Abstract
Chemical cues and pheromones mediate fish reproduction, aggregation, risk assessment, and kin recognition. To better understand the chemical communication of conspecific fish, the behavioral responses to bile acids (BAs), their source, and reception investigated in large yellow croaker (Larimichthys crocea). Behavioral experimental results indicated that juvenile fish were attracted to intestinal contents (ICs) emanating from conspecifics, regardless of whether the fish were feeding. IC BA-targeted metabolomics revealed that cholic acid (CA), taurocholic acid (TCA), taurochenodeoxycholic acid (TCDCA), chenodeoxycholic acid (CDCA), and taurodeoxycholic acid (TDCA) were the top five categories. Tests with and without fasting yielded similar categories and proportions of BAs, indicating that the intestinal BA profiles were generally stable. At the nanomolar level, CA led to significant preference behavior (p < 0.01). The electrophysiological results supported the hypothesis that the top five BAs were potent odorants in L. crocea. Moreover, inhibition of adenylate cyclase–cyclic adenosine monophosphate (AC–cAMP) signaling and phospholipase C (PLC) signaling reduced the electro-olfactogram (EOG) responses to CA and CDCA. Collectively, the findings of this study indicate that conspecific individuals could be attracted by ICs unrelated to feeding. As a key intestinal BA, CA led to fish preference behaviors and olfactory responses relying on cAMP and PLC transduction cascades.
1. Introduction
Chemical cues and pheromones play an indispensable role in mediating social interactions that are associated with reproduction, aggregation, risk assessment, and kin recognition [,]. Chemical cues are sometimes more critical to fish than visual and auditory signals [,]. A possible explanation is that the aquatic environment is often turbid, dim, and turbulent. Pheromones are chemicals released by animals that can evoke physiological or/and behavioral responses among conspecific individuals []. The biology and chemistry of insect chemical communication and pheromones are particularly well described, whereas it is difficult to extract and identify putative pheromones substances in most fish species, especially marine fishes []. Conspecific body fluids (e.g., intestinal fluids, urine and skin mucus) have been shown to serve as sources of pheromones in many fish species [,]. For example, L-kynurenine in the urine of ovulated female masu salmon elicits specific behavioral responses to spermiating males, and spermine in the semen of male sea lamprey attracts ovulatory females [,]. Metabolomics, in addition to biology-driven screening and bioassay-guided fractionation, has emerged as a novel and accessible approach to identify pheromones in many organisms, but not fish [,].
Bile acids (BAs) are among the few structurally identified fish pheromones, although the majority of them are still unknown [,,]. As major end-metabolites of cholesterol, BAs function in the solubilization of fats during digestion, endocrine signaling and cholesterol homeostasis [,]. Their structural diversity and stability make BAs perfect aquatic pheromone molecules. The significant diversity and complexity displayed by BAs and their derivatives ensure the communication signal’s specificity [], and excreted BAs are often conjugated with taurine or glycine, and thus highly water soluble and accessible to the olfactory organ of fish. Their half-life of approximately one day suggests that BAs likely have enough time to act as pheromones and do not persist in a problematic way []. Olfactory sensitivity to BAs has been reported across fish species, such as the European eel (Anguilla anguilla), goldfish (Carassius auratus) and Mozambique tilapia (Oreochromis mossambicus) [], pintado catfish (Pseudoplatystoma corruscans) [], rainbow trout (Oncorhynchus mykiss) [] and lake char (Salvelinus namaycush) []. Recent evidence has indicated that lithocholic acid (LCA), cholinic acid (CLA), and taurocholic acid (TCA) at low concentrations mediate the behavioral preference of fish [,]. Additionally, it has been suggested that BAs may be involved in spawning and migration, as has been proven in the sea lamprey [,].
When ligands bind to specific receptors expressed in olfactory receptor neurons (ORNs), the reception of odorants begins []. It has previously been observed that different types of mammalian ORNs respond to odorants via different families of receptor molecules and signal transduction pathways []. Wong et al suggested that BA-sensitive receptors are expressed by vomeronasal sensory neurons in the mouse accessory olfactory system []. Fish employ olfactory epithelia in the olfactory organ having odorant receptors (ORs), vomeronasal type receptors (VRs) and trace-amine associated receptors (TAARs) []. Senegalese sole (Solea senegalensis) have been shown to utilize the cyclic adenosine monophosphate (cAMP) and phospholipase C (PLC) signal pathways to detect bile salt odorants, whereas catfish (Ictalurus punctatus) only employ the cAMP transduction cascade [,]. These results imply that marine fish and freshwater fish have different bile acid olfactory pathways.
It is difficult to find evidence that BAs, BA salts and their derivatives are used as effective chemosensory signals for fish. The most popular and established method for assessing olfactory receptor neuronspopulation responses to various odorants is the electrophysiological method. An electro-olfactogram (EOG) represents the summed generator potential of the olfactory sensory neurons. A series of cross-adaptation and pharmacological studies were used to demonstrate that different groups of receptors or olfactory pathways were employed by various odorants []. However, the electrophysiological experiment results were not enough to prove the effect of odorants on fish, and direct behavioral verification is needed. More trustworthy and significant results would be gained if electrophysiological and behavioral approaches could be utilized simultaneously on the same species of fish.
The large yellow croaker (Larimichthys crocea) is an important commercial fish species in southeast China. Due to overfishing, the species was assessed for The IUCN Red List of Threatened Species in 2016 and listed as Critically Endangered under criteria A2bd []. In recent years, efforts have been made in the establishment of a fishing moratorium as well as breeding, release, and construction of marine ranching; nevertheless, further measures are required. To better understand the chemical communication of large yellow croaker, the present research was conducted from three aspects: behavioral response, source, and reception of BAs. First, different behavioral responses were found to be elicited by different body fluids in large yellow croakers. Next, an attractive intestinal component was identified by targeted metabolomics combined with behavior assays. Finally, the electrophysiological response was tested to determine the olfactory effectiveness of the attractive component and confirm the involved olfactory transduction mechanisms.
2. Materials and Methods
All animal experiments were conducted according to the Guide for the Care and Use of Laboratory Animals formulated by the Ministry of Science and Technology of China. The study was approved by the Committee on the Ethics of Animal Experiments of Ningbo University and was performed in compliance with the ethical principles and standards of fishes. Before sampling, fish were anesthetized with 20 mg/L tricaine methanesulfonate (MS-222, Sigma Aldrich, St. Louis, MO, USA) orally until the respiratory opercula stopped moving.
