How the luxR Gene Affects the Pathogenicity of Pseudomonas plecoglossicida and the Immune Response of Epinephelus coioides
Abstract
1. Introduction
2. Experimental Materials and Methodology
2.1. Cultivation Procedures and Bacterial Varieties
2.2. The Creation of a P. plecoglossicida RNAi Strain
2.3. Infection of Animals
2.4. Extracting RNA, Creating a cDNA Library, and Performing Sequencing
2.5. Analysis of Transcriptome Data
2.6. Validation Using Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Statistical Analyses
2.8. Accessibility of Data
3. Results
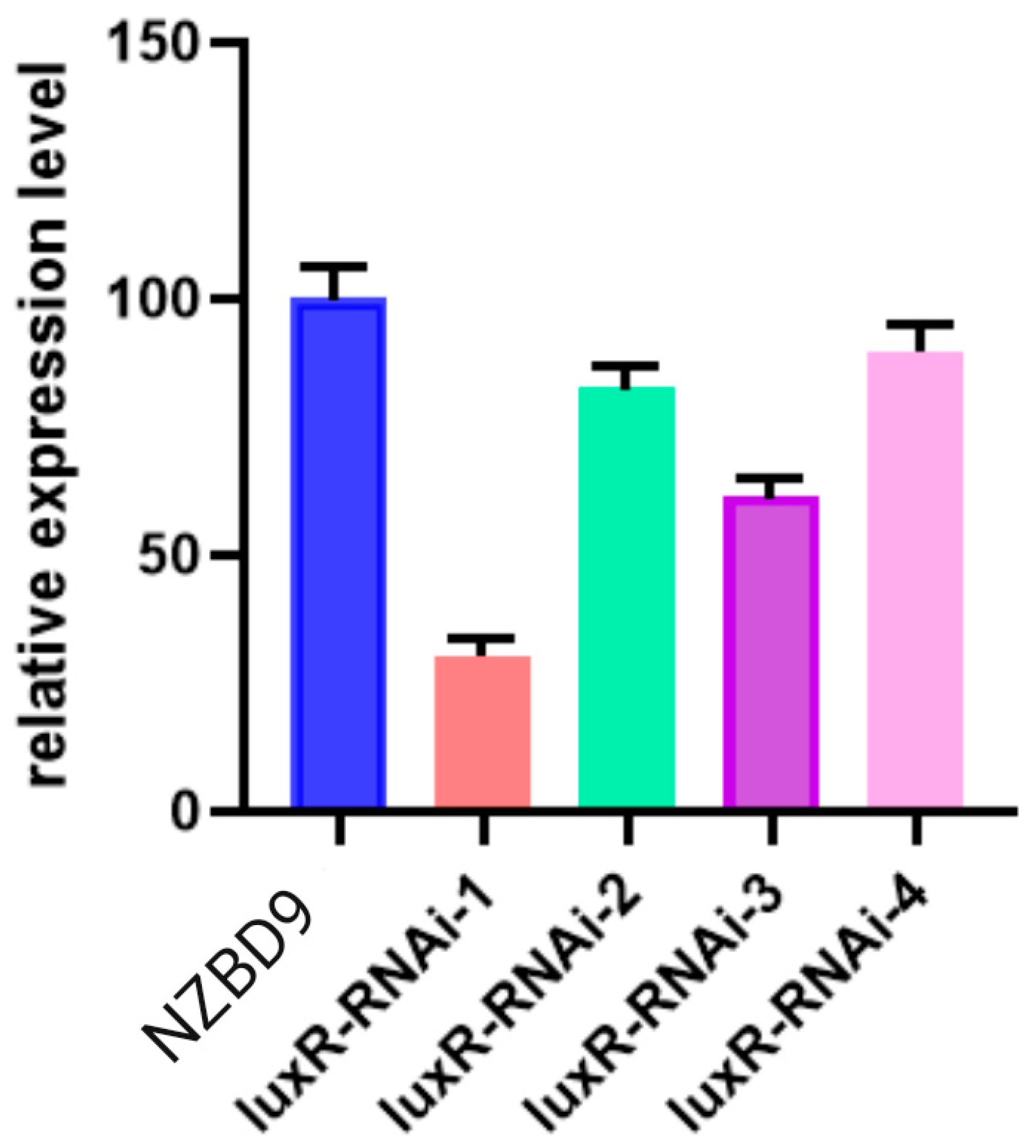
3.1. Construction of the luxR-RNAi Strain
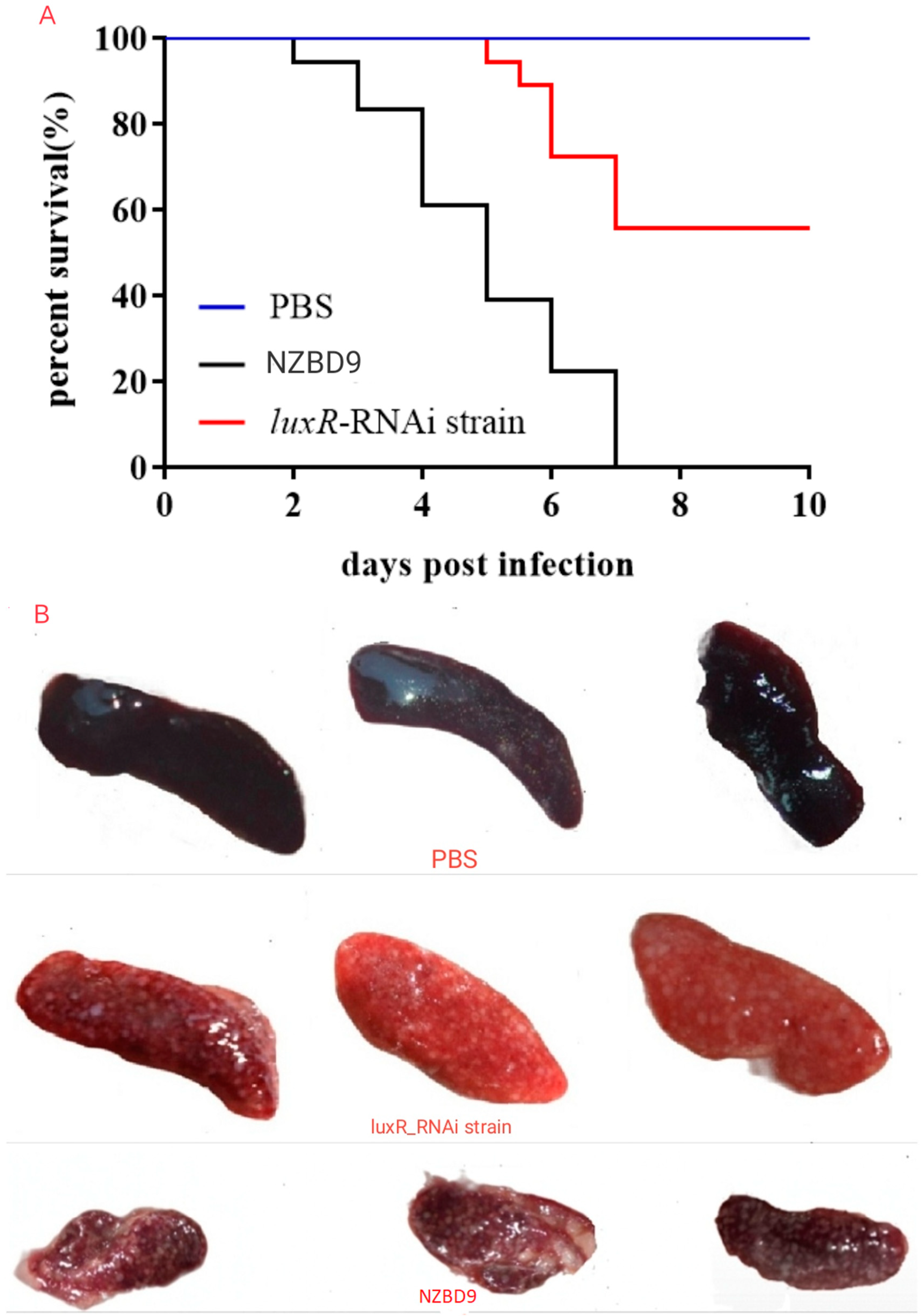
3.2. Impact of luxR on P. plecoglossicida Virulence
3.3. Interpretation of Gene Transcription Data
3.3.1. Evaluating the Fidelity and Precision of Transcriptomic Information
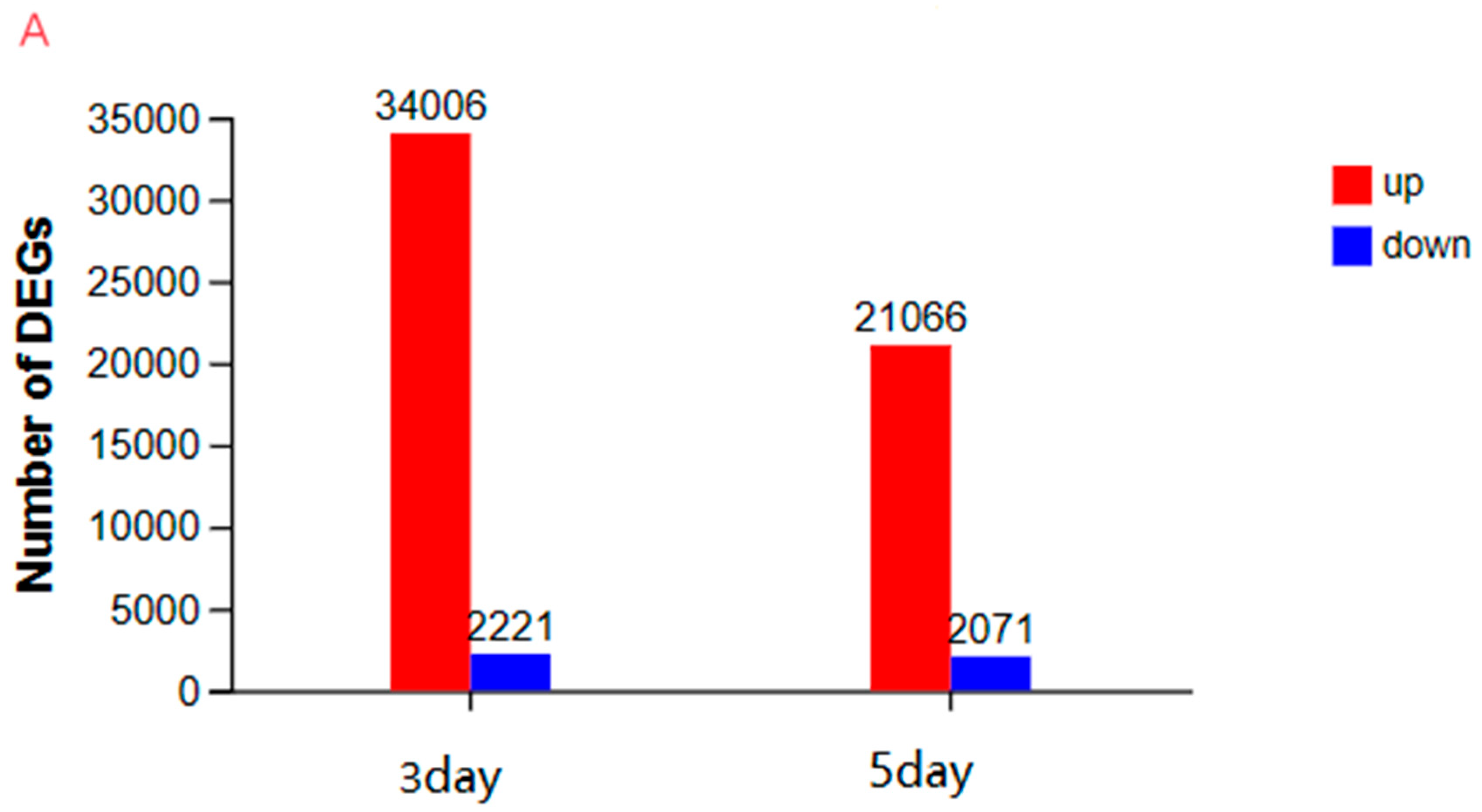
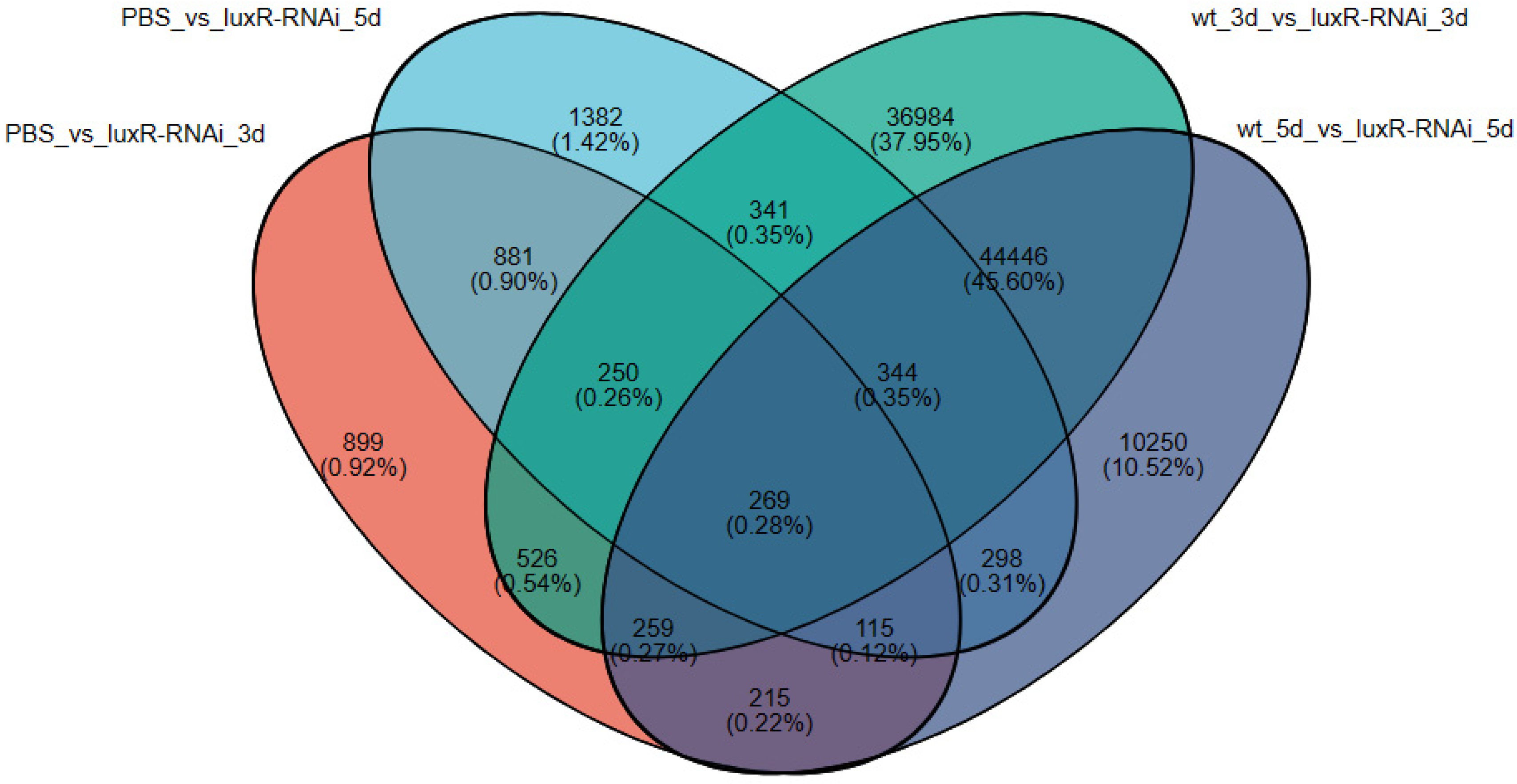
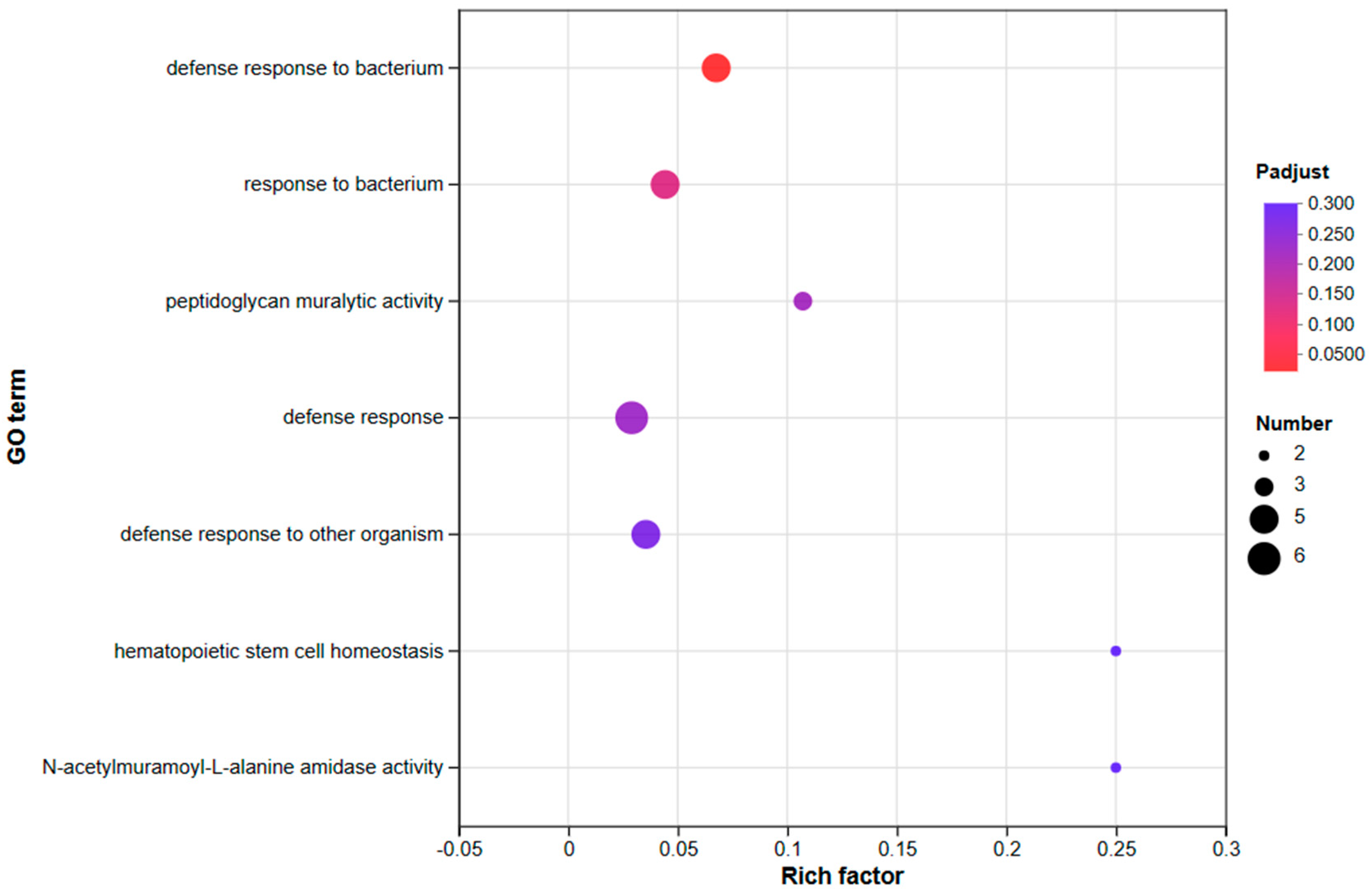
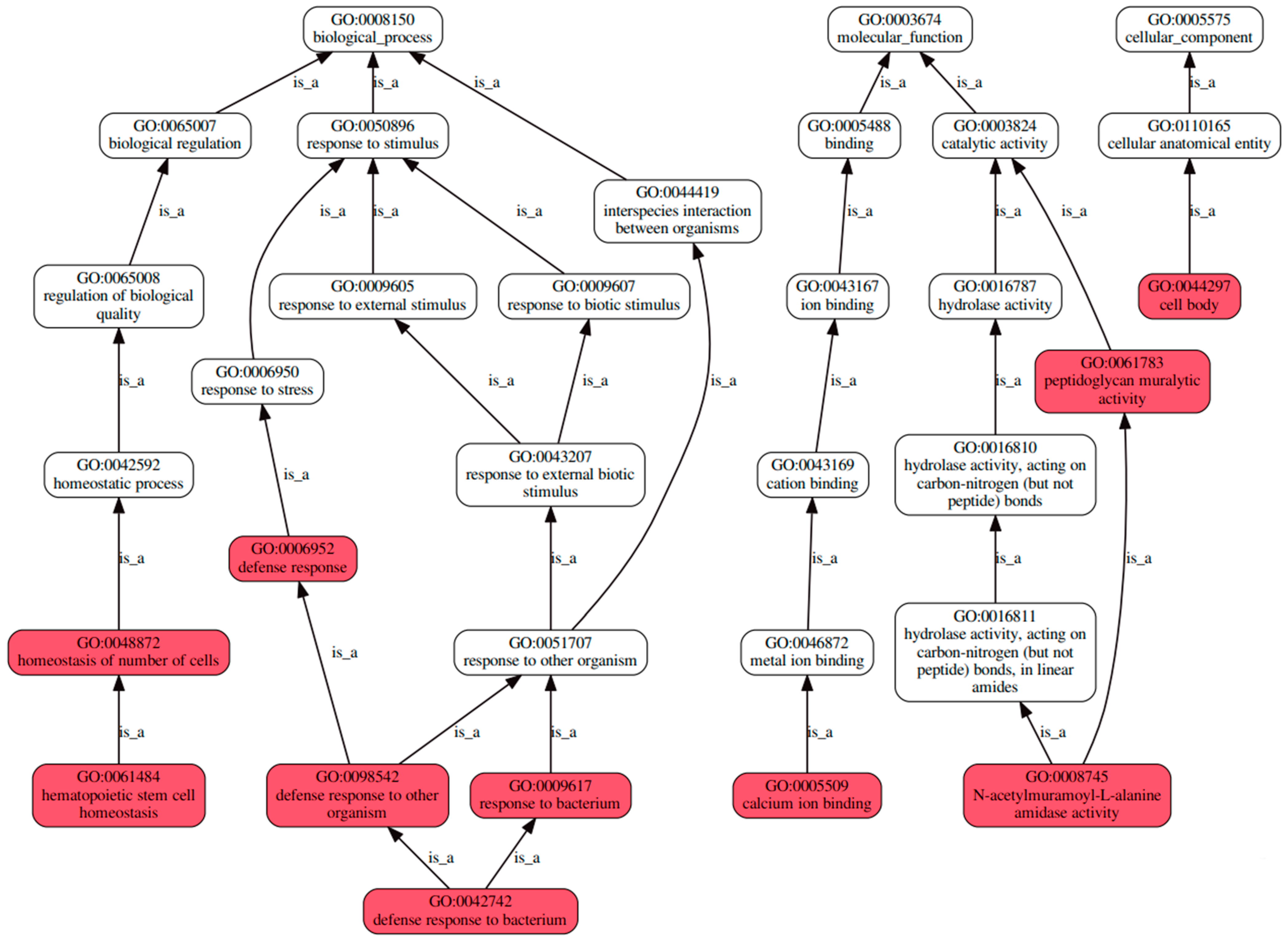
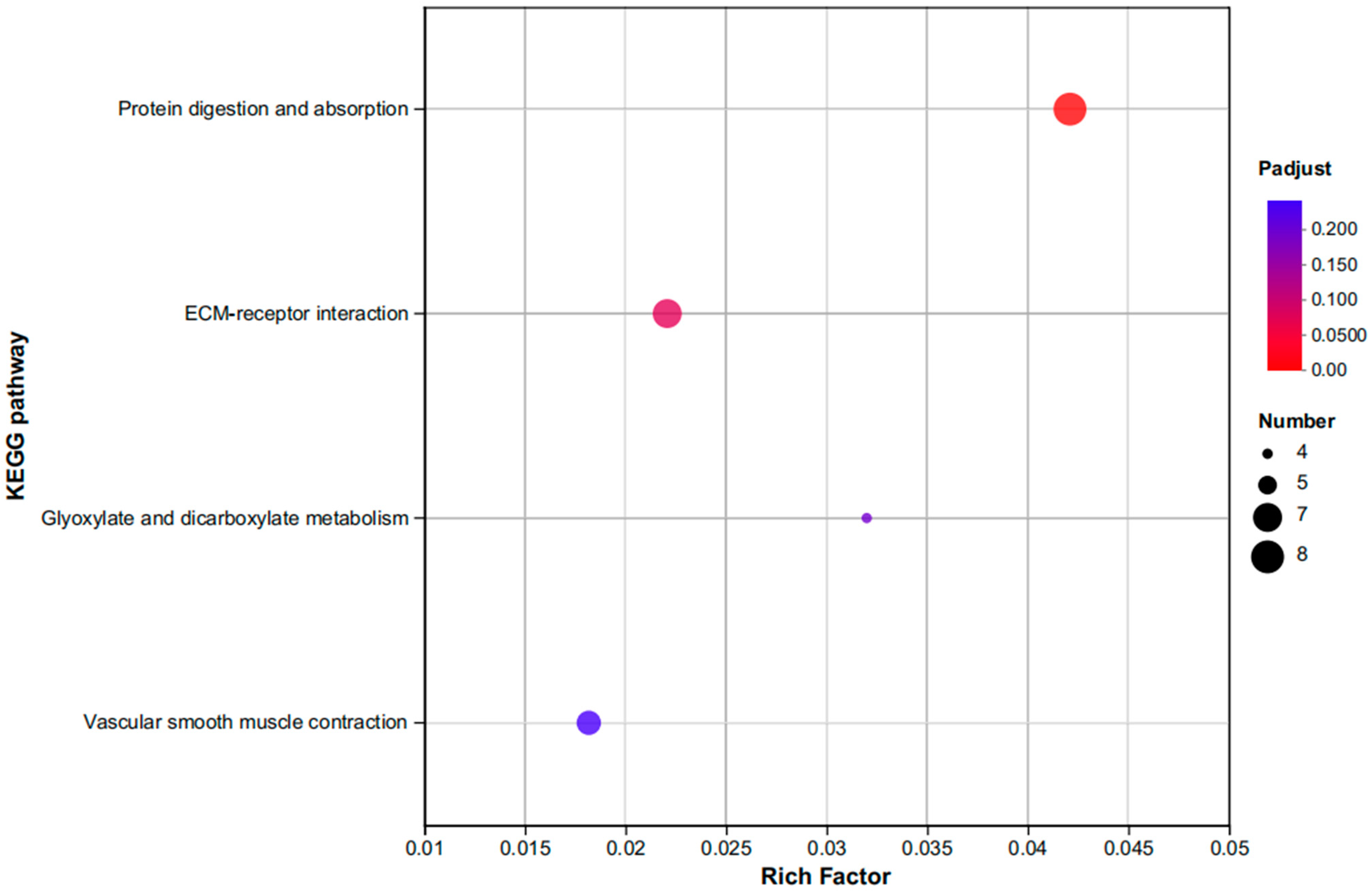
3.3.2. An Investigation into Genes Exhibiting Differential Expression Patterns via Transcriptomic Analysis
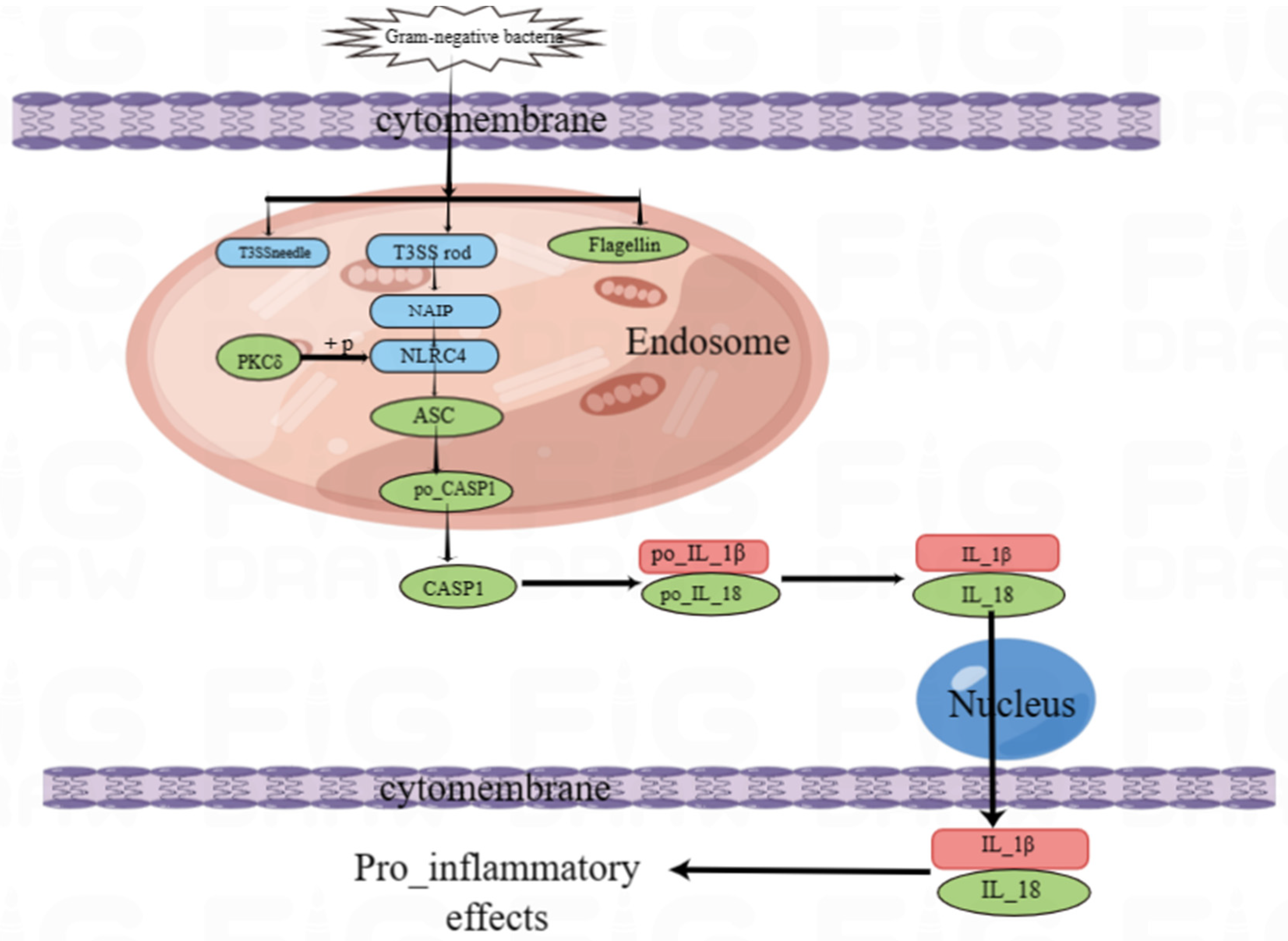
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Park, S.B.; Aoki, T. Pseudomonas plecoglossicida and the bacterial haemorrhagic ascites of ayu, Plecoglossus altivelis. Microbiology 2015, 161, 2115–2123. [Google Scholar] [CrossRef]
- Ma, Y.; Li, X. Pseudomonas plecoglossicida: A major threat to fish health in aquaculture. J. Fish Dis. 2018, 41, 951–960. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; Cui, L. Analysis of the Status of Important Aquatic Animal Diseases in China; China Agricultural Press: Beijing, China, 2022. [Google Scholar]
- Wakabayashi, H.; Hikima, J.I.; Masumura, K. Isolation of Pseudomonas sp. and Vibrio sp. from natural outbreaks of bacterial ascites of ayu Plecoglossus altivelis. Fish Pathol. 1992, 27, 199–205. [Google Scholar] [CrossRef]
