Abstract
The development of specific diets for the juvenile stage is a main target for culture intensification of tench (Tinca tinca). Aquafeeds still rely heavily on the use of fishmeal (FM) but concerns about economic and ecological sustainability make the use of alternative protein sources necessary. Insect meals are considered a promising substitute to replace FM. In a 90-day experiment, 6 diets with different replacement levels of FM by partially defatted black soldier fly larvae meal (BSFLM): 0%, 15%, 30%, 45%, 60% and 75%, were tested on juvenile tench. Survival rates were high (95.8–100%) without differences between treatments. Diet with 45% FM replacement allowed for best growth performance in terms of total length (TL = 66.4 mm) and weight (W = 4.19 g), without differences with 60% and 75% of replacement. A cubic relationship was evidenced between the level of FM replacement and growth. From the regression equations, the estimated optimal level of FM replacement was 47% (356 g BSFLM kg−1 diet). Externally visible deformities were always under 0.05%. The whole-body lipid content of the fish had a significantly negative linear regression with BSFLM (r2 = 0.80). The content of the essential amino acids (EAA) arginine, leucine, lysine, phenylalanine, methionine, and threonine in diets decreased with dietary BSFLM inclusion. However, it did not have a negative effect on growth performance, suggesting that EAA requirements were covered. The amount of essential amino acids in whole-body juveniles was similar independently of the diet provided. The results allow considering BSFLM as a sustainable protein source for juvenile tench feeding.
1. Introduction
In many countries, a policy objective for aquaculture development is diversification. This term not only applies to searching for new species, but also to increasing the production of those species currently farmed in small quantities [] such as tench, Tinca tinca. Tench is a freshwater fish belonging to the Cyprinidae family that originally occurred in the waters of Europe and Siberia and today is present in the inland waters of all continents []. Tench is commonly cultured in extensive systems where growth is often limited and production usually poor [,]. Considering that this species is appreciated by consumers as a tasty fish with healthy meat [,] and attractive for anglers, the development of intensive rearing systems is required to satisfy the demand. A major obstacle to increasing tench production is the deficit of young fishes for further growth or to restock open waters for sport fishing [,]. Some relevant advances have been achieved in juvenile tench rearing techniques under controlled conditions, focusing mainly on feed as an essential factor. In this sense, González-Rodríguez et al. [] established the protein requirements for juvenile tench between 48 and 52% and later, García et al. [] proposed a practical diet which allows good survival and growth performance, setting the basis for further nutritional studies.
Fishmeal (FM) is the most nutritious and digestible source of protein in aquatic diets, which is the biggest consumer sector with a global use in 2019 of 78% []. In 2031, world production of fishmeal is expected to reach 5.6 Mt of which 71% will come from forage species []. The environmental footprint derived from its use in feedstuffs is a main concern to reach sustainable growth [], especially when production mainly depends on a finite wild-harvest capture []. In addition to environmental concerns, the upscaling demand and price for FM have awakened the search for alternative ingredients, specifically byproducts and those obtained by recycling nutrients, to support a circular economy within aquaculture [].
Among different possibilities, there is a recent interest in insects as a food source for animal feeding. Insect culture is considered sustainable as insects do not need large areas or much water and they contribute to waste recycling [,]. The review by Henry et al. [] highlighted the good potential of insect meal to replace FM in fish diets due to their high content of protein and adequate profile of essential amino acids (EAA). However, only a few species of insects have the potential to be reared at a large scale []. In addition, there are regulatory obstacles to use insect protein for animal feeding. The recently approved UE regulations, 2017/893/EC, 2017, and 2021/1925/UE [,] allow the inclusion of protein from eight species of insects in aquafeeds. Among them, the black soldier fly (Hermetia illucens) shows major advantages derived from its ability to turn bio-waste into larvae with valuable nutrient content []. Table 1 includes data on the possibilities to replace FM by black soldier fly meals (BSFLM) in freshwater and marine fish species without negative effects on growth performance. Except for the experiments performed in the turbot (Scophthalmus maximus) [], in the yellowtail (Seriola quinqueradiata) [], and in the gilthead seabream (Sparus aurata) [], where the inclusion of BSFLM negatively affected growth performance, levels of FM replacement between 20% in the dusky kob (Argyrosomus japonicus) [] and 100% were shown to be feasible. However, the dietary amounts of BSFLM usually did not reflect the percentages of FM substitution. Therefore, total replacement of FM was achieved with 600 g kg−1 in diets for Atlantic salmon (Salmo salar) [] and 106 g kg−1 in diets for Jian carp (Cyprinus carpio var. Jian) []. In addition to interspecific differences, several factors must be considered to explain the variability of results, such as the nutritional value of BSFLM including insect growth substrate and further meal processing, life stage of fish, diet formulation, and nutritional composition [].

Table 1.
Fishmeal (FM) replacement level (%) and corresponding dietary amount (g kg−1 diet) of black soldier fly larvae meal (BSFLM) included without negative effects on growth performance in marine and freshwater fish species.
Regarding the effect of FM replacement with BSFLM on body composition, several studies concluded that the inclusion of BSFLM did not affect the final whole-body or fish fillet proximate composition. Meanwhile, others reported significant increases in dry matter and lipid [,] or protein content [].
To the best of our knowledge, no studies have reported the effect of replacing FM by BSFLM in the diet of juvenile tench; therefore, the present study aimed to evaluate the effects of increasing substitution level of FM with BSFLM on survival, growth performance, incidence of externally visible deformities, and whole-body composition.
2. Materials and Methods
2.1. Ethics Statement
According to Spanish law (RD 53/2013) and an EU directive (2010/63/EU), the Ethic Committee of the University of León approved the experiment conducted in this study (Approval reference ULE_16_2015). Fish health, welfare, and the environmental conditions in the experimental tanks were checked twice daily by visual observation of animal behavior. Water quality parameters, such as oxygen saturation, temperature, and water flow were periodically measured (see Section 2.2.). The necessary number of fish to analyze whole-body composition were euthanized with an over-dose of tricaine methanesulfonate (MS222, Ortoquímica S.L., Barcelona, Spain) by prolonged immersion. At the end of the experiment, the remaining animals were transported to the fish farm where breeder fish come from.
