1. Introduction
MS-222, also known as tricaine methane sulphonate, tricaine mesylate and TMS, is commonly used in both the anaesthesia and euthanasia of some species of fish. As an agent for euthanasia, standard protocols involve immersion in a neutral solution of MS-222 followed by a secondary physical method such as pithing, where a metal spike is used to destroy the brain [
1]. Being structurally close to other local anaesthetics including benzocaine, MS-222 works via a similar mechanism, altering the properties of voltage gated sodium channels to depress the central nervous system. The use of MS-222 is well established and effective [
2,
3], although it is not the only method of euthanasia approved and used in fish.
The side effects of MS-222 as an anaesthetic have been well studied [
2] and can include the induction of apoptosis and oxidative stress in fish gills [
4], effects on the cardiovascular system [
5], and reduced membrane fluidity and mitochondrial functionality in the sperm of the zebrafish [
6]. However, any physiological effects of using MS-222 for fish euthanasia have not yet been characterised. With a growing body of work assessing mitochondrial respiration in fish, including studies of thermal sensitivity and in particular mitochondrial responses to increased temperatures and altered environments due to climate change [
7,
8,
9], it is vital to take into account the consequences of using a prior overdose of anaesthetic such as MS-222. Knowing how MS-222 alters mitochondrial respiration in fish would ensure that any observed changes to respiration are in fact a consequence of the study conditions being assessed and not due to the anaesthetic itself, and they would allow more inciteful comparison of studies implementing different methods of euthanasia.
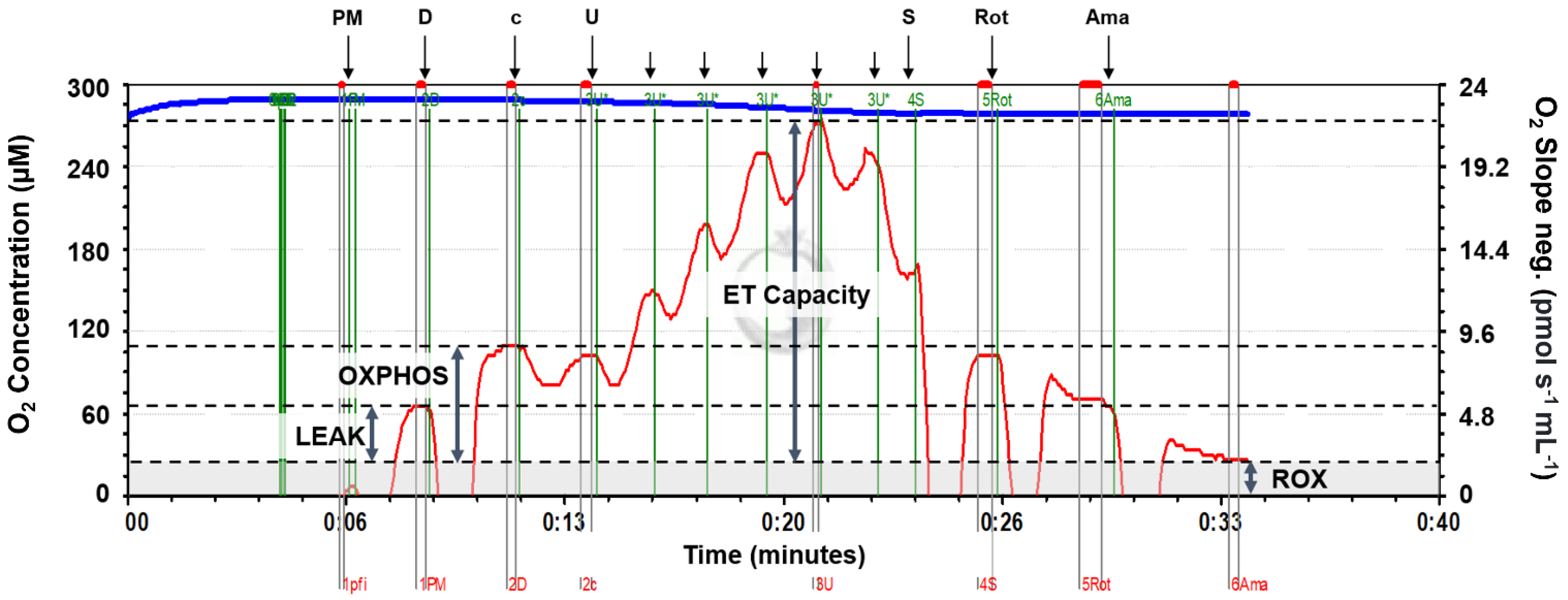
High-resolution respirometry (HRR) measures respiration in mitochondrial preparations, allowing analysis of the mitochondrial respiratory pathways. Respiration states including LEAK, OXPHOS and ET capacity can be measured and compared between treatment groups to give an overview of how MS-222 affects mitochondrial respiration. LEAK state is the non-phosphorylating resting state where oxygen flux is maintained only to compensate for the proton leak back across the inner mitochondrial membrane, which does not contribute to ATP generation. In the mitochondrion, electron transfer is coupled to the phosphorylation of ADP to ATP, so with ADP present, the respiratory capacity of mitochondria in the oxidative phosphorylation (OXPHOS) state can be measured. ET capacity, the electron-transfer capacity of mitochondria, is the maximum capacity of the non-coupled electron-transfer system, which is reached when ADP phosphorylation is uncoupled from electron transfer by the addition of a protonopore. These states can be attained experimentally by the addition of different substrate, inhibitor and uncoupler combinations.