- Nishimura, S.; Miyoshi, S.; Sugino, H. Pseudomonas plecoglossicida sp. nov., the causative agent of bacterial haemorrhagic ascites of ayu, Plecoglossus altivelis. Int. J. Syst. Bacteriol. 1996, 46, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Xia, H.; Zhang, Y.; Xu, P.; Li, Y. Complete genome sequence of Pseudomonas plecoglossicida strain NBRC 103064, the causative agent of bacterial hemorrhagic ascites of ayu fish. Genome Announc. 2013, 1, e00587-13. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, R.; Liu, J.; Wang, Q.; Xu, P.; Li, Y. Comparative genomics and transcriptomics of Pseudomonas plecoglossicida and its closely related Pseudomonas species. BMC Genom. 2014, 15, 724. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Wei, L.; Huang, X.; Sun, Z.; Lv, J. Genome analysis of Pseudomonas plecoglossicida N12-1221, a pathogenic strain isolated from diseased ayu fish in China. Microbiol. Res. Announc. 2021, 10, e01335-20. [Google Scholar] [CrossRef]
- Huang, L.; Liu, W.; Jiang, Q.; Zuo, Y.; Su, Y.; Zhao, L.; Qin, Y.; Yan, Q. Integration of transcriptomic and proteomic approaches reveals the temperature-dependent virulence of Pseudomonas plecoglossicida. Front. Cell. Infect. Microbiol. 2018, 8, 207. [Google Scholar] [CrossRef]
- Fuqua, C.; Winans, S.C.; Greenberg, E.P. Quorum sensing in bacteria: The LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 1994, 176, 269–275. [Google Scholar] [CrossRef]
- Hanzelka, B.L.; Greenberg, E.P. Quorum sensing in Vibrio fischeri: Evidence that S-adenosylmethionine is the amino acid substrate for autoinducer synthesis. J. Bacteriol. 1996, 178, 5291–5294. [Google Scholar] [CrossRef]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Whitehead, N.A.; Barnard, A.M.; Slater, H.; Simpson, N.J.; Salmond, G.P. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol. Rev. 2001, 25, 365–404. [Google Scholar] [CrossRef] [PubMed]
- Engebrecht, J.; Nealson, K.; Silverman, M. Bacterial bioluminescence: Isolation and genetic analysis of functions from Vibrio fischeri. Cell 1983, 32, 773–781. [Google Scholar] [CrossRef]