2.2. Fish, Facilities, and Experimental Procedures
Tench larvae were obtained by hatching using artificial reproduction techniques [] and reared in outdoor tanks. After 4 months, 540 juvenile tench from a homogenous pool were randomly distributed as groups of 30 fish in 18 fiberglass tanks (0.5 × 0.25 × 0.25 m) containing 25 L of water to obtain replicates corresponding to the different feeding treatments. Prior to distribution, 100 juveniles were anesthetized with tricaine methanesulfonate (MS-222; Ortoquímica S.L., Barcelona, Spain) to measure initial total length (TL) and weight (W). Values of 30.7 ± 0.28 mm and 0.39 ± 0.02 g (mean ± SEM) were obtained. TL was measured with a digital caliper (to the nearest 0.01 mm) and, after removing excess water with tissue paper, W was determined by a precision balance (to the nearest 0.001 g). Total biomass of each tank was weighed. Following a monofactorial design, diet was the experimental factor with three replicates per level of treatment. The juveniles were previously acclimated to experimental conditions for 4 days.
Artesian well water was supplied in an open system (flow-through system) and each tank had a water inlet (inflow 0.30 L min−1) and outlet (provided with a 250 µm mesh filter) and light aeration. Measures of the incoming water quality, ammonia, nitrites, hardness, and total suspended solids were performed once a week with a spectrophotometer HACH DR2800 (Hach Lange GMBH, Vigo, Spain). Dissolved oxygen in tanks was measured with a multi-meter HACH HQ30d (Hach Lange GMBH, Vigo, Spain). Mean values of water quality were pH 7.6, hardness 5.3 German degrees (calcium 32.8 mg L−1), total suspended solids 34.0 mg L−1, dissolved oxygen ranged between 5.7 and 7.3 mg L−1, ammonia < 0.10 mg L−1, and nitrites < 0.010 mg L−1.
Water temperature (measured twice a day) was 26 ± 1 °C and a 16 h light:8 h dark photoperiod was maintained throughout the experiment. Tanks were cleaned of feces and uneaten feed every two days. The experiment lasted for 90 days.
2.3. Diets and Feeding
Based on the results of our research group [,], different diets (50% crude protein) were formulated and prepared to test the effects of different substitution levels of FM by black soldier fly (H. illucens) meal. A partially defatted BSFLM from Hermetia Deutschland GmbH & Co. KG (Baruth/Mark, Germany), obtained by processing larvae reared on a vegetable byproducts substrate, was included. According to the producer, partial defatting was made by a mechanical process using high pressure and without any solvents. Proximate composition and amino acid profiles of FM and BSFLM are in Table 2.

Table 2.
Proximate composition and amino acid profiles of fishmeal (FM) and black soldier fly larvae meal (BSFLM) (g kg−1).
A total of 6 diets (nearly isonitrogenous and isoenergetic) with different replacement levels of FM by BSFLM were formulated: 0% (control), 15%, 30%, 45%, 60%, or 75%, corresponding to 0, 117, 232, 348, 464, or 579 g of BSFLM kg−1 diet, respectively (Table 3). Ingredients were ground in a rotary mill BRABENDER (Brabender GmbH & Co. KG, Duisburg, Germany), mixed in a mixer STEPHAN UMC5 (Stephan Food Service Equipment, Hameln, Germany) and extruded using a stand-alone extruder BRABENDER KE19/25D (Brabender GmbH & Co. KG, Duisburg, Germany) at a temperature range between 100 °C and 110 °C. Pellets (1 mm diameter) were dried during 24 h at 30 °C and after receiving a coating of cod liver oil. Fish were fed manually three times a day (at 10:00, 14:00, and 18:00 h) to apparent satiation.

Table 3.
Formulation of the practical diets with different levels of replacement of fishmeal (FM) by black soldier larvae fly meal (BSFLM) (g kg−1 diet).
2.4. Chemical Analysis of Diets and Fish
Juveniles were fasted for 14 h before sampling. Samples of diets and juveniles were stored at −30 °C at the beginning and at the end of the experiment. Analyses were performed in duplicate by Analiza Calidad laboratory (Burgos, Spain) following Commission Regulation (EC) 152/2009. Moisture was determined by drying at 105 °C, crude protein was determined according to the Kjeldahl method, crude lipid was determined by extraction with light petroleum and further distillation, ash was determined by calcination at 550 °C, and gross energy was determined according to EU regulation 1169/2011. The content of nitrogen-free extract was calculated by subtracting moisture, protein, lipid, and ash content from the wet weight.
Amino acid profiles were analyzed by HPLC using AccQTag method from Waters (Milford, MA, USA). Amino acids were derivatized with 6-aminoquinolyl-N-hydrosysuccinimidyl carbamate reagent (AQC) by the method of Cohen and Michaud [] and Cohen and De Antonis [] and detected by Dual λ Absorbance Detector Waters 2487 from Waters (Milford, MA, USA) at 254 nm. Quantification was carried out with Empower Pro 2.0 software from Waters (Milford, MA, USA). All analyses were performed in duplicate.
2.5. Data Collection
Juvenile tench behavior was observed and registered after cleaning, feeding, and measuring the water quality parameters.
Every 30 days a sample of 15 fish per tank (45 per treatment, 50% of total) were anesthetized to be individually weighed and measured to have information about the growth performance evolution. TL and W were measured as described in Section 2.1., and afterwards juveniles were gently returned to their tanks.
At the end of the experiment, surviving fishes were anesthetized and observed one by one using a magnifying glass to detect externally visible deformities affecting spinal axis, operculum, mouth and tail fin. W and TL were measured individually and total biomass per tank was weighed. The following indices were calculated:
- -
- Survival rate (%) = (final number of juveniles/initial number of juveniles) × 100;
- -
- Specific growth rate, SGR (% d−1) = [(ln final W − ln initial W)/days)] × 100;
- -
- Fulton’s coefficient or condition factor, K = 100 × [final W/(TL3)];
- -
- Biomass gain, BG (g) = (final biomass/tank − initial biomass/tank);
- -
- Feed conversion ratio, FCR = (total feed provided per tank/BG [].
