Effects of Dietary Blend of Algae Extract Supplementation on Growth, Biochemical, Haemato-Immunological Response, and Immune Gene Expression in Labeo rohita with Aeromonas hydrophila Post-Challenges
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Culture of Algae
2.2. Preparation of Experimental Diet
2.3. Proximate Analysis
2.4. Experimental Design, Animal, and Maintenance
2.5. Blood Sample and Measurements
Collecting Blood and Separating the Serum
2.6. Growth Measurements
2.7. Serum Biochemical Assays
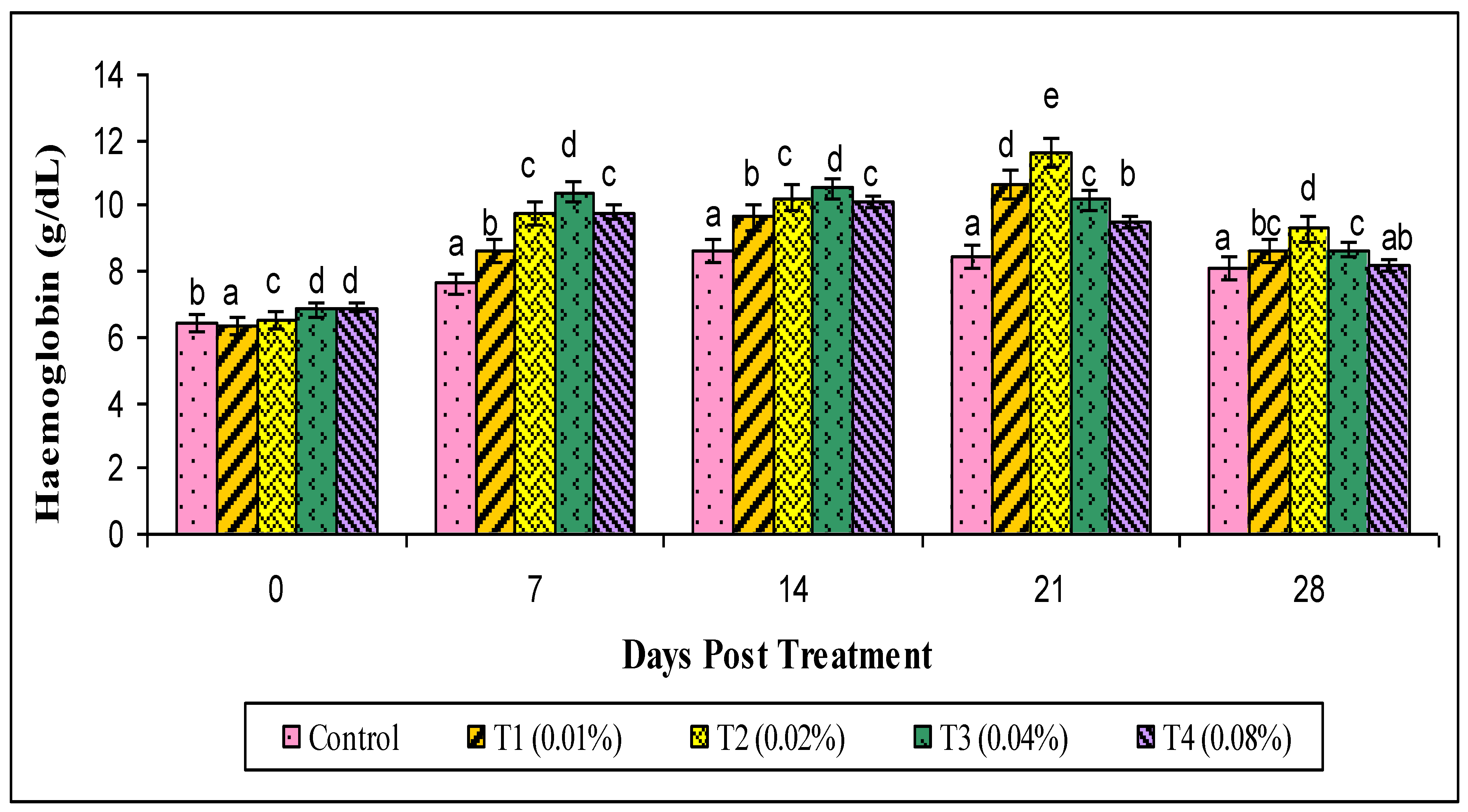
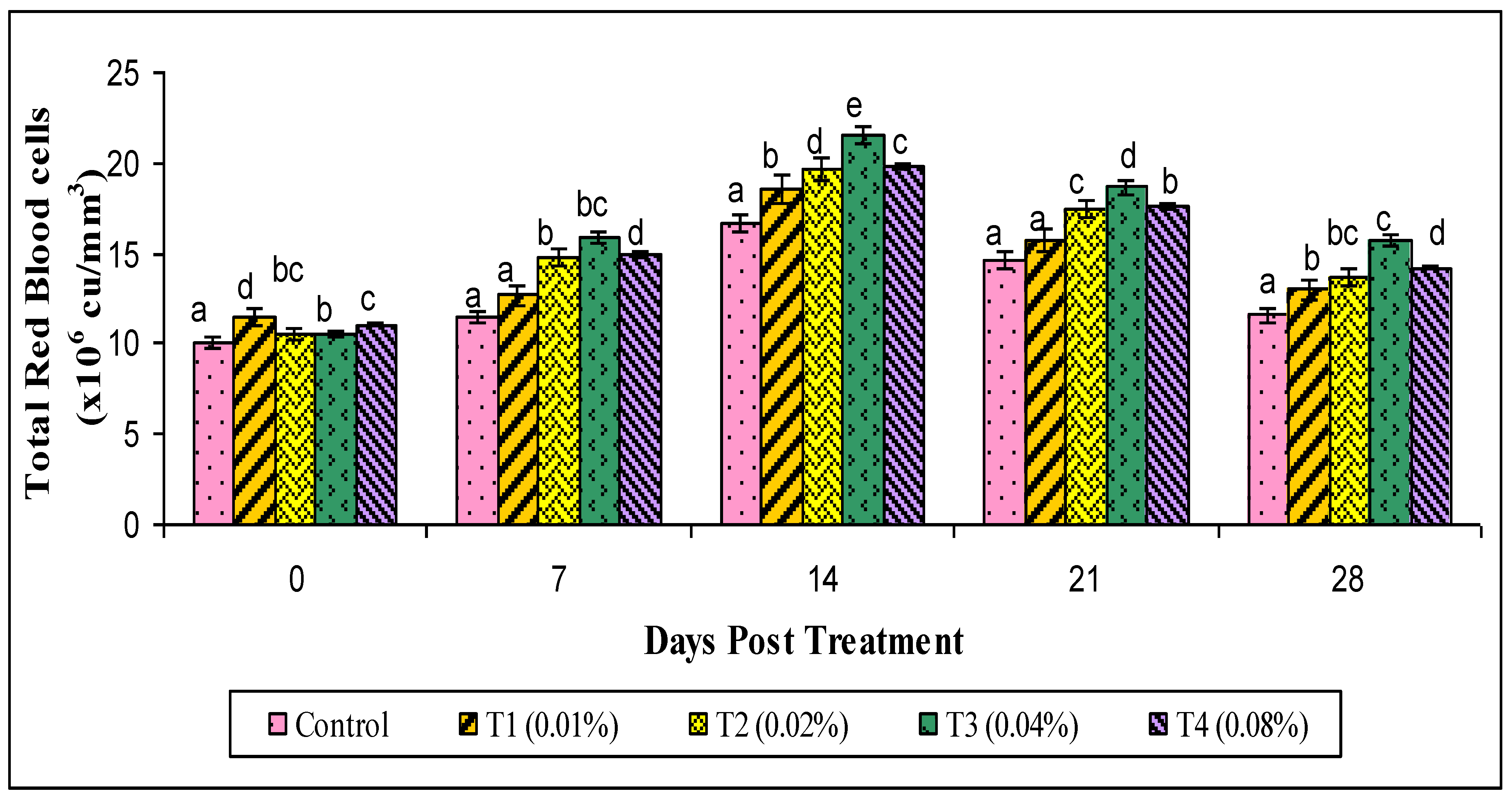
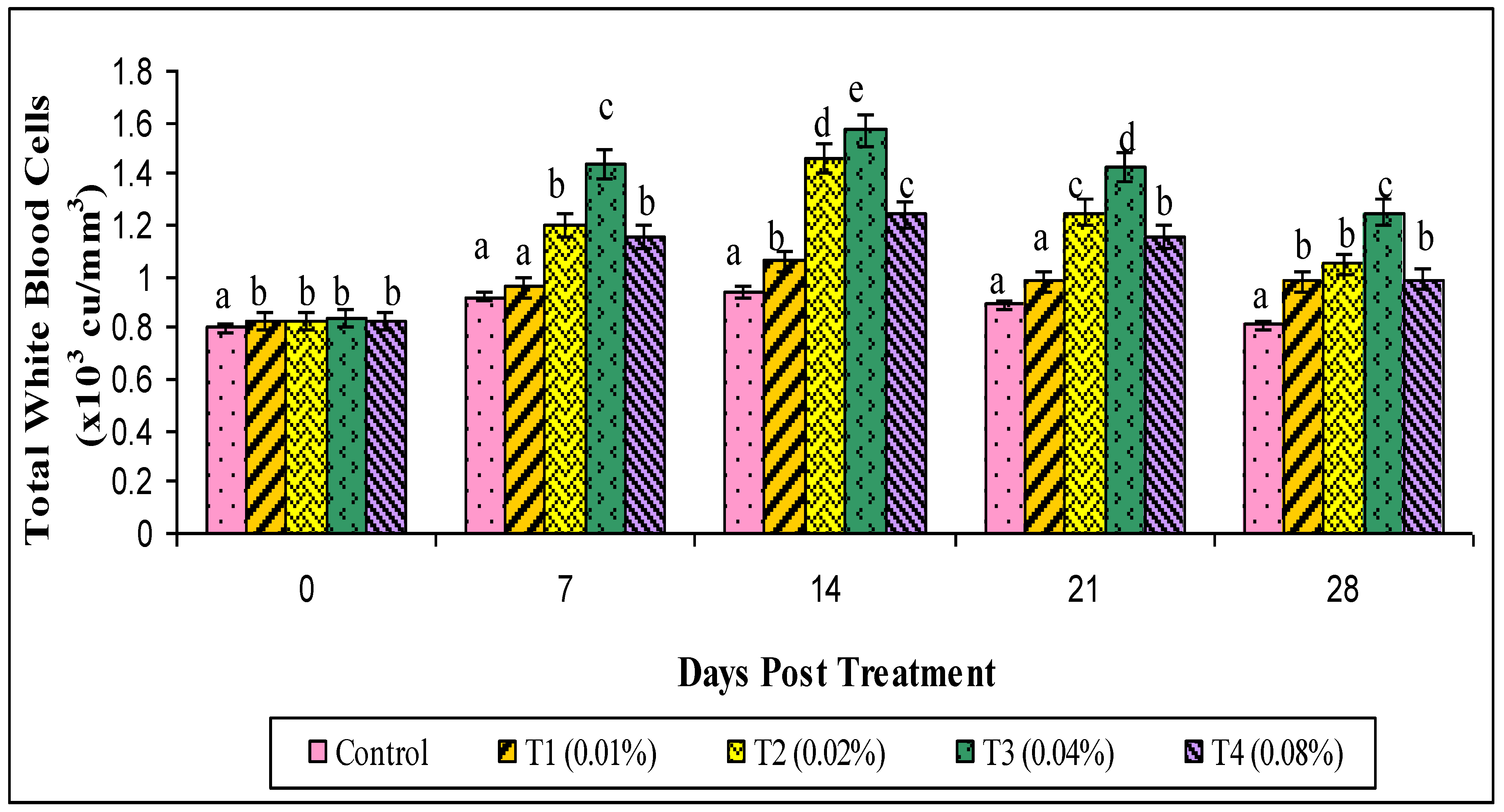
2.8. Hematological Parameters
2.9. Immunological Assays
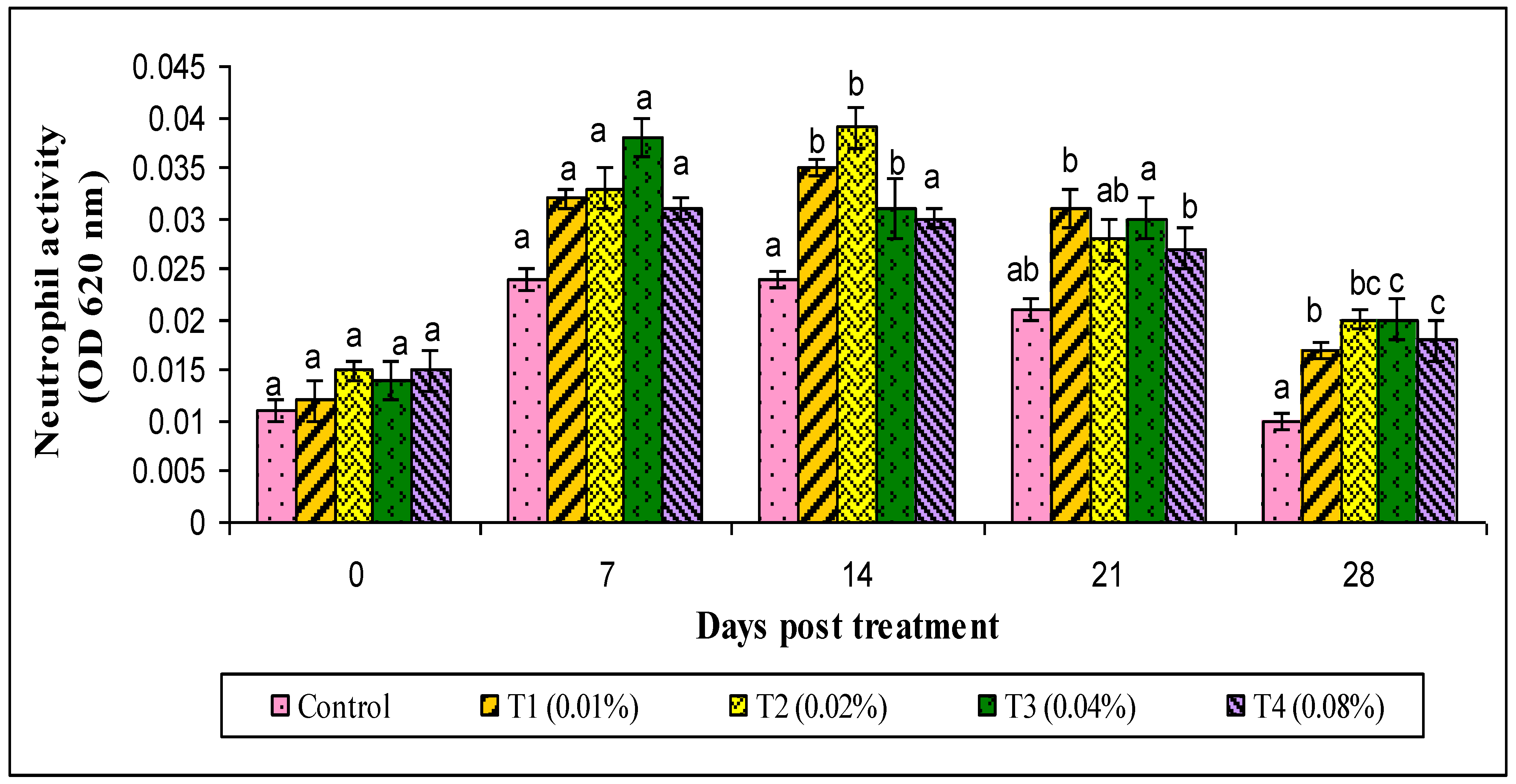
2.9.1. Respiratory Burst Assay (Neutrophil Activity)
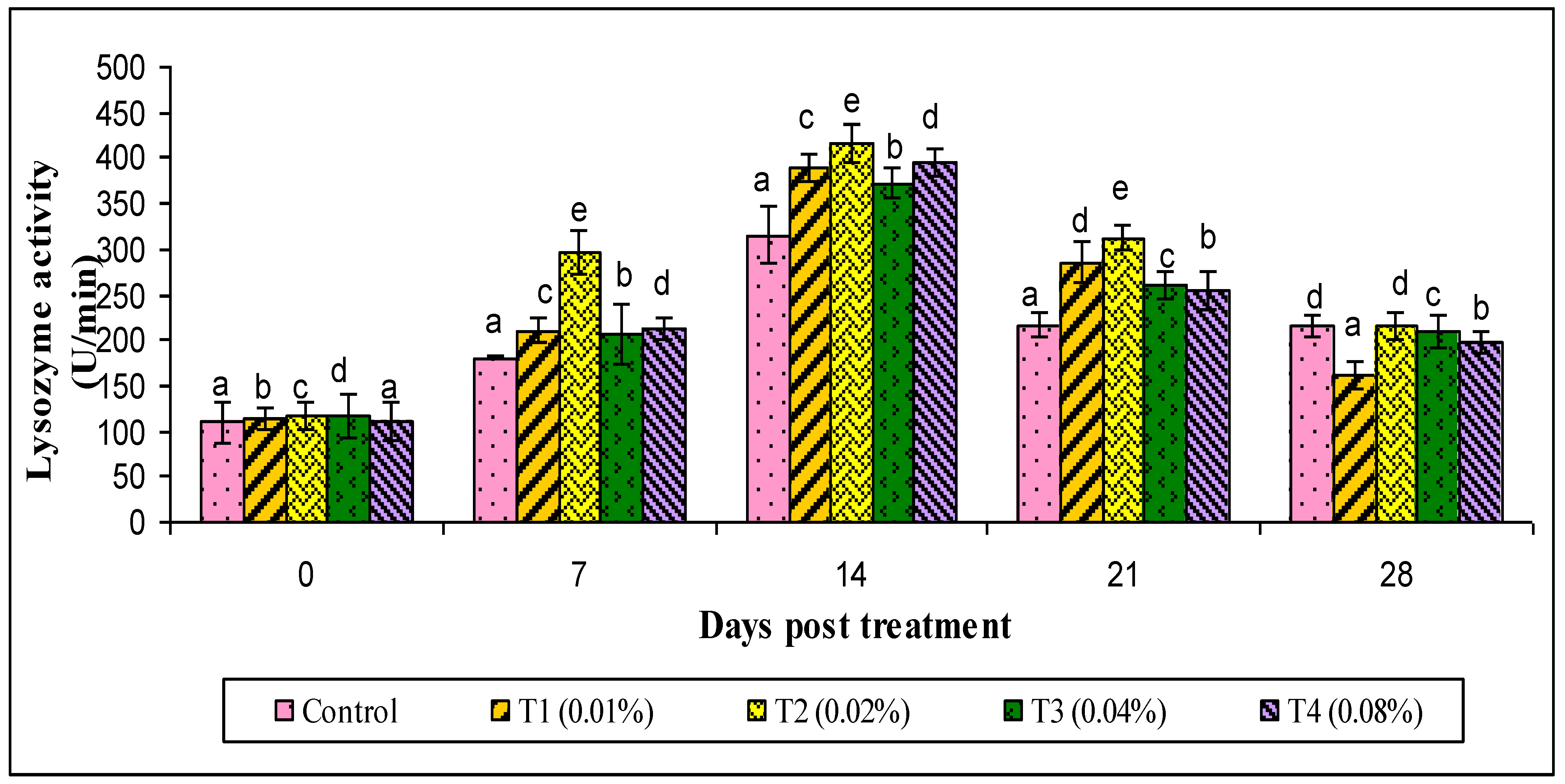
2.9.2. Lysozyme Assay
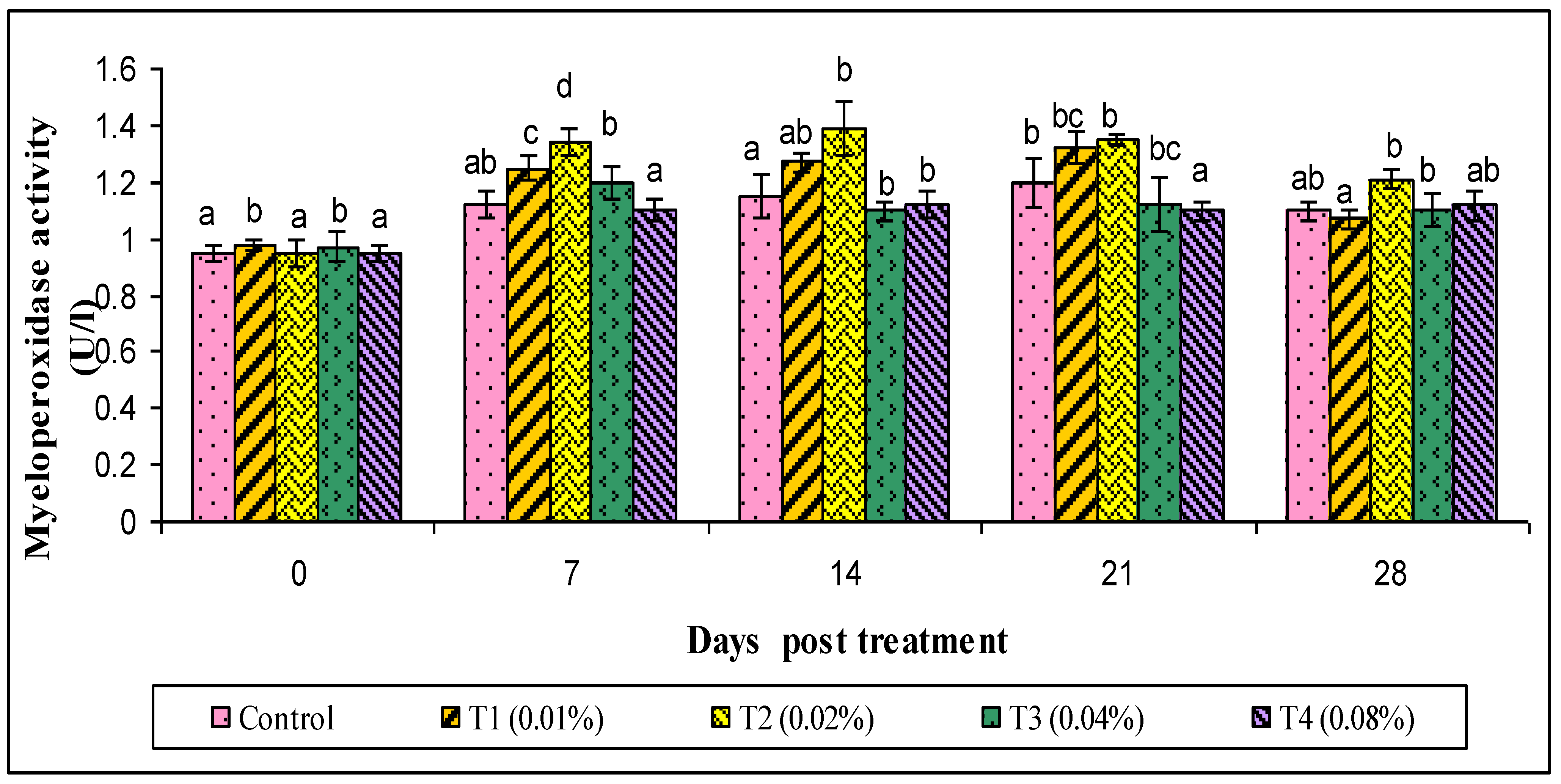
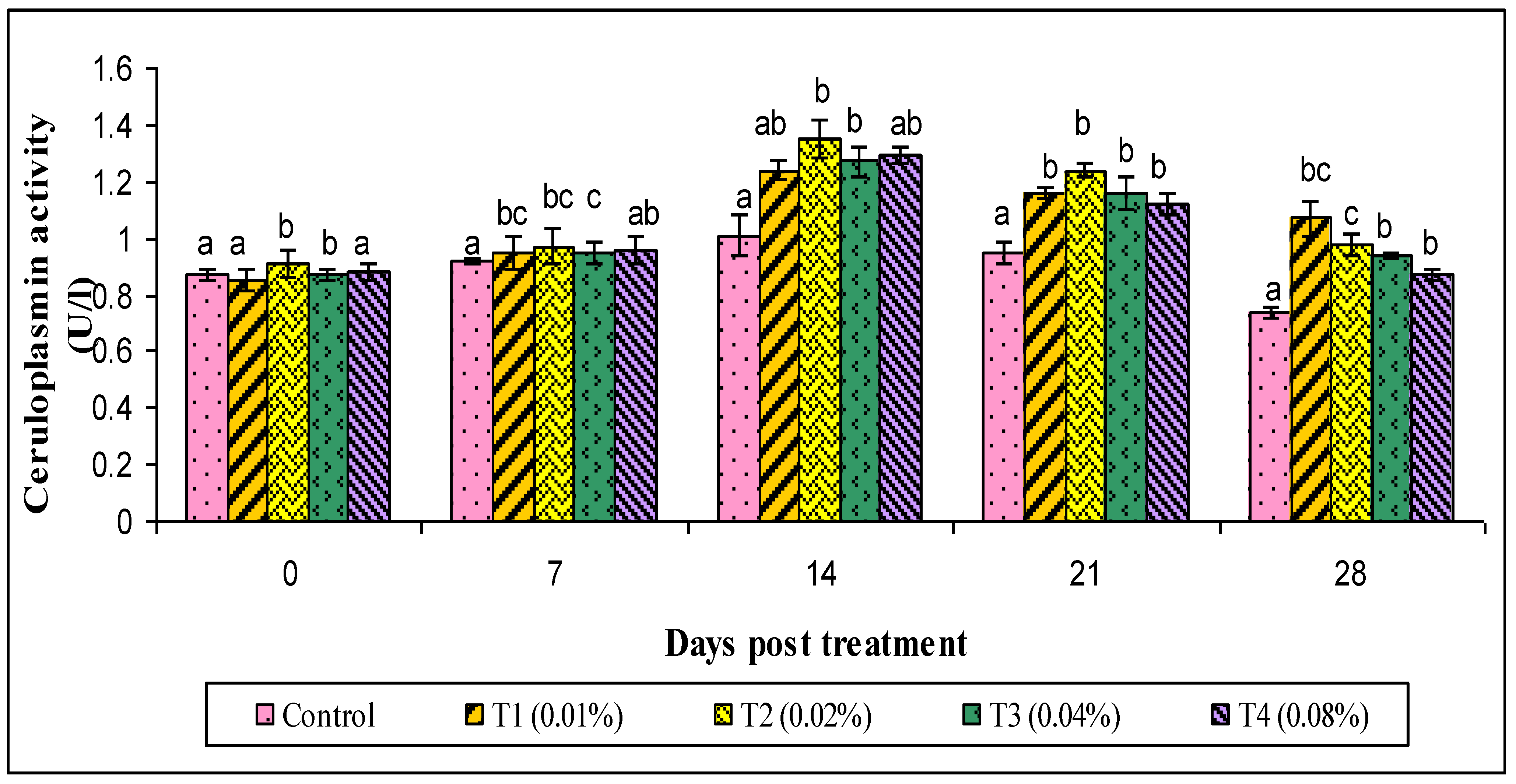
2.9.3. Ceruloplasmin and Myeloperoxidase (MPO) Assay
2.9.4. Bactericidal Assay
2.9.5. Serum Antiprotease Assay
2.10. Challenge Test
2.11. Immune-Related Gene Expression Analysis
Synthesis of cDNA, PCR and Expression Analysis
2.12. Statistical Analysis
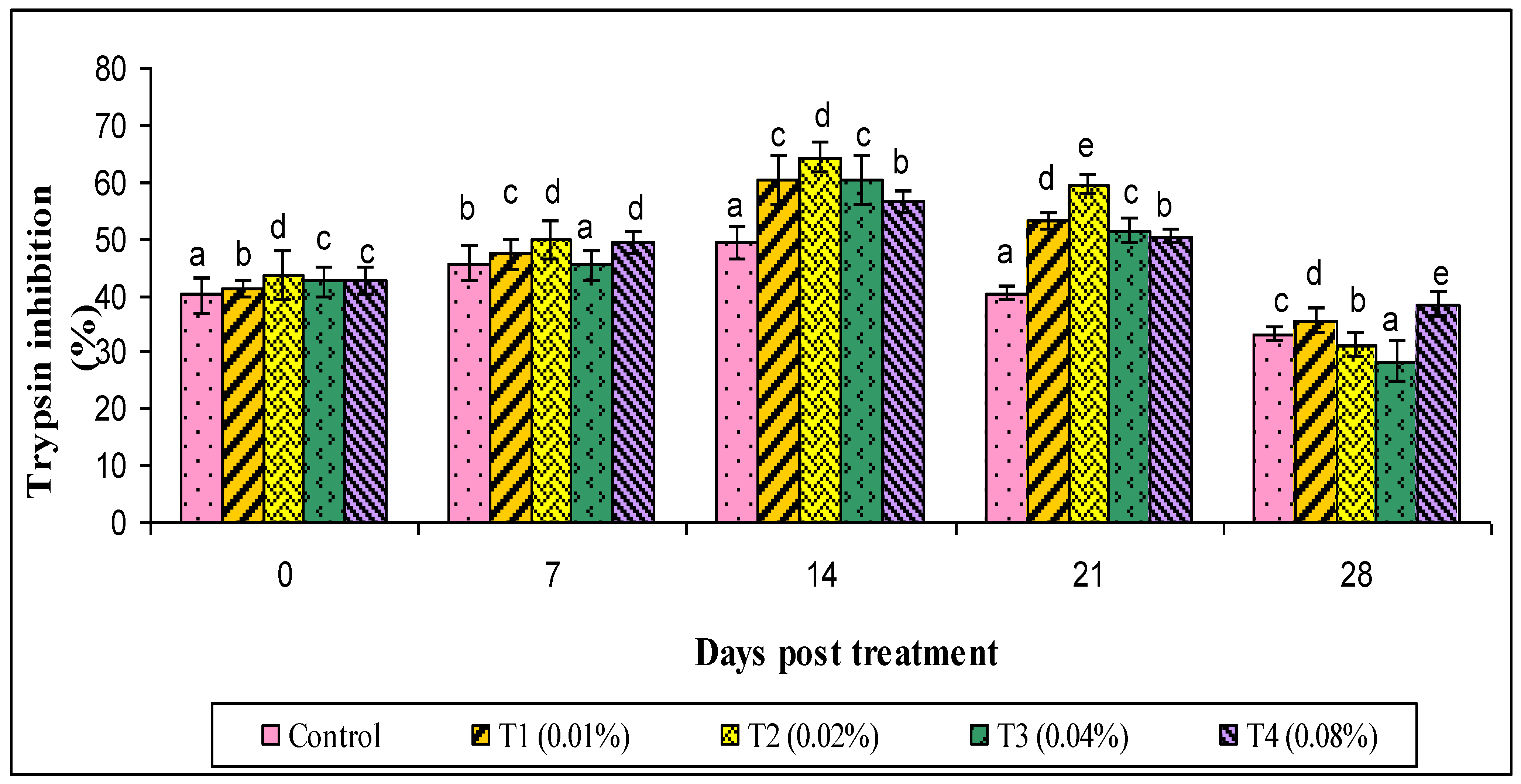
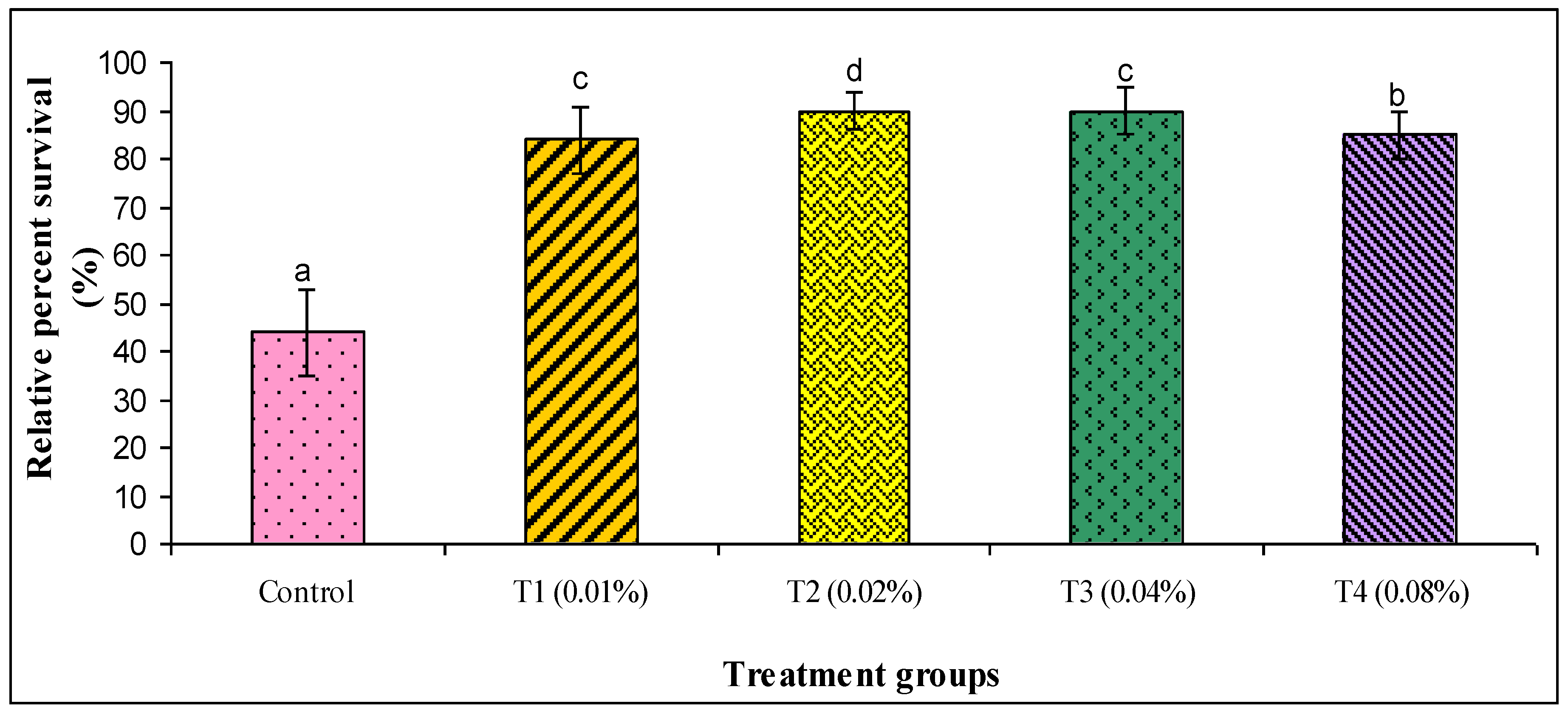
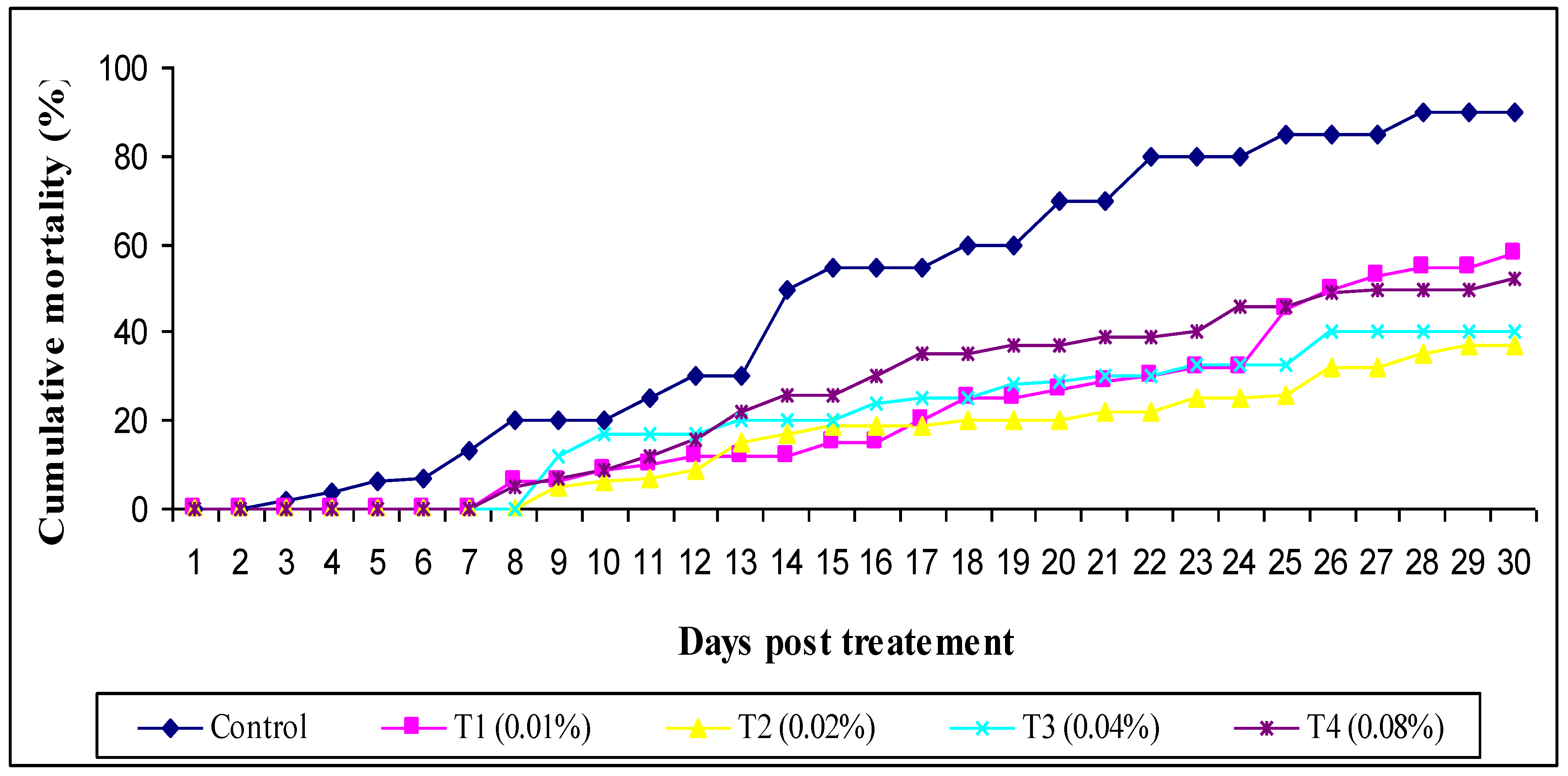
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoseinifar, S.H.; Yousefi, S.; Capillo, G.; Paknejad, H.; Khalili, M.; Tabarraei, A.; Van Doan, H.; Spanò, N.; Faggio, C. Mucosal immune parameters, immune and antioxidant defence elated genes expression and growth performance of zebrafish (Danio rerio) fed on Gracilaria gracilis powder. Fish Shellfish Immunol. 2018, 83, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Wan, A.H.; Davies, S.J.; Soler-Vila, A.; Fitzgerald, R.; Johnson, M. Macroalgae as a sustainable aquafeed ingredient. Rev. Aquac. 2018, 11, 458–492. [Google Scholar] [CrossRef]
- Guardiola, F.; Porcino, C.; Cerezuela, R.; Cuesta, A.; Faggio, C.; Esteban, M. Impact of date palm fruits extracts and probiotic enriched diet on antioxidant status, innate immune response and immune-related gene expression of European seabass (Dicentrarchus labrax). Fish Shellfish Immunol. 2016, 52, 298–308. [Google Scholar] [CrossRef]
- Sakai, M. Current research status of fish immunostimulants. Aquaculture 1999, 172, 63–92. [Google Scholar] [CrossRef]
- Wassef, E.A.; El Masry, M.H.; Mikhail, F.R. Growth enhancement and muscle structure of striped mullet, Mugil cephalus L., fingerlings by feeding algal meal-based diets. Aquac. Res. 2001, 32, 315–322. [Google Scholar] [CrossRef]
- Zeraatpisheh, F.; Firouzbakhsh, F.; Khalili, K.J. Effects of the macroalga Sargassum angustifolium hot water extract on hematological parameters and immune responses in rainbow trout (Oncohrynchus mykiss) infected with Yersinia rukeri. J. Appl. Phycol. 2018, 30, 2029–2037. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y. The growth performance and nonspecific immunity of juvenile grass carp (Ctenopharyngodon idella) affected by dietary Porphyra yezoensis polysaccharide supplementation. Fish Shellfish Immunol. 2019, 87, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications seaweed Ulva rigida C. Agardh. Int. Immunopharmacol. 2011, 7, 879–888. [Google Scholar]
- Fleurence, J. Seaweed Proteins. In Proteins in Food Processing; Yada, R.Y., Ed.; Woodhead Publishing: Cambridge, UK, 2004; pp. 197–213. [Google Scholar]
- Van Doan, H.; Lumsangkul, C.; Hoseinifar, S.H.; Hung, T.Q.; Stejskal, V.; Ringø, E.; Dawood, M.A.; Esteban, M. Administration of watermelon rind powder to Nile tilapia (Oreochromis niloticus) culture under biofloc system: Effect on growth performance, innate immune response, and disease resistance. Aquaculture 2020, 528, 735574. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; El-Araby, D.A. Immune and antioxidative effects of dietary licorice (Glycyrrhiza glabra L.) on performance of Nile tilapia, Oreochromis niloticus (L.) and its susceptibility to Aeromonas hydrophila infection. Aquaculture 2021, 530, 735828. [Google Scholar] [CrossRef]
