Abstract
Firstly, a linoleic and linolenic acid emulsion were incubated with petroleum ether extract, ethyl acetate extract (EAE), ethanol extract and aqueous extract of Ginkgo biloba leaves. The flavonoids content, total antioxidant capacity (T-AOC) and metal-chelating ability (MCA) were determined in EGbs above. Results showed that the extracts of Ginkgo biloba leaves (EGbs) inhibited the lipid oxidation in material above. Of all of EGbs, EAE showed the strongest T-AOC, MCA and protective effects against the lipid oxidation. Next, fish feeds were incubated with graded levels of EAE. The results showed that EAE inhibited lipid oxidation in fish feeds. The optimal inclusion levels of EAE for minimizing lipid oxidation were 4.26 g kg−1 feeds. The effect of EGbs on the lipid oxidation may be closely associated with their flavonoid content. Finally, juvenile carp (14.8 ± 0.4 g) were fed with EAE at concentrations ranging from 0.0 to 6.0 g kg−1 for 60 days. Current data displayed that dietary EAE increased the growth performance of fish. This result of EAE may be ascribed to its enhancing effect on the activity of digestive and absorptive enzymes and antioxidant capacity in digestive organs of fish. Furthermore, dietary EAE decreased the hot-drying-induced lipid oxidation in fish meat through inhibiting the induction effect of hemoglobin in erythrocytes. Our study suggests that EGb can be considered as a potential natural antioxidant for fish and fish feed.
Keywords:
ginkgo leaf; lipid oxidation; fish feedstuff; growth performance; fish meat; natural antioxidant Key Contribution:
A. Extract of ginkgo leaves decreases lipid oxidation in fish feed and meat. B. Extract of ginkgo leaves improves fish growth performance and antioxidant capacity.
1. Introduction
Fish feed contains high amounts of lipids as well as unsaturated fatty acids [1]. In fish feed, under the action of reactive oxygen species (ROS) and excessive redox metal ions, unsaturated fatty acids are easily oxidized to produce oxidation products such as peroxide, malondialdehyde and conjugated diene [2]. On the one hand, fish meat is prone to lipid oxidation due to the presence of high amounts of polyunsaturated fatty acids (PUFAs) [3]. On the other hand, in fish processing, blood is not always removed, retaining a high level of hemoglobin (Hb) in fish meat [4]. ROS can be generated by the auto-oxidation of Hb, which induces heme iron release in erythrocytes and lipid oxidation in fish meat [5]. Meanwhile, lipid oxidation may be harmful to fish meat quality, such as breakdown of nutritional components, generation of toxic metabolites, and degradation of color, aroma and taste, as well as reducing the shelf life [6]. Furthermore, dietary oxidized lipid had a negative effect on fish growth performance as manifested by the decreased weight gain ratio and special growth ratio [7].
Synthetic antioxidants (ethoxyquin, butylated hydroxytoluene and butylated hydroxyanisole) are commonly used in the foods, cosmetics and therapeutic industries for lipid oxidation prevention [6]. Recently, our laboratory reported that ethoxyquin and butylated hydroxytoluene inhibited lipid oxidation of fish feed [8]. However, these synthetic antioxidants can accumulate in tissues and would be toxic and carcinogenic to animals [9], and then are transmitted to humans via the consumption of these animals [6,8]. Based on the detrimental effects on animals and humans, the application of these synthetic antioxidants in the food industry should be under strict regulation [10]. In this sense, natural ingredients may be the promising substitute for synthetic antioxidants [11].
Ginkgo biloba L. (Gb), a Chinese-origin tree, has been cultivated as a gardening plant and used in medicine in China, with leaves that are rich in the main bioactive chemical components, such as terpenes and flavonoids [12]. Tan et al. (2018) reported that extract of Ginkgo biloba leaves (EGb) increased hepatic antioxidant enzyme (superoxide dismutase and catalase) activities and decreased hepatic malonaldehyde content in hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀) [13]. EGb761, a standardized extract of Ginkgo biloba leaves, is an antioxidative natural product [14]. Previous investigations have established that EGb effectively inhibits lipid peroxidation, a capacity demonstrated in both edible and animal feed matrices under in vitro conditions [8]. Given these findings, EGb presents itself as a promising candidate for a natural antioxidant within aquaculture systems.
In China, Jian carp (Cyprinus carpio var. Jian) is the most widely cultivated fish species because it grows faster and has higher meat quality than common carp [15]. This investigation assessed the impact of EGb on the oxidative stability of linoleic acid and linolenic acid emulsions and its effects on the growth performance and antioxidant capacity of Jian carp when incorporated into their diet at varying concentrations over a period of 60 days. The findings offer a foundation for the potential inclusion of EGb as an antioxidant agent in the diets of aquatic species.
2. Materials and Methods
2.1. Chemical Reagent
Reagents such as Tween 20, petroleum ether, ethyl acetate, and ethanol of analytical reagent (AR) grade were sourced from Chengdu Kelong Chemical Reagent Factory, located in Chengdu, China. High-purity linoleic acid and linolenic acid, with purities exceeding 97% and 95%, respectively, were procured from Shanghai Biochemical Reagent Co., Ltd., based in Shanghai, China. The remainder of the chemicals utilized in this study conformed to analytical reagent (AR)-grade standards.
2.2. EGbs Preparation and Composition Analyses
Ginkgo leaves were harvested from Gb in the vicinity of Neijiang Normal University, Neijiang, China, during the month of November. The extraction of EGbs followed methodologies previously established by our research collective [8]. Extracts derived from the leaves using dry petroleum ether, ethyl acetate (EAE), ethanol, and water were stored in darkness within airtight containers at a temperature maintained at −80 °C pending subsequent analysis. The characterization of the EAE was conducted in adherence to analytical protocols delineated in prior publications by our team [16].
2.3. Measurement of Flavonoids Content, Total Antioxidant Capacity and Metal-Chelating Ability
The flavonoids content, total antioxidant capacity and metal-chelating ability of EGbs were determined according to the methods of Zou et al. [17], Cao et al. [18] and Zhao et al. [19], respectively.
2.4. Measurement of Lipid Oxidation in Linoleic Acid and Linolenic Acid Emulsion
The antioxidative impact of EGb on an emulsified mixture of linoleic acids or linolenic acids was evaluated following a slightly adapted procedure from Yuan et al. [20]. An emulsion was constituted by blending 0.1 mL each of linoleic acids or linolenic acids with 9.9 mL of chilled phosphate buffer (with a pH of 7.0 and concentration of 0.02 M) and 50 μL of Tween 20, followed by homogenization on ice using a FJ200-SH homogenizer (Shanghai, China) for two intervals of 10 s at a centrifugal force of 21,000× g. Different concentrations of EGbs, namely 0 (as a control) and 1.0 mg mL−1, were then solubilized into the emulsion. Post an 8-day incubation period at 45 °C, the resultant levels of malondialdehyde, conjugated diene, and peroxide were quantified employing the methodologies previously described by Maqsood and Benjakul [21].
2.5. Measurement of Lipid Oxidation in Fish Feed
Our diet preparation protocol adhered to the established methods within our group [22]. As indicated in Table 1, the fundamental diet’s composition included 34.30% of crude protein and 6.25% crude lipid. This feed underwent treatment employing a method analogous to the emulsification process used for linoleic acids or linolenic acids as previously detailed.

Table 1.
Composition and nutrients content of the basal diet
2.6. Feeding Trial
Juvenile Jian carp were acquired from an aquaculture facility in Neijiang, China, and were subsequently housed under conditions that replicated their natural light environment in our laboratory [22]. Groups of juvenile Jian carp, averaging 14.8 ± 0.4 g, were assigned randomly across 7 treatment sets. Each set comprised 4 tanks, with 20 fish in each tank. Following the formulation techniques delineated by Chen et al. [15], seven distinct experimental diets were produced. Over a course of sixty days, the carp were fed diets augmented with varying concentrations of EAE: specifically, 0.0, 1.0, 2.0, 3.0, 4.0, 5.0, and 6.0 g per kilogram of feed. Vital growth parameters such as survival rate, feed intake, body mass increment, feed conversion efficiency, and specific growth rate were computed in alignment with methodologies established in our preceding research [22].
Consistent with the protocol outlined in our previous work [16], at the experiment’s conclusion, a quintet of fish from each aquarium was subjected to desiccation at 105 °C for 48 h, adapting the technique prescribed by Li et al. [8] with minor adjustments. Subsequent to this drying phase, these specimens were utilized to assess malondialdehyde, conjugated diene, and peroxide levels, employing the analytical methods established by Maqsood and Benjakul [21]. For further investigation, five fish from each tank were exsanguinated and sectioned, followed by preservation at −20 °C for a span of two months. Additionally, an equivalent number of fish were sedated using a benzocaine solution at 50 mg L−1 before being humanely sacrificed via cervical dissection. Post-mortem, vital organs such as the hepatopancreas, intestine and gills were meticulously harvested and subsequently stored at an ultra-low temperature of −80 °C for impending analyses.
In accordance with our established procedures, the activities of several enzymes, including Na+/K+-ATPase, amylase, lipase, trypsin, and alkaline phosphatase, were evaluated. Additionally, we measured the concentrations of hydrogen peroxide, reduced glutathione, and malondialdehyde, as well as the enzymatic activities of glutathione S-transferase, catalase, glutathione peroxidase, superoxide dismutase, and the capacity for anti-hydroxyl radical and anti-superoxide anion action. Blood samples were collected from 15 fish in each experimental group using a heparinized syringe via caudal puncture. Subsequently, these samples were centrifuged at 1000× g for 3 min at 4 °C within one hour of collection. The plasma was then separated from the erythrocytes to assay the aforementioned parameters, aligning with the methods detailed by Li et al. [23]. Within the erythrocyte fraction, we determined the activities of Na+/K+-ATPase, glutamate-pyruvate transaminase, superoxide dismutase, catalase, glutathione reductase, lactate dehydrogenase, and the content of malondialdehyde, reduced glutathione, met-hemoglobin, superoxide anion, and hydrogen peroxide. Furthermore, we quantified the activities of glutamate-oxaloacetate transaminase and glutamate-pyruvate transaminase in the plasma, and the plasma’s ammonia levels.