2.1. Fish Husbandry
Disease-free one-year-old large yellow croakers from the same batch (28.7 ± 4.6 g, 16.2 ± 3.5 cm) were obtained from the Fishery Research Institute of Zhoushan (Zhejiang, China). Before the experiment began, 1000 fish were randomly divided into five tanks, with 200 fish per tank. The fish were acclimatized in a flowing seawater aquaculture system (water temperature 25.0 ± 2.0 °C, pH ~8.2, dissolved oxygen > 7.0 mg/L, salinity 26.0 ± 1.0 g/L, unionized ammonia nitrogen < 0.05 mg/L). During the experiment, the fish were fed a commercial diet twice daily (6:00 and 17:00) until apparent satiety was reached.
2.2. Behavioral Experiments
2.2.1. Y-Maze Choice Apparatus
The Y-maze choice apparatus was a Y-shaped polypropylene tank with three arms (100 × 40 cm) Two choice arms were respectively connected to a pump to maintain the seawater flow in the test aquarium during the experiment. The height of the tank was 30 cm, but it was filled with seawater only to a depth of 15 cm (Figure 1A). Dye (1g/L KMnO4) was added to one of the choice arms and allowed to gradually diffuse into the mix arm. The dye did not flow back into the other choice arm when the flow rate was adjusted to 600 mL/min using pumps. The fish freely explored the choice arms and the mixing arm. A video camera was positioned directly above the maze, and the behavior of the experimental fish was recorded.
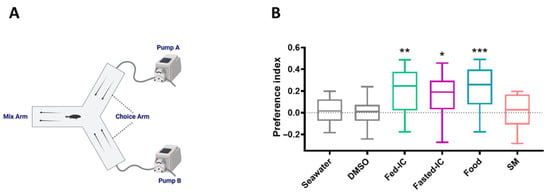
Figure 1.
Behavioral responses to conspecific body fluids. (A) Schematic diagram of the Y-maze choice apparatus. The fish could freely explore each arm and the mixing zone. Pumps A and B established identical flow rates, and the delivery of solutions with different odorants was established in the choice arms. The arrows show the direction of flow. (B) The behavioral responses of large yellow croaker to intestinal contents (ICs) with regular feeding (Fed-ICs, represented by green box), ICs after a 24 h fast (Fasted-ICs, represented by purple box), food (commercial pellet feed, represented by blue box), and skin mucus (SM, represented by orange box). The preference index for an odorant is presented as the mean ± S.E.M.; n = 20. Student’s t-test was used to analyze all treatment data versus the seawater control data. * p < 0.05, ** p < 0.01, *** p < 0.001.
2.2.2. Solution Preparations
Ten fish fed with a regular diet and ten fish fasted for 24 h were used for extraction of body fluids. Intestinal contents (ICs) were extracted from the posterior 10 cm of the intestine, and skin mucus (SM) was obtained by scraping the skin surface of the body section with a scalpel. Fed-ICs and Fasted-ICs represented ICs from fish with regular feeding and fasted for 24 h, respectively. Body fluids were diluted in dimethylsulfoxide (DMSO, 1:2, v/v), homogenized thoroughly, centrifuged, aliquoted, and stored at −20 °C. Fish food odor was prepared by incubating commercial pellet feed (Tongwei, Chengdu, China) in seawater [20 g (feed)/L, 2 h] and by removing solid debris by centrifugation. Body fluids and food odor were diluted with filtered seawater to 1:10,000 before use. Stock solutions of TCA, TCDCA, CA, CDCA, and TDCA (10−2 mol/L, Macklin, Shanghai, China) were prepared with DMSO and stored at 4 °C. Test solutions of BAs were diluted with filtered seawater to 10−8 mol/L before the behavior assay. Filtered seawater and DMSO served as controls.
2.2.3. Behavior Assay
Behavioral testing was conducted between 10:00 and 16:00. After a 24 h fast, five randomly selected fish were placed in the Y-maze tank and allowed to acclimatize for 20 min. Then, the test solutions were added to the choice arm with fewer fish by an experimenter not visible to the fish, while the other choice arm remained unchanged. Specifically, the tube connecting to the pump was swiftly moved from the filtered saltwater to the previously prepared test solution by the experimenter. The flow entering the apparatus changed from seawater to the test solution after the flow was interrupted for two seconds. During an entire trial, about 3 L test solution was introduced into the aquarium. A camera above the Y-maze tank was used to take a snapshot of the moving fish every 5 seconds before and after the switch for 5 minutes. The researcher counted and summed the number of fish in the experimental (Be) and control choice arms (Bc) before the odorant was introduced and the number of fish in the experimental (Ae) and control choice arms (Ac) after the odorant was introduced. To measure the attraction of the odorant to test fish, the scores were used to calculate a preference index (PI) = [Ae/(Ae + Be) − Ac/(Ac + Bc)]. The PI ranged between 1 (complete attraction) and −1 (complete aversion). The apparatus was washed in 5% bleach (sodium hypochlorite), rinsed thoroughly with tap water and filtered seawater after every trial; new test fish were used for each trial. The test of each odorant was repeated at least 20 times.
2.3. Ba-Targeted Metabolomics
2.3.1. Sample Collection and Pretreatment
Three regular fed and three fish fasted for 24 h were utilized for extraction of body fluids. Bile fluids (BFs) were taken directly from the gallbladder, and ICs were extracted from the posterior 10 cm of the intestine. Fed-BFs and Fed-ICs represented BFs and ICs from fish with regular feeding, while Fasted-BFs and Fasted-ICs were sampled from the fish that had fasted for 24 h. All samples were stored at −80 °C until ultrahigh-performance liquid chromatography coupled to tandem mass spectrometry (UHPLC-MS/MS) analysis was performed.
The samples were homogenized with 500 μL of acetonitrile/methanol (8:2, v/v, Thermo Fisher Scientific, Waltham, MA, USA) and further centrifuged at 12,000 rpm for 20 min to remove the protein. Then, the supernatant was blown with nitrogen until dry. The precipitate was reconstituted with 100 μL of water/acetonitrile (8:2, v/v, including 0.1% formic acid). Finally, the supernatant (2 μL) was subjected to UHPLC-MS/MS analysis.