- Bilen, S.; Altunoglu, Y.C.; Ulu, F.; Biswas, G. Innate immune and growth promoting responses to caper (Capparis spinosa) extract in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2016, 57, 206–212. [Google Scholar] [CrossRef]
- Gabriel, N.N.; Qiang, J.; Ma, X.Y.; He, J.; Xu, P.; Liu, K. Dietary Aloe vera improves plasma lipid profile, antioxidant, and hepatoprotective enzyme activities in GIFT-tilapia (Oreochromis niloticus) after Streptococcus iniae challenge. Fish Physiol. Biochem. 2015, 41, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Abtahi, B.; Adineh, H.; Hoseinifar, S.H.; Mirghaed, A.T.; Paolucci, M.; Van Doan, H. Effects of dietary arginine supplementation on cytokine- and antioxidant-related gene expressions in common carp (Cyprinus carpio) fingerling during ammonia toxicity. Aquac. Res. 2021, 52, 2751–2758. [Google Scholar] [CrossRef]
- Xu, A.; Shang-Guan, J.; Li, Z.; Gao, Z.; Huang, Y.C.; Chen, Q. Effects of dietary Chinese herbal medicines mixture on feeding attraction activity, growth performance, nonspecific immunity and digestive enzyme activity of Japanese seabass (Lateolabrax japonicus). Aquac. Rep. 2020, 17, 100304. [Google Scholar] [CrossRef]
- Khan, M.F.; Tang, H.; Lyles, J.T.; Pineau, R.; Mashwani, Z.-U.; Quave, C.L. Antibacterial properties of medicinal plants from Pakistan against multidrug-resistant ESKAPE pathogens. Front. Pharmacol. 2018, 9, 815. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Behrendt, U.; Ahmad, P.; Berg, G. Antimicrobial activity of medicinal plants correlates with the proportion of antagonistic endophytes. Front. Microbiol. 2017, 8, 199. [Google Scholar] [CrossRef]
- Sheikhzadeh, N.; Mousavi, S.; Oushani, A.K.; Firouzamandi, M.; Mardani, K. Spirulina platensis in rainbow trout (Oncorhynchus mykiss) feed: Effects on growth, fillet composition, and tissue antioxidant mechanisms. Aquac. Int. 2019, 27, 1613–1623. [Google Scholar] [CrossRef]
- Raji, A.A.; Jimoh, W.A.; Abu Bakar, N.H.; Taufek, N.H.M.; Muin, H.; Alias, Z.; Milow, P.; Razak, S.A. Dietary use of Spirulina (Arthrospira) and Chlorella instead of fish meal on growth and digestibility of nutrients, amino acids and fatty acids by African catfish. J. Appl. Phycol. 2020, 32, 1763–1770. [Google Scholar] [CrossRef]
- Al-Deriny, S.H.; Dawood, M.A.; Zaid, A.A.A.; El-Tras, W.F.; Paray, B.A.; Van Doan, H.; Mohamed, R.A. The synergistic effects of Spirulina platensis and Bacillus amyloliquefaciens on the growth performance, intestinal histomorphology, and immune response of Nile tilapia (Oreochromis niloticus). Aquac. Rep. 2020, 17, 100390. [Google Scholar] [CrossRef]
- Teles, R.M.B.; Graeber, T.G.; Krutzik, S.R.; Montoya, D.; Schenk, M.; Lee, D.J.; Komisopoulou, E.; Kelly-Scumpia, K.; Chun, R.; Iyer, S.S.; et al. Type I Interferon Suppresses Type II Interferon-Triggered Human Anti-Mycobacterial Responses. Science 2013, 339, 1448–1453. [Google Scholar] [CrossRef]
- Ravelonandro, P.H.; Ratianarivo, D.H.; Joannis-Cassan, C.; Isambert, A.; Raherimandim, M. By Influence of light quality and intensity in the cultivation of Spirulinaplatensis from Toliara (Madagascar) in a closed system. J. Chem. Technol. Biotechnol. 2008, 83, 842–848. [Google Scholar] [CrossRef]
- Das, B.K.; Pradhan, J.; Pattnaik, P.; Samantaray, B.R.; Samal, S.K. Production of antibacterials from the freshwater alga Euglena viridis (Ehren). World, J. Microbiol. Biotechnol. 2005, 21, 45–50. [Google Scholar] [CrossRef]
- Vijayavel, K.; Anbuselvam, C.; Balasubramanian, B. Antioxidant effect of the marine algae Chlorellavulgaris against naphthalene-indcued oxidative stress in the albino rats. Mol. Cell. Biocehm. 2007, 303, 39–44. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Animal Feed. In Association of Official Analytical Chemists, Official Methods of Analysis of Official Analytical Chemists International, 18th ed.; Horwitz, W., Latimer, G.W., Jr., Eds.; Association of Official Analytical Chemists: Washington, DC, USA, 2005; pp. 1–52. [Google Scholar]
