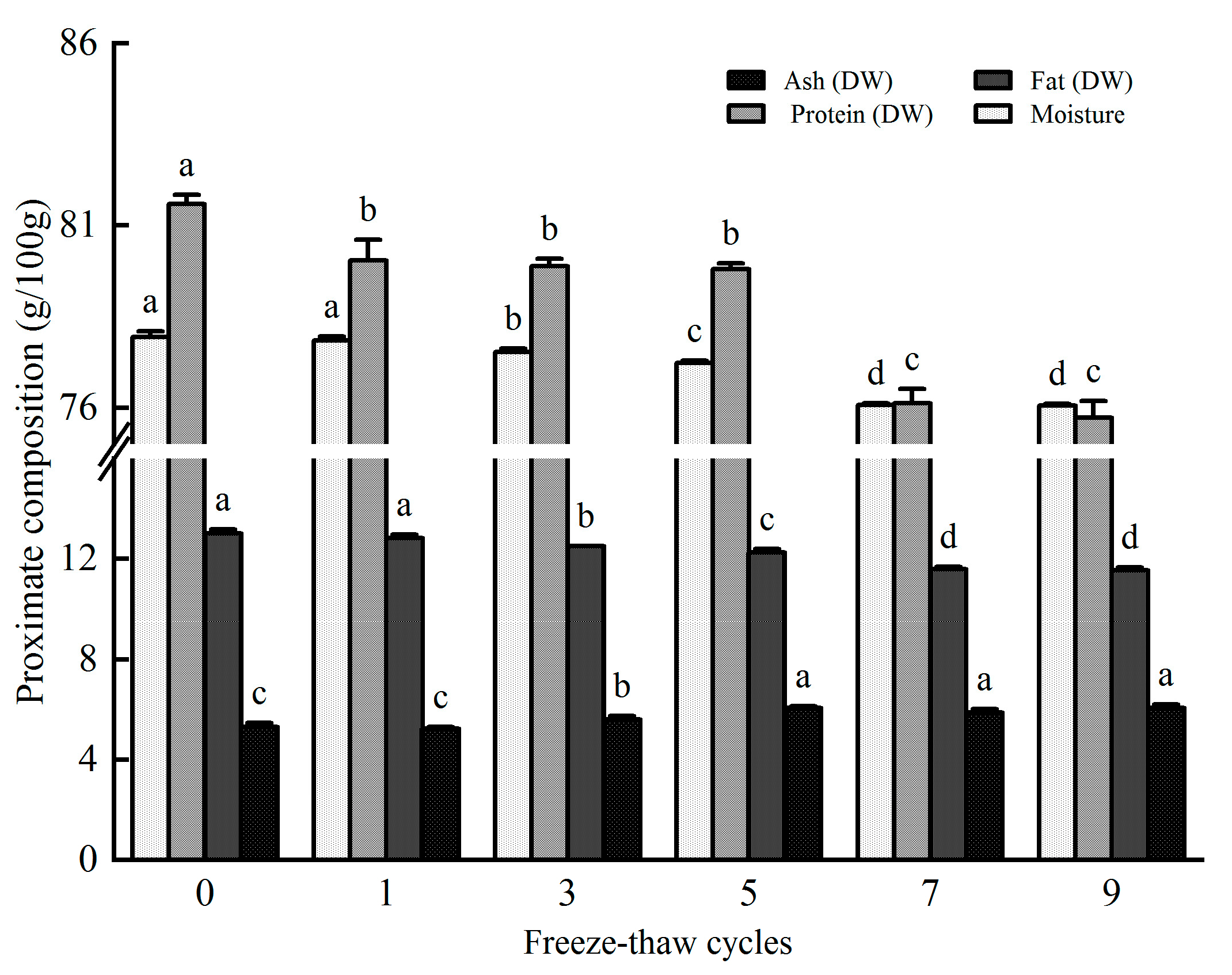
3.5. Effect of Freeze-Thaw Cycles on the Proximate Nutrition Components of Rainbow Trout
3.6. Effect of Freeze-Thaw Cycles on Amino Acid Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Selli, S.; Rannou, C.; Prost, C.; Robin, J.; Serot, T. Characterization of aroma-active compounds in rainbow trout (Oncorhynchus mykiss) eliciting an off-odor. J. Agric. Food Chem. 2006, 54, 9496–9502. [Google Scholar] [CrossRef]
- Rebolé, A.; Velasco, S.; Rodríguez, M.; Treviño, J.; Alzueta, C.; Tejedor, J.; Ortiz, L. Nutrient content in the muscle and skin of fillets from farmed rainbow trout (Oncorhynchus mykiss). Food Chem. 2015, 174, 614–620. [Google Scholar] [CrossRef]
- Molony, B.; Molony, B. Environmental Requirements and Tolerances of Rainbow Trout (Oncorhynchus mykiss) and Brown Trout (Salmo trutta) with Special Reference to Western Australia: A Review; Department of Fisheries, Government of Western Australia: Perth, Australia, 2001. [Google Scholar]
- Sampaio, F.D.; Freire, C.A. An overview of stress physiology of fish transport: Changes in water quality as a function of transport duration. Fish Fish. 2016, 17, 1055–1072. [Google Scholar] [CrossRef]
- Chávez-Mendoza, C.; García-Macías, J.A.; Alarcón-Rojo, A.D.; Ortega-Gutiérrez, J.Á.; Holguín-Licón, C.; Corral-Flores, G. Comparison of fatty acid content of fresh and frozen fillets of rainbow trout (Oncorhynchus mykiss) Walbaum. Braz. Arch. Biol. Technol. 2014, 57, 103–109. [Google Scholar] [CrossRef]
- Lisitsyn, A.B.; Chernukha, I.M.; Lunina, O.I. To the question about meat freezing. review. Theory Pract. Meat Process. 2019, 4, 27–31. [Google Scholar] [CrossRef]
- Nakazawa, N.; Okazaki, E. Recent research on factors influencing the quality of frozen seafood. Fish. Sci. 2020, 86, 231–244. [Google Scholar] [CrossRef]
- Du, X.; Chang, P.; Tian, J.; Kong, B.; Sun, F.; Xia, X. Effect of ice structuring protein on the quality, thermal stability and oxidation of mirror carp (Cyprinus carpio L.) induced by freeze-thaw cycles. LWT 2020, 124, 109140. [Google Scholar] [CrossRef]
- Li, F.; Wang, B.; Kong, B.; Shi, S.; Xia, X. Decreased gelling properties of protein in mirror carp (Cyprinus carpio) are due to protein aggregation and structure deterioration when subjected to freeze-thaw cycles. Food Hydrocoll. 2019, 97, 105223. [Google Scholar] [CrossRef]