2.7. Biochemical Analysis
Enzymatic assays were conducted to evaluate the activities of Na+/K+-ATPase, lipase, alkaline phosphatase, amylase, and trypsin, as well as the transaminases glutamate-oxaloacetate and glutamate-pyruvate, replicating methods from our preceding research [15]. Determinations of the anti-superoxide anion capacity, hydrogen peroxide concentration, plasma ammonia levels, and the presence of glutathione and malondialdehyde, in conjunction with assays for superoxide dismutase, catalase, glutathione peroxidase, lactate dehydrogenase, and glutathione reductase, were carried out in alignment with procedures reported by Chen et al. [22]. Measurement protocols for superoxide anion and met-hemoglobin levels adhered to those established by Li et al. [24]. Moreover, the activities of glutathione S-transferase and the anti-hydroxyl radical capacity were quantified following the techniques detailed by Jiang et al. [25].
2.8. Statistical Analysis
Results are presented as the means ± S.D. Statistical analyses were performed using a one-way analysis of variance (ANOVA) with the software SPSS, version 15.0. Post hoc comparisons to determine significant differences among groups were made using Duncan’s multiple range tests.
3. Results
3.1. Flavonoids Content, Total Antioxidant Capacity and Metal-Chelating Ability in EGbs
As shown in Table 2, the flavonoids content was significantly higher in EAE than other extracts (p < 0.05). Flavonoids content of petroleum ether extract of Ginkgo biloba leaves was significantly lower than other extracts (p < 0.05). Flavonoids content of ethanol extract of Ginkgo biloba leaves was higher than aqueous extract (p < 0.05). The total antioxidant capacity was significantly higher in EAE of Ginkgo biloba leaves than other extracts (p < 0.05), followed by ethanol, aqueous and petroleum ether extract. A similar trend was found in metal-chelating ability when treated with different EGbs. Power regression of flavonoids content in EGbs on total antioxidant capacity and metal-chelating ability were presented in Figure 1. The total antioxidant capacity and metal-chelating ability were positively correlated with the flavonoid contents of EGbs.

Table 2.
The flavonoids content, total antioxidant capacity (T-AOC) and metal-chelating ability (MCA) of petroleum ether extract (PEE), ethyl acetate extract (EAE), ethanol extract (EE) and aqueous extract (AQE) of Ginkgo biloba leaves.
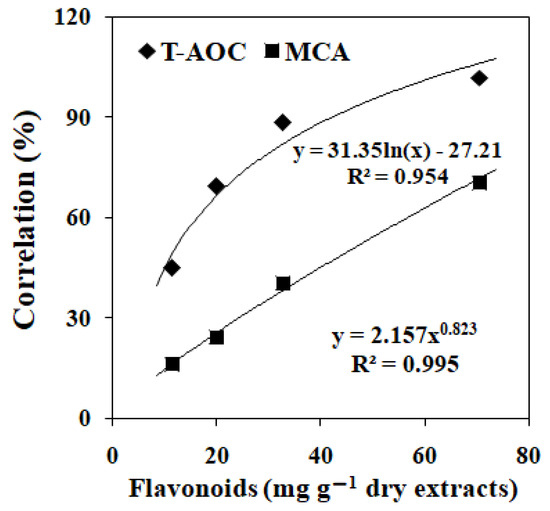
Figure 1.
The correlations of flavonoids content with the total antioxidant capacity (T-AOC) and metal-chelating ability (MCA) in the extracts of Ginkgo biloba leaves. Values are the means of three replicates.
3.2. Effects of EGbs on the Lipid Oxidation in Linoleic Acid and Linolenic Acid
The relationship between EGbs and the peroxide, conjugated diene and malonaldehyde levels in a linoleic acid emulsion was shown in Table 3. The peroxide level was lower when treated with EAE, followed by ethanol, aqueous and petroleum ether extract (p < 0.05). The conjugated diene content of EAE was significantly lower than other extracts (p < 0.05). The conjugated diene content of ethanol extract was lower than aqueous and petroleum ether extract (p < 0.05). A similar trend was found in malonaldehyde level when treated with different EGb. Of the examined compounds, the levels of peroxide, conjugated diene and malonaldehyde were higher when treated with aqueous and petroleum ether extract, suggesting that these two extracts had the lowest inhibitory effects on the oxidation of linoleic acid (p < 0.05).

Table 3.
The peroxide (PO), conjugated diene (CD) and malonaldehyde (MDA) levels in a linoleic acid emulsion treated with 1.0 mg mL−1 of petroleum ether extract (PEE), ethyl acetate extract (EAE), ethanol extract (EE) and aqueous extract (AQE) of Ginkgo biloba leaves.
The relationship between EGbs and the peroxide, conjugated diene and malonaldehyde levels in a linolenic acid emulsion was shown in Table 4. The peroxide level was significantly lower when treated with EAE, followed by ethanol, aqueous and petroleum ether extract (p < 0.05). The conjugated diene level was lower when treated with EAE (p < 0.05), followed by ethanol and aqueous extract. The level of conjugated diene was higher when treated with petroleum ether extract. The malonaldehyde level of EAE was significantly lower than other extracts (p < 0.05). The malonaldehyde level of ethanol extract was lower than aqueous and petroleum ether extract (p < 0.05). Of the examined compounds, the levels of peroxide, conjugated diene and malonaldehyde were higher when treated with petroleum ether extract, suggesting the petroleum ether extract had the lowest inhibitory effects on the oxidation of linolenic acid (p < 0.05).

Table 4.
The peroxide (PO), conjugated diene (CD) and malonaldehyde (MDA) levels in a linolenic acid emulsion treated with 1.0 mg mL−1 of petroleum ether extracts (PEE), ethyl acetate extracts (EAE), ethanol extract (EE) and aqueous extract (AQE) of Ginkgo biloba leaves.
The correlations of flavonoids content in EGb on peroxide, conjugated diene and malonaldehyde levels in linoleic acid and linolenic acid emulsion were presented in Figure 2. The peroxide, conjugated diene and malonaldehyde levels were negatively correlated with the flavonoids contents of EGbs.
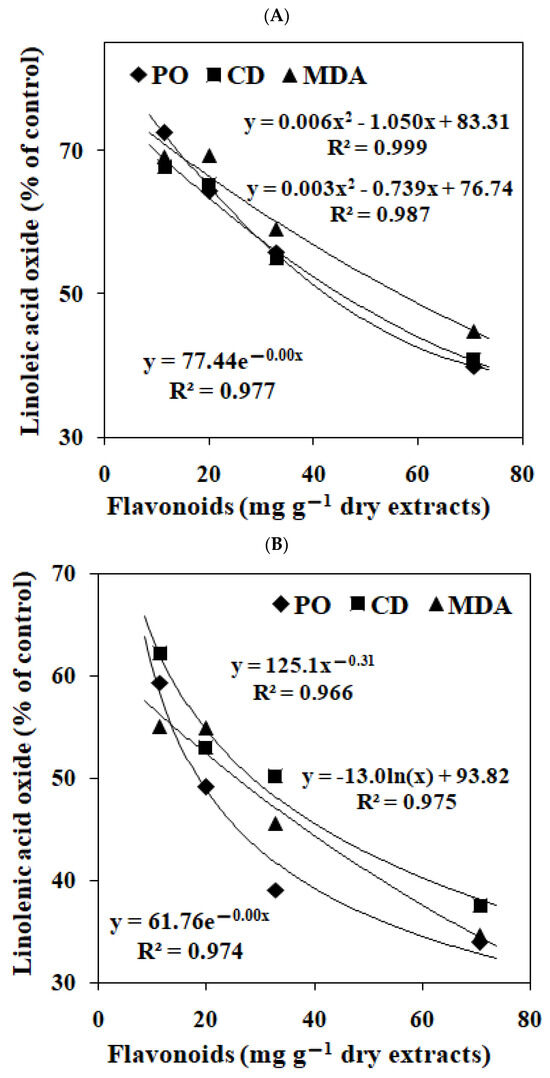
Figure 2.
The correlations of flavonoids content in the extracts of Ginkgo biloba leaves with the levels of peroxide (PO), conjugated diene (CD) and malonaldehyde (MDA) in linoleic acid emulsion (A) and linolenic acid emulsion (B). The data represent the means of three replicates.
3.3. The EAE Composition
As showed in Figure 3 and Table 5, there are some components in EAE, including 1,5,6,7-tetramethylbicyclo[3.2.0]hept-6-en-3-one, 1,4-cyclohexadiene, 3,3,6,6-tetramethyl-, acetic acid 3,7,11,15-tetramethyl-2-hexadecenyl and 1,2-dihexylcyclopropene.
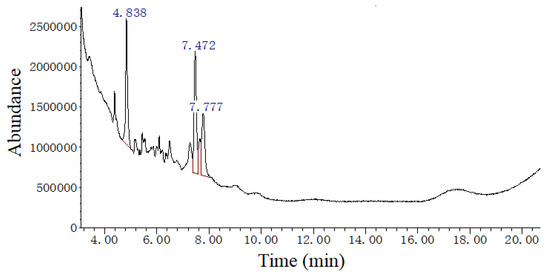
Figure 3.
The gas chromatogram of ethyl acetate extract of Ginkgo biloba leaves (EAE). This experiment was repeated three times with similar results achieved.

Table 5.
The composition analyses of ethyl acetate extract of Ginkgo biloba leaves (EAE) by gas chromatograph–mass spectrometry (GC–MS).
