First Mitogenome of Endangered Enteromius thysi (Actinopterygii: Cypriniformes: Cyprinidae) from Africa: Characterization and Phylogeny
Abstract
1. Introduction
2. Materials and Methods
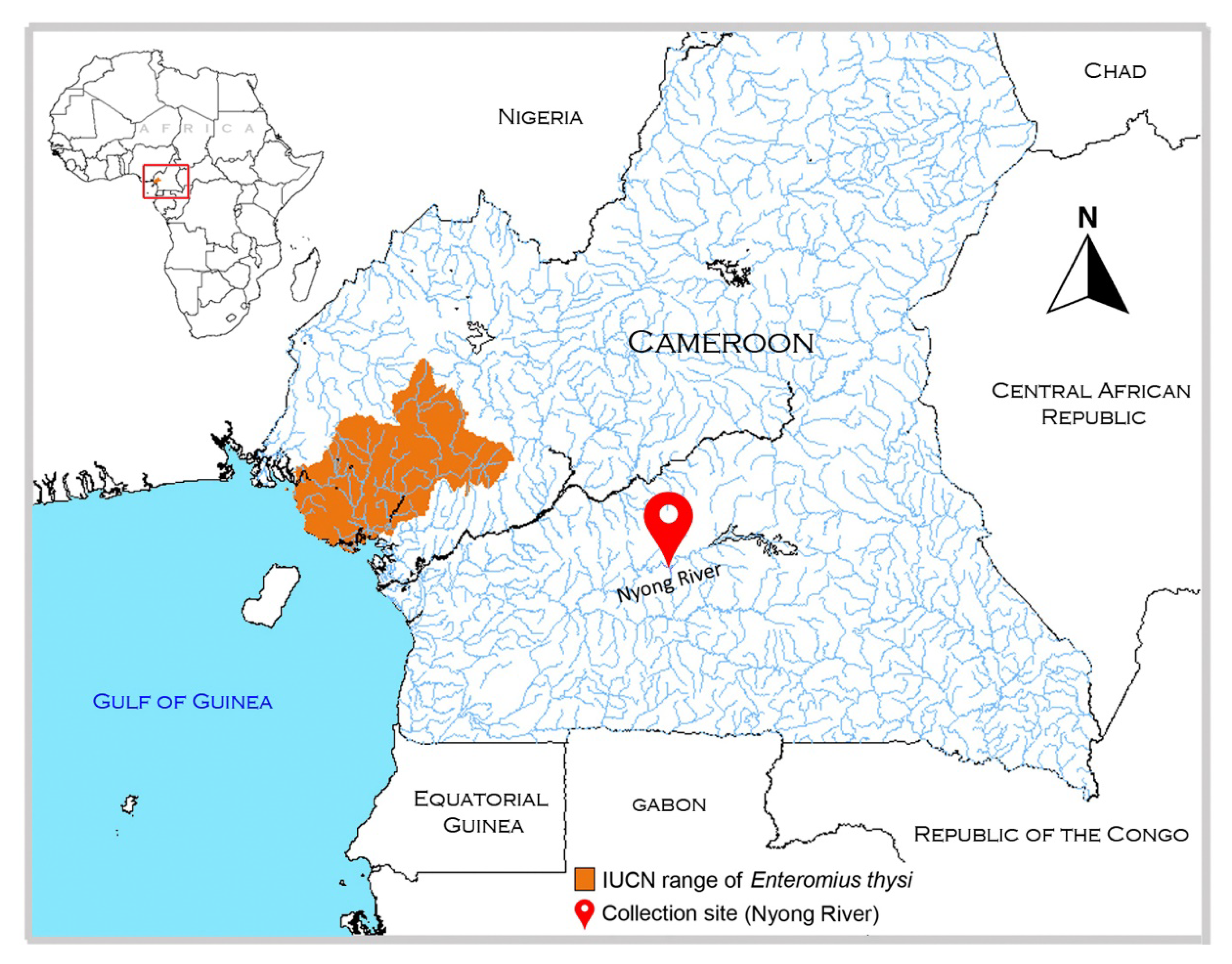
2.1. Sampling and Species Identification
2.2. DNA Extraction, Sequencing, and Assembly