- Yasemi, M. Prevention of denaturation of freshwater crayfish muscle subjected to different freeze-thaw cycles by gelatin hydrolysate. Food Chem. 2017, 234, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Prior, B. Measurement of water activity in foods: A review. J. Food Prot. 1979, 42, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Novellino, E.; Armini, V.; Ritieni, A. State of the art of Ready-to-Use Therapeutic Food: A tool for nutraceuticals addition to foodstuff. Food Chem. 2013, 140, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Vilgis, T.A. Soft matter food physics-the physics of food and cooking. Rep. Prog. Phys. 2015, 78, 124602. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.B.; Silva, M.V.D.; Lannes, S.C.D.S. Lipid oxidation in meat: Mechanisms and protective factors-a review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef]
- Qian, S.; Li, X.; Wang, H.; Sun, Z.; Zhang, C.; Guan, W.; Blecker, C. Effect of sub-freezing storage (−6, −9 and −12° C) on quality and shelf life of beef. Int. J. Food Sci. Technol. 2018, 53, 2129–2140. [Google Scholar] [CrossRef]
- Pan, N.; Dong, C.; Du, X.; Kong, B.; Sun, J.; Xia, X. Effect of freeze-thaw cycles on the quality of quick-frozen pork patty with different fat content by consumer assessment and instrument-based detection. Meat Sci. 2021, 172, 108313. [Google Scholar] [CrossRef] [PubMed]
- Sabow, A.B.; Sazili, A.Q.; Aghwan, Z.A.; Zulkifli, I.; Goh, Y.M.; Ab Kadir, M.Z.A.; Nakyinsige, K.; Kaka, U.; Adeyemi, K.D. Changes of microbial spoilage, lipid-protein oxidation and physicochemical properties during post mortem refrigerated storage of goat meat. Anim. Sci. J. 2016, 87, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Kaale, L.D.; Eikevik, T.M. Changes of proteins during superchilled storage of Atlantic salmon muscle (Salmo salar). J. Food Sci. Technol. 2016, 53, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Zhao, Y.; Hu, X.; Zhang, X.; Xie, J. Effects of carrageenan oligosaccharide on lipid, protein oxidative changes, and moisture migration of Litopenaeus vannamei during freeze-thaw cycles. J. Food Process. Preserv. 2020, 44, e14675. [Google Scholar] [CrossRef]
- Hu, C.; Xie, J. The effect of multiple freeze-thaw cycles on protein oxidation and quality of Trachurus murphyi. J. Food Process. Preserv. 2021, 45, e15998. [Google Scholar] [CrossRef]
- Sáez, M.I.; Suárez, M.D.; Cárdenas, S.; Martínez, T.F. Freezing and freezing-thawing cycles on textural and biochemical changes of meagre (Argyrosomus regius, L) fillets during further cold storage. Int. J. Food Prop. 2015, 18, 1635–1647. [Google Scholar] [CrossRef]
- Miyawaki, O. Water and freezing in food. Food Sci. Technol. Res. 2018, 24, 1–21. [Google Scholar] [CrossRef]
- Cao, Y.; He, S.; Yu, Q.; Han, L.; Zhang, W.; Zou, X. Effects of multiple freeze-thaw cycles on meat quality, nutrients, water distribution and microstructure in bovine rumen smooth muscle. Int. J. Food Sci. Technol. 2022, 57, 3001–3011. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Wang, J.; Li, X.; Li, J.; Cui, F.; Yi, S.; Xu, Y.; Zhu, W.; Mi, H. Effects of ultrasound-assisted freezing on the quality of large yellow croaker (Pseudosciaena crocea) subjected to multiple freeze-thaw cycles. Food Chem. 2023, 404, 134530. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Shi, W.; Wang, X.; Wang, Z. Effect of ice water pretreatment on the quality of Pacific White Shrimps (Litopenaeus vannamei). Food Sci. Nutr. 2019, 7, 645–655. [Google Scholar] [CrossRef]