3.4. The Effects of EAE on the Lipid Oxidation in Fish Feed
Figure 4 illustrates the impact of escalating levels of EAE in the diet on the quantities of peroxides, conjugated dienes, and malonaldehyde within the fish feed. A significant reduction in these compounds was observed concomitant with increased EAE inclusion in the diet up to 4.0 g kg−1, as evidenced by p-values less than 0.05. This declining trend plateaued when EAE concentrations in the diet exceeded this amount, indicated by p-values greater than 0.05. The broken line analysis suggested that the optimal inclusion levels of EAE for minimizing peroxides, conjugated dienes, and malonaldehyde were 4.26, 4.18, and 4.22 g kg−1 of the diet, respectively.
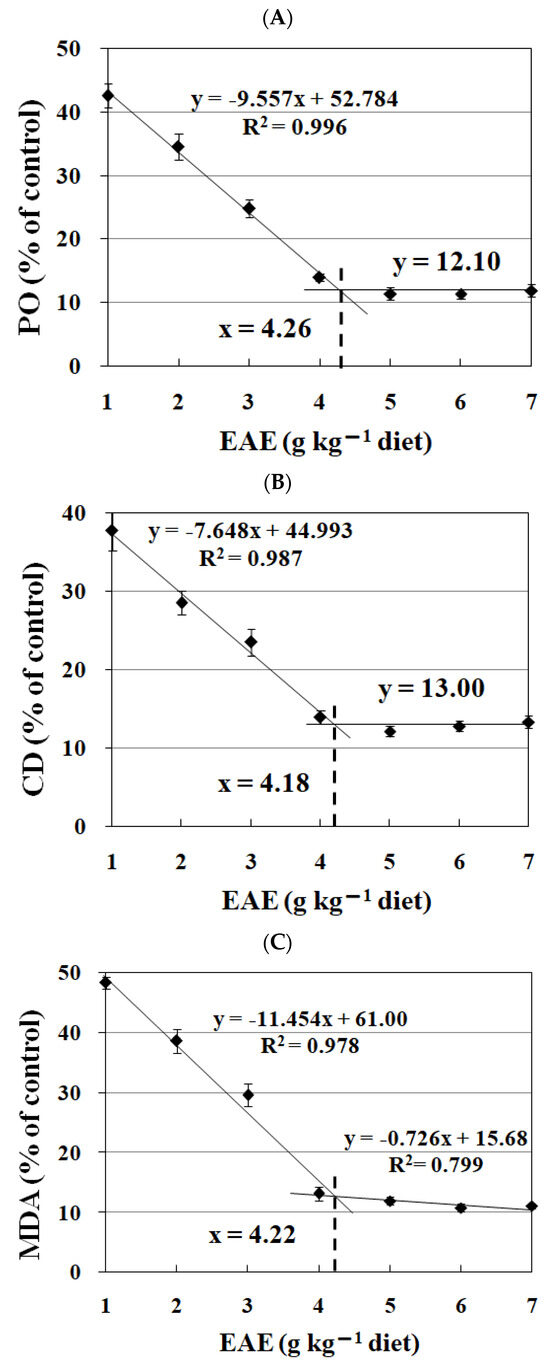
Figure 4.
Broken-line analysis of peroxide (PO, (A)), conjugated diene (CD, (B)) and malondialdehyde (MDA, (C)) levels in fish feeds treated with graded levels of ethyl acetate extract (EAE) of Ginkgo biloba leaves. Values are the means ± S.D. of three replicates.
3.5. Effects of Dietary EAE on Fish Growth Performance
Table 6 delineates the lack of significant alterations in the fish feed efficiency and survival rates upon varying the levels of EAE in the diet (with p-values exceeding 0.05). Incremental increases in dietary EAE up to 2.0 g kg−1 were associated with a notable enhancement in weight gain, which reached a threshold as p-values exceeded 0.05 beyond this concentration. Subsequent increases to 4.0 g kg−1 did not yield further benefits and, in fact, led to a diminution in weight gain with higher EAE levels. Analogous patterns were observed regarding final body weight and specific growth rate, with both metrics experiencing similar trends. Feed intake exhibited an increase with rising dietary EAE levels up to 1.0 g kg−1, reached a steady state with continued elevation to 4.0 g kg−1 (p-values again surpassing 0.05), and subsequently decreased significantly when dietary EAE levels were augmented further (p-values falling below 0.05).

Table 6.
Initial body weight (IBW), final body weight (FBW), weight gain (WG), specific growth rate (SGR), feed intake (FI), feed efficiency (FE) and survival ratio (SR) of Jian carp fed diets containing graded levels of ethyl acetate extract of Ginkgo biloba leaves (EAE) for 60 days.
Polynomial regression analysis pinpointed the optimal dietary EAE supplementation for Jian carp at 2.75 g kg−1 of the diet, as extrapolated from weight gain data (refer to Figure 5).
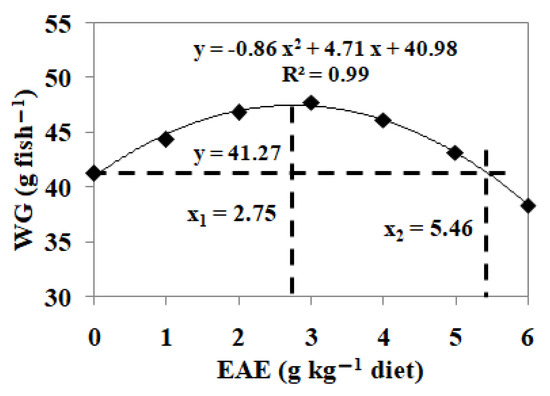
Figure 5.
Polynomial regression analysis of weight gain (WG) for Jian carp fed diets containing graded levels of ethyl acetate extract (EAE) of Ginkgo biloba leaves for 60 days. Values are the means of four replicates, with 20 fish in each replicate.
3.6. Effects of Dietary EAE on the Biochemical Parameters in Hepatopancreas of Jian Carp
Table 7 presents a correlation between the elevation of EAE in the diet and enzymatic activities. It was noted that trypsin activity witnessed a significant rise with increments in EAE up to 2.0 g kg−1 of the diet, indicated by a p-value less than 0.05, followed by a decline upon further elevation in EAE. Enzymes lipase and amylase similarly showed significant increases in their activities at EAE concentrations of 4.0 and 2.0 g kg−1 of the diet, respectively, with p-values below 0.05. No significant changes in their activities were observed once the concentrations of EAE exceeded these levels, as the p-values went above 0.05. Enhanced activity levels of antioxidant enzymes such as anti-superoxide anion, anti-hydroxyl radical, superoxide dismutase, catalase, and glutathione peroxidase were recorded at 4.0, 3.0, 3.0, 4.0, and 4.0 g kg−1 EAE in the diet, respectively. In contrast, the malonaldehyde concentration in the hepatopancreas was lower when the diet was supplemented with 4.0 g kg−1 EAE. Additionally, a decrease in hydrogen peroxide was observed with increasing dietary EAE up to 1.0 g kg−1 (p < 0.05), with levels stabilizing with further increments (p > 0.05). The reduced glutathione level showed a significant upsurge with increased dietary EAE up to 3.0 g kg−1 (p < 0.05), but further increases in EAE resulted in a significant reduction in its concentration (p < 0.05).

Table 7.
The activities of trypsin, lipase, amylase, anti-superoxide anion (ASA), anti-hydroxyl radical (AHR), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione S-transferase (GST) and the levels of hydrogen peroxide (H2O2), malondialdehyde (MDA) and reduced glutathione (GSH) in hepatopancreas of Jian carp fed diets containing different levels of ethyl acetate extract of Ginkgo biloba leaves (EAE) for 60 days.
3.7. Effects of Dietary EAE on the Biochemical Parameters in Intestine of Jian Carp
According to the data in Table 8, it was observed that dietary incorporation of EAE influenced several enzymatic activities in the intestinal tissue of fish. Specifically, trypsin and amylase activity levels were augmented in correlation with EAE increments in the diet up to a threshold of 1.0 g kg−1, as indicated by a p-value less than 0.05. These enzymatic activities reached a stable state when the EAE concentration in the diet was enhanced to between 5.0 g kg−1 and 3.0 g kg−1, after which a p-value greater than 0.05 was noted and subsequent declines in activity were observed with an increased EAE concentration (p < 0.05). The anti-superoxide anion capacity and glutathione S-transferase activity followed a similar pattern, showing elevated activity up to EAE concentrations of 1.0 g kg−1 and 2.0 g kg−1, respectively, before stabilizing. Superoxide dismutase activity increased with EAE up to 1.0 g kg−1, plateaued up to a dietary content of 3.0 g kg−1, and then decreased. Catalase activity showed significant enhancement with EAE levels reaching 2.0 g kg−1, which then plateaued with no significant changes upon further increase in EAE (p > 0.05). Additionally, Na+/K+-ATPase, alkaline phosphatase, and glutathione peroxidase activities were positively influenced at EAE supplementation levels of 2.0, 3.0, and 4.0 g kg−1 of the diet, respectively. A notable increase in glutathione content was observed with EAE supplementation at 6.0 g kg−1 of the diet. Hydrogen peroxide concentrations in the fish intestine decreased concomitant with EAE levels up to 1.0 g kg−1 of the diet (p < 0.05) and remained constant up to an EAE content of 5.0 g kg−1 (p > 0.05), beyond which an increase was recorded.

Table 8.
The activities of trypsin, amylase, Na+/K+-ATPase, alkaline phosphatase (AKP), antisuperoxide anion (ASA), glutathione S-transferase (GST), superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) and the content of hydrogen peroxide (H2O2), malondialdehyde (MDA) and reduced glutathione (GSH) in intestine of Jian carp fed diets containing different levels of ethyl acetate extract of Ginkgo biloba leaves (EAE) for 60 days.