All treatments were replicated three times and the experimental unit was a tank.
2.6. Statistical Analysis
After confirmation of normality and homogeneity of variance, statistical analysis of growth performance and whole-body composition data were conducted by one-way analysis of variance (ANOVA) and polynomial contrasts with the SPSS16.0 computer program (SPSS, Chicago, IL, USA). Significant differences between means were estimated by Tukey’s multiple range test. p < 0.05 was used for rejection of null hypothesis.
3. Results
3.1. Diets
Proximate composition and amino acid profile of practical diets are provided in Table 4. Lipid and carbohydrate contents in diets tended to increase with the inclusion of BSFLM. On the contrary, ash content decreased. Amino acid profile was also affected since the content of the essential amino acids (EAA) such as arginine, leucine, lysine, methionine phenylalanine, and threonine decreased as the dietary content of FM did. Only isoleucine increased when BSFLM was included in diets. A reduction in total NEAA was also observed as FM replacement level increased.

Table 4.
Proximate composition (g kg−1 diet) and amino acid profiles of practical diets with different levels of replacement of fishmeal (FM) by black soldier fly larvae meal (BSFLM).
3.2. Growth Performance
Since the beginning, juvenile tench readily accepted all practical diets independently of the amount of BSFLM. There were no statistical differences between treatments neither in length nor in weight after 30 days (mean value range: 35.0–36.9 mm and 0.74–0.85 g), whereas after 60 days juveniles fed the 45%, 60%, and 75% BSFLM diets reached higher total length than those fed the control diet. Mean TL increases of 17% and 63.6% were achieved after 30 and 60 days, respectively. From initial weight, mean W was almost 2 times higher after 30 days, while at 60 days it was almost 5 times higher.
Final values (90 days) of survival, growth, feed conversion ratio, and percentages of fish with externally visible deformities are in Table 5. Survival ranged from 95.8% to 100% without significant differences between diets. Juveniles fed on a practical diet with 45% BSFLM reached a significant (p > 0.05) higher total length and weight than those fed with the control diet or lower levels of FM substitution. Compared with the control diet, juveniles fed 45% BSMLF reached the higher TL, W, SGR and BG values, and lower FCR (p < 0.05). In comparison with the 45% BSFLM diet, a significant reduction in SGR and BG was evidenced in juveniles fed the 75% BSFLM diet. No differences in TL and W of juveniles fed 45%, 60%, and 75% replacement level diets were found. From a polynomial contrast study, a cubic relationship between TL (r2 = 0.69) and W (r2 = 0.67) and BSFLM content was stablished. Figure 1 and Figure 2 show the regression curves between the level of FM substitution and TL and W, respectively. From regression equations, the optimal dietary inclusion of BSFLM was estimated in 356 g BSFLM kg−1 (47% of FM replacement).

Table 5.
Survival, growth performance, and percentages of juvenile tench with externally visible deformities fed practical diets with different levels of substitution of fishmeal (FM) by black soldier fly larvae meal (BSFLM) over 90 days.
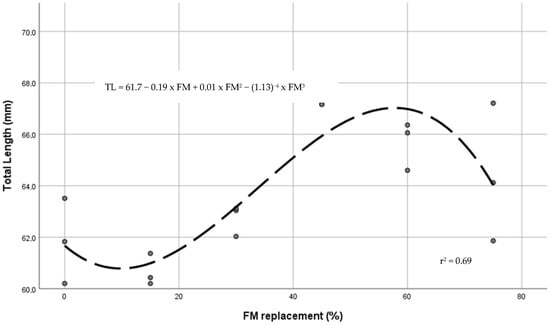
Figure 1.
Regression curve between dietary BSFLM and juvenile tench total length (TL).
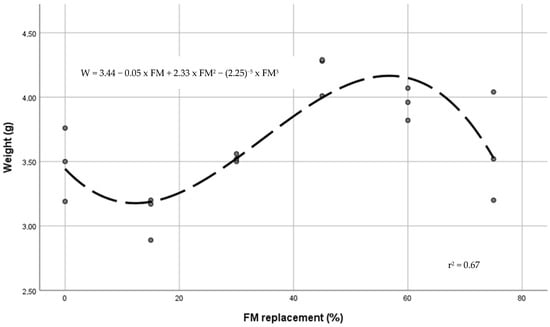
Figure 2.
Regression curve between dietary BSFLM and juvenile tench weight (W).
The condition factor (K) ranged from 1.27 to 1.38, being significantly higher in tench fed the control diet. Regarding FCR, the lower value (1.08) corresponds to fish fed with the 45% FM substitution. The percentages of fish with externally visible deformities was low, ranging from 0% to 0.04%. Body deformities affected the spinal column and caudal peduncle (break in the tail axis).
3.3. Juvenile Whole-Body Composition
The proximate composition and the amino acid profiles of the whole-body of juvenile tench at the beginning and at the end of the study are provided in Table 6. The whole-body lipid content had a significantly linear negative regression (r2 = 0.80), showing decreasing values as inclusion of BSFLM increased. Juvenile tench fed the 30%, 45%, 60%, and 75% BSFLM diets had lower lipid content than the control and 15% BSFLM diets. Although a content reduction in most EAA was evidenced with increasing inclusion of BSFLM in diets (see Table 5), in juvenile it was similar independently of the diet provided, except for isoleucine. With respect to non-essential amino acids (NEAA) in whole-body juvenile tench, diets affected the content of alanine, aspartate, glutamate, glycine, proline, and tyrosine.

Table 6.
Proximate composition and amino acid profiles (g kg−1) of the whole-body of juvenile tench fed practical diets with different levels of replacement of fishmeal (FM) by black soldier fly larvae meal (BSFLM).
4. Discussion
Insects are part of the natural diet of many freshwater fish species and, thus, the inclusion of insect meals could be advantageous to ease feed intake []. Tench feed on zooplankton and other small invertebrates in natural habitats, such as some insect larvae [,,]. Although black soldier fly is a terrestrial insect, the natural feeding habits of tench would help voluntary ingestion of diets independently of the different content of BSFLM, indicating that substitution of FM did not affect its palatability. This agrees with most experiments on partial or total FM replacement by BSFLM, where diets including insect meal were attractive to fishes. However, Kroeckel et al. [] reported a reduction in growth in turbot which was partially attributed to a decrease in feed intake due to a lower palatability of diets including BSFLM.