- Sattanathan, G.; Thanapal, P.; Padmapriya, S.; Vijaya Anand, A.; Park, S.; Kim, I.H.; Balasubramanian, B. Influences of dietary inclusion of algae Chaetomporphaaerea enhanced growth performance, immunity, haematological response and disease resistance of Labeorohita challenged with Aeromonas hydrophila. Aquac. Rep. 2020, 100353, 100353. [Google Scholar] [CrossRef]
- Michael, R.D.; Srinivas, S.D.; Sailendri, K. A rapid method for repetitive bleeding in fish. Indian J. Exp. Biol. 1994, 32, 838–839. [Google Scholar]
- Ricker, W.K. Growth Rates and Models. In Fish Physiology Volume VIII Bioenergetics and Growth; Hoar, W.S., Randall, D.J., Brett, J.R., Eds.; Academic Press: New York, NY, USA, 1979; pp. 677–743. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein. Anal. Biochem. 1976, 72, 248. [Google Scholar] [CrossRef] [PubMed]
- Doumas, B.T.; Bayse, D.D.; Carter, R.J.; Peters, T.; Schaffer, R. A candidate reference method for determination of total protein in serum. I. Development and validation. Clin. Chem. 1981, 27, 1642–1650. [Google Scholar] [CrossRef]
- Stasiack, A.S.; Bauman, C.P. Neutrophil activity as a potent indicator for concomitant analysis. Fish Shellfish Immunol. 1996, 37, 539–545. [Google Scholar]
- Sahoo, P.K.; Rauta, P.R.; Mohanty, B.R.; Mahapatra, K.D.; Saha, J.N.; Rye, M.; Eknath, A.E. Selection for improved resistance to Aeromonas hydrophila in Indian major carp Labeorohita: Survival and innate immune responses in first generation of resistant and susceptible lines. Fish Shellfish Immunol. 2011, 31, 432–438. [Google Scholar] [CrossRef]
- Sattanathan, G.; Tamizhazhagan, V.; Padmapriya, S.; Wen-Chao, L.; Balamuralikrishnan, B. Effect of Green Algae Chaetomorpha antennina Extract on Growth, Modulate Immunity, and Defenses against Edwardsiellatarda Infection in Labeo rohita. Animals 2020, 10, 2033. [Google Scholar] [CrossRef]
- Rao, Y.V.; Chakrabarti, R. Enhanced anti-protease in Labeorohita fed with diet containing herbal ingredients. Indian J. Clin. Biochem. 2004, 19, 132–134. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Srivastava, P.K.; Verma, N.; Sharma, J. Effect of seeds of Achyranthes aspera on the immune responses and expression of some immune-related genes in carp Catla catla. Fish Shellfish. Immunol. 2014, 41, 64–69. [Google Scholar] [CrossRef]
- Sattanathan, G.; Shyamala, V.; Deepa, B.; Keerthiga, R. Dietary administration of Pleurotussajorcaju mushroom extract on Growth, Immune Response and Disease Resistance in Gibelioncatla against Aeromonas hydrophila. LS Int. J. Life Sci. 2018, 7, 142–150. [Google Scholar] [CrossRef]
- Chen, G.; Liu, Y.; Jiang, J.; Jiang, W.; Kuang, S.; Tang, L.; Tang, W.; Zhang, Y.-A.; Zhou, X.; Feng, L. Effect of dietary arginine on the immune response and gene expression in head kidney and spleen following infection of Jian carp with Aeromonas hydrophila. Fish Shellfish Immunol. 2015, 44, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Huttenhuis, H.B.; Grou, C.P.; Taverne-Thiele, A.J.; Taverne, N.; Rombout, J.H. Carp (Cyprinus carpio L.) innate immune factors are present before hatching. Fish Shellfish Immunol. 2006, 20, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.; Sahoo, P. Immune responses and expression profiles of some immune-related genes in Indian major carp Labeo rohita to Edwardsiella tarda infection. Fish Shellfish Immunol. 2010, 28, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.F.; Buchmann, K.; Nielsen, M.E. Real-time gene expression analysis in carp (Cyprinus carpio L.) skin: Inflammatory responses caused by the ectoparasite Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2007, 22, 641–650. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K.; Mohanty, B.R.; Mohanty, J.; Jena, J.K.; Mukherjee, S.C.; Sarangi, N. Modulation of the innate immune response of rohu Labeo rohita (Hamilton) by experimental freshwater lice Argulus siamensis (Wilson) infection. Aquac. Res. 2010, 41, e326–e335. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Soltani, M.; Pourgholam, R. Lysozyme activity of grass carp (Ctenopharingodon idella) following exposure to sublethal concentrations of organophosphate, diazinon. J. Vet. Res. 2007, 62, 49–52. [Google Scholar]