3.8. Effects of EAE on the Biochemical Parameters in Gills of Jian Carp
In the investigation detailed by Table 9, the anti-superoxide anion capability in the gill tissue of fish was enhanced when their feed was supplemented with 4.0 g kg−1 of EAE. Concurrently, there was a noted decrease in malondialdehyde levels with the elevation of EAE in the diet up to 2.0 g kg−1, marked by a statistically significant threshold (p < 0.05). This downward trend in malondialdehyde content reached a plateau with no notable differences at EAE concentrations of up to 4.0 g kg−1, and an upsurge in levels occurred with further increments in EAE. Regarding antioxidative enzymatic activities and associated compounds in fish gills, this study found an uptick in the presence of superoxide dismutase, catalase, and glutathione peroxidase, with their activities peaking when the diet contained 3.0, 4.0, and 3.0 g kg−1 of EAE, respectively. The reduced glutathione content was observed to increase in diets containing 2.0 g kg−1 of EAE. Furthermore, glutathione S-transferase activity rose in tandem with higher dietary EAE supplementation, with the most substantial activities recorded at EAE concentrations of 5.0 and 6.0 g kg−1.

Table 9.
The level of malondialdehyde (MDA) and reduced glutathione (GSH) and the activities of antisuperoxide anion (ASA), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione S-transferase (GST) in gills of Jian carp fed diets containing different levels of ethyl acetate extract of Ginkgo biloba leaves (EAE) for 60 days.
3.9. Effects of EAE on the Biochemical Parameters in Plasma and Erythrocytes of Jian Carp
Table 10 reveals that the activities of both glutamate-oxaloacetate transaminase and glutamate-pyruvate transaminase in plasma showed a significant reduction as the levels of EAE in the diet were increased (p < 0.05). This trend persisted until reaching a dietary EAE concentration of 3.0 g kg−1, beyond which no significant changes were observed, with activities rising upon further EAE enrichment. Additionally, plasma ammonia concentrations were observed to be lower in fish whose diet included an EAE supplement of 6.0 g kg−1.

Table 10.
The activities glutamate-oxaloacetate transaminase (GOT) and glutamate-pyruvate transaminase (GPT) in plasma and plasma ammonia content (PAC) of Jian carp fed diets containing different levels of ethyl acetate extract of Ginkgo biloba leaves (EAE) for 60 days.
Table 11 illustrates an increase in the activities of Na+/K+-ATPase in fish erythrocytes correlating with a rise in dietary EAE up to 2.0 g kg−1 (p < 0.05), with a subsequent decline noted with additional EAE inclusion. The activities of glutamate-pyruvate transaminase escalated with an increase in EAE up to 5.0 g kg−1, and continued to rise with further EAE supplementation. Erythrocytes exhibited lower lactate dehydrogenase activity when the fish diet was supplemented with 2.0 g kg−1 of EAE. Moreover, the levels of met-hemoglobin, superoxide anion, and hydrogen peroxide in fish erythrocytes were reduced in diets supplemented with EAE at 4.0, 4.0, and 3.0 g kg−1, respectively. Malondialdehyde content showed a significant decrease in conjunction with elevated dietary EAE up to 2.0 g kg−1 (p < 0.05), with a stabilization of levels observed upon reaching an EAE concentration of 3.0 g kg−1, followed by an increase with higher EAE amounts. The activities of superoxide dismutase and catalase, along with the reduced glutathione levels in fish erythrocytes, were higher in diets containing EAE at 3.0, 4.0, and 6.0 g kg−1, respectively. Glutathione reductase activity was enhanced with increased dietary EAE up to 3.0 g kg−1 (p < 0.05), plateauing with subsequent EAE increments.

Table 11.
The activities of Na+/K+-ATPase, glutamate-pyruvate transaminase (GPT) and lactate dehydrogenase (LDH) in erythrocytes, superoxide dismutase (SOD), catalase (CAT) and glutathione reductase (GR) and the content of met-hemoglobin (Met-Hb), superoxide anion (O2•–), hydrogen peroxide (H2O2), malondialdehyde (MDA) and reduced glutathione (GSH) in erythrocytes of Jian carp fed diets containing different levels of ethyl acetate extract of Ginkgo biloba leaves (EAE) for 60 days.
3.10. Effects of EAE on the Biochemical Parameters in Meat of Jian Carp
Upon examination of Table 12, it was observed that the concentration of hydrogen peroxide and malondialdehyde in fish tissues diminished as dietary EAE was augmented to 4.0 g kg−1 and 3.0 g kg−1, respectively. Concurrently, there was a significant elevation in the capability to neutralize hydroxy radicals, the activity of glutathione S-transferase, and the reduced glutathione concentration when the diet was supplemented with EAE up to 4.0, 4.0, and 3.0 g kg−1, respectively (p < 0.05). However, these metrics saw a decline when the levels of dietary EAE were increased beyond these points. Additionally, the catalase activity in fish meat showed a progressive increment correlating with higher dietary EAE concentrations.

Table 12.
The level of hydrogen peroxide (H2O2), malondialdehyde (MDA) and reduced glutathione (GSH) and the activities of anti-hydroxy radical (AHR), catalase (CAT) and glutathione S-transferase (GST) in the meat of Jian carp fed diets containing different levels of ethyl acetate extract of Ginkgo biloba leaves (EAE) for 60 days.
3.11. Effects of Dietary EAE on the Hot-Drying-Induced Lipid Oxidation in Meat of Jian Carp
Figure 6 illustrates the impact of varying EAE supplementation on peroxide, conjugated diene, and malondialdehyde levels within piscine muscle tissues. A reduction in peroxide concentration was observed with EAE inclusion in the diet up to 4.0 g kg−1, followed by an ascent upon further elevation of EAE levels. This pattern was also mirrored in the measurements of conjugated diene and malondialdehyde. Regressive analysis deduced that the optimal inclusion rates of dietary EAE, calculated from the concentrations of peroxide, conjugated diene, and malondialdehyde in fish tissues, were 3.72 g kg−1, 3.05 g kg−1, and 3.73 g kg−1, respectively.
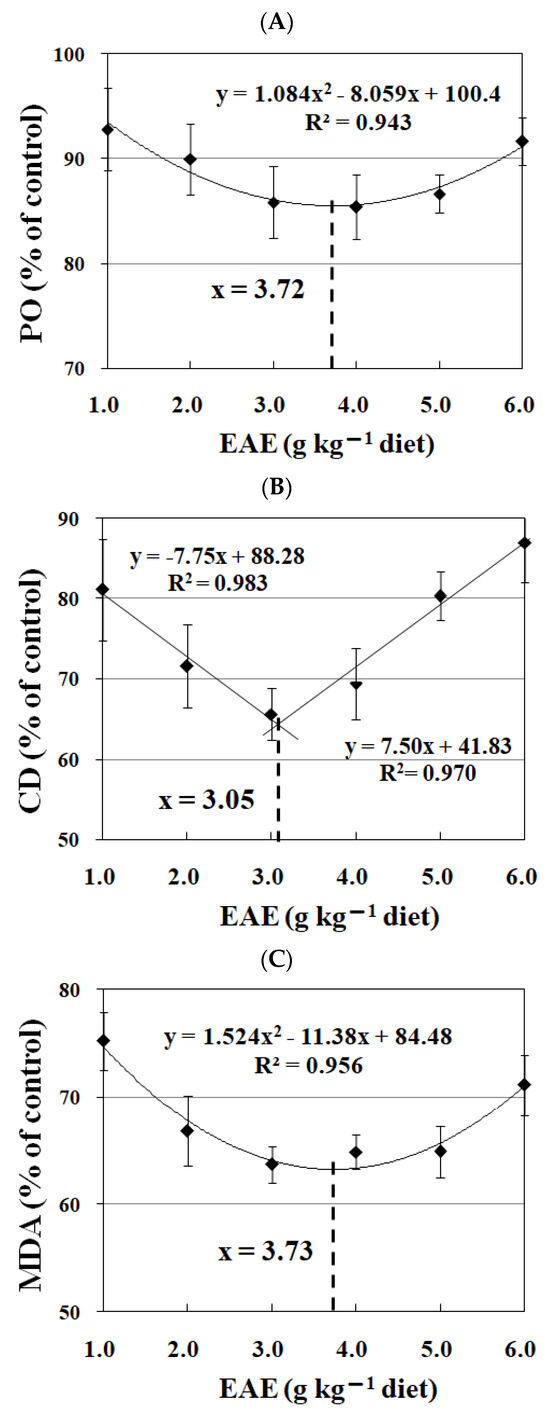
Figure 6.
Regressive analysis of peroxide (PO, (A)), conjugated diene (CD, (B)) and malondialdehyde (MDA, (C)) levels in fish fed diets containing graded levels of ethyl acetate extract (EAE) of Ginkgo biloba leaves, following drying at 105 ℃ for 48 h. Values are the means ± SD of four replicates.
4. Discussion
4.1. EGb Inhibited the Lipid Oxidation in Fish Feed
Lipid oxidation is a common problem in foods and feeds [8]. In this study, EAE reduced peroxide, conjugated diene and malondialdehyde levels in fish feed. Similarly, malondialdehyde level was relieved in fish erythrocytes by EAE in vitro [8]. The investigation revealed that EGb could potentially serve as an inhibitor of lipid oxidation within aqua feed. By employing a broken-line model for peroxide, conjugated diene, and malondialdehyde, it was ascertained that the optimal levels of EAE supplementation in aqua feed are 4.26 g kg−1. Lipid oxidation within aqua feed is likely linked to the presence of unsaturated fatty acids. Peroxides and hydro-peroxides are known as the principal products in the initial phase of lipid oxidation in unsaturated fatty acids [26]. The molecular architecture of hydro-peroxides, characterized by conjugated dienes, arises via the resonance of methylene-interrupted double bonds in unsaturated fatty acids, upon the assault of free radicals [27]. Consequently, peroxide and conjugated diene are acknowledged as precise indicators of early oxidation [28]. In the subsequent phase, peroxides and hydro-peroxides undergo reactions with oxygen or redox-active metals, culminating in the formation of malondialdehyde [29]. Essential unsaturated fatty acids like linolenic acid (18:3n − 3) and linoleic acid (18:2n − 6) are vital in freshwater fish nutrition [30]. Our study noted that EGbs diminishes the levels of peroxide, conjugated diene, and malondialdehyde within linoleic and linolenic acid emulsions, indicating its capability to mitigate lipid oxidation in aqua feed by disrupting both the initiation and propagation stages of the lipid oxidation process. Out of all extracts tested, EAE was identified as the most efficacious in attenuating lipid oxidation in these fatty acid emulsions. This aligns with prior observations where EAE inhibited lipid oxidation, verified via thiocyanate and thiobarbituric acid reactive substance assays [8].