In order to assess whether MS-222 has any effect on mitochondrial respiration, we conducted HRR on the brain and skeletal muscle of the three-spined stickleback (Gasterosteus aculeatus), which is a model organism in evolutionary ecology. We found that LEAK state respiration was reduced in the brain of fish euthanized with MS-222 in comparison to those without use of any anaesthetic. As MS-222 is altering some parameters of respiration, we conclude that the method used for euthanasia is an important factor to be considered when conducting HRR in fish.
2. Materials and Methods
To assess the effect of MS-222 anaesthetic, which is commonly used in the euthanasia of fish species, on mitochondrial respiration, respiration states were compared between three-spined sticklebacks culled with MS-222 (n = 9) and those culled without the use of any anaesthetic (n = 8). The Oroboros Oxygraph-2k Respirometer (O2k; Oroboros
® Instruments, Innsbruck, Austria) was used for HRR, which allows an analysis of bioenergetics and oxidative phosphorylation in small amounts of biological samples, such as tissue homogenates which were used here [
7,
10,
11].
2.1. Fish Husbandry
Three-spined sticklebacks (
Gasterosteus aculeatus) were raised in the aquarium. Fish of the same backcross from populations on the Scottish island of North Uist, hatched between 20th and 25th July 2018, were used for analysis [
12,
13]. A balanced experiment was set up: two fish from the same tank were analysed; one fish was culled by submersion in MS-222 (400 mg/L) followed by pithing, and the other was killed by severing the spinal cord (here in referred to as decapitation) under UK Home Office project licence PP5421721. Fish were weighed and placed immediately on ice.
2.2. Dissection
Immediately prior to respirometry analysis with an Oroboros O2k, the brain of each stickleback was removed and weighed. The brain was then homogenised in ice cold MiRO5 buffer (Oroboros Instruments GmbH; 0.5 mM EGTA, 3 mM MgCl2, 60 mM Lactobionic acid, 20 mM Taurine, 10 mM KH2PO4, 20 mM HEPES, 110 mM D-Sucrose, 1g/L BSA) using 20 crushes with an Eppendorf and micropestle (200 uL buffer per 3.5 mg of brain). Then, 3.5 mg of homogenised brain was added per 2 mL chamber of the O2k.
Analysis of muscle was always conducted in sequence after the brain. Tail skeletal muscle was taken from the left side of the fish, and the skin was carefully removed. Muscle was cut into fine pieces (<1 mm) and homogenised using a scalpel before a subsample was weighed and homogenised with 25 crushes using an Eppendorf and micropestle in ice-cold MiRO5 buffer (200 uL per 5 mg muscle). Then, 5 mg of muscle was added per 2 mL volume chamber. Where possible, technical replicates were conducted for both muscle and brain samples.
2.3. High-Resolution Respirometry
The O2k has two chambers, so two samples can be run simultaneously. In most cases, experimental design allowed one MS-222 sample and one decapitated sample to be analysed at the same time for direct comparison of fish from the same tank/family. HRR was conducted using an O2k (Oroboros® Instruments, Innsbruck, Austria). Electrodes were calibrated at the start of each day. Analysis was conducted at 14 °C, as this is the approximate body temperature of the stickleback raised in the aquarium. Then, 2 mL MiRO5 was added to each O2k chamber prior to the brain or muscle sample.