- Jiang, Q.; Nakazawa, N.; Hu, Y.; Osako, K.; Okazaki, E. Changes in quality properties and tissue histology of lightly salted tuna meat subjected to multiple freeze-thaw cycles. Food Chem. 2019, 293, 178–186. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, Y.; Li, Z.; Wang, Y.; Yang, W.; Xue, C. Effects of ozone on the removal of geosmin and the physicochemical properties of fish meat from bighead carp (Hypophthalmichthys nobilis). Innov. Food Sci. Emerg. Technol. 2016, 34, 16–23. [Google Scholar] [CrossRef]
- Soyer, A.; Özalp, B.; Dalmış, Ü.; Bilgin, V. Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem. 2010, 120, 1025–1030. [Google Scholar] [CrossRef]
- Benjakul, S.; Seymour, T.A.; Morrissey, M.T.; An, H. Physicochemical changes in Pacific whiting muscle proteins during iced storage. J. Food Sci. 1997, 62, 729–733. [Google Scholar] [CrossRef]
- Qi, J.; Li, C.; Chen, Y.; Gao, F.; Xu, X.; Zhou, G. Changes in meat quality of ovine longissimus dorsi muscle in response to repeated freeze and thaw. Meat Sci. 2012, 92, 619–626. [Google Scholar] [CrossRef]
- Cheng, H.; Song, S.; Jung, E.-Y.; Jeong, J.-Y.; Joo, S.-T.; Kim, G.-D. Comparison of beef quality influenced by freeze-thawing among different beef cuts having different muscle fiber characteristics. Meat Sci. 2020, 169, 108206. [Google Scholar] [CrossRef]
- Cai, L.; Tong, F.; Tang, T.; Ao, Z.; Wei, Z.; Yang, F.; Shu, Y.; Liu, S.; Mai, K. Comparative evaluation of nutritional value and flavor quality of muscle in triploid and diploid common carp: Application of genetic improvement in fish quality. Aquaculture 2021, 541, 736780. [Google Scholar] [CrossRef]
- Da Silva, M.C.A.; Leite, J.S.A.F.; Barreto, B.G.; dos Anjos Neves, M.V.; Silva, A.S.; de Viveiros, K.M.; Passos, R.S.F.T.; Costa, N.P.; da Silva, R.V.; Cavalheiro, C.P. The impact of innovative gluten-free coatings on the physicochemical, microbiological, and sensory characteristics of fish nuggets. LWT 2021, 137, 110409. [Google Scholar] [CrossRef]
- Ma, X.; Mei, J.; Xie, J. Effects of multi-frequency ultrasound on the freezing rates, quality properties and structural characteristics of cultured large yellow croaker (Larimichthys crocea). Ultrason. Sonochemistry 2021, 76, 105657. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, L.; Shi, J.; Wang, Z.; Luo, Y. Effects of frozen storage on physicochemical characteristics of bighead carp (Aristichthys nobilis) fillets. J. Food Process. Preserv. 2019, 43, e14141. [Google Scholar] [CrossRef]