ROS and redox metal ions play an important role in lipid oxidation of unsaturated fatty acids [8]. The total antioxidant capacity is an indicator of ROS scavenging activity, which reflects the overall antioxidative capability in hydrophilic or lipophilic substances [31,32]. Lipid oxidation is propagated under the catalytic actions of iron and other redox metal ions [8]. In this study, EGb displayed the strong metal chelation capabilities and total antioxidant capacity that was determined by ABTS assay and similar to trolox. Previous studies in our laboratory reported that EGb decreased ROS production in fish erythrocytes caused by hydroxyl radical [8]. Moreover, study in vitro showed that EGb can act as primary and/or secondary antioxidants, and free radical scavengers in prevention of lipid oxidation [33]. The current finding is consistent with the reports mentioned above. These results suggested that EGb may prevent unsaturated fatty acids of fish feed from undergoing lipid oxidation through enhancing metal inactivation and free radical scavenging activity.
The beneficial effects of EGbs on lipid oxidation in fish feed may be closely associated with their chemical constituents. The flavonoids content of Ginkgo biloba leaves is concerned with total antioxidant activity, which was determined by Near-infrared spectroscopy method [34]. In vitro, the ethyl acetate extract and methanol extract from Ginkgo biloba leaves evidenced a marked radical scavenging activity, which was based on the metal-chelating activity of flavonoids [35]. Within the ambit of radical-scavenging mechanisms, flavonoids are equipped with intramolecular hydrogen bonds that serve to enhance the antioxidative efficacy of hydroxyl functionalities, wherein the hydroxyl groups act as receptors of hydrogen bonds, notably at the 4′-OH position [36]. In addition, flavonoids compounds were showed to protect the polyunsaturated fatty acid from oxidation [37]. The hydroxyl groups in flavonoids compound are the crucial structure for antioxidant capacity, which could not only provide hydrogen atoms to lipid radicals, but also generate stable lipid derivatives and antioxidant radicals [38]. In the present study, not only the close correlations of flavonoids content in EGbs with the levels of peroxide, conjugated diene and malonaldehyde in linoleic acid and linolenic acid, but also with their total antioxidant capacity and the metal-chelating activity were observed. This is in good agreement with the report that there are four possible antioxidant mechanisms for flavonoids, namely inhibiting free radical generation of related enzymes, chelating metals, quenching free radical elements as well as stimulating the internal antioxidant enzymes [39]. These results confirmed that the antioxidant activity of EGb in fish feed might be caused by the flavonoids compounds.
4.2. Dietary EAE Supplementation Improved Fish Growth Performance
In piscine mitochondria, the presence of aminotransferases such as glutamate-oxaloacetate transaminase and glutamate-pyruvate transaminase is notable, given their extensive distribution across various tissues including the liver, muscle, heart, and kidney [40]. Tissue cell damage triggers the release of these enzymes into the bloodstream [41], rendering the levels of glutamate-oxaloacetate transaminase and glutamate-pyruvate transaminase in plasma reliable biomarkers for assessing fish health [42]. Findings from the current investigation indicate that inclusion of dietary EAE has led to a reduction in the activity of both aminotransferases in fish plasma, implying that EAE in the diet could potentially bolster fish health by mitigating liver damage. The positive impacts observed from EGb supplementation on piscine well-being may be attributable to its flavonoid content [43]. Nonetheless, elucidation of the precise mechanisms by which EGb influences fish health warrants additional research.
In the present study, dietary EAE increased feed intake, weight gain and specific growth rate, which indicates that dietary EGb improves Jian carp growth performance. Based on polynomial regression analysis of weight gain, the optimum dietary EAE for Jian carp was 2.75 g kg−1 diet. Similarly, these results were consistent with the report on Nile tilapia (Oreochromis niloticus) [44]. Similar observations have been reported in Penaeus vannamei and hybrid grouper [13,45]. Furthermore, EGb can improve energy metabolism in rats, partly because it might increase the expression of ghrelin, which was an endogenous ligand of the growth hormone [46,47]. However, to date few studies have investigated the growth-promoting effect of EGb on fish.
The growth performance of fish is closely related to the digestion of nutrients, which has been attributed to the levels of digestive enzymes activities [15]. Digestive enzymes (amylase, trypsin, chymotrypsin and lipase) are synthesized in fish exocrine pancreas and secreted into the intestine [48,49]. In this study, dietary EAE increased activities of trypsin, lipase and amylase, indicates that dietary EAE supplementation improved fish digestive ability. Meanwhile, the growth performance of fish is also closely related to the absorption of nutrients, which has been attributed to the levels of brush-border membrane enzymes activities [22]. Na+/K+-ATPase is able to create potential energy of Na gradient to transport nutrients into cells, such as glucose, phosphate and amino acids [50]. Alkaline phosphatase, employed to be a general marker of fish absorption capacity, is an important enzyme in absorption of food molecules [51]. In this study, dietary EAE increased activities of alkaline phosphatase and Na+/K+-ATPase, which indicates that dietary EGb improved fish absorptive ability. This is in line with the reports that the intestinal length is increased in Nile tilapia after feeding EGb [44].
The structure and function of tissues and organs in aquatic animals are largely determined by their antioxidant status [52]. The integrity of intestinal morphology is the foundation for the digestive and absorptive process for fish [15]. ROS, which are produced as a result of aerobic metabolism and exposure to exogenous agents, have been shown to be damaging to lipids and proteins [53]. Malonaldehyde is used as a sensitive biomarker for lipid peroxidation [52]. In this study, dietary EAE decreased malonaldehyde content in hepatopancreas and intestine, which indicates that dietary EGb decreases lipid peroxidation in fish digestive and absorptive organs. These results were in conformity to the report that short-term oral administration of Ginkgo biloba extract reduces malonaldehyde level in washed platelets of type 2 diabetic patients [54]. Oral ingestion of Ginkgo biloba extract protected the liver injury by blunting the rises of malonaldehyde in rats [55].
In addition, the increased generation of ROS, including superoxide anion and hydroxyl radical, can induce lipid oxidation [25]. In this study, dietary EAE supplementation increased anti-superoxide anion capacity in hepatopancreas and intestine, which indicates that dietary EAE enhanced superoxide anion-scavenging ability in fish digestive and absorptive organs. These results were in conformity the report that EGb decreased the superoxide anion levels of in carp erythrocytes under hydroxyl radical stress [8]. In vitro study showed that Ginkgo biloba leaves exerted antioxidant properties by scavenging of superoxide anions [56]. Furthermore, it was reported that Ginkgo biloba extract showed a good capability of scavenging ROS in intestine of rats [57]. The effects of EAE may be linked to the chemical structure of their flavonoid compounds. Seyoum et al. reported that the flavonoids compound had free radical-scavenging ability [58]. In the meantime, it was demonstrated that uptake of flavonoids could decrease the activity of ROS in human colon adenocarcinoma Caco-2 cells [59].
The antioxidative systems in fish, inclusive of non-enzymatic and enzymatic components, have been refined through evolution to mitigate or rectify the damage inflicted by ROS on piscine tissues and organisms [60]. Within the enzymatic segment of this defense system, superoxide dismutase emerges as the initial enzyme to interact with the superoxide anion, facilitating its conversion into H2O2 and dioxygen [52]. Subsequently, catalase and glutathione peroxidase act on H2O2 to decompose it further [61]. Current research demonstrates that inclusion of EAE in the diet elevates the activity levels of superoxide dismutase, catalase, and glutathione peroxidase within the hepatopancreas, signifying an enhancement in the antioxidant capabilities of fish owing to EGb inclusion in feed. These findings align with studies showing that EGb-supplemented diets increase the activities of hepatic antioxidant enzymes such as superoxide dismutase and catalase in hybrid grouper [13]. The advantageous influence of EAE could be attributed to its flavonoid constituents. For instance, in Channa punctata, quercetin, a flavonoid supplement, was reported to alleviate stress induced by deltamethrin, as evidenced by restored activities of liver enzymes including superoxide dismutase, glutathione peroxidase, and catalase [62]. Furthermore, the significance of fish antioxidative defense mechanisms is paramount in counteracting oxidative stress [60]. The glutathione-dependent antioxidant system, pivotal in cell damage prevention caused by radicals, encompasses glutathione S-transferase and glutathione reductase, in concert with glutathione itself, a tripeptide of low molecular weight [63,64]. Glutathione S-transferase represents a family of multifunctional enzymes that facilitate the binding of electrophilic metabolites to glutathione’s thiol group [13]. Meanwhile, glutathione reductase is responsible for the regeneration of reduced glutathione from its oxidized form, utilizing NADPH as the electron source [65]. In the present study, augmenting the diet with EAE was found to boost both glutathione reductase activity and reduced glutathione levels in the intestinal tissue, indicative of amelioration in enzymatic antioxidative function brought on by EGb. This is corroborated by laboratory evidence showing that EGb reinstated glutathione reductase activity in piscine erythrocytes when subjected to hydroxyl radical stress [8]. Consequently, this study posits that EAE augments the antioxidative defense of digestive organs, thereby preserving their structure and functionality, which in turn supports digestive efficiency and, consequently, fish growth performance. Nonetheless, the mechanisms through which EAE exerts its regulatory effect on fish growth remain to be elucidated further.
