1. Introduction
Globally, aquaculture has played a significant role in improving the economic status of farmers and other key players (actors at each node) in the fish value chain [
1]. In the past two decades, the aquaculture sector has seen rapid development due to increased demand for fish as an affordable source of animal protein [
2]. Due to new technologies, fish culture methods have become more intensive, leading to higher yields per unit area [
3]. However, the increase in aquaculture production has been accompanied by huge losses because of high fish mortality caused by disease outbreaks [
4]. Outbreaks due to diseases such as tilapia lake virus (TiLV), streptococcosis, and motile aeromonad septicaemia have been reported in several intensive tilapia producing regions around the world [
5,
6,
7]. The loss of revenue due to fish diseases is estimated at USD 6 billion per year globally [
8]. Therefore, fish disease management remains a significant factor in the growth of the sector.
Fish diseases may be divided into infectious diseases, caused by pathogenic organisms present in the environment [
9], and non-infectious diseases, caused by environmental problems, nutritional deficiencies, or genetic anomalies [
10]. The presence of pathogens in a fish population does not always result in disease and mortality, and individuals can remain asymptomatic under favourable conditions [
11]. However, many factors are associated with the development and progress of fish disease in intensive production facilities. These include poor husbandry practices and inadequate biosecurity systems [
12]. External stressors, such as high stocking densities, poor water quality and improper nutrition, may exacerbate the development of clinical disease, which sometimes leads to high incidences of mortality and low productivity [
13]. Globally, biocide and antibiotic treatments are used widely in intensive fish production systems against infectious pathogens that cause disease and are present in the aquatic environments where fish are reared [
3]. In many developed aquaculture-producing countries, the constant exposure of fish on farms to antimicrobials has contributed to increased antibiotic resistance in aquatic animals and adjacent ecosystems, and this resistance has spread to terrestrial animals and humans [
14]. The farmers’ knowledge of the clinical signs indicating a disease and the relevant biosecurity measures, such as the collection and disposal of dead fish, has an impact on the outcome of disease outbreaks [
12]. Furthermore, prevention and control strategies are critical to preventing the onset of disease and reducing losses from disease when it occurs [
15]. Therefore, preventing disease outbreaks by managing risk factors remains cardinal for the sustainability and growth of the aquaculture sector.
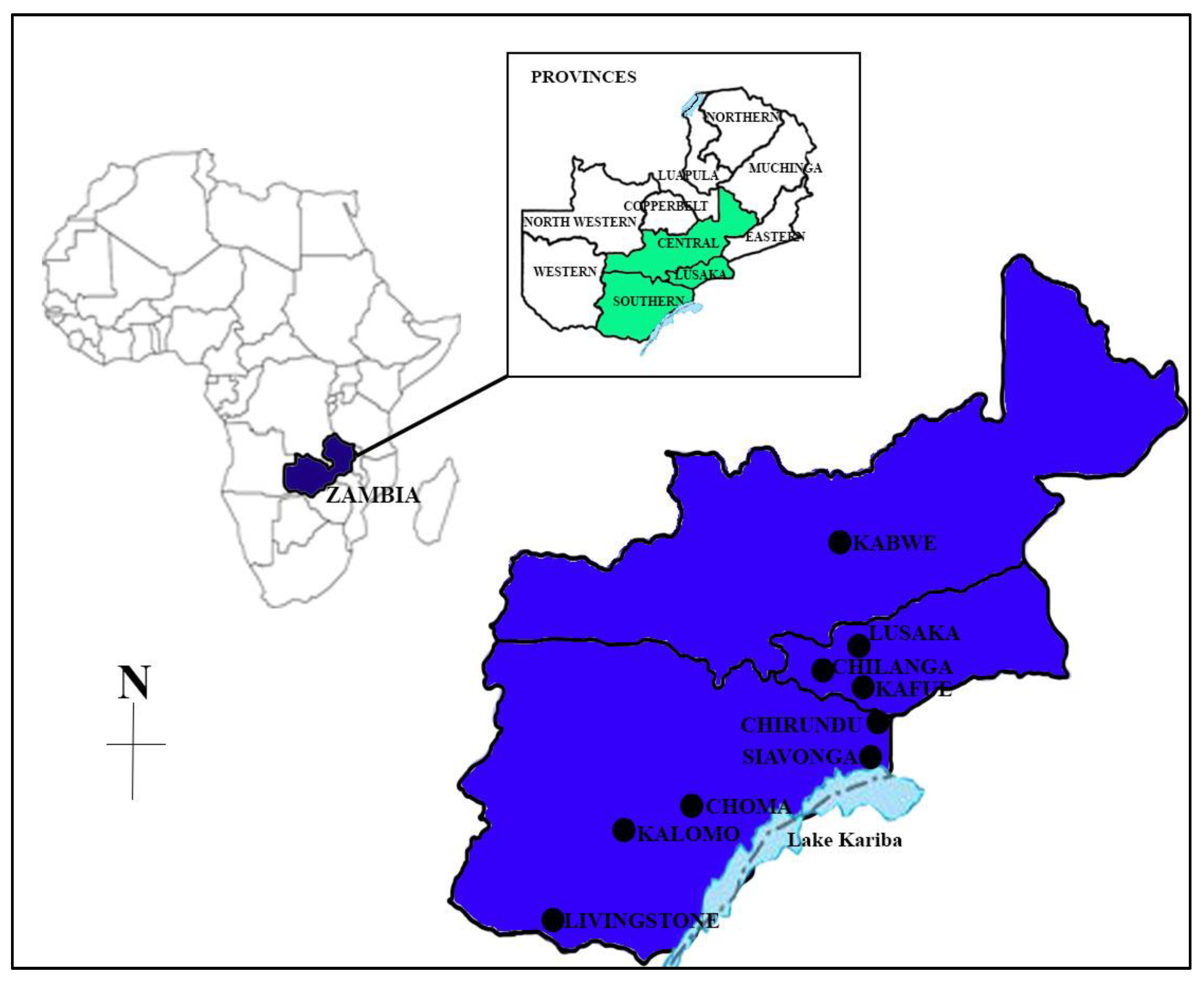
Similar to many developing countries, Zambia has reported the rapid growth of the aquaculture industry, supported by increased investment in the sector, which has allowed farmers to adopt improved aquaculture practices [
16]. With intensified aquaculture production, diseases such as streptococcosis and lactococcosis have already been reported on some fish farms in Zambia [