- Jung, S.; Ghoul, M.; de Lamballerie-Anton, M. Influence of high pressure on the color and microbial quality of beef meat. LWT-Food Sci. Technol. 2003, 36, 625–631. [Google Scholar] [CrossRef]
- Abuelfatah, K.; Zuki, A.; Goh, Y.; Sazili, A. Effects of enriching goat meat with n-3 polyunsaturated fatty acids on meat quality and stability. Small Rumin. Res. 2016, 136, 36–42. [Google Scholar] [CrossRef]
- Xia, X.; Kong, B.; Liu, Q.; Liu, J. Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze-thaw cycles. Meat Sci. 2009, 83, 239–245. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Rosenthal, A. Human oral processing and texture profile analysis parameters: Bridging the gap between the sensory evaluation and the instrumental measurements. J. Texture Stud. 2019, 50, 369–380. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Z.; Wang, S.; Qian, Y.-F. Textural and quality changes of hairtail fillets (Trichiurus haumela) related with water distribution during simulated cold chain logistics. Food Sci. Technol. Int. 2020, 26, 291–299. [Google Scholar] [CrossRef]
- Lan, W.; Hu, X.; Sun, X.; Zhang, X.; Xie, J. Effect of the number of freeze-thaw cycles number on the quality of Pacific white shrimp (Litopenaeus vannamei): An emphasis on moisture migration and microstructure by LF-NMR and SEM. Aquac. Fish. 2020, 5, 193–200. [Google Scholar] [CrossRef]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Oxidative stability of previously frozen ostrich Muscularis iliofibularis packaged under different modified atmospheric conditions. Int. J. Food Sci. Technol. 2011, 46, 1171–1178. [Google Scholar] [CrossRef]
- Shamshad, S.; Riaz, M.; Zuberi, R.; Qadri, R. Shelf life of shrimp (Penaeus merguiensis) stored at different temperatures. J. Food Sci. 1990, 55, 1201–1205. [Google Scholar] [CrossRef]
- Li, D.; Jia, S.; Zhang, L.; Wang, Z.; Pan, J.; Zhu, B.; Luo, Y. Effect of using a high voltage electrostatic field on microbial communities, degradation of adenosine triphosphate, and water loss when thawing lightly-salted, frozen common carp (Cyprinus carpio). J. Food Eng. 2017, 212, 226–233. [Google Scholar] [CrossRef]
- Li, R.; Guo, M.; Liao, E.; Wang, Q.; Peng, L.; Jin, W.; Wang, H. Effects of repeated freezing and thawing on myofibrillar protein and quality characteristics of marinated Enshi black pork. Food Chem. 2022, 378, 131994. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, Y.; Shi, C.; Shen, H. Effect of different thawing methods and multiple freeze-thaw cycles on the quality of common carp (Cyprinus carpio). J. Aquat. Food Prod. Technol. 2015, 24, 153–162. [Google Scholar] [CrossRef]