4.3. Dietary EAE Decreased Lipid Oxidation in Fish Meat through Inhibiting the Induction Effect of Hemoglobin in Erythrocyte
Lipid oxidation is a key factor that contributes to the quality deterioration in muscle foods [4]. In fish, the content of polyunsaturated fatty acids was commonly higher than in mammalian [3]. In addition, fish is prone to lipid oxidation not only due to the amounts but also the types of polyunsaturated fatty acids. The most common polyunsaturated fatty acids present in mammalian are arachidonic acid, and in fish is eicosa pentanoic acid, which is more susceptible to lipid oxidation [66]. Furthermore, the drying process significantly accelerated lipid oxidation in fish [67]. In this study, dietary EAE supplementation decreased peroxide, conjugated diene and malonaldehyde levels, which indicates that EGb could decrease the lipid oxidation in fish meat induced by hot-drying process. Similar observations have been reported in hybrid grouper showed that dietary supplementation with EGb alleviated lipid peroxidation in fish liver as manifested the decrease in malonaldehyde content [13]. However, the mechanism by which EGb alleviated lipid oxidation in fish needs to be further explored. Based on regressive analysis of peroxide, conjugated diene and malonaldehyde, optimum dietary EAE supplementation were 3.72, 3.05 and 3.73 g kg−1 diet, respectively.
Lipid oxidation in fish foods may be similar to fish muscle. Fish muscle is susceptible to oxidative stress, which led to reduce flesh quality [68]. In this study, dietary EAE supplementation decreased hydrogen peroxide levels and enhanced capacity of anti-hydroxyl radical (a maker of hydroxyl radical-scavenging ability), which indicates that EGb could reduce ROS production in fish meat. Similarly, EGb reduced ROS levels in mice lymphocytes [69]. ROS have been shown to be damaging to fish lipids [53]. In this study, dietary EAE supplementation decreased malonaldehyde levels in fish meat, which indicates that EGb could decrease lipid oxidation in fish meat. The beneficial effects of EAE on antioxidant status in fish meat may be linked to its flavonoid compound. Study in Nile tilapia showed that levels of hydrogen peroxide and malonaldehyde in fish flesh decreased by dietary inclusion of quercetin, a kind of flavonol glycoside in Ginkgo biloba leaves [70,71]. In this study, dietary supplementation of EGb increased reduced glutathione content and activities of catalase and glutathione S-transferase, indicates that dietary EGb could regulate antioxidant defense systems in fish meat, therefore decreasing lipid oxidation and improving the capacity to cope with ROS. Study in broiler chickens showed that dietary inclusion of fermented Ginkgo biloba leaves could improve antioxidant properties in thigh meat by enhancing the antioxidant enzyme system [72].
Lipid oxidation of fish meat may be related to the auto-oxidation of hemoglobin. Fish erythrocytes are important for all tissues and organs because they carried hemoglobin to them for oxygen supply [73]. Erythrocytes exhibit a heightened sensitivity to reactive oxygen species (ROS) due to the elevated tension of oxygen, significant iron content from heme groups, and the presence of polyunsaturated fatty acids within their membranes, which are inherently prone to oxidative damage [74,75]. While hemoglobin principally functions as an oxygen-transporting protein, its susceptibility to oxidative stress leads to its conversion into met-hemoglobin, a form incapable of carrying oxygen [74]. Studies have identified hemoglobin as a significant pro-oxidant, catalyzing lipid peroxidation in piscine muscle [76]. The propensity for lipid oxidation in fish tissue is exacerbated by two factors: the substantial hemoglobin content in fish meat, which remains largely unextracted before processing, and the autoxidation of hemoglobin, a continuous process yielding ROS and releasing iron ions within erythrocytes, facilitating further oxidation of lipids in fish muscle [4,5]. The present investigation revealed that incorporating of EAE in fish diets led to reduced levels of met-hemoglobin, as well as diminished concentrations of malondialdehyde, superoxide radicals, and hydrogen peroxide in piscine erythrocytes. This suggests that EAE intake curtails ROS generation and lipid peroxidation within erythrocytes, subsequently mitigating the lipid oxidation process in fish muscle tissue. Similar observations have been reported that Ginkgo biloba extract reduced H2O2-induced oxidative stress in human erythrocytes [77]. Furthermore, dietary supplementation of EAE increased content of reduced glutathione, and activities of glutathione reductase, superoxide dismutase and catalase, indicates that dietary EAE could regulate antioxidant defense systems in fish erythrocytes, therefore decreasing lipid oxidation and ROS production. So, the effects of dietary EGb on the lipid oxidation in fish meat may be attributed to its inhibition on the induction of hemoglobin in erythrocytes of fish.
Fish gills were easier attacked by the ROS than other organs not only because they always exposed to environmental stress but also as a dynamic osmoregulatory and respiratory organ, which gathered high amounts of erythrocytes as well as hemoglobin [78]. ROS have been shown to be damaging to lipids in fish [53]. In this study, dietary EAE supplementation decreased malonaldehyde levels, which indicates that EGb could decrease lipid oxidation in fish gills. In addition, dietary EAE supplementation enhanced anti-superoxide anion capacity, which suggested EGb could reduce ROS production in fish gills. Fishes have developed non-enzymatic antioxidants (such as reduced glutathione) and ROS scavenging enzymes to prevent or repair the harm triggered by ROS [60]. In this study, dietary EAE supplementation increased the reduced glutathione content and the activities of superoxide dismutase, glutathione peroxidase, catalase and glutathione S-transferase, indicates that dietary EGb could regulate antioxidant defense systems in fish gills, therefore decreasing lipid oxidation and ROS production. Study in tilapia (Oreochromis niloticus) showed that dietary inclusion of Ginkgo biloba extract could alleviate oxidative stress by enhancing activities of superoxide dismutase and catalase, and reduced glutathione levels in fish gills induced by glyphosate exposure [79]. The effects of dietary EGb on fish gills confirmed our hypothesis of its effects on fish meat about the role of hemoglobin.
5. Conclusions
Therefore, we conclude that EGb could decrease lipid oxidation in fish feed through protecting unsaturated fatty acids from oxidation. The effect of EGbs on the unsaturated fatty acids may be closely associated with their metal chelation capabilities and total antioxidant capacity. Broken-line analysis of peroxide, conjugated diene and malonaldehyde showed that the optimum dietary EAE supplementation was 4.26 g kg−1 in fish feed. Meanwhile, current results displayed that dietary EGb increased the growth performance of fish, which may be ascribed to its enhancing effect on the activity of digestive and absorptive enzymes and antioxidant capacity in digestive organs of fish. Based on polynomial regression analysis of weight gain, optimum dietary EAE supplementation for Jian carp was 2.75 g kg−1 diet. In addition, dietary EAE supplementation inhibits the induction effect of hemoglobin in erythrocyte, therefore decreasing the hot-drying-induced lipid oxidation in fish meat. The antioxidant capacity of EGb was closely related to their flavonoid content. Our present study indicates that EGb can be considered as a potential natural antioxidant for fish and fish feed.
Author Contributions
Conceptualization, G.C. and H.L.; methodology, G.C., J.X., Q.Y., L.F. and M.W.; formal analysis, J.X. and M.W.; validation, G.C., J.X., Q.Y., L.F. and H.L.; data curation, G.C. and M.W.; resources, H.L. and L.F.; writing—original draft preparation, G.C., J.X., Q.Y. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Sichuan Science and Technology Program [grant number 2018JY0214]; the Patent Project of Neijiang Normal University [grant number Z2019027]; and the Talent Program of Neijiang Normal University [grant number R2019015].