7]. Many fish farms in Zambia face several challenges related to health management practices that make them highly vulnerable to disease outbreaks [
17]. Some of the challenges are a lack of knowledge of health management due to inadequate extension services, a lack of basic biosecurity measures and a lack of proper diagnostic tools [
17].
Studies on fish diseases conducted in Zambia so far have been limited to the diagnosis and detection of pathogens [
7,
18,
19]. However, so far, there has been no study on the potential risk factors associated with disease outbreaks in aquaculture farms. This study, therefore, investigated the management practices that can contribute to disease outbreaks in farmed fish in Lusaka and central and southern provinces in Zambia.
4. Discussion
This study was conducted to assess management practices and the potential risk factors that contribute to disease outbreaks in tilapia farms in Zambia. The results indicated that pond size and type, water source, stocking density, fish species, control of piscivorous birds, and disposal methods of dead fish were the main contributing factors to the cumulative mortality rate per batch of fish produced and the duration of high-mortality episodes.
Tilapias are among the most important warm-water fishes used for aquaculture production [
20]. In this study, the common tilapia species the respondents reared were Nile tilapia, threespot tilapia, and greenhead tilapia, and the majority of the respondents reared Nile tilapia, indicating that at that time it was probably the most reared fish in that region of the country. In 2019, the Food and Agriculture Organisation of the United Nations (FAO) ranked Nile tilapia ninth among the aquatic species (plants and animals) reared globally, it being the most popular species group farmed in 127 countries [
21]. The fish adapts well in water temperatures between 23 and 30 °C, making the tropical and subtropical conditions of Zambia conducive for Nile tilapia production [
22]. In our study, we found a positive correlation between the rearing of Nile tilapia and high total mortality rates on the farms. However, this result is in contrast with research findings that have demonstrated that Nile tilapia is less susceptible to disease development compared to other species. Nile tilapia has been found to be more resistant to
Streptococcus spp. compared to blue tilapia (
Oreochromis aureus) and Mozambique tilapia (
Oreochromis mossambicus) and their hybrids [
23]. Furthermore,
Edwardsiella tarda has been shown to cause mild to moderate lesions and mortality in Nile tilapia but not in African catfish (
Clarias gariepnus) [
24]. In 2009, in an infection experiment, Songe demonstrated that compared with threespot tilapia and straightfin barb (
Barbus paludinosus), Nile tilapia was more resistant to
Aphanomyces invadans infections, not showing any clinical signs even after 32 days of inoculation [
19]. Therefore, because of its comparative resistance to a number of disease pathogens, Nile tilapia is an excellent culture species for the developing aquaculture industry in Zambia. Furthermore, the positive correlation of Nile tilapia to cumulative mortality could also be attributed to it being the overrepresented species in this study.