2.3.2. Solution Preparation and BA Quantification
All 33 BA/BA salt standards and six stable isotope-labeled standards were obtained from Zhenzhun Standards Co., Ltd. (Shanghai, China). The ammonium acetate (Sigma Aldrich, St. Louis, MO, USA) was of analytical grade. Methanol, acetonitrile, and formic acid (Optima LC-MS) were purchased from Thermo-Fisher Scientific (Waltham, MA, USA). The BA stock solution was mixed in BA-free matrices to obtain BA calibrators at concentrations of 1.5, 2.5, 5, 15, 25, 50, 250, 500, 2500, 5000, 15000, and 25000 ng/mL. Glycocholic acid-d4 (GCA-d4), ursodeoxycholic acid-d4 (UDCA-d4), glycochenodeoxycholic acid-d4 (GCDCA-d4), CA-d4, LCA-d4, and CDCA-d4 were compounded and mixed as an internal standard (IS). The stock solutions and working solutions were stored in a freezer at −20 °C until use.
The samples were analyzed on a UHPLC-MS/MS system (ExionLC™ AD UHPLC-QTRAP 6500+, AB SCIEX Corp., Boston, MA, USA) to quantitatively identify BAs. The mobile phase consisted of 0.1% formic acid in water (solvent A) and acetonitrile (solvent B). Separation was achieved using a Waters ACQUITY UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm) and the following gradient program at a flow rate of 0.30 mL/min for 17 min at 50 °C: initial 20% B for 0.5 min, increased to 100% B from 0.5 to 13 min and maintained until 15 min, and decreased to 20% B from 15 to 15.1 min and maintained to 17 min. The injection volume was 10 μL. Multiple reaction monitoring (MRM) transitions and related parameters are listed in Table S1. The parameters were as follows: ion spray voltage (−4500 V), curtain gas (35 psi), ion source temperature (550 °C), and ion source gas of 1 and 2 (60 psi).
2.4. Electrophysiological Response of the Olfactory Epithelium
2.4.1. Experimental Fish Preparation
Before the experiment, the fish were anesthetized with MS-222 orally and intramuscularly injected with gallamine tri-iodide (5 mg/kg body weight) for immobilization. Then, the fish were wrapped with absorbent tissue and secured in a holding apparatus while seawater was pumped over their gills (0.5 mL/s) via a silicon tube placed in their mouths. The filtered seawater or test solutions bathing the olfactory epithelium were delivered through separate tubes at a flow rate of 5–7 mL/min. Minor surgery was performed to remove the skin and connective tissue superficial to the olfactory epithelium to facilitate electrode placement.
2.4.2. EOG Measurements
EOG is a negative electrical potential that was recorded in the water above the fish olfactory epithelium in immediate response to an odorant stimulus; thus, the EOG represents the summed generator potential of the olfactory sensory neurons []. Before the experiment, test solutions of an amino acid (L-serine, Macklin, Shanghai, China) and bile salts (TCA, TCDCA, CA, CDCA, and TDCA, Macklin, Shanghai, China) were diluted with filtered seawater to experimental concentrations from stock solutions (10−2 mol/L, with DMSO solute, stored at 4 °C). Tests were performed at least twice for each concentration of the stimulus. The olfactory epithelium was allowed to recover for 5 min between stimuli, as this time permitted complete recovery. If the two responses were quite different, a third test was conducted.
Differential EOG responses were recorded using two Ag/AgCl electrodes (World Precision Instruments, Sarasota, FL, USA) bridged to capillaries filled with 3 mol/L KCl, 0.15 mol/L NaCl, and 0.5% agar (tip diameter 150 µm). The recording electrode was positioned above the olfactory epithelium, whereas the reference electrode was placed against the skin between the eyes. Electrical signals were amplified and digitized using a BL-420F data acquisition and analysis system (TaiMeng Software Design, Chengdu, China) and displayed on a computer.
2.4.3. Cross-Adaptation
Cross-adaptation was used to compare the EOG responses to the test solution before and during adaptation to an adapting compound. The following steps were included in each experiment: (1) the response to a test odorant was measured when the olfactory epithelium was immersed in filtered seawater; (2) the background seawater superfusing the olfactory epithelium was replaced by an adapting solution (CA) until the response declined and stabilized, and then the response to a test odorant was measured, and (3) the flow bathing the olfactory epithelium was returned to filtered seawater, and the odorant was retested to confirm reversible adaptation.
2.4.4. Pharmacological Treatments
To identify the intracellular pathway involved in olfactory signal transduction, the EOG responses to the test stimuli were recorded in the presence of the drugs forskolin and U-73122 (MedChemExpress, Monmouth Junction, NJ, USA), which selectively inhibit the cAMP and PLC pathways, respectively. Forskolin and U-73122 were dissolved in DMSO and added to filtered seawater to generate 10−4 mol/L stock solutions, which were then stored at −20 °C. The concentrations of DMSO controls were adjusted to match those used to dissolve the pharmacological agents.
Similar to cross-adaptation, three stages were also needed. During stage 1, the olfactory epithelium was continuously immersed in filtered seawater for a minimum of 10 min before the stimulus was applied. During stage 2, the olfactory epithelium was immersed in the drug solutions at the adjusted concentrations (which resulted in an EOG response with approximately the same relative magnitude as the EOG response to the odorant stimulus) continuously for a minimum of 10 min before the stimulus was applied. All odorants tested during stage 2 were prepared in the presence of the drug at the same concentration as the water superfusing the olfactory epithelium. During stage 3, the olfactory epithelium was continuously immersed in filtered seawater for 10 min before the stimulus was applied. If the EOG responses in stage 3 were significantly different from those described in stage 1, the experiment was repeated.
2.5. Data Analysis and Statistics
All data were processed with GraphPad version 9.0.0 for Windows (USA, www.graphpad.com) and presented as the mean ± standard error of the mean (S.E.M.). Student’s t-test for unpaired data was used to compare the PI of the test solution to the seawater control in the behavior assay. The analytes were identified by comparing the elution time and mass spectra to those of standard samples. Principal component analysis (PCA) and heatmap construction were performed with the R packages: FactoMineR, RColorBrewer, and pheatmap. EOG response magnitudes were normalized as percentages of the response to 10−5 mol/L L-serine. In the cross-adaptation and pharmacological treatments, the EOG amplitudes of fish on the treatment were compared to respective controls using Student’s t-test for paired data. In all analyses, p < 0.05 was taken to represent statistical significance.
3. Results
3.1. Behavioral Response to Conspecific Body Fluids
The behavior assay showed that food odor (p < 0.001) as a fish attractant evoked an increase in the number of fish on the side with odorant addition (Figure 1). Interestingly, large yellow croakers showed pronounced preference behavior for both conspecific ICs with regular feeding (p < 0.01) and ICs after a 24 h fast (p < 0.05), whereas the seawater control, skin mucus and DMSO had no effect. During the entire test, the fish swam freely in the device, and no clustered behavior, feeding response or abnormal states (e.g., dashing, tachypnoea, or nervousness) were found. When the food or ICs were added, a few fish showed exploratory behavior and stayed more time in the mix or choice arm to which the test solution was added.