2.3. Mitogenome Characterization and Phylogenetic Analyses
3. Results and Discussion
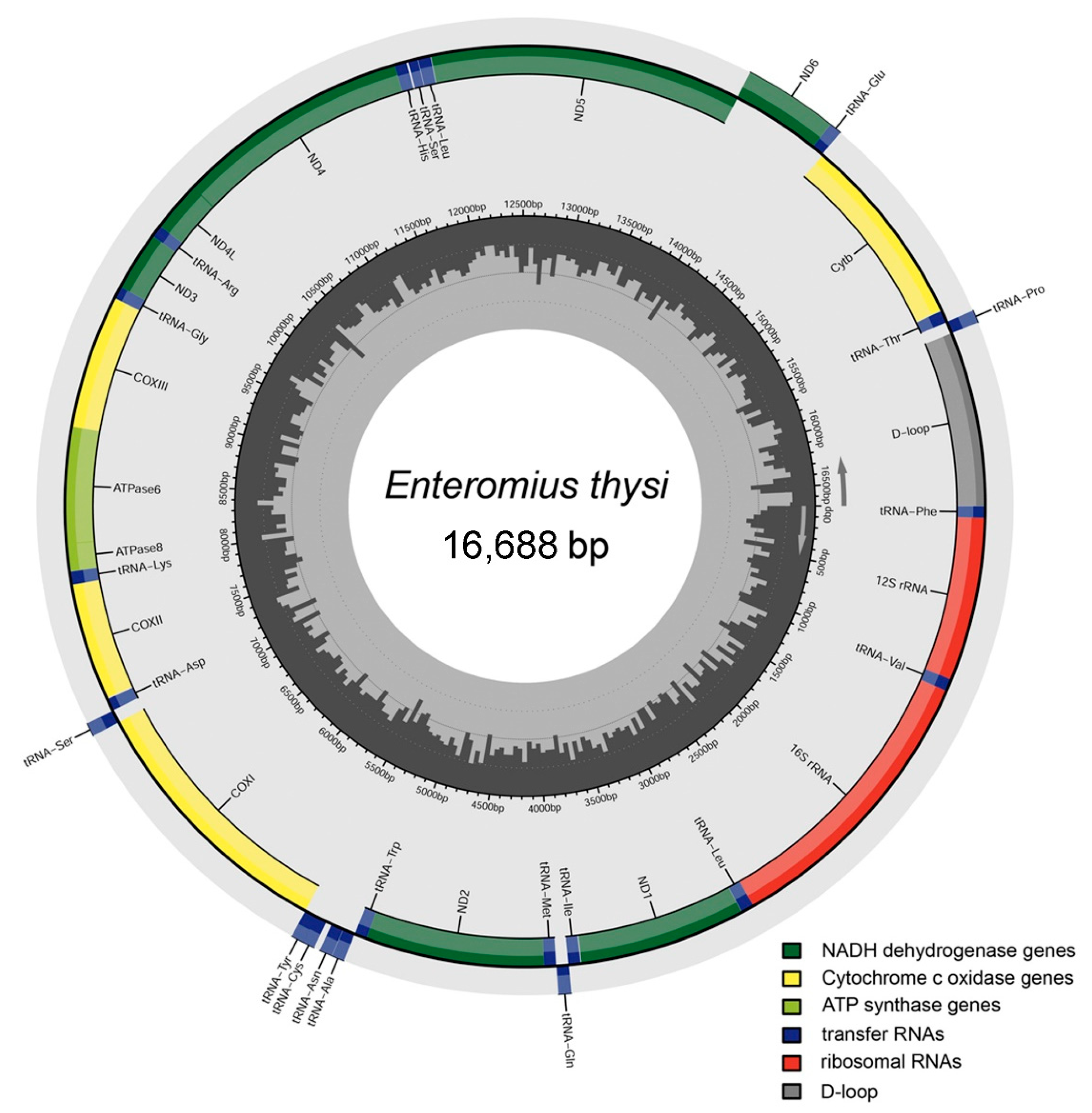
3.1. Mitogenome Structure and Organization
3.2. Protein-Coding Genes
3.3. Ribosomal RNA and Transfer RNA Genes
3.4. Features of Control Region
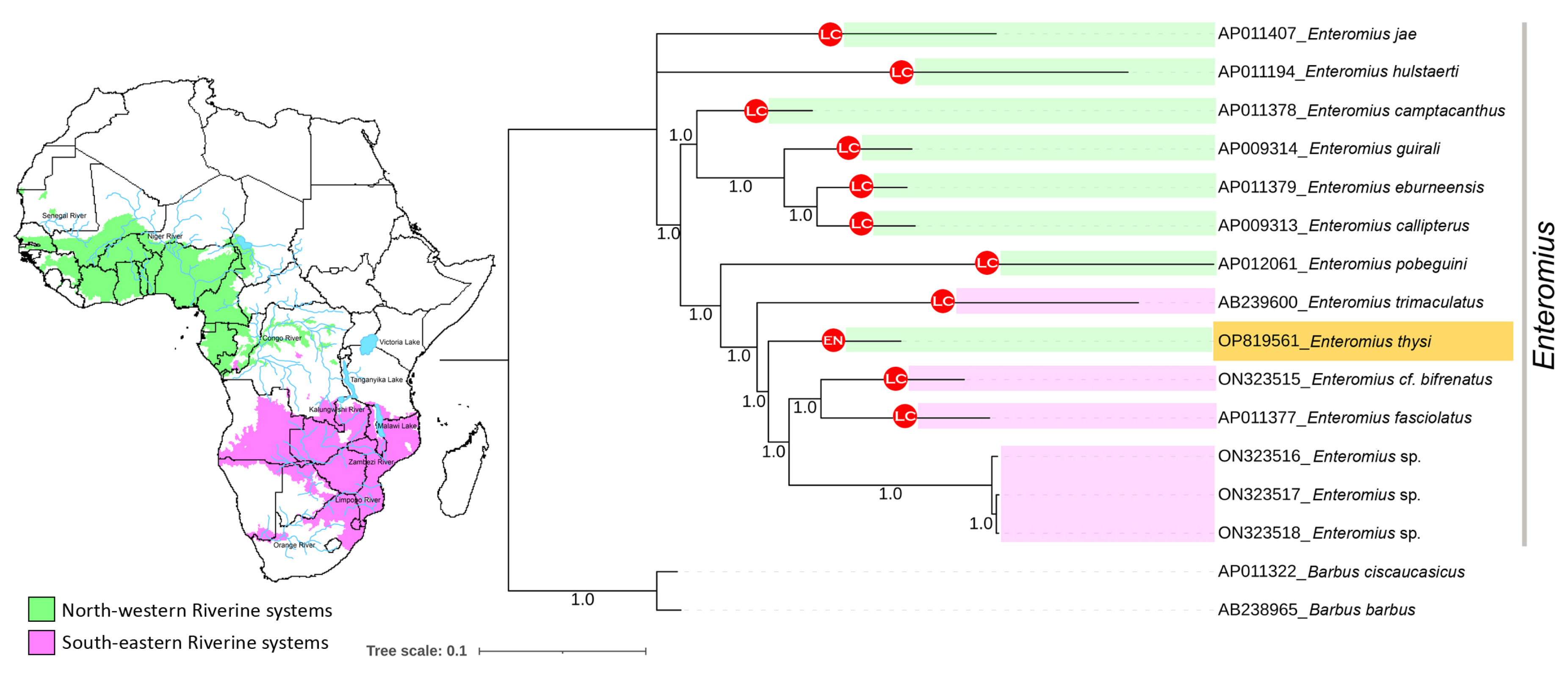
3.5. Phylogenetic Relationship of Enteromius
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fricke, R.; Eschmeyer, W.N.; Van der Laan, R. (Eds.) Eschmeyer’s Catalog of Fishes: Genera, Species. 2022. Electronic Version. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 16 November 2022).