A substrate–uncoupler–inhibitor–titration (SUIT) protocol ideal for assessing oxidative phosphorylation with NADH-linked substrates (complex I) and S-pathway substrates (complex II) was used (SUIT-004 [
14]). This allowed analysis of LEAK state respiration, oxidative phosphorylation (OXPHOS) and ET capacity. This protocol was selected as the substrates tested resulted in altered oxygen flux in tissue homogenates used, the protocol gave a good overview of many parameters of respiration including complex I and complex II respiration, and it was relatively short (<1 h), so it prevented sample deterioration. All substrate and inhibitor concentrations were optimized for fish tissue homogenate used in this study with final concentrations in parenthesis below. After the addition of a substrate or inhibitor, oxygen flux was allowed to reach equilibrium before marking the value and moving onto the next stage (
Figure 1). All chemical reagents were supplied by Sigma-Aldrich (Merck, Darmstadt, Germany) except ADP which was supplied by Calbiochem (Merck, Darmstadt, Germany).
LEAK state was attained and measured by addition of the complex I substrates pyruvate (5 mM) and malate (2 mM) in the absence of ADP. In the mitochondria, pyruvate is converted to acetyl-CoA by pyruvate-dehydrogenase, and acetyl-CoA enters the TCA cycle where electrons are donated to NAD+, producing NADH. Malate is oxidized to oxaloacetate in the TCA cycle, also forming NADH. NADH enters the electron transport system (ETS) at complex I, and electron transfer to oxygen then occurs via the ETS enzyme complexes. However, without ADP addition, no phosphorylation of ADP can occur.
OXPHOS state respiration was measured by the addition of ADP (7.5 mM). To check the mitochondrial outer membrane integrity, cytochrome c (0.01 mM) was added. If the membrane had been damaged during prior tissue preparation, cytochrome c, which is part of the electron transport system, would have been lost from the membrane, so the addition of cytochrome c would increase the respiration rate. Experimental runs where a percentage increase of greater than 20% was observed were excluded from analysis.
ET capacity was measured by the stepwise addition of an uncoupler (carbonyl cyanide m-chlorophenyl hydrazone (CCCP), a protonophore, added in 1 μL increments of a 1 mM stock solution) to reach maximum oxygen flux. To test combined complex I and II respiration, succinate (10 mM) was added to allow the measurement of ET capacity with complex I and II reducing substrates. Succinate is converted to fumarate by complex II and succinate dehydrogenase while reducing FAD to FADH2. To measure the contribution of complex II alone, a complex I inhibiter (rotenone; 0.5 uM) was added, which prevents electron transfer through complex I. Finally, complex III inhibitor antimycin A (2.5 uM) prevents electron transfer to give a measure of background oxygen flux (ROX) due to any oxidative side reactions.
2.4. Data Analysis
Raw data outputs for each stage were obtained from DatLab (DatLab v7, Oroboros, Innsbruck, Austria). Background corrected oxygen flux was calculated by subtracting ROX from the oxygen flux measured at each stage. Oxygen flux is expressed per 3.5 or 5 milligrams of tissue homogenate used for brain and muscle, respectively. Although the amount of brain or muscle tissue was kept constant between samples, the number of mitochondria may vary between samples; to account for this, an internal normalization was conducted by calculating the Flux Control Ratio (FCR), which expresses respiratory control independent of mitochondrial content [
15,
16]. FCRs normalise each sample to the maximum oxygen flux reached by that sample, allowing comparison between samples from different individuals [
16]. For samples with technical replicates, mean values were calculated, and these were used in further analysis, which was completed using R (R Core Team 2022, v4.2.2). Unpaired
t-tests were conducted to determine if there were any differences in mean oxygen flux and FCRs with each substrate combination tested between decapitated and MS-222 treated fish.
Mean weights of the two treatment groups were 2.12 ± 0.59 grams for MS-222 treated and 2.14 ± 0.64 grams for decapitated. These did not differ significantly (Unpaired Student’s
t-test: tdf = 15 = 0.0647,
p = 0.949). As fish were from different crosses, each raised in a separate tank, a one-way ANOVA was conducted to determine any effects on mitochondrial function: the cross/tank had no effect at any mitochondrial stage. To analyse all data together, a linear mixed model was fitted with oxygen flux as the dependent variable, the euthanasia method and respiration state nested within tissue type as fixed effects, and the cross/tank as a random effect (
Table S1). This revealed that the tissue type and respiration state affected oxygen flux as expected; however, the euthanasia method did not, therefore not providing additional information on the consequences of MS-222 on the different parameters of mitochondrial respiration.