Institutional Review Board Statement
All procedures were approved by the Neijiang Normal University Institutional Animal Care and Use Committee(Approval Code: JM2016-11).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to generate the results in this manuscript can be made available if requested from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Godwin, A.; Prabhu, H.R. Lipid peroxidation of fish oils. Indian. J. Clin. Biochem. 2006, 21, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Chen, S.N.; Cao, J.Y.; Zhou, J.Y.; Chen, Y.Z.; Jamali, M.A.; Zhang, Y.W. Hydrolysis and oxidation of protein and lipids in dry-salted grass carp (Ctenopharyngodon idella) as affected by partial substitution of NaCl with KCl and amino acids. RSC Adv. 2019, 9, 39545–39560. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; García García, B.; Jordán, M.J.; Hernández, M.D. Natural antioxidants in extruded fish feed: Protection at different storage temperatures. Anim. Feed. Sci. Technol. 2014, 195, 112–119. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S. Effect of bleeding on lipid oxidation and quality changes of Asian seabass (Lates calcarifer) muscle during iced storage. Food. Chem. 2011, 124, 459–467. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S.; Kamal-Eldin, A. Haemoglobin-mediated lipid oxidation in the fish muscle: A review. Trends Food Sci. Technol. 2012, 28, 33–43. [Google Scholar] [CrossRef]
- Błaszczyk, A.; Augustyniak, A.; Skolimowski, J. Ethoxyquin: An Antioxidant Used in Animal Feed. Int. J. Food Sci. 2013, 1–12. [Google Scholar] [CrossRef]
- Song, C.; Liu, B.; Xu, P.; Xie, J.; Ge, X.; Zhou, Q.; Sun, C.; Zhang, H.; Shan, F.; Yang, Z. Oxidized fish oil injury stress in Megalobrama amblycephala: Evaluated by growth, intestinal physiology, and transcriptome-based PI3K-Akt/NF-κB/TCR inflammatory signaling. Fish Shellfish Immunol. 2018, 81, 446–455. [Google Scholar]
- Li, H.; Zhou, X.; Gao, P.; Li, Q.; Li, H.; Huang, R.; Wu, M. Inhibition of lipid oxidation in foods and feeds and hydroxyl radical-treated fish erythrocytes: A comparative study of Ginkgo biloba leaves extracts and synthetic antioxidants. Anim. Nutr. 2016, 2, 234–241. [Google Scholar] [CrossRef]
- Ito, N.; Fukushima, S.; Tsuda, H. Carcinogenicity and modification of the carcinogenic response by BHA, BHT, and other antioxidants. Crit. Rev. Toxicol. 1985, 15, 109–150. [Google Scholar]
- EFSA. Safety and efficacy of ethoxyquin (6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline) for all animal species. EFSA J. 2015, 13, 4272. [Google Scholar]
- Aksoy, L.; Kolay, E.; Agilonu, Y.; Aslan, Z.; Kargioglu, M. Free radical scavenging activity, total phenolic content, total antioxidant status, and total oxidant status of endemic Thermopsis turcica. Saudi J. Biol. Sci. 2013, 20, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lou, K.; Wu, G.; Wu, X.; Zhou, X.; Feng, Y.; Zhang, H.; Yu, P. Bioactive metabolites in of Ginkgo biloba leaves: Variations by seasonal, meteorological and soil. Braz. J. Biol. 2019, 80, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Sun, Z.; Liu, Q.; Ye, H.; Zou, C.; Ye, C.; Wang, A.; Lin, H. Effects of dietary Ginkgo biloba leaf extract on growth performance, plasma biochemical parameters, fish composition, immune responses, liver histology, and immune and apoptosis-related genes expression of hybrid grouper (Epinephelus lanceolatus♂× Epinephelus fuscoguttatus♀) fed high lipid diets. Fish Shellfish Immun. 2018, 72, 399–409. [Google Scholar]
- Mahadevan, S.; Park, Y. Multifaceted therapeutic benefifits of Ginkgo biloba L.: Chemistry, effificacy, safety, and uses. J. Food. Sci. 2008, 73, R14e9. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.F.; Feng, L.; Kuang, S.Y.; Liu, Y.; Jiang, J.; Hu, K.; Jiang, W.D.; Li, S.H.; Tang, L.; Zhou, X.Q. Effect of dietary arginine on growth, intestinal enzyme activities and gene expression in muscle, hepatopancreas and intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Br. J. Nutr. 2012, 108, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Long, J.; Li, H.; Xu, J.; Yuan, J.; Yang, Q.; Feng, L.; Wu, M.; Jiang, J. The Protective Effect of a Dietary Extract of Mulberry (Morus alba L.) Leaves against a High Stocking Density, Copper and Trichlorfon in Crucian Carp (Carassius auratus). Animals 2023, 13, 2652. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.P.; Lu, Y.H.; Wei, D.Z. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J. Agric. Food. Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Zhao, J.; Posa, D.K.; Kumar, V.; Hoetker, D.; Kumar, A.; Ganesan, S.; Riggs, D.W.; Bhatnagar, A.; Wempe, M.F.; Baba, S.P. Carnosine protects cardiac myocytes against lipid peroxidation products. Amino Acids 2018, 51, 123–128. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Bone, D.E.; Carrington, M.F. Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem. 2005, 91, 485–494. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S. Comparative studies of four different phenolic compounds on in vitro antioxidative activity and the preventive effect on lipid oxidation of fish oil emulsion and fish mince. Food Chem. 2010, 119, 123–132. [Google Scholar] [CrossRef]
- Chen, G.; Wu, M.; Li, H.; Xu, J.; Liu, H.; Du, W.; Yang, Q.; Feng, L.; Jiang, J. Scoparia dulcis L. Extract Relieved High Stocking Density-Induced Stress in Crucian Carp (Carassius auratus). Animals 2023, 13, 2522. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Wu, M.; Wang, J.; Qin, C.J.; Long, J.; Zhou, S.S.; Yuan, P.; Jing, X.Q. Protective role of Angelica sinensis extract on trichlorfon-induced oxidative damage and apoptosis in gills and erythrocytes of fish. Aquaculture 2020, 519, 734895. [Google Scholar] [CrossRef]
- Li, H.T.; Feng, L.; Jiang, W.D.; Liu, Y.; Jiang, J.; Li, S.H.; Zhou, X.Q. Oxidative stress parameters and anti-apoptotic response to hydroxyl radicals in fish erythrocytes: Protective effects of glutamine, alanine, citrulline and proline. Aquat. Toxicol. 2013, 126, 169–179. [Google Scholar] [CrossRef]
- Jiang, W.D.; Wu, P.; Kuang, S.Y.; Liu, Y.; Jiang, J.; Hu, K.; Li, S.H.; Tang, L.; Feng, L.; Zhou, X.Q. Myo-inositol prevents copper-induced oxidative damage and changes in antioxidant capacity in various organs and the enterocytes of juvenile Jian carp (Cyprinus carpio var. Jian). Aquat. Toxicol. 2011, 105, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Meng, Y.; Zhao, X.; Fan, W.; Yi, T.; Wang, X. Sunflower oil flavored by essential oil from Punica granatum cv. Heyinshiliu peels improved its oxidative stability and sensory properties. LWT 2019, 111, 55–61. [Google Scholar] [CrossRef]
- Recknagel, R.O.; Glende, E.A. Spectrophotometric detection of lipid conjugated dienes. Method Enzymol. 1984, 105, 331–337. [Google Scholar]
- Martínez-Yusta, A.; Goicoechea, E.; Guillén, M.D. A review of thermo-oxidative degradation of food lipids studied by 1HNMR spectroscopy: Influence of degradative conditions and food lipid nature. Compr. Rev. Food Sci. F 2014, 13, 838–859. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Zu, Y.G.; Yang, L.; Lu, Q.; Wang, W. Antioxidant effects of rosemary extracts on sunflower oil compared with synthetic antioxidants. Int. J. Food Sci. Technol. 2014, 49, 385–391. [Google Scholar] [CrossRef]
- El-Husseiny, O.M.; Abdul-Aziz, G.M.; Goda, A.S.; Suloma, A. Effect of altering linoleic acid and linolenic acid dietary levels and ratios on the performance and tissue fatty acid profiles of Nile tilapia Oreochromis niloticus fry. Aquac. Int. 2010, 18, 1105–1119. [Google Scholar] [CrossRef]
- Wei, X.B.; Liu, H.Q.; Sun, X.; Fu, F.; Zhang, X.; Wang, J.; An, J.; Ding, H. Hydroxysafflor yellow A protects rat brains against ischemia-reperfusion injury by antioxidant action. Neurosci. Lett. 2005, 386, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, S.; Duan, X.; Feng, X.; Yang, Y. The interaction effects of coke oven emissions exposure and metabolic enzyme Gene variants on total antioxidant capacity of workers. Environ. Toxicol. Pharmacol. 2019, 70, 103197. [Google Scholar] [CrossRef] [PubMed]
- Lugasi, A.; Dworschak, E.; Horvatovich, P. Additional information to the in vitro antioxidant activity of Ginkgo biloba L. Phytother. Res. 1999, 13, 160–162. [Google Scholar] [CrossRef]
- Zhao, L.J.; Liu, W.; Xiong, S.H.; Tang, J.; Lou, Z.H.; Xie, M.X.; Xia, B.H.; Lin, L.M.; Liao, D.F. Determination of total flavonoids contents and antioxidant activity of Ginkgo biloba leaf by near-infrared reflectance method. Int. J. Anal. Chem. 2018, 8195784. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.K.; Jung, H.A.; Chung, H.Y.; Choi, J.S. In vitro peroxynitrite scavenging activity of 6-hydroxykynurenic acid and other flavonoids from Gingko biloba yellow leaves. Arch. Pharm. Res. 2006, 29, 1074–1079. [Google Scholar] [CrossRef]
- Ni, Y.; Duan, Z.; Zhou, D.; Liu, S.; Wan, H.; Gui, C.; Zhang, H. Identification of Structural Features for the Inhibition of OAT3-Mediated Uptake of Enalaprilat by Selected Drugs and Flavonoids. Front. Pharmacol. 2020, 11, 802. [Google Scholar] [CrossRef]
- Fuhrman, B.; Aviram, M. Flavonoids protect LDL from oxidation and attenuate atherosclerosis. Curr. Opin. Lipidol. 2001, 12, 41–48. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Chan, P.T.; Ho, K.Y.; Fung, K.P.; Wang, J. Antioxidant activity of natural flavonoids is governed by number and location of their aromatic hydroxyl groups. Chem. Phys. Lipids 1996, 79, 157–163. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Rather, A.A. Biochemical responses induced by sub lethal concentrations of carbaryl and parathion on certain enzymes of fresh water catfish Clarias batrachus. Int. J. Biol. Sci. 2015, 4, 52–56. [Google Scholar]