- Li, Z.H.; Zlabek, V.; Velisek, J.; Grabic, R.; Machova, J.; Kolarova, J.; Li, P. Acute toxicity of carb amazepine to juvenile rainbow trout (Oncorhynchus mykiss): Effects on antioxidant responses, hematological parameters and hepatic. Ecotoxicol. Environ. Saf. 2011, 74, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Sattanathan, G.; Tamizhazhagan, V.; Raza, N.; Shah, S.Q.A.; Hussain, M.Z.; Kim, K.-H. Effects of Green Alga, Chaetomorphaaerea Extract on Non-Specific Immune Responses and Disease Resistance against Edwardsiellatarda Infection in Labeo rohita. Appl. Sci. 2021, 11, 4325. [Google Scholar] [CrossRef]
- Adel, M.; Yeganeh, S.; Dadar, D.; Sakai, M.; Dawood, M.A.O. Effects of dietary Spirulina platensis on growth performance, humoral and mucosal immune responses and disease resistance in juvenile great sturgeon (Husohuso Linnaeus, 1754). Fish Shellfish Immunol. 2016, 56, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.R.; Reda, R.M.; Awad, A. Efficacy of Spirulina platensis diet supplements on disease resistance and immune-related gene expression in Cyprinus carpio L. exposed to herbicide atrazine. Fish Shellfish. Immunol. 2017, 67, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Kumar, M.; Reddy, C.R.K.; Jha, B. Algal Lipids, Fatty Acids and Sterols. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 87–134. [Google Scholar]
- Siwicki, A.K.; Anderson, D.P. Nonspecific Defense Mechanisms Assay in Fish: II. Potential Killing Activity of Neutrophils and Macrophages, Lysozyme Activity in Serum and Organs and Total Immunoglobulin Level in Serum. In Fish Disease Diagnosis and Prevention Methods; Wydawnictwo Instytutu Rybactwa Srodladowego: Olsztyn, Poland, 1993; pp. 105–112. [Google Scholar]
- Mohammadi, G.; Rafiee, G.; El Basuini, M.F.; Van Doan, H.; Ahmed, H.A.; Dawood, M.A.; Abdel-Latif, H.M. Oregano (Origanum vulgare), St John’s-wort (Hypericum perforatum), and lemon balm (Melissa officinalis) extracts improved the growth rate, antioxidative, and immunological responses in Nile tilapia (Oreochromis niloticus) infected with Aeromonas hydrophila. Aquac. Rep. 2020, 18, 100445. [Google Scholar] [CrossRef]
- Bai, S.C.; Koo, J.W.; Kim, K.W.; Kim, S.K. Effects of Chlorella powder as a feed additive on growth performance in juvenile Korean rock fish, Sebastesschlegeli (Hilgendorf). Aquac. Res. 2001, 32, 92–98. [Google Scholar] [CrossRef]
- Pradhan, J.; Kumardas, B. Effect of dietary Chlorella vulgaris on liver enzymatic profiles of rohu Labeo rohita (Hamilton, 1822). Indian J. Fish. 2015, 62, 132–136. [Google Scholar]
- Svobodová, Z.; Máchová, J.; Drastichová, J.; Groch, L.; Lusková, V.; Poleszczuk, G.; Kroupová, H. Haematological and biochemical profiles of carp blood following nitrite exposure at different concentrations of chloride. Aquac. Res. 2005, 36, 1177–1184. [Google Scholar] [CrossRef]
- Ozovehe, B.N. Growth performance, haematological indices and some biochemical enzymes of juveniles Clarias gariepinus (Burchell 1822) fed varying levels of Moringa oleifera leaf meal diet. J. Aquac. Res. Dev. 2013, 4, 145–150. [Google Scholar] [CrossRef]
- Dienye, H.; Olumuji, O. Growth performance and haematological responses of African mud catfish Clarias gariepinus fed dietary levels of Moringa oleifera leaf meal. Net J. Agric. Sci. 2014, 2, 79–88. [Google Scholar]