In their review, Barragán-Fonseca et al. [] reported that composition of BSFLM depends on body composition of fly larvae, which varies among the rearing substrates, but also on further processing. Black soldier fly larvae have a high fat content, reaching in non-defatted meals a lipid content average of 353.2 g kg−1 [] making their inclusion in aquafeeds difficult. Thus, BSFLM is processed to obtain partially defatted meal with high protein content, which allows high inclusion levels of insect meal in fish diets without reducing the technical quality of extruded diets []. According to the producer, the BSFLM of this experiment was partially defatted and its high protein content (54.7%) makes possible dietary amounts of up to 579 g kg−1 (75% FM replacement). As the high content of saturated and monosaturated fatty acids in black soldier fly larvae is associated to a decline in growth of aquatic species [], the defatting process could have beneficial effects in the nutritional composition of diets.
Considering that aquaculture is diverse in terms of cultured species, production systems, and culture conditions, it is difficult to establish accurate comparisons between studies of FM replacement by BSFLM. In most cases, relative data (% FM replacement) are provided but correspond to different inclusion amounts. Therefore, total FM replacement were achieved in Jin carp, with 106 g kg diet−1 [] and 140 g kg diet−1 [], whereas in rainbow trout [] and Atlantic salmon [], amounts were 450 and 600 g kg−1, respectively. In this study with juvenile tench, which have similar protein requirements than rainbow trout and Atlantic salmon, high amounts of BSFLM (579 g kg diet−1) did not affect survival and growth performance compared with the control diet.
The nutritional quality of black soldier fly products must be considered to accurately interpret the response of fish. Available data on the nutritional value of H. illuscens larvae meal showed that the EAA profile, an important indicator of protein quality [], does not differ much between studies []. In agreement with Henry et al. [] and Maurer et al. [], the profile of EAA of BSFLM in the practical diets was similar to the FM (Table 1), with the exception of arginine, lysine, phenylalanine, and methionine (60%, 49%, 69.2%, and 33.6% less than FM, respectively). Despite the reduction in the mentioned amino acids in BSFLM diets (Table 4), juvenile growth was unaffected by inclusion of BSFLM, leading us to consider that EEA requirements were fully covered. According to Hua et al. [], the relationship between BSFLM incorporation level and the growth response is best described by a simple negative linear equation. In this experiment, we found a cubic relationship, showing an increasing growth (TL, W, SGR, FCR, and BG) from the control diet to 45% BSFLM and, with inclusion levels above this threshold, a trend to growth reduction. The negative effects of insect meal on fish growth could be partly due to a lower nutrient digestibility []. Some authors speculated that the content of chitin in insect meals is closely related to a reduction in protein and fat digestibility [,,]. Considering this, the reduction in growth observed in juvenile tench from inclusion BSFLM over 348 g kg −1 could be associated to an increasing content of chitin.
Despite the defatting process, the lipid content of BSFLM was higher than in FM, determining an increase in fat in diets from 107.5 g kg−1 (control diet) to 123 g kg−1 (75% BSFLM diet). The increase in lipid content in diets is usually correlated with an increase in fish body fat [,], but in opposition to this statement and similarly to results reported on turbot [] and Jian carp [,], whole-body tench lipid content decreased with the increase in dietary BSFLM. Kroeckel et al. [] and Li et al. [] hypothesized that this fact could be related to a reduction in lipid digestibility due to the content of chitin provided by the insect meal. In agreement with Ferrer-Llagostera et al. [], the information on the effects of chitin are still discussed and further research is required to clarify its role in aquatic species.
Kamisnki et al. [] reported one of the highest values of SGR in juvenile tench, 3.69% day−1, with initial weight (W) of 0.24 g and at five-months old. In our study, SGR’s ranged between 2.20 and 2.45% day−1 using six-month old juveniles with an initial W of 0.46 g. Considering that SGR slows down with age [], the lower values could be partially attributed to differences in age and initial weight.
As in former experiments performed by our research team, the control diet enabled acceptable growth and high survival and no external visible deformities. External visible body deformities are mainly associated to inadequate feeding, especially during early development [,]. In juvenile tench, a relationship between feeding commercial diets for other species and fish with elevated condition coefficients (between 1.3 and 1.4) and presence of body deformities has been suggested [,,,]. Although the K factor for some diets have shown values above 1.3, the percentage of deformed fish was insignificant (<0.05) showing that diets were well balanced for tench.
From this preliminary study, BSFLM should be regarded as a good protein source for these critical early stages of tench, being feasible for inclusion in high levels of FM dietary replacement without negative effects on survival and growth. Since the high protein quality requirements during this early growth stage were covered by BSFLM, it would be expected that FM saving would increase through inclusion of this insect meal in feedstuffs for further outgrowing phases, whenever the development of efficient insect rearing and processing systems allow for market availability at reduced cost. Under this consideration, further research on the effects of dietary BSFLM in nutritional and sensory quality in tench reared to commercial size should be performed.
5. Conclusions
Compared with the control diet, 75% of fishmeal by black soldier fly larvae meal did not affect survival and growth performance of juvenile tench. Juvenile tench fed a diet where 45% of fishmeal was replaced with BSFLM reached significantly better growth performance than those fed the other diets. The optimal level of fishmeal substitution for juvenile growth performance was 47%, corresponding to 356 g of black soldier fly larvae meal kg−1 diet. Whole-body lipid content showed a negative linear regression with dietary black soldier fly larvae meal. Although a reduction in most essential amino acids was evidenced with increasing inclusion of black soldier fly larvae meal in diets, the content in whole-body juveniles was similar independently of the diet provided.
Author Contributions
J.M.C. contributed to funding acquisition, project administration, conceptualization, methodology, supervision, statistical analysis, and writing—original draft preparation. M.S.-R. contributed to conceptualization, methodology, supervision, statistical analysis, and writing—original draft preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Ministerio de Economía y Competitividad (MINECO) and the Fondo Europeo de Desarrollo Regional (FEDER) through the Research Project AGL2015-64202-R.