3.2. Ba-Targeted Metabolomics
UHPLC-MS/MS was applied to analyze BA profiles of the intestine and gallbladder in large yellow croaker. Separation of the 33 BA/BA salt standards was completed in a single run of 16 min, including column equilibration (Table S1 and Figure 2). The matrix effects, precision, accuracy, and stability of the method were within the required ranges (Table S2). The BA compositions of large yellow croakers are shown in Figure 3 and Table 1. Twenty-seven BAs were detected, with the exceptions of HCA, 23norDCA, isoLCA, 12-ketoLCA, βUDCA, and β-MCA, which were not detected or were below the quantification limits.
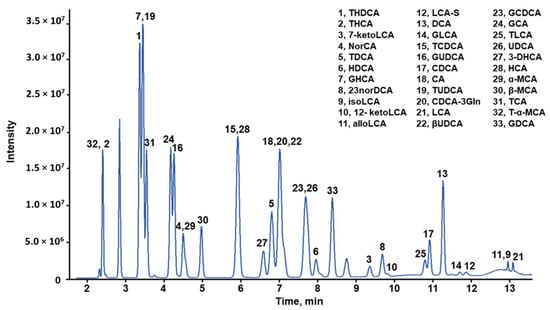
Figure 2.
Chromatogram of 33 BAs/BA salts produced by ultrahigh-performance liquid chromatography coupled to tandem mass spectrometry (UHPLC–MS/MS). The sample concentration was 100 ng/mL. Abbreviations are listed in Table 1.
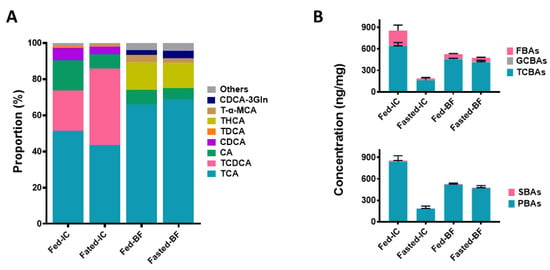
Figure 3.
Compositions of BA profiles in large yellow croaker (Larimichthys crocea). (A) The amounts of the top five BAs are presented as a proportion of the total pool. (B) The classification and statistics of BAs in different groups. Ethanolic extracts of ICs with regular feeding (Fed-ICs) and after a 24 h fast (Fasted-ICs) and bile fluids with regular feeding (Fed-BFs) and after a 24 h fast (Fasted-BFs) were analyzed by UHPLC-MS/MS. Data are presented as the mean ± S.E.M.; n = 5. TCA, taurocholic acid; TCDCA, sodium salt of taurochenodeoxycholic acid; CA, cholic acid; CDCA, chenodeoxycholic acid; TDCA, sodium salt of taurodeoxycholic acid; THCA, sodium salt of taurohyocholic acid; T-α-MCA, sodium salt of tauro-alpha-muricholic acid; CDCA-3Gln, chenodeoxycholic acid-3-β-D-glucuronide; THDCA, sodium salt of taurohyodeoxycholic acid; others, other BAs; TCBAs, taurine-conjugated BAs; GCBAs, glycine-conjugated BAs; FBAs, free BAs; PBAs, primary BAs; SBAs, secondary BAs.

Table 1.
The concentrations of 33 BAs/BA salts in different samples (ng/mg).
Twenty-six different BAs were detected in the intestines, among which TCA, TCDCA, CA, CDCA, and TDCA were the top five. The concentration of total BAs in the ICs with regular feeding (Fed-ICs) (855.83 ng/mg) significantly decreased to 187.42 ng/mg after 24 h fasting (Fasted-ICs). The proportions of BAs (except TCDCA) showed relatively small differences in the Fed-ICs and Fasted-ICs. The two BA profiles of ICs (Fed-ICs and Fasted-ICs) were categorized into one group in the heatmap, although PCA showed that Fed-ICs and Fasted-ICs could be distinguished from each other. In contrast to the results for ICs, only 19 BAs were detected for varying concentrations of BAs in gallbladders. TCA, THCA, CA, CDCA-3Gln, and T-α-MCA accounted for more than 95% of the total BA pools in both Fed-BFs and Fasted-BFs. The 24 h fasting treatment had little effect on the BA profile of the gallbladder, as little change in composition and proportion was observed, and the decrease was only 51.7 ng/mg (Figure 3B and Table 1). These findings agreed with the results of PCA and multiclass analysis, indicating that Fed-BFs clustered with Fasted-BFs. The PCA and heatmap directly showed the differences between the BA profiles of the gallbladder and intestine (Figure 4). However, in both the gallbladder and the intestine, conjugated BAs accounted for more than 75% of the total BA pools. Furthermore, taurine-conjugated BAs had an absolute advantage over glycine-conjugated BAs. Primary BAs were more abundant than secondary BAs in both types of tissues (Figure 3B).
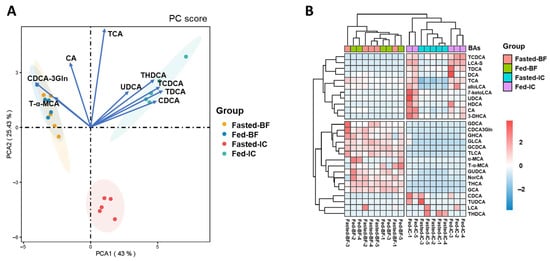
Figure 4.
Comparisons of BA profiles in large yellow croaker (Larimichthys crocea). (A) Principal component analysis (PCA) of BA profiles with each of the 27 BAs (all the BAs that appeared in large yellow croaker). The 95% confidence interval is marked by oval circles. The contributions of the top five BAs are indicated by arrows (the projection of the arrows on the coordinate axes shows the contribution). (B) Heatmap depicting a multiclass analysis of the four groups of BA profiles. The intensity of the color indicates the expression level of BAs (dark red, high expression; sky blue, low expression). Fed-IC, IC of fish fed regularly; Fasted-IC, IC of fish fasted for 24 h; Fed-BF, BF of fish fed regularly; Fasted-BF, BF of fish fasted for 24 h.
3.3. Behavioral Response to BAs
The Y-maze choice apparatus was employed to determine whether the top five BAs in the ICs were attractive to large yellow croakers. As shown in Figure 5, fish had a significant preference for 10−8 mol/L CA (p < 0.01) over the negative control (seawater and DMSO). In contrast, the PI scores showed no effect of adding TCA, TCDCA, CDCA, or TDCA (p > 0.05). All the fish behaved normally during the experiment. A few fish showed exploratory behavior rather than feeding-related behavior in the CA solution.