- Bassler, B.L.; Wright, M.; Showalter, R.E.; Silverman, M.R. Intercellular signalling in Vibrio harveyi: Sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 1993, 9, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Liu, Z.X.; Zhao, L.M.; Huang, L.X.; Qin, Y.X.; Su, Y.Q.; Nie, P. Dual RNA-seq provides novel insight into the roles of dksA from Pseudomonas plecoglossicida in pathogen-host interactions with large yellow croakers (Larimichthys crocea). Zool. Res. 2020, 41, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Tang, R. Preliminary Study of Whole-Bacterial Vaccine against Pseudomonas Mutans. Master’s Thesis, Jimei University, Xiamen, China, 2017. [Google Scholar]
- Luo, G.; Sun, Y.; Huang, L.; Su, Y.; Zhao, L.; Qin, Y.; Xu, X.; Yan, Q. Time-Resolved dual RNA-seq of tissue uncovers Pseudomonas plecoglossicida key virulence genes in host-pathogen interaction with Epinephelus coioides. Environ. Microbiol. 2020, 22, 677–693. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chang, D.; Wang, X.; Chen, L.; Yan, X.; Zhang, Y.; Guo, Q. Interactions of gut microbiota with host physiology and innate immune system. J. Cell. Physiol. 2021, 236, 319–330. [Google Scholar] [CrossRef]
- Wlodarska, M.; Finlay, B.B. Host immune response to antibiotic perturbation of the microbiota. Mucosal Immunol. 2010, 3, 100–103. [Google Scholar] [CrossRef]
- Sommer, F.; Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, L.; Zhang, L. luxR family members are key regulators of quorum sensing in Vibrio harveyi. Front. Cell. Infect. Microbiol. 2022, 12, 790839. [Google Scholar]
- Kim, J.; Kim, H.; Kim, Y.J. Characterization of luxR homologues in Vibrio parahaemolyticus and their roles in quorum sensing. J. Microbiol. Biotechnol. 2022, 32, 237–348. [Google Scholar]
- Gao, Y.; Zhao, X.; Zhou, L.; Zhang, L. Regulation of luxR -type transcriptional regulators in bacterial quorum sensing. Front. Microbiol. 2021, 12, 800539. [Google Scholar]
- Zhang, L.; Du, P.; Li, L. luxR family transcription factors play crucial roles in bacterial quorum sensing and pathogenesis. Front. Cell. Infect. Microbiol. 2021, 11, 699628. [Google Scholar]
- Li, S.; Li, J.; Liang, J.; Li, Y.; Zheng, M. luxR -type transcriptional regulators in bacterial quorum sensing: Diversity and functions. Front. Microbiol. 2021, 12, 647035. [Google Scholar]