Institutional Review Board Statement
According to Spanish law (RD 53/2013) and an EU directive (2010/63/EU), the Ethic Committee of the University of León approved the experiment conducted in this study (Approval reference ULE_16_2015).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on corresponding author request.
Acknowledgments
We thank the collaboration of Cargill España (Madrid) and Skretting España S.A. (Burgos) for providing us with most of the ingredients for the diets. We also thank Hermetia Deutschland GmbH & Co. KG for providing the black soldier fly meal.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. Planning for Aquaculture Diversification: The Importance of Climate Change and Other drivers. In Proceedings of the FAO Technical Workshop, Bangkok, Thailand, 23–25 June 2016; Harvey, B., Soto, D., Carosfeld, J., Beveridge, M., Bartley, D.M., Eds.; FAO: Roma, Italy, 2017. 145p. [Google Scholar]
- Freyhof, J.; Kottelat, M. Tinca tinca. The IUCN Red List of Threatened Species 2008, e.T21912A9339248. Available online: https://www.iucnredlist.org/species/21912/9339248 (accessed on 21 November 2022).
- Kamiński, R.; Sikorska, J.; Wolkini, J. Diet and water temperature affect growth and body deformities in juvenile tench Tinca tinca (L.) reared under controlled conditions. Aquac. Res. 2017, 48, 1327–1337. [Google Scholar] [CrossRef]
- Pula, H.J.; Trenzado, C.E.; García-Mesa, S.; Fallola, C.; Sanz, A. Effects of different culture systems on growth, immune status, and other physiological parameters of tench (Tinca tinca L.). Aquaculture 2018, 485, 101–110. [Google Scholar] [CrossRef]
- Wedekind, H.; Rennert, B.; Kohlmann, K. Product quality in different strains of tench (Tinca tinca L.) tested under controlled environmental conditions. J. Appl. Ichthyol. 2003, 19, 174–176. [Google Scholar] [CrossRef]
- González-Rodríguez, Á.; Celada, J.D.; Carral, J.M.; Sáez-Royuela, M.; Fuertes, J.B. Effects of varying protein level in practical diets on survival, growth, feed utilization and body composition of juvenile tench (Tinca tinca L.). Aquac. Int. 2014, 22, 1723–1735. [Google Scholar] [CrossRef]
- Celada, J.D.; Aguilera, A.; García, V.; Carral, J.M.; Saez-Royuela, M.; González, R.; González, A. Rearing juvenile tench (Tinca tinca L.) under controlled conditions using Artemia nauplii as supplement to a dry diet. Aquac. Int. 2009, 17, 565–570. [Google Scholar] [CrossRef]
- García, V.; Celada, J.D.; Carral, J.M.; Sáez-Royuela, M.; González, R.; González, Á. Decapsulated Artemia cysts: A suitable dietary supplement for juvenile tench (Tinca tinca L.). J. Appl. Aquac. 2010, 22, 57–65. [Google Scholar] [CrossRef]
- González-Rodríguez, Á.; Celada, J.D.; Carral, J.M.; Sáez-Royuela, M.; Fuertes, J.B. Evaluation of a practical diet for juvenile tench (Tinca tinca L.) and substitution possibilities of fish meal by feather meal. Anim. Feed. Sci. Technol. 2014, 187, 61–67. [Google Scholar] [CrossRef]
- García, V.; Celada, J.D.; González, R.; Carral, J.M.; Sáez-Royuela, M.; González, Á. Response of juvenile tench (Tinca tinca L.) fed practical diets with different protein contents and substitution levels of fish meal by soybean meal. Aquac. Res. 2015, 46, 28–38. [Google Scholar] [CrossRef]
- EUMOFA—European Market Observatory for Fisheries and Aquaculture Products. Fishmeal and Fish Oil: Production and Trade Flows in the UE; Publications Office of the European Union: Luxembourg, 2021; 31p. [Google Scholar]
- OECD/FAO. OECD-FAO Agricultural Outlook 2022–2031; OECD Publishing: Paris, France, 2022; 363p. [Google Scholar] [CrossRef]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.J.; Teletchea, F.; Tomasso, J.R.; et al. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Cottrell, R.S.; Blanchard, J.L.; Halpern, B.S.; Metian, M.; Froehlichet, H.E. Global adoption of novel aquaculture feeds could substantially reduce forage fish demand by 2030. Nat. Food 2020, 1, 301–308. [Google Scholar] [CrossRef]
- Campanati, C.; Willer, D.; Schubert, J.; Aldridge, D.C. Sustainable intensification of aquaculture through nutrient recycling and circular economies: More fish, less waste, blue growth. Rev. Fish. Sci. Aquac. 2022, 30, 143–169. [Google Scholar] [CrossRef]
- Tschirner, M.; Kloas, W. Increasing the sustainability of aquaculture systems: Insects as alternative protein source for fish diets. GAIA-Ecol. Perspect. Sci. Soc. 2017, 26, 332–340. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.-J.; Barroso, F.G.; Manzano-Agugliaro, F. Insect meal as renewable source of food for animal feeding: A review. J. Clean. Prod. 2014, 65, 16–27. [Google Scholar] [CrossRef]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed. Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Gasco, L.; Gai, F.; Maricchiolo, G.; Genovese, L.; Ragonese, S.; Bottari, T.; Caruso, G. Fishmeal Alternative Protein Sources for Aquaculture Feeds. In Feeds for the Aquaculture Sector; Current Situation and Alternative Sources; Springer Briefs in Molecular Science: Chemistry of Foods; Springer Nature: Cham, Switzerland, 2018; pp. 1–28. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2017/893 of 24 May 2017 amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as Regards the Provisions on Processed Animal Protein. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0893&rid=1 (accessed on 21 November 2022).
- Commission Regulation (EU) 2021/1925 of 5 November 2021 Amending Certain Annexes to Regulation (EU) No 142/2011 as Regards the Requirements for Placing on the Market of Certain Insect Products and the Adaptation of a Containment Method. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32021R1925 (accessed on 21 November 2022).