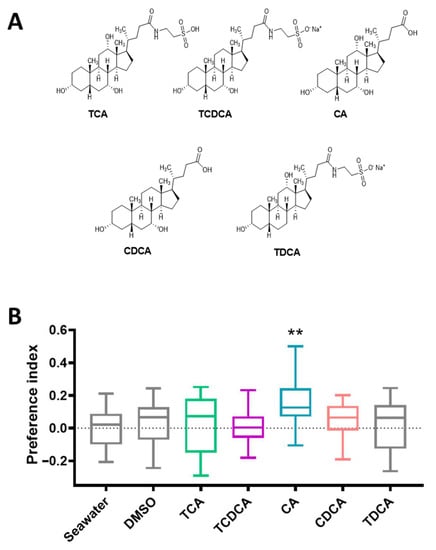
Figure 5.
Behavioral responses to the top five BAs of ICs. (A) Structures of the top five BAs of ICs. (B) The preference index of 10−8 mol/L BAs is presented as the mean ± S.E.M.; n = 20. Student’s t-test was used to analyze all data versus DMSO data. ** p < 0.01.
3.4. Electrophysiological Response to BAs
The EOG responses to TCA, TCDCA, CA, CDCA, and TDCA, the top five BAs in the ICs in large yellow croaker, were recorded to determine the relative effectiveness of BAs as odorant stimuli (Figure 6A,B). The concentration-response (C-R) relationships of BAs were divided into two groups according to molecular structure. EOG amplitudes in response to all five BAs were strongly concentration-dependent, and the response magnitudes grew continuously with increasing concentrations of BAs. The BAs conjugated with taurine, TCA, TCDCA, and TDCA had similar C–R curves, with lower detection thresholds of 10−11, 10−12, and 10−12, respectively. The free BAs CA and CDCA had similar, nearly parallel C–R curves with detection thresholds of 10−11 and 10−12 mol/L, respectively. Remarkably, compared to their respective conjugated forms, the unconjugated forms of the BAs were consistently less potent.
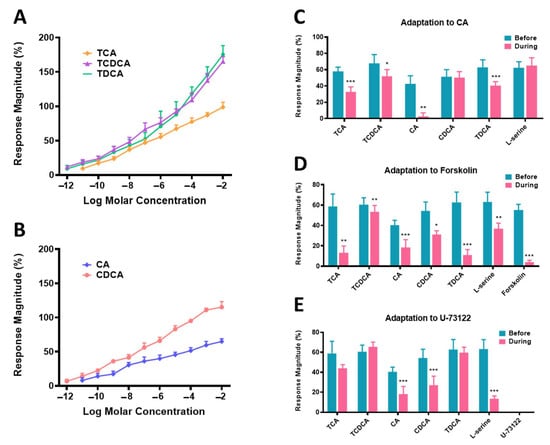
Figure 6.
Electro-olfactogram responses of large yellow croaker (Larimichthys crocea) to BAs. (A) Concentration-response relationships of conjugated and free BAs. (B) Response magnitudes of BAs before and during adaptation to 10−8 mol/L CA. (C–E) The effects of 10−7 mol/L forskolin and U-73122 on 10−8 mol/L odorant-evoked responses. * p < 0.05, ** p < 0.01, *** p < 0.001; without an asterisk indicates no significant difference according to the paired t-test (p > 0.05). Response magnitudes are normalized as percentages of the response to 10−5 mol/L L-serine (mean ± S.E.M., n = 5). TCA, taurocholic acid; TCDCA, sodium salt of taurochenodeoxycholic acid; TDCA, sodium salt of taurodeoxycholic acid; CA, cholic acid; CDCA, chenodeoxycholic acid.
When used as an adapting stimulus (self-adaptation), CA completely suppressed the EOG response to itself (p < 0.01, Figure 6C), implying that the interaction of odorant stimuli with olfactory receptors was reversible. Next, under cross-adaptation to CA, the response magnitudes of TCA, TCDCA, and TDCA were reduced by 43.56% (p < 0.001), 22.49% (p < 0.05), and 35.43% (p < 0.001), respectively. Nevertheless, those of CDCA and L-serine were not affected.
To equalize the potencies of the test stimulus to the cAMP signaling pathway, the concentration of forskolin was adjusted to produce an EOG response approximately equivalent to the EOG responses to the odorant stimuli. As shown in Figure 6D, the 10−7 mol/L forskolin treatment significantly reduced olfactory responses to TCA (p < 0.01), TCDCA (p < 0.01), CA (p < 0.001), CDCA (p < 0.05), TDCA (p < 0.001), and L-serine (p < 0.01) by 11.77% to 77.36%. The results indicate the involvement of the cAMP signaling cascade in the top five BAs’ olfactory transduction. U-73122 elicited a background EOG response when applied to the olfactory epithelium (Figure S1). Therefore, the concentrations of U-73122 were equivalent to those used during the forskolin treatment. In addition, compared to the control amplitude of olfactory responses, 10 min of bathing the PLC blocker U-73122 over the olfactory epithelium significantly reduced the amplitudes to 10−8 mol/L CA, CDCA and L-serine by 56.07% (p < 0.001), 51.23% (p < 0.001) and 78.30% (p < 0.001), respectively (Figure 6E).
4. Discussion
Metabolic byproducts released by fish can be components of pheromones []. This phenomenon could help explain how 30,000 species of fish have developed unique pheromones. Chemical signals or pheromones are frequently carried via body fluids, such as intestinal fluids [], urine [], and skin mucus []. Due to the diversity in osmoregulatory strategy, marine fish excrete urine less frequently and in smaller quantities compared to their freshwater counterparts. []. As a result, marine fish primarily do not emit pheromones through urine. In this study, the behavioral results showed that ICs other than skin mucus could cause preference responses in large yellow croakers implying that ICs (i.e., feces) were the most likely source of attractive active substances in large yellow croakers. Moreover, ICs after a 24 h fast elicited similar attraction behavior in this species, indicating that attractive substances in the intestine were not affected by feeding. This provided additional evidence for the stable properties required for the generation and release of individual conspecific signals, given that intestinal fluids participate in fish chemical communication [,,].