4. Discussion
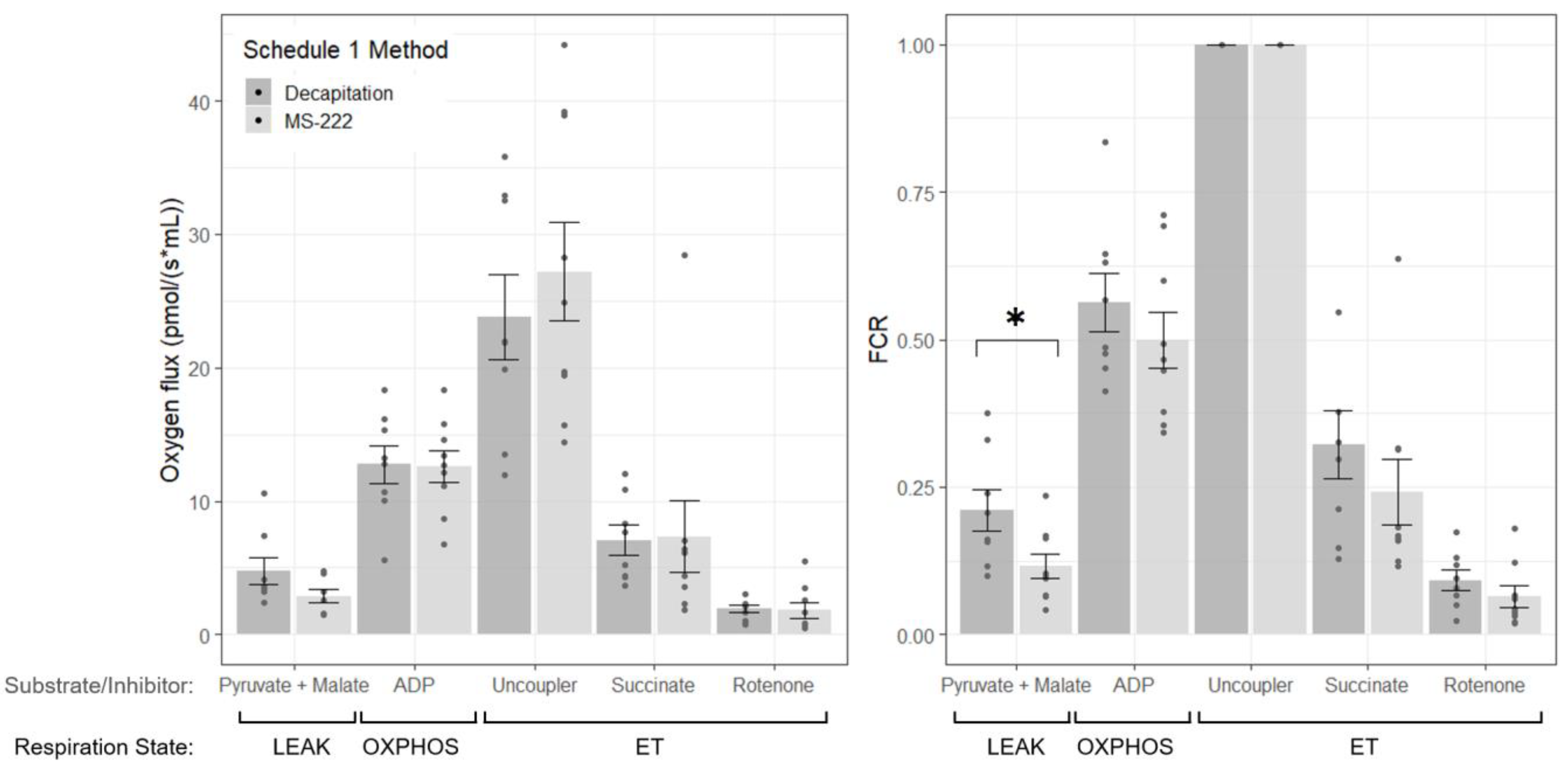
The effects of MS-222 as an agent for euthanasia on mitochondrial respiration in the three-spined stickleback have not been quantified previously. This study has shown that euthanasia with MS-222 can alter mitochondrial respiration in the brain of the stickleback, generally reducing respiration in comparison to in fish where no MS-222 was used. In particular, treatment with MS-222 caused a significant reduction of LEAK state respiration with complex I substrates pyruvate and malate. In the muscle, there were no differences between MS-222 treated and decapitated fish, which was perhaps due to the lower overall oxygen flux in muscle compared to brain. The smaller LEAK state oxygen flux (although note that this is not significantly different between groups) and FCR in the MS-222 treated group suggests that MS-222 can reduce the amount of proton leak across the membrane and decrease the contribution of this non-phosphorylating state to respiration. In the decapitated group, on average, LEAK respiration contributes to 21.0% of maximum respiration. This is in agreement with previous studies where approx. 20–25% of respiration has been found to drive mitochondrial proton leak in mammalian hepatocytes [
17]. With MS-222 treatment, this was reduced to 11.6% (
Table 1) in the present study. As no significant differences in OXPHOS and ET states were found between treatment groups, MS-222 may specifically alter LEAK state respiration with pyruvate and malate as substrates. As proton leak is mainly a function of the inner mitochondrial membrane and is dependent on protonmotive force [
18], the anaesthetic may alter mitochondrial membrane properties and reduce proton leak into the mitochondrial matrix [
19]. Further examination of mitochondrial respiration and membrane potential with different substrate combinations would be required to provide further information on the effect of MS-222 specifically on LEAK state respiration.