- Martin, M.B.; Chakona, A. Designation of a neotype for Enteromius pallidus (Smith, 1841), an endemic cyprinid minnow from the Cape Fold Ecoregion, South Africa. Zookeys 2019, 848, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sado, T.; Vincent Hirt, M.; Pasco-Viel, E.; Arunachalam, M.; Li, J.; Wang, X.; Freyhof, J.; Saitoh, K.; Simons, A.M.; et al. Phylogeny and polyploidy: Resolving the classification of cyprinine fishes (Teleostei: Cypriniformes). Mol. Phylogenet. Evol. 2015, 85, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Van Ginneken, M.; Decru, E.; Verheyen, E.; Snoeks, J. Morphometry and DNA barcoding reveal cryptic diversity in the genus Enteromius (Cypriniformes: Cyprinidae) from the Congo basin, Africa. Eur. J. Taxon. 2017, 310, 1–32. [Google Scholar]
- Schmidt, R.C.; Bart, H.L., Jr.; Nyingi, W.D. Multi-locus phylogeny reveals instances of mitochondrial introgression and unrecognized diversity in Kenyan barbs (Cyprininae: Smiliogastrini). Mol. Phylogenet. Evol. 2017, 111, 35–43. [Google Scholar] [CrossRef]
- Schmidt, R.C.; Bart, H.L.J.; Nyingi, W.D. Integrative taxonomy of the red-finned barb, Enteromius apleurogramma (Cyprininae: Smiliogastrini) from Kenya, supports recognition of E. amboseli as a valid species. Zootaxa 2018, 4482, 566–578. [Google Scholar] [CrossRef]
- Hayes, M.M.; Armbruster, J.W. The taxonomy and relationships of the African small barbs (Cypriniformes: Cyprinidae). Copeia 2017, 105, 348–362. [Google Scholar] [CrossRef]
- Katemo Manda, B.; Snoeks, J.; Decru, E.; Bills, R.; Vreven, E. Enteromius thespesios (Teleostei: Cyprinidae): A new minnow species with a remarkable sexual dimorphism from the south-eastern part of the Upper Congo River. J. Fish Biol. 2020, 96, 1160–1175. [Google Scholar] [CrossRef]
- Kambikambi, M.J.; Kadye, W.T.; Chakona, A. Allopatric differentiation in the Enteromius anoplus complex in South Africa, with the revalidation of Enteromius cernuus and Enteromius oraniensis, and description of a new species, Enteromius mandelai (Teleostei: Cyprinidae). J. Fish Biol. 2021, 99, 931–954. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2018-2; IUCN: Gland, Switzerland, 2018; Available online: www.iucnredlist.org (accessed on 16 November 2022).
- Ren, Q.; Mayden, R.L. Molecular phylogeny and biogeography of African diploid barbs, ‘Barbus’, and allies in Africa and Asia (Teleostei: Cypriniformes). Zool. Scr. 2016, 45, 642–649. [Google Scholar] [CrossRef]
- Miya, M.; Kawaguchi, A.; Nishida, M. Mitogenomic exploration of higher teleostean phylogenies: A case study for moderate-scale evolutionary genomics with 38 newly determined complete mitochondrial DNA sequences. Mol. Biol. Evol. 2001, 18, 1993–2009. [Google Scholar] [CrossRef]
- Satoh, T.P.; Miya, M.; Mabuchi, K.; Nishida, M. Structure and variation of the mitochondrial genome of fishes. BMC Genom. 2016, 17, 719. [Google Scholar] [CrossRef] [PubMed]
- Kappas, I.; Vittas, S.; Pantzartzi, C.N.; Drosopoulou, E.; Scouras, Z.G. A Time-Calibrated Mitogenome Phylogeny of Catfish (Teleostei: Siluriformes). PLoS ONE 2016, 11, e0166988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhu, K.; Liu, Y.; Zhang, H.; Gong, L.; Jiang, L.; Liu, L.; Lü, Z.; Liu, B. Novel gene rearrangement in the mitochondrial genome of Muraenesox cinereus and the phylogenetic relationship of Anguilliformes. Sci. Rep. 2021, 11, 2411. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Guo, Y.; Gao, Y. Natural selection drives the evolution of mitogenomes in Acrossocheilus. PLoS ONE 2022, 17, e0276056. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, W.; Fukunaga, T.; Isagozawa, R.; Yamada, K.; Maeda, Y.; Satoh, T.P.; Sado, T.; Mabuchi, K.; Takeshima, H.; Miya, M.; et al. MitoFish and MitoAnnotator: A mitochondrial genome database of fish with an accurate and automatic annotation pipeline. Mol. Biol. Evol. 2013, 30, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Miya, M.; Fukunaga, T.; Sado, T.; Iwasaki, W. MitoFish and MiFish Pipeline: A Mitochondrial Genome Database of Fish with an Analysis Pipeline for Environmental DNA Metabarcoding. Mol. Biol. Evol. 2018, 35, 1553–1555. [Google Scholar] [CrossRef]
- Saitoh, K.; Sado, T.; Mayden, R.L.; Hanzawa, N.; Nakamura, K.; Nishida, M.; Miya, M. Mitogenomic evolution and interrelationships of the Cypriniformes (Actinopterygii: Ostariophysi): The first evidence toward resolution of higher-level relationships of the world’s largest freshwater fish clade based on 59 whole mitogenome sequences. J. Mol. Evol. 2006, 63, 826–841. [Google Scholar] [CrossRef]
- Schedel, F.D.B.; Musilova, Z.; Indermaur, A.; Bitja-Nyom, A.R.; Salzburger, W.; Schliewen, U.K. Towards the phylogenetic placement of the enigmatic African genus Prolabeops Schultz, 1941. J. Fish Biol. 2022, 101, 1333–1342. [Google Scholar] [CrossRef]
- Baldwin, C.C.; Mounts, J.H.; Smith, D.G.; Weight, L.A. Genetic identification and color descriptions of early life-history stages of Belizean Phaeoptyx and Astrapogon (Teleostei: Apogonidae) with comments on identification of adult Phaeoptyx. Zootaxa 2009, 2008, 1–22. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo Metazoan Mitochondrial Genome Annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Perna, N.T.; Kocher, T.D. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 1995, 41, 353–359. [Google Scholar] [CrossRef]
- Laslett, D.; Canbäck, B. ARWEN, a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics 2008, 24, 172–175. [Google Scholar] [CrossRef]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Vences, M.; Miralles, A.; Brouillet, S.; Ducasse, J.; Fedosov, A.; Kharchev, V.; Kostadinov, I.; Kumari, S.; Patmanidis, S.; Scherz, M.D.; et al. iTaxoTools 0.1: Kickstarting a specimen-based software toolkit for taxonomists. Megataxa 2021, 6, 77–92. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New Methods for Selecting Partitioned Models of Evolution for Molecular and Morphological Phylogenetic Analyses. Mol. Biol. Evol. 2016, 34, 772–773. [Google Scholar] [CrossRef]
- Miller, M.A.; Schwartz, T.; Pickett, B.E.; He, S.; Klem, E.B.; Scheuermann, R.H.; Passarotti, M.; Kaufman, S.; O’Leary, M.A. A RESTful API for Access to Phylogenetic Tools via the CIPRES Science Gateway. Evol. Bioinform. 2015, 11, 43–48. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 2007, 23, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Kumar, V.; Tyagi, K.; Chandra, K. The complete mitochondrial genome of the endangered Assam Roofed Turtle, Pangshura sylhetensis (Testudines: Geoemydidae): Genomic features and phylogeny. PLoS ONE 2020, 15, e0225233. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Alam, I.; Maheswaran, G.; Tyagi, K.; Kumar, V. Complete Mitochondrial Genome of Great Frigatebird (Fregata minor): Phylogenetic Position and Gene Rearrangement. Biochem. Genet. 2022, 60, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, R.R.; Johnson, W.E.; O’Brien, S.J.; Ramos, M.J.; Antunes, A. The adaptive evolution of the mammalian mitochondrial genome. BMC Genom. 2008, 9, 119. [Google Scholar] [CrossRef]