The majority of BAs are involved in enterohepatic circulation, while only a small portion is excreted into the external environment via feces or other ways [,]. Targeted metabolomics has shown that the intestine and gallbladder contain large amounts of BAs, serving as release and storage sites. The BA profiles of different fish species are highly similar, which could be attributed to evolutionary conservation []. TCA, TCDCA, CA, and CDCA were the main BAs in this study, similar to those in other fish species [,,]. Taurine-conjugated BAs predominate in the BA profile of marine fish, and CA is more prevalent in freshwater fish []. Significantly higher CA content was detected in large yellow croakers than in other marine fish, suggesting CA could be crucial for nutrient digestion assistance or signal transduction. Moreover, BA profiles were relatively stable in a given tissue regardless of feeding. Likewise, previous studies showed that BA profiles are generally stable and are not directly correlate with diets []. Furthermore, CA was found to evoke significant preference behavior, indicating intestinal CA was an attractive chemical in this species.
Some evidence has shown that BAs stimulate olfactory receptors as well as the nuclear receptor FXR (farnesoid X receptor) and the membrane receptor TGR5 (Taken G protein-coupled receptor 5) [,]. In the present study, the concentration-response relationship, cross-adaptation, and pharmacological results showed that different kinds of BAs could be detected in the olfactory epithelium of this species. EOG recordings have shown that freshwater and marine fish species detect BAs at nanomolar concentrations [,]. Large yellow croakers were sensitive to all five BAs with lower detection thresholds at 10−11 or 10−12 mol/L. A possible explanation for this is that more than 100 receptor genes are expressed in the olfactory epithelial tissues []. To identify thousands of odorant molecules in the surroundings, the olfactory system adopts a complex pattern in which an odorant is recognized by more than one receptor, and a receptor recognizes several odorants []. The crossover of olfactory receptors is more likely to occur for substances with similar chemical structures [,]. Consistent with these findings, the present cross-adaptation results showed that CA shared olfactory receptors with TCA, TCDCA, and TDCA. Nevertheless, these results conflict with previous findings that CDCA and CA share the same olfactory receptors in rainbow trout []. This discrepancy is probably due to the diversity of olfactory reception across species. Treatment with either forskolin or U-73122 led to partial attenuation of EOG responses, indicating that the cAMP signaling cascade together with the PLC pathway was triggered by CA, CDCA and L-serine. These results are consistent with previous assumptions that cAMP and PLC signaling are involved in marine fish sensing, whereas cAMP signaling mediates the olfactory reception of BAs in freshwater fish [,,]. This highlights how marine fish and freshwater fish have different molecular mechanisms for olfactory reception. Further evidence that salinity influences the olfactory transduction process comes from the recent discovery that the seabass’s olfactory system is more sensitive in saltwater than in freshwater []. Furthermore, there is evidence that ORs and VRs ae both sensitive to BAs [,]. Sodium cholates treatment up-regulated expression of 59 ORs and two VRs in olfactory epithelium tissues as our previous finding reported []. Therefore, the reception of BAs in large yellow croaker is mediated by ORs via the cAMP transduction cascade combined with VRs through the PLC signal pathway. This shows that different types of olfactory receptors and different types of signal transduction mechanisms can be employed for BAs. Further experiments are warranted to clarify the specific molecular mechanism of BAs reception in marine fish.
Intra-species and inter-species chemical communication is very important in the life history of fish, including predation, reproduction, aggregation, and risk assessment. Our behavior experiment results proved for the first time that large yellow croaker can be attracted by ICs of conspecific individuals. This finding is consistent with the importance of feces in scent marking and chemical communication of many fish species []. Fish preferred not only Fed-ICs but also Fasted-ICs, disproving the theory that fish were attracted by undigested food in the intestine. Due to the experimental large yellow croakers being juveniles that had not reached sexual maturity, the explanation of mutual attraction between male and female individuals did not apply to the results. Therefore, attractive ICs may carry and transmit information related to conspecific identification or aggregation. Large yellow croaker, in a group, may cluster in response to signals transmitted through ICs. Most studies about BAs in large yellow croakers have only focused on their function as exogenous feed additives or phagostimulants [,,]. A series of experimental results demonstrated that CA released from the conspecific intestine could be exploited as endogenous chemical signals or even aggregation pheromones.
5. Conclusions
To better understand chemical communication between conspecific fish, behavioral observation, BA-targeted metabolomics analysis, and electrophysiological response were investigated in large yellow croaker. Our major findings were the following: (1) a behavioral study demonstrated for the first time that ICs evoked preferential fish behaviors regardless of whether the fish fasted for 24 h; (2) TCA, TCDCA, CA, CDCA, and TDCA were the top five BAs in the ICs, regardless of fish feeding; (3) CA evoked behavioral preferences and the top five BAs were potent odorants in L. crocea; (4) Cross-adaptation tests showed that CA shared the olfactory receptors with TCA, TCDCA, and TDCA, and (5) the cAMP and PLC pathways are both involved in the olfactory signal transduction of BAs.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/fishes8010026/s1, Figure S1. Electro-olfactogram responses of large yellow croaker to forskolin and U-73122. Response magnitudes are normalized as percentages of the response to 10−5 mol/L L-serine (mean ± S.E.M., n = 3), Table S1. The mass spectrometry conditions of different Bas, Table S2. The matrix effects, precision, accuracy, and stability of the LC/MS/MS method.
Author Contributions
A.Z.: Data curation, formal analysis, investigation, software, validation, writing—original draft, writing—review & editing. X.Z.: data curation, formal analysis. X.Y.: conceptualization, data curation, funding acquisition, project administration, supervision, validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Research & Development Project of Zhejiang Province (grant number: 2020C02004).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Ningbo University (protocol code 202104070304 and date of approval 7 April 2021).
Data Availability Statement
Raw data were generated at Ningbo University, China. Derived data supporting the findings of this study are available from Prof. Xiaojun Yan (yanxiaojun@nbu.edu.cn) upon request.