- Sequeira, R.P.; McDonald, J.A.K.; Marchesi, J.R.; Clarke, P.; Hill, K.E.; Nally, J.E. Differential modulation of host gene expression in response to decreased expression of the bacterial quorum sensing regulator luxR. Microb. Pathog. 2022, 161, 105345. [Google Scholar]
- Dantas-Barbosa, C.; Duarte, P.; Carriço, J.A.; Freitas, A.T.; Ferreira, R.B.; Mira, N.P. The regulatory impact of luxR gene knockdown on Burkholderia thailandensis gene expression. Genes 2021, 12, 1591. [Google Scholar]
- Venugopal, A.; Patel, P.; Stabb, E.V. Differential gene expression in the squid-Vibrio symbiosis in response to decreased expression of the quorum sensing regulator luxR. Front. Microbiol. 2021, 12, 704472. [Google Scholar]
- Lai, Y.; Lu, X.; Wu, Z.; Li, X.; Wu, Q.; Zhang, Y.; Cao, X. Downregulation of the luxR gene in Vibrio parahaemolyticus enhances the host immune response and reduces bacterial virulence. Microb. Pathog. 2022, 163, 105476. [Google Scholar]
- Han, J.E.; Jeong, S.Y.; Choi, S.H.; Lee, K.H.; Lee, S.J. Down-regulation of luxR -type transcriptional regulator gene affects expression of virulence genes in Vibrio vulnificus. J. Microbiol. Biotechnol. 2021, 31, 1675–1682. [Google Scholar]
- Huang, H.; He, Y.; Wu, X.; Xie, J.; Xu, Z.; Zhang, Y.; Chen, S. The down-regulation of luxR in Vibrio alginolyticus triggers immune responses in Japanese flounder (Paralichthys olivaceus) against bacterial infection. Fish Shellfish Immunol. 2021, 114, 527–536. [Google Scholar]
- Ma, R.; Wang, X.; Yang, Z.; Liu, Y.; Zhang, X.; Gao, W.; Gao, P. The down-regulation of luxR in Vibrio anguillarum enhances the activation of immune response in fish macrophages. Fish Shellfish Immunol. 2021, 112, 135–142. [Google Scholar]
- Cheng, J.; Zhang, L.; Sun, L. The down-regulation of luxR in Vibrio splendidus enhances the immune response of scallops (Chlamys farreri) against bacterial infection. Fish Shellfish Immunol. 2021, 109, 231–238. [Google Scholar]
- Decho, A.W.; Gutierrez, T. Microbial Quorum Sensing in Marine Environments. Annu. Rev. Mar. Sci. 2021, 13, 231–256. [Google Scholar]
- Li, Y.; Li, M.; Huang, T. luxR -Type Quorum Sensing Systems in Bacteria. Int. J. Mol. Sci. 2021, 22, 2578. [Google Scholar]