- Ojala, D.; Montoya, J.; Attardi, G. tRNA punctuation model of RNA processing in human mitochondria. Nature 1981, 290, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Foote, A.D.; Morin, P.A.; Durban, J.W.; Pitman, R.L.; Wade, P.; Willerslev, E.; Gilbert, M.T.; da Fonseca, R.R. Positive selection on the killer whale mitogenome. Biol. Lett. 2011, 7, 116–118. [Google Scholar] [CrossRef]
- Garvin, M.R.; Bielawski, J.P.; Gharrett, A.J. Positive Darwinian selection in the piston that powers proton pumps in complex I of the mitochondria of Pacific salmon. PLoS ONE 2011, 6, e24127. [Google Scholar] [CrossRef]
- Hill, J.; Enbody, E.D.; Pettersson, M.E.; Sprehn, C.G.; Bekkevold, D.; Folkvord, A.; Laikre, L.; Kleinau, G.; Scheerer, P.; Andersson, L. Recurrent convergent evolution at amino acid residue 261 in fish rhodopsin. Proc. Natl. Acad. Sci. USA 2019, 116, 18473–18478. [Google Scholar] [CrossRef]
- Sato, N.S.; Hirabayashi, N.; Agmon, I.; Yonath, A.; Suzuki, T. Comprehensive genetic selection revealed essential bases in the peptidyl-transferase center. Proc. Natl. Acad. Sci. USA 2006, 103, 15386–15391. [Google Scholar] [CrossRef]
- Varani, G.; McClain, W.H. The G-U wobble base pair: A fundamental building block of RNA structure crucial to RNA function in diverse biological systems. EMBO Rep. 2000, 1, 18–23. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, X.; Nie, L. Organization and variation of mitochondrial DNA control region inpleurodiran turtles. Zoologia 2011, 28, 495–504. [Google Scholar] [CrossRef]
- Lee, W.J.; Conroy, J.; Howell, W.H.; Kocher, T.D. Structure and evolution of teleost mitochondrial control regions. J. Mol. Evol. 1995, 41, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Weijers, J.W.H.; Schefuß, E.; Stouten, S.; Damasté, J.S.S. Coupled thermal and hydrological evolution of tropical Africa over the last glaciation. Science 2007, 315, 1701–1704. [Google Scholar] [CrossRef] [PubMed]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Balderas, S.C.; Bussing, W.; et al. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. BioScience 2008, 58, 403–414. [Google Scholar] [CrossRef]
- Elmer, K.R.; Reggio, C.; Wirth, T.; Verheyen, E.; Salzburger, W.; Meyer, A. Pleistocene desiccation in East Africa bottlenecked but did not extirpate the adaptive radiation of Lake Victoria haplochromine cichlid fishes. Proc. Natl. Acad. Sci. USA 2009, 106, 13404–13409. [Google Scholar] [CrossRef]
- O’Reilly, C.; Alin, S.; Plisnier, P.; Cohen, A.; McKee, B. Climate change decreases aquatic ecosystem productivity of Lake Tanganyika, Africa. Nature 2003, 424, 766–768. [Google Scholar] [CrossRef]
- Butchart, S.H.M.; Walpole, M.; Collen, B.; van Strien, A.; Scharlemann, J.P.W.; Almond, R.E.A.; Baillie, J.E.M.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global biodiversity: Indicators of recent declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef]
- Nyboer, E.A.; Liang, C.; Chapman, L.J. Assessing the vulnerability of Africa’s freshwater fishes to climate change: A continent-wide trait-based analysis. Biol. Conserv. 2019, 236, 505–520. [Google Scholar] [CrossRef]

| Gene | Start | End | Strand | Size (bp) | Intergenic | Anticodon | Start Codon | Stop Codon |
|---|---|---|---|---|---|---|---|---|
| tRNA-Phe (F) | 1 | 69 | + | 69 | . | GAA | . | . |