- Barragán-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Nutritional value of the black soldier fly (Hermetia illuscens L.) and its suitability as animal feed—A review. J. Insects Food Feed. 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Kroeckel, S.; Harjes, A.G.E.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a turbot catches a fly: Evaluation of a pre-pupae meal of the black soldier fly (Hermetia illucens) as fish meal substitute—growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 2012, 364–365, 345–352. [Google Scholar] [CrossRef]
- Ido, A.; Ali, M.-F.-Z.; Takahashi, T.; Miura, C.; Miura, T. Growth of yellowtail (Seriola quinqueradiata) fed on a diet including partially or completely defatted black soldier fly (Hermetia illucens) larvae meal. Insects 2021, 12, 722. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Daskalopoulou, E.; Vogiatzis, I.; Rumbos, C.; Mente, E.; Athanassiou, C.G. Substitution of fishmeal by fly Hermetia illuscens prepupae meal in the diet of gilthead seabream (Sparus aurata). In Proceedings of the HydroMedit 2014, Volos, Greece, 13–15 November 2014; pp. 110–114. [Google Scholar]
- Madibana, M.J.; Mwanza, M.; Lewis, B.R.; Fouché, C.H.; Toefy, R.; Mlambo, V. Black Soldier Fly Larvae Meal as a Fishmeal Substitute in Juvenile Dusky Kob Diets: Effect on feed utilization, growth performance, and blood parameters. Sustainability 2020, 12, 9460. [Google Scholar] [CrossRef]
- Belghit, I.; Liland, N.S.; Waagbø, R.; Biancarosa, I.; Pelusio, N.; Li, Y.; Krogdahl, Å.; Lock, E.-J. Potential of insect-based diets for Atlantic salmon (Salmo salar). Aquaculture 2018, 491, 72–81. [Google Scholar] [CrossRef]
- Li, S.; Ji, H.; Zhang, B.; Zhou, J.; Yu, H. Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): Growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological structure. Aquaculture 2017, 477, 62–70. [Google Scholar] [CrossRef]
- Hua, K. A Meta-analysis of the effects of replacing fish meals with insect meals on growth performance of fish. Aquaculture 2020, 530, 735732. [Google Scholar] [CrossRef]
- Fabrikov, D.; Vargas-García, M.d.C.; Barroso, F.G.; Sánchez-Muros, M.J.; Cacua Ortíz, S.M.; Morales, A.E.; Cardenete, G.; Tomás-Almenar, C.; Melenchón, F. Effect on intermediary metabolism and digestive parameters of the high substitution of fishmeal with insect meal in Sparus aurata feed. Insects 2021, 12, 965. [Google Scholar] [CrossRef]
- Magalhães, R.; Sánchez-López, A.; Silva Leal, R.; Martínez-Llorens, S.; Oliva-Teles, A.; Peres, H. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 2017, 476, 79–85. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Khalil, R.H.; Metwally, A.A.; Shakweer, M.S.; Khallaf, M.A.; Abdel-Latif, H.M.R. Effects of black soldier fly (Hermetia illucens L.) larvae meal on growth performance, organs-somatic indices, body composition, and hemato-biochemical variables of European sea bass, Dicentrarchus labrax. Aquaculture 2020, 522, 735136. [Google Scholar] [CrossRef]
- Wang, G.; Peng, K.; Hu, J.; Yi, C.; Chen, X.; Wu, H.; Huang, Y. Evaluation of defatted black soldier fly (Hermetia illucens L.) larvae meal as an alternative protein ingredient for juvenile Japanese seabass (Lateolabrax japonicus) diets. Aquaculture 2019, 507, 144–154. [Google Scholar] [CrossRef]
- Li, X.; Qin, C.; Fang, Z.; Sun, X.; Shi, H.; Wang, Q.; Zhao, H. Replacing dietary fish meal with defatted black soldier fly (Hermetia illucens) larvae meal affected growth, digestive physiology and muscle quality of Tongue Sole (Cynoglossus semilaevis). Front. Physiol. 2022, 13, 855957. [Google Scholar] [CrossRef] [PubMed]
- Takakuwa, F.; Tanabe, R.; Nomura, S.; Inui, T.; Yamada, S.; Biswas, A.; Tanaka, H. Availability of black soldier fly meal as an alternative protein source to fish meal in red sea bream (Pagrus major, Temminck & Schlegel) fingerling diets. Aquac. Res. 2022, 53, 36–49. [Google Scholar] [CrossRef]
- Lock, E.R.; Arsiwalla, T.; Waagbø, R. Insect larvae meal as an alternative source of nutrients in the diet of Atlantic salmon (Salmo salar) postsmolt. Aquac. Nutr. 2016, 22, 1202–1213. [Google Scholar] [CrossRef]
- Belghit, I.; Liland, N.S.; Gjesdal, P.; Biancarosa, I.; Menchetti, E.; Li, Y.; Waagbø, R.; Krogdahl, Å.; Lock, E.J. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture 2019, 503, 609–619. [Google Scholar] [CrossRef]
- St-Hilaire, S.; Sheppard, C.; Tomberlin, J.K.; Irving, S.; Newton, L.; McGuire, M.A.; Mosley, E.E.; Hardy, R.W.; Sealey, W. Fly prepupae as a feedstuff for rainbow trout (Oncorhynchus mykiss). J. World Aquac. Soc. 2007, 38, 59–67. [Google Scholar] [CrossRef]
- Sealey, W.M.; Gaylord, T.G.; Barrows, F.T.; Tomberlin, J.K.; McGuire, M.A.; Ross, C.; St-Hilaire, S. Sensory analysis of rainbow trout, Oncorhynchus mykiss, fed enriched black soldier fly prepupae, Hermetia illucens. J. World Aquac. Soc. 2011, 42, 34–45. [Google Scholar] [CrossRef]