- Erhunmwunse, N.; Ainerua, M. Characterization of some blood parameters of African Catfish (Clarias gariepinus). Am. Eurasian J. Toxicol. Sci. 2013, 5, 72–76. [Google Scholar]
- Taufek, N.M.; Aspani, F.; Muin, H.; Raji, A.A.; Razak, S.A.; Alias, Z. The effect of dietary cricket meal (Gryllus bimaculatus) on growth performance, antioxidant enzyme activities, and haematological response of African catfish (Clarias gariepinus). Fish Physiol. Biochem. 2016, 42, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Yeganeh, S.; Teimouri, M.; Amirkolaie, A.K. Dietary effects of Spirulina platensis on hematological and serum biochemical parameters of rainbow trout (Oncorhynchus mykiss). Res. Vet. Sci. 2015, 101, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Khani, M.; Soltani, M.; Mehrjan, M.S.; Foroudi, F.; Ghaeni, M. Short communication: The effects of Chlorella vulgaris supplementation on growth performance, blood characteristics, and digestive enzymes in Koi (Cyprinus carpio). Iran. J. Fish. Sci. 2017, 16, 832–843. [Google Scholar]
- Magnadóttir, B. Innate immunity of fish (overview). Fish Shellfish Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef]
- Wassef, E.A.; El-Sayed, A.-F.M.; Sakr, E.M. Pterocladia (Rhodophyta) and Ulva (Chlorophyta) as feed supplements for European seabass, Dicentrarchus labrax L., fry. J. Appl. Phycol. 2013, 25, 1369–1376. [Google Scholar] [CrossRef]
- Van Muiswinkel, W.B.; Nakao, M. A short history of research on immunity to infectious diseases in fish. Dev. Comp. Immunol. 2014, 43, 130–150. [Google Scholar] [CrossRef]
- Zhao, J.G.; Zhou, L.; Jin, J.Y.; Zhao, Z.; Lan, J.; Zhang, Y.B.; Zhang, Q.Y.; Gui, J.F. Antimicrobial activity-specific to Gram-negative bacteria and immune modulation-mediated NF-κB and Sp1 of a medaka β-defensin. Dev. Comp. Immunol. 2009, 33, 624–637. [Google Scholar] [CrossRef]
- Wan, A.H.L.; Soler-Vila, A.; O’Keeffe, D.; Casburn, P.; Fitzgerald, R.; Johnson, M.P. The inclusion of Palmaria palmata macroalgae in Atlantic salmon (Salmo salar) diets: Effects on growth, haematology, immunity and liver function. J. Appl. Phycol. 2016, 28, 3091–3100. [Google Scholar] [CrossRef]
- Gabrielsen, B.O.; Austreng, E. Growth, product quality and immune status of Atlantic salmon, Salmo salar L., fed wet feed with alginate. Aquac. Res. 1998, 29, 397–401. [Google Scholar] [CrossRef]
- Prabu, D.L.; Sahu, N.P.; Pal, A.K.; Dasgupta, S.; Narendra, A. Immunomodulation and interferon gamma gene expression in sutchi catfish, Pangasianodon hypophthalmus: Effect of dietary fucoidan rich seaweed extract (FRSE) on pre and post challenge period. Aquac. Res. 2016, 47, 199–218. [Google Scholar] [CrossRef]
- Sajina, K.A.; Sahu, N.P.; Varghese, T.; Jain, K.K. Fucodan-rich Sargassum wightii extract supplemented with Alpha amylase improve growth and immune responses of Labeo rohita (Hamilton, 1822) fingerlings. J. Appl. Phcology. 2019, 31(4), 2469–2480. [Google Scholar] [CrossRef]
- Yua, W.; Wena, G.; Lina, H.; Yanga, Y.; Huanga, X.; Zhoua, C.H.; Zhangc, Z.; Duana, Y.; Huanga, Z.H.; Lia, T. Effects of dietary Spirulina platensis on growth performance, hematological and serum biochemical parameters, hepatic antioxidant status, immune responses and disease resistance of coral trout Plectropomus leopardus (Lacepede, 1802). Fish Shellfish Immunol. 2018, 74, 649–655. [Google Scholar] [CrossRef]
- Rajendran, P.; Subramani, P.A.; Michael, D. Polysaccharides from marine macroalga, Padina gymnospora improve the nonspecific and specific immune responses of Cyprinus carpio and protect it from different pathogens. Fish Shellfish Immunol. 2016, 58, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Bhavan, P.S.; Seenivasan, C.; Shanthi, R.; Muralisankar, T. Replacement of fishmeal with Spirulina platensis, Chlorella vulgaris and Azolla pinnata on non-enzymatic and enzymatic antioxidant activities of Macrobrachium rosenbergii. J. Basic Appl. Zoöl. 2014, 67, 25–33. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Guo, H.; Wu, J.; Xue, Y. Effects of water-soluble fractions of diesel oil on the antioxidant defenses of the goldfish, Carassius auratus. Ecotoxicol. Environ. Saf. 2004, 58, 110–116. [Google Scholar] [CrossRef]