About half of the farmers in this study reared fish in large ponds (501–600 m
2), although it has been previously reported that 80% of the farmers in Zambia and 90% of the farmers in Sub-Saharan Africa use medium-sized ponds [
25,
26]. The correlation between pond size and total or cumulative mortality rates was only seen in small ponds (<300 m
2). Pond size and water surface area have been shown to have a relationship with the levels of dissolved oxygen. Impaired respiration in fish is more pronounced in small ponds compared to larger ones, where midnight dissolved oxygen readings were lower in the former (1.65 mg/L) than in the latter (3.18 mg/L) [
27]. In contrast to the observations of this study, higher incidences of septicaemia and columnaris disease have previously been reported in fish from large ponds, attributed to high intensive culture practices, such as feeding rates [
28].
Stocking density has a huge influence on the growth rate, productivity, and incidence of disease outbreak in aquaculture production facilities. The FAO recommends a stocking density of 4 to 8 fish/m
2 for pond culture [
29]. In this study, fewer than half the farmers had the ideal stocking density. Stocking density (4 to 8 fish/m
2) showed a positive correlation with the duration of a high-mortality episode. This result is in contrast to studies that have demonstrated that fish reared in high stocking densities (>8 fish/m
2) are susceptible to high mortalities. High stocking densities have been shown to reduce feeding activity and growth rates in farmed fish as well as increase the level of metabolites, such as urine and faeces, in pond water [
30]. Accumulating metabolites change water quality, subjecting fish to chemical stressors in addition to the chronic stress caused by social dominance [
31,
32]. Several studies have demonstrated that high stocking density exacerbates disease development and transmission and mortality rates [
33,
34,
35]. A significant relationship exists between high stocking density, infectious dose (cfu/mL) of
Streptococcus iniae in Nile tilapia and outbreaks of
Streptococcus agalactiae in tilapia cultured in a high-stocking-density environment, poor-quality water, and water temperatures above 28 °C [
33,
35]. In Ecuador, the severity of Tilapia lake virus outbreak was positively associated with high stocking densities apart from other risk factors [
34].
The source of water used in aquaculture production has a direct impact on the quality of the water that the fish is exposed to. In this study, among the water sources, lake water was the only potential risk factor in relation to the duration of disease outbreak. Despite rivers and lakes providing a ready supply of water for fish production, studies have shown that in high-aquaculture-production regions, water quality parameters such as physicochemical parameters (ammonia, phosphates, and heavy metals) and microbiological parameters are high [
36,
37]. Depending on the number of farms upstream, pathogens might enter the water body (river, stream, or lake), which will serve as a vehicle for transmitting the pathogens to farms downstream. Pathogens such as
Saprolegnia spp. have shown a strong correlation with fish farms in downstream locations receiving water from infected farms upstream [
38]. We can postulate that lake water was a potential risk in this study because of the increasing number of farms on lakes currently growing fish in these lakes, thereby increasing pathogen presence in the areas.
An essential best practice in aquaculture production is the routine monitoring of water quality parameters such as dissolved oxygen and pH. This study revealed that the majority of the farmers in the study region did not monitor water quality in their rearing units at all. In other words, once the fish were stocked in the ponds, dissolved oxygen, temperature, pH, ammonia, turbidity, and others were not measured throughout the production cycle. Only a small proportion of the farmers monitored water quality parameters daily. High levels of chemicals, such as ammonia, have a toxic effect on fish, leading to their inability to extract energy from feed and lethargy among the fish on chronic exposure [
39]. High ammonia levels also promote the proliferation of ectoparasites and disease pathogens in the pond water [
39,
40]. Therefore, the measurement of ammonia and other water-quality variables provides a snapshot of conditions at the time the water sample was collected. Routine monitoring of water quality provides information to the farmers on the suitability of the aquatic environment for fish rearing, allowing the farmers to change the water when the parameters are above permissible limits. Furthermore, it serves as a fish welfare assessment tool that helps prevent fish exposure to chemical stress that may predispose the fish to stress and possible opportunistic disease development and outbreaks.