- Renna, M.; Schiavone, A.; Gai, F.; Dabbou, S.; Lussiana, C.; Malfatto, V.; Prearo, M.; Capucchio, M.T.; Biasato, I.; Biasibetti, E.; et al. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. J. Anim. Sci. Biotechnol. 2017, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Dumas, A.; Raggi, T.; Barkhouse, J.; Lewis, E.; Weltzien, E. The oil fraction and partially defatted meal of black soldier fly larvae (Hermetia illucens) affect differently growth performance, feed efficiency, nutrient deposition, blood glucose and lipid digestibility of rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 492, 24–34. [Google Scholar] [CrossRef]
- Bruni, L.; Secci, G.; Husein, Y.; Faccenda, F.; Lira de Medeiros, A.C.; Parisi, G. Is it possible to cut down fishmeal and soybean meal use in aquafeed limiting the negative effects on rainbow trout (Oncorhynchus mykiss) fillet quality and consumer acceptance? Aquaculture 2021, 543, 736996. [Google Scholar] [CrossRef]
- Caimi, C.; Renna, M.; Lussiana, C.; Bonaldo, A.; Gariglio, M.; Meneguz, M.; Dabbou, S.; Schiavone, A.; Gai, F.; Elia, A.C.; et al. First insights on Black Soldier Fly (Hermetia illucens L.) larvae meal dietary administration in Siberian sturgeon (Acipenser baerii Brandt) juveniles. Aquaculture 2020, 515, 734539. [Google Scholar] [CrossRef]
- Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. Black soldier fly full-fat larvae meal as an alternative to fish meal and fish oil in Siberian sturgeon nutrition: The effects on physical properties of the feed, animal growth performance, and feed acceptance and utilization. Animals 2020, 10, 2119. [Google Scholar] [CrossRef]
- Muin, H.; Taufek, N.M.; Kamarudin, M.S.; Razak, S.A. Growth performance, feed utilization and body composition of Nile tilapia, Oreochromis niloticus (Linnaeus, 1758) fed with different levels of black soldier fly, Hermetia illucens (Linnaeus, 1758) maggot meal diet. Iran. J. Fish. Sci. 2017, 16, 567–577. [Google Scholar]
- Devic, E.; Leschen, W.; Murray, F.; Little, D.C. Growth performance, feed utilization and body composition of advanced nursing Nile tilapia (Oreochromis niloticus) fed diets containing Black Soldier Fly (Hermetia illucens) larvae meal. Aquac. Nutr. 2018, 24, 416–423. [Google Scholar] [CrossRef]
- Wachira, M.N.; Osuga, I.M.; Munguti, J.M.; Ambula, M.K.; Subramanian, S.; Tanga, C.M. Efficiency and improved profitability of insect-based aquafeeds for farming Nile tilapia fish (Oreochromis niloticus L.). Animals 2021, 11, 2599. [Google Scholar] [CrossRef]
- Tippayadara, N.; Dawood, M.A.O.; Krutmuang, P.; Hoseinifar, S.H.; Doan, H.V.; Paolucci, M. Replacement of fish meal by black soldier fly (Hermetia illucens) larvae meal: Effects on growth, haematology, and skin mucus immunity of Nile tilapia, Oreochromis niloticus. Animals 2021, 11, 193. [Google Scholar] [CrossRef]
- Adeoye, A.A.; Akegbejo-Samsons, Y.; Fawole, F.J.; Davies, S.J. Preliminary assessment of black soldier fly (Hermetia illucens) larval meal in the diet of African catfish (Clarias gariepinus): Impact on growth, body index, and hematological parameters. J. World Aquac. Soc. 2020, 51, 1024–1033. [Google Scholar] [CrossRef]
- Fawole, F.J.; Adeoye, A.A.; Tiamiyu, L.O.; Ajala, K.I.; Obadara, S.O.; Ganiyu, I.O. Substituting fishmeal with Hermetia illucens in the diets of African catfish (Clarias gariepinus): Effects on growth, nutrient utilization, haemato-physiological response, and oxidative stress biomarker. Aquaculture 2020, 518, 734849. [Google Scholar] [CrossRef]
- Vongvichith, B.; Morioka, S.; Sugita, T.; Phousavanh, N.; Phetsanghanh, N.; Chanthasone, P.; Pommachan, P.; Nakamura, S. Evaluation of the efficacy of aquaculture feeds for the climbing perch Anabas testudineus: Replacement of fishmeal by black soldier fly Hermetia illucens prepupae. Fish. Sci. 2020, 86, 145–151. [Google Scholar] [CrossRef]
- Kattakdad, S.; Suratip, N.; Yuangsoi, B.; Kasamawut, K.; Udduang, S. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in climbing perch (Anabas testudineus) diet. Aquacult. Aquar. Conserv. Legis. 2022, 15, 68–82. [Google Scholar]
- Xiao, X.; Jin, P.; Zheng, L.; Cai, M.; Yu, Z.; Yu, J.; Zhang, J. Effects of black soldier fly (Hermetia illucens) larvae meal protein as a fishmeal replacement on the growth and immune index of yellow catfish (Pelteobagrus fulvidraco). Aquac. Res. 2018, 49, 1569–1577. [Google Scholar] [CrossRef]
- Khieokhajonkhet, A.; Uanlam, P.; Ruttarattanamongkol, K.; Aeksiri, N.; Tatsapong, P.; Kaneko, G. Replacement of fish meal by black soldier fly larvae meal in diet for goldfish Carassius auratus: Growth performance, hematology, histology, total carotenoids, and coloration. Aquaculture 2022, 561, 738618. [Google Scholar] [CrossRef]
- Kamalii, A.; Antony, C.; Ahilan, B.; Uma, A.; Prabu, E. Dietary protein replacement of fish meal with black soldier fly larvae meal: Effects on growth, whole-body composition, digestive enzyme activity, muscle-growth-related gene expression and haemato-biochemical responses of juvenile goldfish, Carassius auratus. Turk. J. Fish. Aquat. Sci. 2023, 23, 21837. [Google Scholar] [CrossRef]
- Zhou, J.S.; Liu, S.S.; Ji, H.; Yu, H.B. Effect of replacing dietary fish meal with black soldier fly larvae meal on growth and fatty acid composition of Jian carp (Cyprinus carpio var. Jian). Aquac. Nutr. 2018, 24, 424–433. [Google Scholar] [CrossRef]