- Zhang, X.; Xu, Y.; Qiu, J.; Shen, Y. Quorum sensing and bacterial virulence. Int. J. Oral Sci. 2021, 13, 9. [Google Scholar]
- Sheng, L.; Ren, X.; Gao, Y.; Zhang, R. Quorum sensing and bacterial virulence regulation: A comprehensive review. Front. Cell. Infect. Microbiol. 2021, 11, 621594. [Google Scholar]
- Li, L.; Li, H.; Zhang, X.X. luxR -Type Quorum Sensing in Aquatic Bacteria: Diversity, Mechanisms, and Applications. Front. Microbiol. 2022, 12, 794220. [Google Scholar]
- Low, K.Y.; Gubbi, S. luxR -type quorum sensing regulators are required for metabolic fitness in Chromobacterium piscinae. Microbiol. 2019, 165, 416–428. [Google Scholar] [CrossRef]
- Chen, C.; Lin, J. luxR -type regulators in pathogenic bacteria: Pleiotropic regulators or virulence factors? J. Microbiol. Immunol. Infect. 2020, 53, 631–638. [Google Scholar] [CrossRef]
- Chen, X.; Ji, Z.; Webber, A.T.; Luo, Y. luxR -type quorum sensing transcriptional regulators affect biofilm formation, motility, and antibiotic resistance in bacteria. Front. Microbiol. 2021, 12, 753244. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, W.; Kim, J.; Lee, Y.J.; Park, J.H. NOD-like receptors in cancer and inflammation. Front. Immunol. 2021, 12, 738303. [Google Scholar]
- Goulielmaki, E.; Papadaki, M. NOD-like receptors: Emerging therapeutic targets for inflammatory diseases. Front. Pharmacol. 2021, 12, 647874. [Google Scholar]
- Wang, L.; Li, Y.; Li, Z.; Li, Y.; Li, X.; Li, L.; Yang, K. NOD-like receptor signaling in innate immunity and inflammation: A comprehensive review. Cell. Mol. Immunol. 2021, 18, 1391–1412. [Google Scholar]
- Chen, K.W.; Groß, C.J.; Sotomayor, F.V.; Stacey, K.J.; Tschopp, J.; Sweet, M.J.; Schroder, K. The neutrophil NLRC4 inflammasome selectively promotes IL-1β maturation without pyroptosis during acute Salmonella challenge. Cell Rep. 2021, 37, 110045. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Lee, J.O. Structural biology of NLRP3 inflammasome activation. Exp. Mol. Med. 2021, 53, 406–417. [Google Scholar]
- He, Q.; Xia, L.; Chen, Y.; Hao, Y.; Li, Y.; Li, J. Crosstalk between the NLRP3 inflammasome and autophagy. Cell. Mol. Immunol. 2022, 19, 22–33. [Google Scholar]