Globally, disease outbreaks have been reported in many aquaculture production regions. Depending on the aetiology and causative pathogens, disease can be reported at any stage of the growth phase of fish. In this study, the majority of the farmers reported high-mortality episodes in fish when the fish weighed 3 to 20 g. In Zambia, hatcheries supply fingerlings at body weights between 2 and 5 g. This means that the farmers reported high mortality rates during the first 14 days after they stocked fish in their ponds [
41]. In one study conducted in Zambia, high early mortalities were reported at a farm on lake Kariba, and the major contributing factor was cumulative stress experienced prior to, during and after the transportation of the fingerlings [
42]. The main stressors were (a) mechanical trauma during grading and counting prior to transportation, (b) undulating water temperature, low dissolved oxygen (DO) levels, high density of fish, changes in water salinity and high turbidity during transportation, and (c) abrupt changes in temperature and water quality and high stocking density at stocking in the cages [
43]. Mortalities immediately post-stocking can be attributed to stress from handling and transportation and will usually last about 3 to 5 days. If mortalities persist for more than 5 days, it is likely a disease outbreak due to primary or secondary pathogens. Some of the disease pathogens known to cause mortality in tilapia fingerlings are
Flavobacterium columnare [
44],
Gyrodactylus sp. and
Trichodina sp. [
45], and tilapia lake virus [
46].
Among the clinical signs observed during mortality episodes, fish gasping for air at the surface of water was the commonest one reported by farmers. This clinical sign is mainly reported in fish in hypoxic conditions, where the dissolved oxygen is insufficient to support respiration. Other clinical signs reported were erratic swimming, reddish lesions of the skin, fin rot or erosion, cotton-like appearance on the skin, lethargy, and corneal opacity. These clinical signs reported by the farmers are consistent with those seen in tilapia diseases such as columnaris, streptococcosis, lactococcosis, motile aeromonad septicaemia, and saprolegniasis [
7,
43,
47]. In Zambia, disease outbreaks caused by
Aeromonas spp.,
Streptococcus agalactiae, and
Lactococcus garvieae have been reported in a number of farms on Lake Kariba rearing Nile tilapia [
7,
18].
Disease control remains an integral component of aquaculture production as intensification increases the risk of outbreaks. Globally, several methods of controlling fish diseases are available and are categorised as either reactive treatments or proactive disease strategies. In this study, farmers reported using salt (sodium chloride), potassium permanganate, or lime as disease treatment options. This study, therefore, confirms that farmers do not use antibiotics to control disease outbreaks in aquaculture production facilities in the central and southern regions of Zambia. It is important to note that the global antimicrobial consumption in aquaculture in 2017 was estimated at 10,259 tons, with the highest consumers being China (57.9%), India (11.3%), Indonesia (8.6%), and Vietnam (5%) [
48]. The non-use of antibiotics in the Zambian aquaculture industry offers an opportunity to develop more sustainable and environmentally acceptable methods of preventing diseases, such as the use of probiotics, vaccines, and ethnoveterinary products. Ethnoveterinary medicine has been used successfully to treat diseases on fish farms in Korea [
49].
Due to losses and low quantity of fish available for sale at the end of each cycle, the cumulative mortality rate reported on fish farms has a huge impact on the revenue expected by the farmer. In this study, a larger proportion of farmers recorded cumulative mortality rates of less than 5% in each production cycle. Only 27.3% of the farmers reported higher cumulative mortality rates (>10%). Disease outbreaks by primary or secondary pathogens have been shown to cause mortality rates higher than 10%. Elsewhere, viral pathogens such as tilapia lake virus (TiLV) and infectious spleen and kidney necrosis virus (ISKNV) have been shown to cause mortality rates of up to 90% [
5,
50].
Lactococcus garvieae,
Streptococcus spp.,
Francisella spp.,
Edwardsiella tarda, and other bacterial pathogen have recorded mortality rates of up to 50% [
7,
51,
52,
53]. The significant potential risk factors associated with the cumulative mortality rate in this study were pond size, fish species reared, methods of disposing of dead fish, and methods of controlling piscivorous birds.