- Velasquez, S.F.; Chan, M.A.; Abisado, R.G.; Traifalgar, R.F.M.; Tayamen, M.M.; Maliwat, G.C.F.; Ragaza, J.A. Dietary Spirulina (Arthrospira platensis) replacement enhances performance of juvenile Nile tilapia (Oreochromis niloticus). J. Appl. Phycol. 2016, 28, 1023–1030. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, H.; Zhang, H. The effect of Sargassum fusiforme polysaccharide extracts on vibriosis resistance and immune activity of the shrimp, Fenneropenaeus chinensis. Fish Shellfish Immunol. 2006, 20, 750–757. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Ahmad, M.H.; Abdel-Hadi, Y.M.; Seden, M.E.A. Use of Spirulina (Arthrospira platensis) as a Growth and Immunity Promoter for Nile Tilapia, Oreochromis niloticus (L.) Fry Challenged with Pathogenic Aeromonas hydrophila. In Proceedings of the 8th International symposium on Tilapia Aquaculture, Cairo, Egypt, 12–14 October 2008; pp. 1015–1032. [Google Scholar]
- Grayfer, L.; Walsh, J.G.; Belosevic, M. Characterization and functional analysis of goldfish (Carassius auratus L.) tumor necrosis factor-alpha. Dev. Comp. Immunol. 2008, 32, 532–543. [Google Scholar] [CrossRef]
- Corripio-Miyar, Y.; Bird, S.; Tsamopoulos, K.; Secombes, C. Cloning and expression analysis of two pro-inflammatory cytokines, IL-1β and IL-8, in haddock (Melanogrammus aeglefinus). Mol. Immunol. 2007, 44, 1361–1373. [Google Scholar] [CrossRef]
- Jimenez-Cantizano, R.M.; Infante, C.; Martin-Antonio, B.; Ponce, M.; Hachero, I.; Navas, J.I.; Manchado, M. Molecular characterization, phylogeny, and expression of c-type and g-type lysozymes in brill Scophthalmus rhombus. Fish Shellfish Immunol. 2008, 25, 57–65. [Google Scholar] [CrossRef]
- Juan, T.S.; Wilson, D.R.; Wilde, M.D.; Darlington, G.J. Participation of the transcription factor C/EBP delta in the acute-phase regulation of the human gene for complement component C3. Proc. Natl. Acad. Sci. USA 1993, 90, 2584–2588. [Google Scholar] [CrossRef] [PubMed]
- Watanuki, H.; Ota, K.; Tassakka, A.C.M.; Kato, T.; Masahiro, S. Immuno stimulant effects of dietary Spirulina platensis on carp, (Cyprinus carpio). Aquaculture 2006, 258, 157–163. [Google Scholar] [CrossRef]
- Yuan, C.; Pan, X.; Gong, Y.; Xia, A.; Wu, G.; Tang, J. Effects of astragalus poly saccharides (APS) on the expression of immune response genes in headkidney, gill and spleen of the common carp, (Cyprinus carpio L.). Int. Immunopharmacol. 2008, 8, 51–58. [Google Scholar] [CrossRef]
- Tort, L.; Balasch, J.; Mackenzie, S. Fish immune system. A crossroads between innate and adaptive responses. Inmunologia. 2003, 22, 277–286. [Google Scholar]
- Heydari, M.; Firouzbakhsh, F.; Paknejad, H. Effects of Mentha longifolia extract on some blood and immune parameters, and disease resistance against yersiniosis in rainbow trout. Aquaculture 2019, 515, 734586. [Google Scholar] [CrossRef]
- Mehrabi, Z.; Firouzbakhsha, F.; Rahimi-Mianjib, G.H.; Paknejad, H. Immunostimulatory effect of Aloe vera (Aloe barbadensis) on non-specific immune response, immune gene expression, and experimental challenge with Saprolegnia parasitica in rainbow trout (Oncorhynchus mykiss). Aquaculture 2019, 503, 330–338. [Google Scholar] [CrossRef]
- Singh, S.T.; Kamilya, D.; Kheti, B.; Bordoloi, B.; Parhi, J. Paraprobiotic preparation from Bacillus amyloliquefaciens FPTB16 modulates immune response and immune relevant gene expression in Catla catla (Hamilton, 1822). Fish Shellfish Immunol. 2017, 66, 35–42. [Google Scholar] [CrossRef]