- Gonçalves, A.V.; De Oliveira, J.R.; Molfetta, J.B. NOD-like receptors and viral infections: Gatekeepers, guardians, and caretakers of the immune system. Front. Immunol. 2021, 12, 752324. [Google Scholar]
- Brennan, M.A.; Cookson, B.T. Salmonella-induced inflammasome activation and IL-1β release in humans. Curr. Opin. Infect. Dis. 2021, 34, 245–250. [Google Scholar]
- Eislmayr, K.; Bestehorn, A.; Morelli, L.; Borroni, M.; Walle, L.V.; Lamkanfi, M.; Kovarik, P. Nonredundancy of IL-1α and IL-1β is defined by distinct regulation of tissues orchestrating resistance versus tolerance to infection. Sci. Adv. 2022, 8, eabj7293. [Google Scholar] [CrossRef]
- Ma, T.; Liu, X.; Cen, Z.; Xin, C.; Guo, M.; Zou, C.; Song, W.; Xie, R.; Wang, K.; Zhou, H.; et al. MicroRNA-302b negatively regulates IL-1β production in response to MSU crystals by targeting IRAK4 and EphA2. Arthritis Res. Ther. 2018, 20, 34. [Google Scholar] [CrossRef]
- Lin, C.C.; Edelson, B.T. New Insights into the Role of IL-1β in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis. J. Immunol. 2017, 198, 4553–4560. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.A.; Stephens, J.C. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef] [PubMed]
- Mauer, J.; Denson, J.L.; Brüning, J.C. Versatile functions for IL-6 in metabolism and cancer. Trends Immunol. 2014, 35, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef]
- Van De Veerdonk, F.L.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A.B. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol. 2011, 32, 110–116. [Google Scholar] [CrossRef]
- Tegtmeyer, N.; Sharafutdinov, I.; Harrer, A.; Backert, S. Campylobacter Virulence Factors and Molecular Host–Pathogen Interactions. Curr. Top. Microbiol. Immunol. 2021, 431, 169–202. [Google Scholar]
- Gebre, M.S.; Brito, L.A.; Tostanoski, L.H.; Edwards, D.K.; Irvine, D.J. Novel approaches for vaccine development. Cell 2021, 184, 1589–1603. [Google Scholar] [CrossRef]
- Wei, Y.; Murphy, E.R.; Larramendy, M.; Broach, J.R. Temperature-dependent regulation of bacterial gene expression by RNA thermometers. In Nucleic Acids—from Basic Aspects to Laboratory Tools; IntechOpen: London, UK, 2016; pp. 157–181. [Google Scholar]