- Dalvi-Isfahan, M.; Hamdami, N.; Le-Bail, A. Effect of freezing under electrostatic field on the quality of lamb meat. Innov. Food Sci. Emerg. Technol. 2016, 37, 68–73. [Google Scholar] [CrossRef]
- Benjakul, S.; Sutthipan, N. Muscle changes in hard and soft shell crabs during frozen storage. LWT-Food Sci. Technol. 2009, 42, 723–729. [Google Scholar] [CrossRef]
- Hematyar, N.; Rustad, T.; Sampels, S.; Kastrup Dalsgaard, T. Relationship between lipid and protein oxidation in fish. Aquac. Res. 2019, 50, 1393–1403. [Google Scholar] [CrossRef]
- Sante-Lhoutellier, V.; Aubry, L.; Gatellier, P. Effect of oxidation on in vitro digestibility of skeletal muscle myofibrillar proteins. J. Agric. Food Chem. 2007, 55, 5343–5348. [Google Scholar] [CrossRef]
- Jommark, N.; Runglerdkriangkrai, J.; Konno, K.; Ratana-arporn, P. Effect of cryoprotectants on suppression of protein structure deterioration induced by freeze-thaw cycle in Pacific white shrimp. J. Aquat. Food Prod. Technol. 2018, 27, 91–106. [Google Scholar] [CrossRef]
- Thanonkaew, A.; Benjakul, S.; Visessanguan, W.; Decker, E.A. The effect of antioxidants on the quality changes of cuttlefish (Sepia pharaonis) muscle during frozen storage. LWT-Food Sci. Technol. 2008, 41, 161–169. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Gavlighi, H.A.; Xu, X.; Regenstein, J.M. Hydrolysates from rainbow trout (Oncorhynchus mykiss) processing by-products: Properties when added to fish mince with different freeze-thaw cycles. Food Biosci. 2019, 30, 100418. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Gan, X.; Li, H. Interrelationship among ferrous myoglobin, lipid and protein oxidations in rabbit meat during refrigerated and superchilled storage. Meat Sci. 2018, 146, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Baron, C.P.; KjÆrsgård, I.V.; Jessen, F.; Jacobsen, C. Protein and lipid oxidation during frozen storage of rainbow trout (Oncorhynchus mykiss). J. Agric. Food Chem. 2007, 55, 8118–8125. [Google Scholar] [CrossRef]
- Chaijan, M. Lipid and myoglobin oxidations in muscle foods. Songklanakarin J. Sci. Technol. 2008, 30, 47–53. [Google Scholar]
- Tan, M.; Lin, Z.; Zu, Y.; Zhu, B.; Cheng, S. Effect of multiple freeze-thaw cycles on the quality of instant sea cucumber: Emphatically on water status of by LF-NMR and MRI. Food Res. Int. 2018, 109, 65–71. [Google Scholar] [CrossRef]
- Watanabe, M.; Suzuki, T.; Ichimaida, K.; Hattori, T.; Ueda, R. Do consumers actually sense that sashimi made from frozen-thawed fish tastes worse than non-frozen one? Int. J. Refrig. 2020, 111, 94–102. [Google Scholar] [CrossRef]
- Zhang, B.; Hao, G.-J.; Cao, H.-J.; Tang, H.; Zhang, Y.-Y.; Deng, S.-G. The cryoprotectant effect of xylooligosaccharides on denaturation of peeled shrimp (Litopenaeus vannamei) protein during frozen storage. Food Hydrocoll. 2018, 77, 228–237. [Google Scholar] [CrossRef]