Acknowledgments
The authors thank Han Tao for his assistance. Large yellow croakers and experimental sites were kindly provided by the Fishery Research Institute of Zhoushan and the Marine Fisheries Research Institute of Zhejiang Province.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sorensen, P.W.; Wisenden, B.D. Fish Pheromones and Related Cues; John Wiley & Sons: New York, NY, USA, 2014. [Google Scholar]
- Thomas, B.; Martin, T. Chemical communication in crustaceans. In A Review of Research in Fish Pheromones; Chung-Davidson, Y.-W., Huertas, M., Li, W., Eds.; Springer Science & Business Media: New York, NY, USA, 2010; pp. 467–482. [Google Scholar]
- Burnard, D.; Gozlan, R.E.; Griffiths, S.W. The role of pheromones in freshwater fishes. J. Fish Biol. 2008, 73, 1–16. [Google Scholar] [CrossRef]
- Tirindelli, R.; Dibattista, M.; Pifferi, S.; Menini, A. From pheromones to behavior. Physiol. Rev. 2009, 89, 921–956. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Buchinger, T.J.; Li, W. Discovery and characterization of natural products that act as pheromones in fish. Nat. Prod. Rep. 2018, 35, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Huertas, M.; Hubbard, P.C.; Canário, A.V.M.; Cerdà, J. Olfactory sensitivity to conspecific bile fluid and skin mucus in the European eel Anguilla anguilla (L.). J. Fish Biol. 2007, 70, 1907–1920. [Google Scholar] [CrossRef]
- Fatsini, E.; Carazo, I.; Chauvigne, F.; Manchado, M.; Cerda, J.; Hubbard, P.C.; Duncan, N.J. Olfactory sensitivity of the marine flatfish Solea senegalensis to conspecific body fluids. J. Exp. Biol. 2017, 220, 2057–2065. [Google Scholar] [CrossRef]
- Yambe, H.; Kitamura, S.; Kamio, M.; Yamada, M.; Matsunaga, S.; Fusetani, N.; Yamazaki, F. L-Kynurenine, an amino acid identified as a sex pheromone in the urine of ovulated female masu salmon. Proc. Natl. Acad. Sci. USA 2006, 103, 15370–15374. [Google Scholar] [CrossRef]
- Scott, A.M.; Zhang, Z.; Jia, L.; Li, K.; Zhang, Q.; Dexheimer, T.; Ellsworth, E.; Ren, J.; Chung-Davidson, Y.W.; Zu, Y.; et al. Spermine in semen of male sea lamprey acts as a sex pheromone. PLoS Biol. 2019, 17, e3000332. [Google Scholar] [CrossRef]
- Lacalle-Bergeron, L.; Goterris-Cerisuelo, R.; Portoles, T.; Beltran, J.; Sancho, J.V.; Navarro-Moreno, C.; Martinez-Garcia, F. Novel sampling strategy for alive animal volatolome extraction combined with GC-MS based untargeted metabolomics: Identifying mouse pup pheromones. Talanta 2021, 235, 122786. [Google Scholar] [CrossRef]
- Izrayelit, Y.; Srinivasan, J.; Campbell, S.L.; Jo, Y.; von Reuss, S.H.; Genoff, M.C.; Sternberg, P.W.; Schroeder, F.C. Targeted metabolomics reveals a male pheromone and sex-specific ascaroside biosynthesis in Caenorhabditis elegans. ACS Chem. Biol. 2012, 7, 1321–1325. [Google Scholar] [CrossRef]
- Li, W.; Scott, A.P.; Siefkes, M.J.; Yan, H.; Liu, Q.; Yun, S.-S.; Gage, D.A. Bile acid secreted by male sea lamprey that acts as a sex pheromone. Science 2002, 296, 138–141. [Google Scholar] [CrossRef]
- Sorensen, P.W.; Fine, J.M.; Dvornikovs, V.; Jeffrey, C.S.; Shao, F.; Wang, J.; Vrieze, L.A.; Anderson, K.R.; Hoye, T.R. Mixture of new sulfated steroids functions as a migratory pheromone in the sea lamprey. Nat. Chem. Biol. 2005, 1, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Kiriyama, Y.; Nochi, H. The biosynthesis, signaling, and neurological functions of bile acids. Biomolecules 2019, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sagada, G.; Wang, C.; Liu, R.; Li, Q.; Zhang, C.; Yan, Y. Exogenous bile acids regulate energy metabolism and improve the health condition of farmed fish. Aquaculture 2023, 562, 738852. [Google Scholar] [CrossRef]
- Hofmann, A.F.; Hagey, L.R.; Krasowski, M.D. Bile salts of vertebrates: Structural variation and possible evolutionary significance. J. Lipid Res. 2010, 51, 226–246. [Google Scholar] [CrossRef] [PubMed]
- Polkinghorne, C.N.; Olson, J.M.; Gallaher, D.G.; Sorensen, P.W. Larval sea lamprey release two unique bile acids to the water at a rate sufficient to produce detectable riverine pheromone plumes. Fish Physiol. Biochem. 2001, 24, 15–30. [Google Scholar] [CrossRef]
- Huertas, M.; Hagey, L.; Hofmann, A.F.; Cerda, J.; Canario, A.V.; Hubbard, P.C. Olfactory sensitivity to bile fluid and bile salts in the European eel (Anguilla anguilla), goldfish (Carassius auratus) and Mozambique tilapia (Oreochromis mossambicus) suggests a ‘broad range’ sensitivity not confined to those produced by conspecifics alone. J. Exp. Biol. 2010, 213, 308–317. [Google Scholar] [CrossRef]
- Giaquinto, P.C.; Barreto, R.E.; Volpato, G.L.; Fernandes-de-Castilho, M.; Gonçalves-de-Freitas, E. Bile acids as potential pheromones in pintado catfish Pseudoplatystoma corruscans (Spix & Agassiz, 1829): Eletrophysiological and behavioral studies. Neotrop. Ichthyol. 2015, 13, 237–244. [Google Scholar] [CrossRef][Green Version]
- Giaquinto, P.C.; Hara, T.J. Discrimination of bile acids by the rainbow trout olfactory system: Evidence as potential pheromone. Biol. Res. 2008, 41, 33–42. [Google Scholar] [CrossRef]
- Zhang, C.; Brown, S.B.; Hara, T.J. Biochemical and physiological evidence that bile acids produced and released by lake char (Salvelinus namaycush) function as chemical signals. J. Comp. Physiol. B 2001, 171, 161–171. [Google Scholar] [CrossRef]
- Cong, X.; Zheng, Q.; Ren, W.; Cheron, J.B.; Fiorucci, S.; Wen, T.; Zhang, C.; Yu, H.; Golebiowski, J.; Yu, Y. Zebrafish olfactory receptors ORAs differentially detect bile acids and bile salts. J. Biol. Chem. 2019, 294, 6762–6771. [Google Scholar] [CrossRef]
- Buchinger, T.J.; Li, W.; Johnson, N.S. Bile salts as semiochemicals in fish. Chem. Senses 2014, 39, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Firestein, S. How the olfactory system makes sense of scents. Nature 2001, 413, 211–218. [Google Scholar] [CrossRef]