- Shimoni, Y.; Friedlander, G.; Hetzroni, G.; Niv, G.; Altuvia, S.; Biham, O.; Margalit, H. Regulation of gene expression by small non-coding RNAs: A quantitative view. Mol. Syst. Biol. 2007, 3, 138. [Google Scholar] [CrossRef]
- Mangan, J.A.; Monahan, I.M.; Butcher, P.D. Gene expression during host—Pathogen interactions: Approaches to bacterial mRNA extraction and labelling for microarray analysis. In Methods in Microbiology; Academic Press: Cambridge, MA, USA, 2002; Volume 33, pp. 137–151. [Google Scholar]
- Han, L.; Gao, Y.; Liu, Y.; Li, Y.; Lu, J.; Guo, L. An Outer Membrane Protein YiaD Contributes to Adaptive Resistance of Meropenem in Acinetobacter baumannii. Microbiol. Spectrum. 2022, 10, e00173-22. [Google Scholar] [CrossRef]
- Blount, Z.D.; Maddamsetti, R.; Grant, N.A.; Ahmed, S.T.; Jagdish, T.; Baxter, J.A.; Sommerfeld, B.A.; Tillman, A.; Moore, J.; Slonczewski, J.L.; et al. Genomic and phenotypic evolution of Escherichia coli in a novel citrate-only resource environment. elife 2020, 9, e55414. [Google Scholar] [CrossRef]
- Ahmed, W. RNA-seq resolving host-pathogen interactions: Advances and applications. Ecol. Genet. Genom. 2020, 15, 100057. [Google Scholar] [CrossRef]
- Jones, P. Multi-Omic Systems Biological Analysis of Host-Microbe Interactions. Ph.D Thesis, University of Tennessee, Knoxville, TX, USA, 2022. [Google Scholar]
- Denzer, L.; Schroten, H.; Schwerk, C. From gene to protein—How bacterial virulence factors manipulate host gene expression during infection. Int. J. Mol. Sci. 2020, 21, 3730. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, T. Inflammasomes, autophagy, and cell death: The trinity of innate host defense against intracellular bacteria. Mediat. Inflamm. 2019, 2019, 2471215. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zheng, W.; Lv, X.; Xin, S.; Sun, Y.; Xu, T. microRNA-144 modulates the NF-κB pathway in miiuy croaker (Miichthys miiuy) by targeting IκBα gene. Dev. Comp. Immunol. 2022, 130, 104359. [Google Scholar] [CrossRef]
- Kumar, A.; Sperandio, V. Indole signaling at the host-microbiota-pathogen interface. mBio 2019, 10, e01031-19. [Google Scholar] [CrossRef]