- Ramnani, P.; Chitarrari, R.; Tuohy, K.; Grant, J.; Hotchkiss, S.; Philp, K.; Campbell, R.; Gill, C.; Rowland, I. In vitro fermentation and prebiotic potential of novel low molecular weight polysaccharides derived from agar and alginate seaweeds. Anaerobe 2012, 18, 1–6. [Google Scholar] [CrossRef]
- Saad, N.; Delattre, C.; Urdaci, M.; Schmitter, J.M.; Bressollier, P. An overview of the last advances in probiotic and prebiotic field. LWT Food Sci. Technol. 2013, 50, 1–16. [Google Scholar] [CrossRef]


| Experimental Diet (g/kg) | |||||
|---|---|---|---|---|---|
| Ingredients (g/kg) | Control (0 %) | T1 (0.01%) | T2 (0.02%) | T3 (0.04%) | T4 (0.08%) |
| Rice bran (g) | 170 | 169 | 168 | 166 | 162 |
| Soybean meal (g) | 550 | 550 | 550 | 550 | 550 |
| Fish meal (g) | 50 | 50 | 50 | 50 | 50 |
| Wheat flour (g) | 20 | 20 | 20 | 20 | 20 |
| Corn flour (g) | 150 | 150 | 150 | 150 | 150 |
| Vegetable oil (v/w) | 25 | 25 | 25 | 25 | 25 |
| Iodized salt (g) | 10 | 10 | 10 | 10 | 10 |
| Vitamin and minerals * | 25 | 25 | 25 | 25 | 25 |
| Mixed algal blend (%) (w/w) | Absent | 0.01 | 0.02 | 0.04 | 0.08 |
| Proximate Composition of Diets (%) | |||||
| Total protein | 35.6 ± 4.54 c | 35.2 ± 4.89 b | 35.6 ± 5.33 c | 35.2 ± 4.21 b | 33.5 ± 4.49 a |
| Total carbohydrate | 94.2 ± 5.35 a | 94.4 ± 4.69 a | 94.3 ± 5.55 a | 94.2 ± 5.33 a | 94.4 ± 5.39 a |
| Total lipid | 120.4 ± 10.43 a | 124 ±10.35 b | 124.2 ±12.55 b | 124.6 ± 9.85 b | 124.9 ± 10.54 b |
| Moisture (%) | 4.0 ± 0.89 b | 3.5 ± 0.84 a | 4.8 ±1.04 d | 4.2 ± 1.90 b | 4.3 ± 0.92 c |
| Ash (%) | 7.5 ± 0.99 a | 9.0 ± 1.40 b | 9.4 ± 0.82 b | 8.7 ± 1.04 b | 9.1 ± 1.03 b |
| Dry matter (%) | 87.4 ± 5.32 a | 98.9 ±4.35 b | 93.0 ± 5.43 b | 91.3 ± 5.52 b | 94.5 ± 4.37 b |
| Proximate Composition of Tissue (%) | |||||
| Total protein | 216 ± 18.8 a | 284 ± 18.54 d | 233 ± 19.54 b | 240 ± 19.54 b | 258 ± 18.95 c |
| Total carbohydrate | 143 ± 12.54 a | 235 ± 21.64 e | 213 ± 22.87 d | 208 ± 23.98 c | 194 ± 22.87 b |
| Total lipid | 98 ± 10.84 d | 77 ± 10.8 b | 89 ± 9.95 c | 69 ± 8.69 a | 98 ± 10.84 d |
| Moisture (%) | 25.7 ± 8.12 b | 47.6 ± 7.95 e | 39.2 ± 9.44 c | 26.7 ± 5.87 b | 43.2 ± 8.65 d |
| Ash (%) | 26.8 ± 5.65 b | 26.4 ± 6.84 b | 29.9 ± 6.35 c | 25.5 ± 6.78 b | 21.3 ± 6.92 a |
| Dry matter (%) | 89.7 ± 12.25 b | 85.8 ± 13.68 a | 98.6 ± 15.84 e | 90.4 ± 16.32 c | 94.8 ± 15.98 d |
| Proximate Composition of Microalgae (%) | |||||
| Proximate composition | Different green micro-algae | ||||
| Spirulina platensis | Euglena viridis | Chlorella vulgaris | Mixed algae blend | ||
| Total protein | 26.84 | 25.59 | 27.65 | 32.70 | |
| Total carbohydrate | 38.45 | 36.07 | 36.95 | 42.58 | |
| Ether extract | 6.54 | 6.85 | 7.22 | 8.24 | |
| Ash (%) | 7.14 | 8.20 | 7.65 | 8.65 | |
| Dry matter (%) | 78.52 | 78.25 | 78.96 | 78.65 | |
| Target Gene | Primer Sequence (5’-3’) | Optimum Annealing Temperature (°C) | Size of PCR Amplification (bp) | Reference |
|---|---|---|---|---|
| NKEF-B | F-ACTGTGACCATCGAGTTC R-TGGGCAAGTAATTGCTG | 49 | 285 | Chen et al. [37] |
| Lysozyme–C | F-GCTGTGATGTTGTCCTATCTTC R-GTAACTTCCCCAGGTATCC | 52.7 | 321 | Huttenhuis et al. [38] |
| Lysozyme G | F-CTTATGCAGGTTGACAAACG R-GGCAACAACATCACTGGAGTAATC | 53.5 | 249 | Mohanty and Sahoo [39] |
| TNF α | F-CCAGGCTTTCACTTCAGG R-GCCATAGGAATCGGAGTAG | 51.6 | 102 | Gonzalez et al. [40] |
| TLR22 | F-TCACCCCATTTCGAGGAATGTC R-GAAGGCGTCGTACTGGCTAACAT | 56.0 | 520 | Saurabh et al. [41] |
| β2M | F-TCCAGTCCCAAGATTCAGGTG R-TGGTGAGGTGAAACTGCCAG | 59.7 | 175 | Mohanty and Sahoo [39] |
| β-actin | F-GACTTCGAGCAGGAGATGG R-CAAGAAGGATGGCTGGAACA | 55.3 | 138 | Mohanty and Sahoo [39] |
| Parameters | Control (0 %) | T1 (0.01%) | T2 (0.02%) | T3 (0.04%) | T4 (0.08%) |
|---|---|---|---|---|---|
| Initial body weight (g) | 100.46 ± 1.38 | 100.19 ± 1.34 | 100.12 ± 2.26 | 100.10 ± 1.68 | 100.60 ± 1.98 |
| Final body weight (g) | 115.98 ± 4.65 a | 118.77 ± 6.77 a | 126.09 ± 5.3 b | 130.41 ± 7.52 c | 124.49 ± 8.68 b |
| Total weight gain (TWG) | 15.51 ± 1.43 a | 18.58 ± 1.64 ab | 25.97 ± 2.56 b | 30.30 ± 1.55 c | 23.89 ± 1.98 b |