- Manzini, I.; Korsching, S. The peripheral olfactory system of vertebrates: Molecular, structural and functional basics of the sense of smell. e-Neuroforum 2011, 17, 68–77. [Google Scholar] [CrossRef]
- Wong, W.M.; Cao, J.; Zhang, X.; Doyle, W.I.; Mercado, L.L.; Gautron, L.; Meeks, J.P. Physiology-forward identification of bile acid-sensitive vomeronasal receptors. Sci. Adv. 2020, 6, eaaz6868. [Google Scholar] [CrossRef]
- Hansen, A.; Zielinski, B.S. Diversity in the olfactory epithelium of bony fishes: Development, lamellar arrangement, sensory neuron cell types and transduction components. J. Neurocytol. 2005, 34, 183–208. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.; Rolen, S.H.; Anderson, K.; Morita, Y.; Caprio, J.; Finger, T.E. Correlation between olfactory receptor cell type and function in the channel catfish. J. Neurosci. 2003, 23, 9328–9339. [Google Scholar] [CrossRef] [PubMed]
- Velez, Z.; Hubbard, P.C.; Barata, E.N.; Canario, A.V. Olfactory transduction pathways in the Senegalese sole Solea senegalensis. J. Fish Biol. 2013, 83, 501–514. [Google Scholar] [CrossRef]
- Scott, J.W.; Scott-Johnson, P.E. The electroolfactogram: A review of its history and uses. Microsc. Res. Tech. 2002, 58, 152–160. [Google Scholar] [CrossRef]
- International Union for Conservation of Nature. Larimichthys crocea. The IUCN Red List of Threatened Species 2020; International Union for Conservation of Nature: Gland, Switzerland, 2020. [Google Scholar]
- Hubbard, P.C.; Barata, E.N.; Canario, A.V. Olfactory sensitivity of the gilthead seabream (Sparus auratus L.) to conspecific body fluids. J. Chem. Ecol. 2003, 29, 2481–2498. [Google Scholar] [CrossRef]
- Matsumura, K.; Matsunaga, S.; Fusetani, N. Phosphatidylcholine profile-mediated group recognition in catfish. J. Exp. Biol. 2007, 210, 1992–1999. [Google Scholar] [CrossRef]
- Velez, Z.; Hubbard, P.C.; Barata, E.N.; Canario, A.V. Differential detection of conspecific-derived odorants by the two olfactory epithelia of the Senegalese sole (Solea senegalensis). Gen. Comp. Endocrinol. 2007, 153, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Apps, P.J.; Weldon, P.J.; Kramer, M. Chemical signals in terrestrial vertebrates: Search for design features. Nat. Prod. Rep. 2015, 32, 1131–1153. [Google Scholar] [CrossRef] [PubMed]
- Romano, N.; Kumar, V.; Yang, G.; Kajbaf, K.; Rubio, M.B.; Overturf, K.; Brezas, A.; Hardy, R. Bile acid metabolism in fish: Disturbances caused by fishmeal alternatives and some mitigating effects from dietary bile inclusions. Rev. Aquac. 2020, 12, 1792–1817. [Google Scholar] [CrossRef]
- Li, K.; Buchinger, T.J.; Bussy, U.; Fissette, S.D.; Johnson, N.S.; Li, W. Quantification of 15 bile acids in lake charr feces by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 1001, 27–34. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Zhou, H.; Li, M.; Wang, M.; Wu, S. An analysis of bile acid composition in different tissues of grass carp (Ctenopharyngodon idellus). Acta Hydrobiol. Sin. 2017, 41, 479–482. [Google Scholar] [CrossRef]
- Goto, T.; Ui, T.; Une, M.; Kuramoto, T.; Kihira, K.; Hoshita, T. Bile salt composition and distribution of the D-cysteinolic acid conjugated bile salts in fish. Fish. Sci. 1996, 62, 606–609. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Q.; Kim, S.K.; Liao, Z.; Wei, Y.; Sun, B.; Jia, L.; Chi, S.; Liang, M. Dietary taurine stimulates the hepatic biosynthesis of both bile acids and cholesterol in the marine teleost, tiger puffer (Takifugu rubripes). Br. J. Nutr. 2020, 123, 1345–1356. [Google Scholar] [CrossRef]
- Hagey, L.R.; Moller, P.R.; Hofmann, A.F.; Krasowski, M.D. Diversity of bile salts in fish and amphibians: Evolution of a complex biochemical pathway. Physiol. Biochem. Zool. 2010, 83, 308–321. [Google Scholar] [CrossRef]
- Ferrell, J.M.; Chiang, J.Y.L. Bile acid receptors and signaling crosstalk in the liver, gut and brain. Liver Res. 2021, 5, 105–118. [Google Scholar] [CrossRef]
- Robinson, T.C.; Sorensen, P.W.; Bayer, J.M.; Seelye, J.G. Olfactory sensitivity of pacific lampreys to lamprey bile acids. Trans. Am. Fish. Soc. 2009, 138, 144–152. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, X.; Xu, S.; Zhu, P.; He, X.; Liu, J. Family structure and phylogenetic analysis of odorant receptor genes in the large yellow croaker (Larimichthys crocea). BMC Evol. Biol. 2011, 11, 237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hara, T.J. Lake char (Salvelinus namaycush) olfactory neurons are highly sensitive and specific to bile acids. J. Comp. Physiol. A 2009, 195, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Velez, Z.; Hubbard, P.C.; Guerreiro, P.M. A Comparison of Olfactory Sensitivity in Seawater- and Freshwater-Adapted Bass, Dicentrarchus labrax. Biol. Life Sci. Forum 2022, 13, 125. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Le, Q.; Yu, N.; Cao, X.; Kuang, S.; Zhang, M.; Gu, W.; Sun, Y.; Yang, Y.; et al. Transcriptome sequencing of olfactory-related genes in olfactory transduction of large yellow croaker (Larimichthy crocea) in response to bile salts. PeerJ 2019, 7, e6627. [Google Scholar] [CrossRef]
- Ding, T.; Xu, N.; Liu, Y.; Du, J.; Xiang, X.; Xu, D.; Liu, Q.; Yin, Z.; Li, J.; Mai, K.; et al. Effect of dietary bile acid (BA) on the growth performance, body composition, antioxidant responses and expression of lipid metabolism-related genes of juvenile large yellow croaker (Larimichthys crocea) fed high-lipid diets. Aquaculture 2020, 518, 734768. [Google Scholar] [CrossRef]
- Du, J.; Xu, H.; Li, S.; Cai, Z.; Mai, K.; Ai, Q. Effects of dietary chenodeoxycholic acid on growth performance, body composition and related gene expression in large yellow croaker (Larimichthys crocea) fed diets with high replacement of fish oil with soybean oil. Aquaculture 2017, 479, 584–590. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).