- Han, X.Y.; Fan, Z.Y.; Cong, J.J.; Yu, T.T.; Yu, L.Z.; Zhou, F.; Wang, X.C. Changes of Taste Substances in Frozen Tilapia (Oreochromis niloticus) Fillets during Freeze-thaw Cycles. Food Sci. 2022, 43, 269–275. [Google Scholar]
- Romotowska, P.E.; Karlsdóttir, M.G.; Gudjónsdóttir, M.; Kristinsson, H.G.; Arason, S. Influence of feeding state and frozen storage temperature on the lipid stability of Atlantic mackerel (Scomber scombrus). Int. J. Food Sci. Technol. 2016, 51, 1711–1720. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, H.; Shahab, U.; Rehman, S.; Rafi, Z.; Khan, M.Y.; Ansari, A.; Siddiqui, Z.; Ashraf, J.M.; Abdullah, S.M. Protein oxidation: An overview of metabolism of sulphur containing amino acid, cysteine. Front. Biosci. Sch. 2017, 9, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Nurhan, U. Change in proximate, amino acid and fatty acid contents in muscle tissue of rainbow trout (Oncorhynchus mykiss) after cooking. Int. J. Food Sci. Technol. 2007, 42, 1087–1093. [Google Scholar] [CrossRef]
- Hang, Y.; Fu, Y.; Jin, C.; Hua, X. Effects of supplemental amino acids and bile acid in a completely replaced fish meal by enzymatically hydrolysed soybean meal diet on growth performance, liver health and fillet quality of rainbow trout (Oncorhynchus mykiss). Aquac. Res. 2022, 53, 3297–3308. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Zhao, F.; Zhuang, P.; Song, C.; Shi, Z.H.; Zhang, L.Z. Amino acid and fatty acid compositions and nutritional quality of muscle in the pomfret, Pampus punctatissimus. Food Chem. 2010, 118, 224–227. [Google Scholar] [CrossRef]
- Hu, C.; Xie, J. The Effect of Multiple Freeze-Thaw Cycles on the Microstructure and Quality of Trachurus murphyi. Foods 2021, 10, 1350. [Google Scholar] [CrossRef]


| Amino acid | Fresh | F–T 1 | F–T 3 | F–T 5 | F–T 7 | F–T 9 |
|---|---|---|---|---|---|---|
| Threonine | 3.68 ± 0.03 a | 3.73 ± 0.11 a | 3.56 ± 0.06 a | 3.43 ± 0.16 b | 3.13 ± 0.09 c | 2.98 ± 0.12 c |
| Valine | 4.13 ± 0.04 a | 3.84 ± 0.08 b | 3.80 ± 0.07 b | 3.60 ± 0.03 c | 3.57 ± 0.12 c | 3.41 ± 0.10 d |
| Methionine | 2.33 ± 0.03 a | 2.31 ± 0.10 a | 2.13 ± 0.07 b | 2.01 ± 0.08 b | 1.51 ± 0.11 c | 1.44 ± 0.06 c |
| Isoleucine | 3.18 ± 0.07 a | 3.20 ± 0.09 a | 3.15 ± 0.04 a | 3.00 ± 0.12 b | 2.93 ± 0.05 b | 2.89 ± 0.07 b |
| Phenylalanine | 3.35 ± 0.20 a | 3.31 ± 0.11 ab | 3.26 ± 0.11 ab | 3.13 ± 0.04 b | 3.04 ± 0.08 b | 2.98 ± 0.07 b |
| Lysine | 6.87 ± 0.08 a | 6.70 ± 0.15 ab | 6.53 ± 0.11 b | 5.95 ± 0.07 c | 4.31 ± 0.13 d | 4.23 ± 0.09 d |
| Leucine | 6.37 ± 0.07 a | 6.29 ± 0.16 a | 6.04 ± 0.07 b | 5.75 ± 0.05 c | 5.22 ± 0.14 d | 5.07 ± 0.09 d |
| Histidine | 1.83 ± 0.03 a | 1.79 ± 0.08 ab | 1.75 ± 0.06 ab | 1.68 ± 0.06 b | 1.63 ± 0.14 b | 1.59 ± 0.04 b |