- Dikshith, T.S.; Datta, K.; Kushwah, H.; Raizada, R. Histopathological and biochemical changes in guinea pigs after repeated dermal exposure to benzene hexachloride. Toxicology 1978, 10, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Johari, S.A.; Sarkheil, M.; Asghari, S.; Haghighat, F.; Dekani, L.; Keyvanshokooh, S. Comparative toxicity of nanoparticulate and ionic copper following dietary exposure to common carp (Cyprinus carpio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 229, 108680. [Google Scholar] [CrossRef] [PubMed]
- Ahmadifar, E.; Yousefi, M.; Karimi, M.; Fadaei Raieni, R.; Dadar, M.; Yilmaz, S.; Dawood, M.; Abdel-Latif, H. Benefits of Dietary Polyphenols and Polyphenol-Rich Additives to Aquatic Animal Health: An Overview. Revi. Fish. Sci. Aquac. 2021, 29, 478–511. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.; Hendam, B.M.; Nofal, M.I.; El-Son, M.A. Ginkgo biloba leaf extract improves growth, intestinal histomorphometry, immunity, antioxidant status and modulates transcription of cytokine genes in hapa-reared Oreochromis niloticus. Fish Shellfish Immunol. 2021, 117, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Wang, F.; Huang, L.; Liu, C.; Dong, W.; Zhuang, X.; Yin, X.; Liu, Y.; Wang, W. Effects of dietary Ginkgo biloba leaf extract on growth performance, immunity and environmental stress tolerance of Penaeus vannamei. Fish Shellfish Immunol. 2023, 132, 108500. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, W.; Lan, T.; Zhang, X. Protective effects of extract of Ginkgo biloba on adriamycin-induced heart failure and its mechanism: Role of ghrelin peptide. Zhongguo Zhong Yao Za Zhi 2009, 34, 2786–2789. [Google Scholar]
- Shi, L.; Du, X.; Jiang, H.; Xie, J. Ghrelin and neurodegenerative disorders—A review. Mol. Neurobiol. 2017, 54, 1144–1155. [Google Scholar] [CrossRef]
- Gilloteaux, J.; Kashouty, R.; Yono, N. The perinuclear space of pancreatic acinar cells and the synthetic pathway of zymogen in Scorpaena scrofa L.: Ultrastructural aspects. Tissue Cell 2008, 40, 7–20. [Google Scholar] [CrossRef]
- Zambonino Infante, J.L.; Cahu, C.L. Ontogeny of the gastrointestinal tract of marine fish larvae. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 130, 477–487. [Google Scholar] [CrossRef]
- Geering, K. Subunit assembly and functional maturation of Na, K-ATPase. J. Membr. Biol. 1990, 115, 109–121. [Google Scholar] [CrossRef]
- Suzer, C.; Aktülün, S.; Çoban, D.; Okan Kamacı, H.; Saka, Ş.; Fırat, K.; Alpbaz, A. Digestive enzyme activities in larvae of sharpsnout seabream (Diplodus puntazzo). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhao, S.; Chen, G.; Jiang, W.; Liu, Y.; Jiang, J.; Hu, K.; Li, S.; Zhou, X. Antioxidant status of serum, muscle, intestine and hepatopancreas for fish fed graded levels of biotin. Fish Physiol. Biochem. 2013, 40, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Gliszczyńska-Świgło, A. Antioxidant activity of water soluble vitamins in the TEAC (trolox equivalent antioxidant capacity) and the FRAP (ferric reducing antioxidant power) assays. Food Chem. 2006, 96, 131–136. [Google Scholar] [CrossRef]
- Kudolo, G.B.; Delaney, D.; Blodgett, J. Short-term oral ingestion of Ginkgo biloba extract (EGb 761) reduces malondialdehyde levels in washed platelets of type 2 diabetic subjects. Diabetes Res. Clin. Pract. 2005, 68, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Gong, Z.; Li, J.; Li, X. Ginkgo biloba extract protects against alcohol-induced liver injury in rats. Phytother. Res. 2007, 21, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Chen, J.; Ding, Y.; Cheng, J.; Yang, S.; Ding, Z.; Dai, Q.; Ding, Z. In vitro antioxidant and immunostimulating activities of polysaccharides from Ginkgo biloba leaves. Int. J. Biol. Macromol. 2019, 124, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.X.; He, W.; Rinne, T.; Liu, Y.; Zhao, M.Q.; Wu, W.K. The effect of Ginkgo biloba extract (EGb 761) pretreatment on intestinal epithelial apoptosis induced by intestinal ischemia/reperfusion in rats: Role of ceramide. Am. J. Chin. Med. 2007, 35, 805–819. [Google Scholar] [CrossRef]
- Seyoum, A.; Asres, K.; El-Fiky, F.K. Structure–radical scavenging activity relationships of flavonoids. Phytochemistry 2006, 67, 2058–2070. [Google Scholar] [CrossRef]
- Yokomizo, A.; Moriwaki, M. Effects of uptake of flavonoids on oxidative stress induced by hydrogen peroxide in human intestinal Caco-2 cells. Biosci. Biotechnol. Biochem. 2006, 70, 1317–1324. [Google Scholar] [CrossRef]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant defenses in fish: Biotic and abiotic factors. Rev. Fish Biol. Fisher. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Ni, M.; Liu, M.; Lou, J.; Mi, G.; Gu, Z. Stocking density alters growth performance, serum biochemistry, digestive enzymes, immune response, and muscle quality of largemouth bass (Micropterus salmoides) in in-pond raceway system. Fish Physiol. Biochem. 2021, 47, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Das, S. Deltamethrin Induced Alteration of Biochemical Parameters in Channa punctata, Bloch and its Amelioration by Quercetin. Bull. Environ. Contam. Toxicol. 2017, 98, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Kirshenbaum, L.A.; Singal, P.K. Increase in endogenous antioxidant enzymes protects hearts against reperfusion injury. Am. J. Physiol. 1993, 265, H484–H492. [Google Scholar] [CrossRef]
- Yim, T.K.; Wu, W.K.; Pak, W.F.; Mak, D.H.F.; Ko, K.M. Myocardial protection against ischaemia- reperfusion injury by a Polygonum multiflorum extract supplemented ‘Dang-Gui decoction for enriching blood’, a compound formulation, ex vivo. Phytother. Res. 2000, 14, 195–199. [Google Scholar] [CrossRef]
- Reed, D.J. Glutathione: Toxicological implications. Annu. Rev. Pharm. Toxicol. 1990, 30, 603–631. [Google Scholar] [CrossRef]
- Sugihara, N.; Tsuruta, Y.; Date, Y.; Furuno, K.; Kohashi, K. High peroxidative susceptibility of fish oil polyunsaturated fatty acid in cultured rat hepatocytes. Toxicol. Appl. Pharmacol. 1994, 126, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Liu, N.; Sun, Y.; Sun, G.; Wang, S.; Yang, M.; Yang, M.; Wang, X.; Zhou, D.; Ge, Y.; et al. Effect of drying processes on the occurrence of lipid oxidation-derived 4-hydroxy-2-hexenal and 4-hydroxy-2-nonenal in Spanish mackerel (Scomberomorus niphonius). Food Sci. Nutri. 2022, 11, 1013–1023. [Google Scholar] [CrossRef]
- Morachis-Valdez, G.; Dublán-García, O.; López-Martínez, L.X.; Galar-Martínez, M.; Saucedo-Vence, K.; Gómez-Oliván, L.M. Chronic exposure to pollutants in Madín Reservoir (Mexico) alters oxidative stress status and flesh quality in the common carp Cyprinus carpio. Environ. Sci. Pollut. Res. 2015, 22, 9159–9172. [Google Scholar] [CrossRef]
- Eckert, A.; Keil, U.; Kressmann, S.; Schindowski, K.; Leutner, S.; Leutz, S.; Muller, W.E. Effects of EGb 761 Ginkgo biloba extract on mitochondrial function and oxidative stress. Pharmacopsychiatry 2003, 36, s15–s23. [Google Scholar]
- Ibrahim, D.; Kishawy, A.T.Y.; Khater, S.I.; Khalifa, E.; Ismail, T.A.; Mohammed, H.A.; Elnahriry, S.S.; Tolba, H.A.; Sherief, W.; Farag, M.; et al. Interactive effects of dietary quercetin nanoparticles on growth, flesh antioxidant capacity and transcription of cytokines and Aeromonas hydrophila quorum sensing orchestrating genes in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2021, 119, 478–489. [Google Scholar] [CrossRef]
- Eisvand, F.; Razavi, B.M.; Hosseinzadeh, H. The effects of Ginkgo biloba on metabolic syndrome: A review. Phytother. Res. 2020, 34, 1798–1811. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Wan, X.L.; Zhang, X.H.; Zhao, L.G.; He, J.T.; Zhang, J.F.; Zhang, L.L.; Wang, T. Effect of supplemental fermented Ginkgo biloba leaves at different levels on growth performance, meat quality, and antioxidant status of breast and thigh muscles in broiler chickens. Poult. Sci. 2017, 96, 869–877. [Google Scholar] [CrossRef]
- Kulkeaw, K.; Sugiyama, D. Zebrafish erythropoiesis and the utility of fish as models of anemia. Stem Cell Res. Ther. 2012, 3, 55. [Google Scholar] [PubMed]
- Çimen, M.Y.B. Free radical metabolism in human erythrocytes. Clin. Chim. Acta 2008, 390, 1–11. [Google Scholar] [CrossRef]
- Clemens, M.R.; Ruess, M.; Bursa, Z.; Waller, H.D. The relationship between lipid composition of red blood cells and their susceptibility to lipid peroxidation. Free Radic. Res. Commun. 1987, 3, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.P.; Hultin, H.O. Contributions of Blood and Blood Components to Lipid Oxidation in Fish Muscle. J. Agric. Food Chem. 2002, 50, 555–564. [Google Scholar] [CrossRef]
- Köse, K.; Dogan, P. Lipoperoxidation Induced by Hydrogen Peroxide in Human Erythrocyte Membranes. J. Int. Med. Res. 1995, 23, 9–18. [Google Scholar] [CrossRef]
- Sinha, A.K.; Zinta, G.; AbdElgawad, H.; Asard, H.; Blust, R.; De Boeck, G. High environmental ammonia elicits differential oxidative stress and antioxidant responses in five different organs of a model estuarine teleost (Dicentrarchus labrax). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 174–175, 21–31. [Google Scholar] [CrossRef]
- Zheng, T.; Jia, R.; Cao, L.; Du, J.; Gu, Z.; He, Q.; Xu, P.; Yin, G. Alleviative effects of Ginkgo biloba extract on oxidative stress, inflammatory response and immune suppression induced by long-term glyphosate exposure in tilapia (Oreochromis niloticus). Aquaculture 2022, 546, 737325. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).