| 12S rRNA | 70 | 1026 | + | 957 | . | . | . | . |
| tRNA-Val (V) | 1027 | 1098 | + | 72 | . | TAC | . | . |
| 16S rRNA | 1099 | 2792 | + | 1694 | . | . | . | . |
| tRNA-Leu (L2) | 2793 | 2868 | + | 76 | 1 | TAA | . | . |
| NAD1 | 2870 | 3844 | + | 975 | 5 | . | ATG | TAA |
| tRNA-Ile (I) | 3850 | 3921 | + | 72 | −2 | GAT | . | . |
| tRNA-Gln (Q) | 3920 | 3990 | − | 71 | 1 | TTG | . | . |
| tRNA-Met (M) | 3992 | 4061 | + | 70 | . | CAT | . | . |
| NAD2 | 4062 | 5107 | + | 1046 | . | . | ATG | TA- |
| tRNA-Trp (W) | 5108 | 5178 | + | 71 | 2 | TCA | . | . |
| tRNA-Ala (A) | 5181 | 5249 | − | 69 | 1 | TGC | . | . |
| tRNA-Asn (N) | 5251 | 5323 | − | 73 | 33 | GTT | . | . |
| tRNA-Cys (C) | 5357 | 5423 | − | 67 | −1 | GCA | . | . |
| tRNA-Tyr (Y) | 5423 | 5493 | − | 71 | 1 | GTA | . | . |
| COI | 5495 | 7045 | + | 1551 | . | . | GTG | TAA |
| tRNA-Ser (S2) | 7046 | 7120 | − | 75 | 1 | TGA | . | . |
| tRNA-Asp (D) | 7122 | 7193 | + | 72 | 6 | GTC | . | . |
| COII | 7200 | 7887 | + | 688 | . | . | ATG | T-- |
| tRNA-Lys (K) | 7888 | 7963 | + | 76 | 1 | TTT | . | . |
| ATP8 | 7965 | 8129 | + | 165 | −7 | . | ATG | TAA |
| ATP6 | 8123 | 8805 | + | 683 | . | . | ATG | TA- |
| COIII | 8806 | 9590 | + | 785 | . | . | ATG | TA- |
| tRNA-Gly (G) | 9591 | 9662 | + | 72 | . | TCC | . | . |
| NAD3 | 9663 | 10,011 | + | 349 | . | . | ATG | T-- |
| tRNA-Arg (R) | 10,012 | 10,081 | + | 70 | . | TCG | . | . |
| NAD4L | 10,082 | 10,378 | + | 297 | −7 | . | ATG | TAA |
| NAD4 | 10,372 | 11,752 | + | 1381 | . | . | ATG | T-- |
| tRNA-His (H) | 11,753 | 11,821 | + | 69 | 10 | GTG | . | . |
| tRNA-Ser (S1) | 11,832 | 11,889 | + | 58 | 2 | GCT | . | . |
| tRNA-Leu (L1) | 11,892 | 11,964 | + | 73 | 3 | TAG | . | . |
| NAD5 | 11,968 | 13,791 | + | 1824 | −4 | . | ATG | TAA |
| NAD6 | 13,788 | 14,309 | − | 522 | . | . | ATG | TAA |
| tRNA-Glu (E) | 14,310 | 14,378 | − | 69 | 5 | TTC | . | . |
| Cyt b | 14,384 | 15,520 | + | 1137 | 3 | . | ATG | TAA |
| tRNA-Thr (T) | 15,524 | 15,595 | + | 72 | −1 | TGT | . | . |
| tRNA-Pro (P) | 15,595 | 15,665 | − | 71 | . | TGG | . | . |
| Control region | 15,666 | 16,688 | + | 1023 | . | . | . | . |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kundu, S.; Binarao, J.D.; De Alwis, P.S.; Kim, A.R.; Lee, S.-R.; Andriyono, S.; Gietbong, F.Z.; Kim, H.-W. First Mitogenome of Endangered Enteromius thysi (Actinopterygii: Cypriniformes: Cyprinidae) from Africa: Characterization and Phylogeny. Fishes 2023, 8, 25. https://doi.org/10.3390/fishes8010025
Kundu S, Binarao JD, De Alwis PS, Kim AR, Lee S-R, Andriyono S, Gietbong FZ, Kim H-W. First Mitogenome of Endangered Enteromius thysi (Actinopterygii: Cypriniformes: Cyprinidae) from Africa: Characterization and Phylogeny. Fishes. 2023; 8(1):25. https://doi.org/10.3390/fishes8010025
Chicago/Turabian StyleKundu, Shantanu, Jerome D. Binarao, Piyumi S. De Alwis, Ah Ran Kim, Soo-Rin Lee, Sapto Andriyono, Fantong Zealous Gietbong, and Hyun-Woo Kim. 2023. "First Mitogenome of Endangered Enteromius thysi (Actinopterygii: Cypriniformes: Cyprinidae) from Africa: Characterization and Phylogeny" Fishes 8, no. 1: 25. https://doi.org/10.3390/fishes8010025
APA StyleKundu, S., Binarao, J. D., De Alwis, P. S., Kim, A. R., Lee, S.-R., Andriyono, S., Gietbong, F. Z., & Kim, H.-W. (2023). First Mitogenome of Endangered Enteromius thysi (Actinopterygii: Cypriniformes: Cyprinidae) from Africa: Characterization and Phylogeny. Fishes, 8(1), 25. https://doi.org/10.3390/fishes8010025