| Specific growth rate (SGR/d) | 0.51 ± 0.03 a | 0.60 ± 0.09 b | 0.82 ± 0.02 d | 0.94 ± 0.05 e | 0.76 ± 0.03 c |
| Feed conversion rate (FCR) | 1.65 ± 0.35 b | 1.53 ± 0.21 a | 1.55 ± 0.43 ab | 1.56 ± 0.32 ab | 1.55 ± 0.09 b |
| Parameters | Treatment Days | Control (0 %) | T1 (0.01%) | T2 (0.02%) | T3 (0.04%) | T4 (0.08%) |
|---|---|---|---|---|---|---|
| Total serum protein (g dL−1) | Initial day | 9.85 ± 0.24 a | 10.98 ± 1.02 a | 11.42 ± 1.49 b | 10.53 ± 1.40 a | 9.84 ± 0.98 b |
| 7 | 9.9 ± 0.12 a | 11.42 ± 0.20 b | 12.42 ± 0.19 c | 13.34 ± 0.14 d | 10.53 ± 0.16 c | |
| 14 | 10.2 ± 0.11 a | 12.54 ± 0.13 b | 15.76 ± 0.09 c | 14.76 ± 0.12 d | 12.54 ± 0.11c | |
| 21 | 9.93 ± 0.84 a | 13.76 ± 0.89 b | 17.65 ± 0.88 c | 15.59 ± 0.95 d | 13.98 ± 0.96e | |
| 28 | 10.1 ± 1.2 a | 12.76 ± 1.5 b | 15.76 ± 1.6 c | 16.65 ± 1.9 d | 14.77 ± 1.2 e | |
| Albumin (g dL−1) | Initial day | 5.34 ± 0.9 c | 6.34 ± 0.04 a | 7.53 ± 0.5 b | 8.0 ± 0.22 a | 6.2 ± 0.25 c |
| 7 | 5.03 ± 0.43 a | 7.34 ± 0.45 b | 8.76 ± 0.38 c | 10.34 ± 0.78 c | 7.53 ± 0.58d | |
| 14 | 5.2 ± 0.35 a | 7.98 ± 0.54 b | 9.7 ± 0.33 c | 8.65 ± 0.32 d | 8.66 ± 0.06c | |
| 21 | 5.04 ± 0.33 a | 8.7 ± 1.45 d | 10.25 ± 1.64 b | 8.59 ± 1.55 b | 9.98 ± 2.54 c | |
| 28 | 5.32 ± 0.54 a | 7.76 ± 1.44 b | 9.76 ± 1.21 c | 9.65 ± 1.32 e | 8.77 ± 1.03 d | |
| Globulin (g dL−1) | Initial day | 4.51 ± 0.93 a | 4.64 ± 0.85 b | 3.89 ± 0.75 b | 2.53 ± 0.96 b | 3.64 ± 0.99 a |
| 7 | 4.87 ± 1.24 b | 4.08 ± 1.43 a | 3.66 ± 1.29 b | 3.0 ± 1.04 d | 3.0 ± 1.02c | |
| 14 | 5.00 ± 1.2 a | 4.56 ± 2.1 b | 6.06 ± 2.2 c | 6.11 ± 1.9 d | 3.88 ± 1.8c | |
| 21 | 4.89 ± 1.4 a | 5.06 ± 1.04 b | 7.4 ± 1.8 c | 7.0 ± 1.4 d | 4.0 ± 1.05d | |
| 28 | 4.78 ± 0.9 a | 5.0 ± 0.8 b | 6.1 ± 0.5 c | 7.0 ± 0.6 d | 6.0 ± 0.9 d | |
| A/G ratio | Initial day | 1.18 ± 0.09 b | 1.36 ± 0.08 a | 1.93 ± 0.06 a | 3.16 ± 0.04 a | 1.70 ± 0.06 b |
| 7 | 1.03 ± 0.4 a | 1.79 ± 0.06 b | 2.39 ± 0.6 b | 3.44 ± 0.2 a | 2.51 ± 0.5b | |
| 14 | 1.04 ± 0.34 c | 1.75 ± 0.4 d | 1.60 ± 0.24 b | 1.41 ± 0.53 a | 2.23 ± 0.43b | |
| 21 | 1.03 ± 0.29 c | 1.71 ± 0.21 d | 1.38 ± 0.22 b | 1.22 ± 0.21 a | 2.49 ± 0.25 b | |
| 28 | 1.11 ± 0.95 a | 1.55 ± 0.89 b | 1.62 ± 0.74 b | 1.37 ± 0.98 b | 1.46 ± 0.76 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sattanathan, G.; Liu, W.-C.; Padmapriya, S.; Pushparaj, K.; Sureshkumar, S.; Lee, J.-W.; Balasubramanian, B.; Kim, I.H. Effects of Dietary Blend of Algae Extract Supplementation on Growth, Biochemical, Haemato-Immunological Response, and Immune Gene Expression in Labeo rohita with Aeromonas hydrophila Post-Challenges. Fishes 2023, 8, 7. https://doi.org/10.3390/fishes8010007
Sattanathan G, Liu W-C, Padmapriya S, Pushparaj K, Sureshkumar S, Lee J-W, Balasubramanian B, Kim IH. Effects of Dietary Blend of Algae Extract Supplementation on Growth, Biochemical, Haemato-Immunological Response, and Immune Gene Expression in Labeo rohita with Aeromonas hydrophila Post-Challenges. Fishes. 2023; 8(1):7. https://doi.org/10.3390/fishes8010007
Chicago/Turabian StyleSattanathan, Govindharajan, Wen-Chao Liu, Swaminathan Padmapriya, Karthika Pushparaj, Shanmugam Sureshkumar, Jang-Won Lee, Balamuralikrishnan Balasubramanian, and In Ho Kim. 2023. "Effects of Dietary Blend of Algae Extract Supplementation on Growth, Biochemical, Haemato-Immunological Response, and Immune Gene Expression in Labeo rohita with Aeromonas hydrophila Post-Challenges" Fishes 8, no. 1: 7. https://doi.org/10.3390/fishes8010007
APA StyleSattanathan, G., Liu, W.-C., Padmapriya, S., Pushparaj, K., Sureshkumar, S., Lee, J.-W., Balasubramanian, B., & Kim, I. H. (2023). Effects of Dietary Blend of Algae Extract Supplementation on Growth, Biochemical, Haemato-Immunological Response, and Immune Gene Expression in Labeo rohita with Aeromonas hydrophila Post-Challenges. Fishes, 8(1), 7. https://doi.org/10.3390/fishes8010007