- Katya, K.; Borsra, M.Z.S.; Ganesan, D.; Kuppusamy, G.; Herriman, M.; Salter, A.; Ali, S.A. Efficacy of insect larval meal to replace fish meal in juvenile barramundi, Lates calcarifer reared in freshwater. Int. Aquat. Res. 2017, 9, 303–312. [Google Scholar] [CrossRef]
- Sudha, C.; Ahilan, B.; Felix, N.; Uma, A.; Prabu, E. Effects of dietary protein substitution of fishmeal with black soldier fly larval meal on growth and physiological responses of juvenile striped catfish, Pangasianodon hypophthalmus. Aquac. Res. 2022, 53, 2204–2217. [Google Scholar] [CrossRef]
- Rodríguez, R.; Celada, J.D.; Sáez-Royuela, M.; Carral, J.M.; Aguilera, A.; Melendre, P.M. Artificial reproduction in 1-year-old tench (Tinca tinca L.). J. Appl. Ichthyol. 2004, 20, 542–544. [Google Scholar] [CrossRef]
- Cohen, S.A.; Michaud, D.P. Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal. Biochem. 1993, 211, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.A.; De Antonis, K.M. Applications of amino acid derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate. J. Chromatogr. A 1994, 661, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Fornshell, G.; Hinshaw, J.M. Better Management Practices for Flow-Through Aquaculture Systems. In Environmental Best Management Practices for Aquaculture; Tucker, C.S., Hargreaves, J.A., Eds.; Blackwell Publishing: Ames, IA, USA, 2008; pp. 331–388. [Google Scholar]
- Nogales-Mérida, S.; Gobbi, P.; Józefiak, D.; Mazurkiewicz, J.; Dudek, K.; Rawski, M.; Kierończyk, B.; Józefiak, A. Insect meals in fish nutrition. Rev. Aquac. 2019, 11, 1080–1103. [Google Scholar] [CrossRef]
- Perrow, M.R.; Jowitt, A.J.D.; Johnsonf, S.R. Factors affecting the habitat selection of tench in a shallow eutrophic lake. J. Fish Biol. 1996, 48, 859–870. [Google Scholar] [CrossRef]
- Pyka, J. Daily feeding cycle tench, Tinca tinca (L.), in larval and fry stages in the conditions of pond culture. An attempt to determine daily food ration. Arch. Pol. Fish. 1997, 5, 279–290. [Google Scholar]
- Alaş, A.; Altindağ, A.; Yılmaz, M.; Kırpık, M.A.; Ak, A. Feeding habits of tench (Tinca tinca L., 1758) in Beyşehir lake (Turkey). Turk. J. Fish. Aquat. Sci. 2010, 10, 187–194. [Google Scholar] [CrossRef]
- Lu, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Sinpru, B.; Sroichak, T.; Archa, P.; Thongpea, S.; Paengkoum, S.; Purba, R.A.P.; et al. Nutritional composition of black soldier fly larvae (Hermetia illucens L.) and its potential uses as alternative protein sources in animal diets: A review. Insects 2022, 13, 831. [Google Scholar] [CrossRef]
- Liland, N.S.; Araujo, P.; Xu, X.X.; Lock, E.J.; Radhakrishnan, G.; Prabhu, A.J.P.; Belghit, I. A meta-analysis on the nutritional value of insects in aquafeeds. J. Insects Food Feed 2021, 7, 743–759. [Google Scholar] [CrossRef]
- Barroso, F.G.; de Haro, C.; Sánchez-Muros, M.-J.; Venegas, E.; Martínez-Sánchez, A.; Pérez-Bañón, C. The potential of various insect species for use as food for fish. Aquaculture 2014, 422–423, 193–201. [Google Scholar] [CrossRef]
- Maurer, V.; Holinger, M.; Amsler, Z.; Früh, B.; Wohlfahrt, J.; Stamer, A.; Leiber, F. Replacement of soybean cake by Hermetia illucens meal in diets for layers. J. Insects Food Feed 2016, 2, 83–90. [Google Scholar] [CrossRef]
- Basto, A.; Matos, I.; Valente, L.M.P. Nutritional value of different insect larvae meals as protein sources for European sea bass (Dicentrarchus labrax) juveniles. Aquaculture 2020, 21, 735085. [Google Scholar] [CrossRef]
- Jobling, M. Nutrient Partitioning and the Influence of Feed Composition on Body Composition. In Food Intake in Fish; Houlihan, D., Boujard, T., Jobling, M., Eds.; Blackwell: New York, NY, USA, 2001; pp. 354–375. [Google Scholar]
- Sargent, J.R.; Tocher, D.R.; Bell, J.G. The Lipids. In Fish Nutrition; Halver, J.E., Hardy, R.W., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 181–257. [Google Scholar]
- Ferrer Llagostera, P.; Kallas, Z.; Reig, L.; Amores de Gea, D. The use of insect meal as a sustainable feeding alternative in aquaculture: Current situation, Spanish consumers’ perceptions and willingness to pay. J. Clean. Prod. 2019, 229, 10–21. [Google Scholar] [CrossRef]
- Lugert, V.; Thaller, G.; Tetens, J.; Schulz, C.; Krieter, J. A review on fish growth calculation: Multiple functions in fish production and their specific application. Rev. Aquac. 2016, 8, 30–42. [Google Scholar] [CrossRef]
- Cahu, C.; Zambonino-Infante, J.; Takeuchi, T. 2003. Nutritional components affecting skeletal development in fish larvae. Aquaculture 2003, 227, 245–258. [Google Scholar] [CrossRef]
- Fontagné, S. The Impact of Nutritional Components on Rainbow Trout. In Control of Malformations in Fish Aquaculture: Science and Practice; Baeverfjord, G., Helland, S., Hough, C., Eds.; RapidPress: Luxembourg, 2009; pp. 73–83. [Google Scholar]
- Kamler, E.; Myszkowski, L.; Kaminski, R.; Korwin-Kossakowski, M.; Wolnicki, J. Does overfeeding affect tench Tinca tinca L. juvenile? Aquac. Int. 2006, 14, 99–111. [Google Scholar] [CrossRef]
- Wolnicki, J.; Myszkowski, L.; Korwin-Kossakowski, M.; Kaminski, R.; Stanny, L.A. Effects of different diets on juvenile tench Tinca tinca (L.) reared under controlled conditions. Aquac. Int. 2006, 14, 89–98. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).