- Febriza, A.; Hatta, M.; Natzir, R.; Kasim, V.N.A.; Idrus, H.H. Activity of antimicrobial peptide; cathelicidin, on bacterial infection. Open Biochem. J. 2019, 13, TOBIOCJ-13-45. [Google Scholar] [CrossRef]
- Dev, A.; Iyer, S.; Razani, B.; Cheng, G. NF-κB and innate immunity. Curr. Top. Microbiol. Immunol. 2011, 349, 115–143. [Google Scholar] [CrossRef]
- Green, J.; Rolfe, M.D.; Smith, L.J. Transcriptional regulation of bacterial virulence gene expression by molecular oxygen and nitric oxide. Virulence 2014, 5, 794–809. [Google Scholar] [CrossRef]
- Maldonado, R.F.; Sá-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef]
- Heithoff, D.M.; Conner, C.P.; Hanna, P.C.; Julio, S.M.; Hentschel, U.; Mahan, M.J. Bacterial infection as assessed by in vivo gene expression. Proc. Nat. Acad. Sci. USA 1997, 94, 934–939. [Google Scholar] [CrossRef]
- Fang, F.C.; Frawley, E.R.; Tapscott, T.; Vázquez-Torres, A. Bacterial Stress Responses during Host Infection. Cell Host Microbe 2016, 20, 133–143. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Huang, L.; Qin, Y.; Yang, D.; Zhang, J.; Zhang, J.; Yan, Q. How the luxR Gene Affects the Pathogenicity of Pseudomonas plecoglossicida and the Immune Response of Epinephelus coioides. Fishes 2023, 8, 507. https://doi.org/10.3390/fishes8100507
Zhao L, Huang L, Qin Y, Yang D, Zhang J, Zhang J, Yan Q. How the luxR Gene Affects the Pathogenicity of Pseudomonas plecoglossicida and the Immune Response of Epinephelus coioides. Fishes. 2023; 8(10):507. https://doi.org/10.3390/fishes8100507
Chicago/Turabian StyleZhao, Lingmin, Lixing Huang, Yingxue Qin, Dou Yang, Jiaonan Zhang, Jiaolin Zhang, and Qingpi Yan. 2023. "How the luxR Gene Affects the Pathogenicity of Pseudomonas plecoglossicida and the Immune Response of Epinephelus coioides" Fishes 8, no. 10: 507. https://doi.org/10.3390/fishes8100507
APA StyleZhao, L., Huang, L., Qin, Y., Yang, D., Zhang, J., Zhang, J., & Yan, Q. (2023). How the luxR Gene Affects the Pathogenicity of Pseudomonas plecoglossicida and the Immune Response of Epinephelus coioides. Fishes, 8(10), 507. https://doi.org/10.3390/fishes8100507