Although no significant differences were observed between the OXPHOS state and ET capacity with the substrate combinations tested, in the brain, FCR values for the MS-222 treatment group tended to be lower than the decapitated group. This suggests that mitochondrial respiration may be reduced when MS-222 is used during euthanasia. To confirm this, HRR measurements with additional individuals and alternative substrate combinations would be required, and further work could determine the precise mechanism of action of MS-222 on the ETS. The focus of the present study, however, was to determine whether MS-222 altered measurements made with HRR in fish to inform future work, and the findings here provide evidence that MS-222 may have an effect on respiration in mitochondria in the brain.
This study also provides a comparison of mitochondrial respiration between tissue types in the three-spined stickleback, which have not often been compared in species of fish. Oxygen flux was consistently higher in the brain compared to the muscle (
Figure 2 and
Figure 3), despite larger amounts of skeletal muscle used for analysis. The maximum ET capacity was higher in the brain, indicating higher maximum respiration rates. Each tissue has differing metabolic demands due to their function or conditions; the heart has previously been found to have the highest oxidative phosphorylation capacity [
20]. In agreement with previous work using cytochrome c oxidase activity in rats as a measure of the OXPHOS system [
20], the brain showed increased specific oxidative phosphorylation activity in comparison to the muscle. Although the ease of homogenisation of the brain tissue likely in part explains this finding in the present study, the increase in energy requirement in the brain may also contribute to this difference.
As mitochondrial respiration has scarcely been measured in fish homogenised tissue compared to other model organisms, this study provides an overview of coupling efficiency in the three-spined stickleback. In the brain, FCRs for OXPHOS state (the coupled respiratory capacity with saturating ADP) with complex I substrates were 0.499 and 0.563 (
Table 1) for the MS-222 and decapitated samples, respectively, agreeing with previous work on the cerebellum of two species of shark:
Hemiscyllium ocellatum and
Chiloscyllium punctatum [
15]. A higher value indicates increased capacity of the phosphorylation system. In the muscle, these values were 0.776 and 0.750, respectively, indicating better coupled oxidative phosphorylation in the muscle compared to the brain, which was perhaps due to the high ATP demand in muscle for swimming. It should also be noted that in four skeletal tail muscle samples, there was an increase in oxygen consumption of greater than 20% with the addition of cytochrome c, indicating some membrane damage had occurred. This is likely due to tissue preparation, as the homogenisation of muscle required more time than homogenisation of the brain.
The deleterious effects of MS-222 as an anaesthetic on fish physiology have previously been studied, including altering blood haemostasis in zebrafish (
Danio rerio) [
21], damaging morphological structure, altering osmoregulation and inducing apoptosis in the gills of the Chinese sea bass (
Lateolabrax maculatus) [
4], and altering antioxidant status in Gilthead sea bream (
Sparus aurata) [
22]. Although any effect of MS-222 on mitochondrial respiration in fish has not previously been tested, these findings hint that MS-222 may alter mitochondrial physiology, including mitochondrial respiration, which was found here. This study therefore adds to the growing body of work highlighting the impact of MS-222 and other anaesthetics on fish physiology.
Overall, we have identified the effects of MS-222 on mitochondrial respiration in the three-spined stickleback, concluding that MS-222 used during euthanasia may alter HRR measurements made on the brain. Importantly, this highlights the need to consider the method of euthanasia used in combination with analysis of the brain with the O2k; this will ensure that any observed differences are due to differing treatments and not a consequence of any anaesthetic used, and they will allow a more inciteful comparison of studies of mitochondrial respirometry in fish. A consistent method of euthanasia and, where possible, avoiding anaesthetics such as MS-222 would prevent any disruption of findings. Despite no differences being observed in the muscle, effects of MS-222 may be smaller and harder to detect due to the overall lower oxygen flux, so caution should still be taken.