The prompt removal and appropriate disposal of moribund and dead fish reduce the chances of an infection spread [
54]. In this study, about a third of the farmers reported not removing dead fish from the pond, and this poor practice perpetuates the cycle of infection. It should be highlighted that the transmission rate of a bacterial fish pathogen (
Flavobacterium columnare) has been demonstrated to be higher from a dead host, most likely a consequence of the higher shedding rates of dead fish when compared to living fish [
55]. Furthermore,
Edwardsiella ictaluri can be transmitted to susceptible fish from infected individuals by cannibalism [
56]. Therefore, leaving dead fish in a pond exacerbates the transmission of pathogens to the susceptible individuals in the population, leading to more mortalities and losses. In this study, we found a negative correlation between the fish burial disposal method and the total mortality rate. For every unit increase in the number of farmers burying dead fish, there was a decrease in the total mortality rate.
In our study, the majority of the farmers did not allow visitors to access their fish production facility and most had perimeter barriers that prevent people or animals from accessing the site. In contrast to our result, a study conducted in Zambia by Hasimuna et al. (2020) reported that the majority of the small-scale farms did not have barrier fences to prevent animals (otters and monitor lizards) and people that may be vectors of some pathogens from accessing the fish production site [
17]. Flores et al. (2015) reported that in Bataan Province in Philippines, 58% of the farmers had perimeter barriers around their ponds [
57]. Only 20.4% of the farmers reported managing biosecurity by placing footbath and handwash stations at the access point to the fish production facility. Bacterial pathogens such as
Streptococcus iniae,
Streptococcus agalactiae,
Lactococcus garvieae, and
Mycobacterium marinum can be transmitted mechanically from fish to humans and vice versa through handling [
58]. However, the risk of humans spreading fish disease pathogens is low unless they are workers working in handling live fish and the activities are conducted within a few hours of two different rearing units.
In our study, only about half of the farmers had some form of programme for disinfecting and cleaning their tools and equipment. The only disinfectants the farmers used were chlorine and quaternary ammonium chloride. In Vietnam, a better developed aquaculture industry, 50% of the farmers in the northern region reportedly used disinfectants in the production facilities, which is consistent with the result of this study [
59]. Although a similar proportion of farmers in both studies used disinfectants, in Vietnam, the farmers used up to 20 different disinfectants, compared to the 2 disinfectants reported in this study [
59]. In a disinfectant susceptibility study, quaternary ammonium compounds and chlorine-based compounds showed mild and poor efficacy towards bacteria isolated from fish farms [
13]. Furthermore, the use of chlorine and quaternary ammonium compounds as a disinfectant have been demonstrated to promote the horizontal transfer of plasmids by natural transformation via the exchange of antimicrobial resistance genes across bacterial genera and leading to the emergence of new antimicrobial-resistant bacteria [
60,
61]. As farmers in this study commonly used quaternary ammonium compounds and chlorine, a susceptibility assessment is warranted to assess the efficacy of these compounds against bacteria on farms.
Piscivorous birds help transmit pathogens between ponds and farms in addition to contributing to economic losses by eating fish from the facility. Pathogens transmitted by birds include digenean parasites,
Francisella spp.,
Edwardsiella tarda, and viral pathogens [
51,
62]. Therefore, the methods of controlling these birds will have an impact on disease transmission as well. In this study, the farmers used bird nets, physical chasing, scarecrows, and fireworks to control birds, with the first two being the commonest methods. Furthermore, there was a correlation between two of the control methods (using scarecrows and chasing away the birds) and an increase in the total mortality rate, indicating that these two methods are not effective enough and, therefore, regardless of their being implemented on the farms, the mortality rates increased, most probably since the birds still had access to the fish and kept feeding on them.
The availability of skilled fish health diagnostic and extension services has an impact on the outcome of disease outbreaks. In this study, the majority of the farmers reported receiving some form of technical help when they experienced high fish mortality in their aquaculture production facilities. Furthermore, most farmers reported receiving fish disease diagnostic services from aquaculturists, who in this study were individuals with some academic qualification in fisheries and aquaculture. An overview of the curriculum of academic institutions offering academic qualification in fisheries and aquaculture revealed little content on fish diseases and diagnosis (personal communication). The aquaculture sectors in other countries, such as Bangladesh and Kenya, have also reported low access of farmers to disease diagnostic services due to unavailability of specialised personnel [
63,
64]. To increase farmers’ accessibility to fish health diagnostic and extension services, in China, it was proposed that a call centre will be created that will help fish farmers to access experts in fish disease diagnosis and treatment via mobiles or telephones [
65]. With the rapidly growing aquaculture sector in Zambia, the adoption of the fish expert call centre strategy will increase farmers’ access to fish disease diagnostic services in the country.