| Arginine | 4.54 ± 0.07 a | 4.60 ± 0.13 a | 4.41 ± 0.10 b | 4.29 ± 0.07 bc | 4.17 ± 0.10 c | 3.95 ± 0.06 d |
| EAAs A | 36.28 ± 0.22 a | 35.77 ± 0.60 a | 34.63 ± 0.37 b | 32.75 ± 0.34 c | 29.51 ± 0.59 d | 28.54 ± 0.36 e |
| Aspartic acid | 7.78 ± 0.03 a | 7.82 ± 0.08 a | 7.75 ± 0.06 a | 7.64 ± 0.04 b | 7.58 ± 0.04 bc | 7.51 ± 0.03 c |
| Glutamic acid | 12.95 ± 0.23 a | 12.53 ± 0.11 b | 12.07 ± 0.34 c | 11.83 ± 0.21 cd | 11.60 ± 0.10 d | 11.53 ± 0.15 d |
| Glycine | 3.73 ± 0.09 ab | 3.84 ± 0.04 a | 3.62 ± 0.08 b | 3.45 ± 0.07 bc | 3.31 ± 0.16 c | 3.29 ± 0.14 c |
| Alanine | 4.49 ± 0.09 ab | 4.61 ± 0.09 a | 4.46 ± 0.09 ab | 4.53 ± 0.12 a | 4.45 ± 0.06 ab | 4.34 ± 0.10 b |
| Serine | 3.40 ± 0.04 a | 3.41 ± 0.06 a | 3.39 ± 0.11 a | 3.37 ± 0.11 a | 3.33 ± 0.09 a | 3.32 ± 0.08 a |
| Cystine | 0.59 ± 0.02 a | 0.54 ± 0.05 a | 0.51 ± 0.07 ab | 0.48 ± 0.08 ab | 0.40 ± 0.09 b | 0.37 ± 0.05 b |
| Tyrosine | 2.94 ± 0.05 a | 2.79 ± 0.09 b | 2.70 ± 0.07 b | 2.57 ± 0.05 c | 2.39 ± 0.05 d | 2.21 ± 0.07 e |
| Proline | 1.68 ± 0.09 a | 1.69 ± 0.03 a | 1.65 ± 0.09 a | 1.57 ± 0.13 ab | 1.43 ± 0.09 b | 1.23 ± 0.10 c |
| NEAAs B | 37.56 ± 0.45 a | 37.23 ± 0.23 a | 36.15 ± 0.52 b | 35.45 ± 0.31 c | 34.49 ± 0.32 d | 33.79 ± 0.19 e |
| TAAs C | 73.84 ± 0.60 a | 73.00 ± 0.82 a | 70.78 ± 0.87 b | 68.20 ± 0.39 c | 64.00 ± 0.62 d | 62.33 ± 0.45 e |
| EAAs/TAAs | 0.49 | 0.49 | 0.49 | 0.48 | 0.46 | 0.46 |
| EAAs/NEAAs | 0.97 | 0.96 | 0.96 | 0.92 | 0.86 | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, N.; Sun, Y.; Chen, Z.; Huang, X.; Li, C.; Gao, L.; Bai, S.; Wang, P.; Hao, Q. Effects of Multiple Freeze-Thaw Cycles on Protein and Lipid Oxidation, Microstructure and Quality Characteristics of Rainbow Trout (Oncorhynchus mykiss). Fishes 2023, 8, 108. https://doi.org/10.3390/fishes8020108
Du N, Sun Y, Chen Z, Huang X, Li C, Gao L, Bai S, Wang P, Hao Q. Effects of Multiple Freeze-Thaw Cycles on Protein and Lipid Oxidation, Microstructure and Quality Characteristics of Rainbow Trout (Oncorhynchus mykiss). Fishes. 2023; 8(2):108. https://doi.org/10.3390/fishes8020108
Chicago/Turabian StyleDu, Ningning, Yanchun Sun, Zhongxiang Chen, Xiaoli Huang, Chenhui Li, Lei Gao, Shuyan Bai, Peng Wang, and Qirui Hao. 2023. "Effects of Multiple Freeze-Thaw Cycles on Protein and Lipid Oxidation, Microstructure and Quality Characteristics of Rainbow Trout (Oncorhynchus mykiss)" Fishes 8, no. 2: 108. https://doi.org/10.3390/fishes8020108
APA StyleDu, N., Sun, Y., Chen, Z., Huang, X., Li, C., Gao, L., Bai, S., Wang, P., & Hao, Q. (2023). Effects of Multiple Freeze-Thaw Cycles on Protein and Lipid Oxidation, Microstructure and Quality Characteristics of Rainbow Trout (Oncorhynchus mykiss). Fishes, 8(2), 108. https://doi.org/10.3390/fishes8020108






