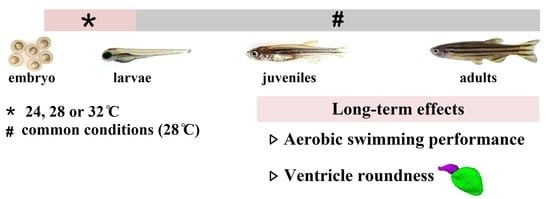
Elevated Embryonic Temperature Has Persistent Adverse Effects on Zebrafish Swimming Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Treatments
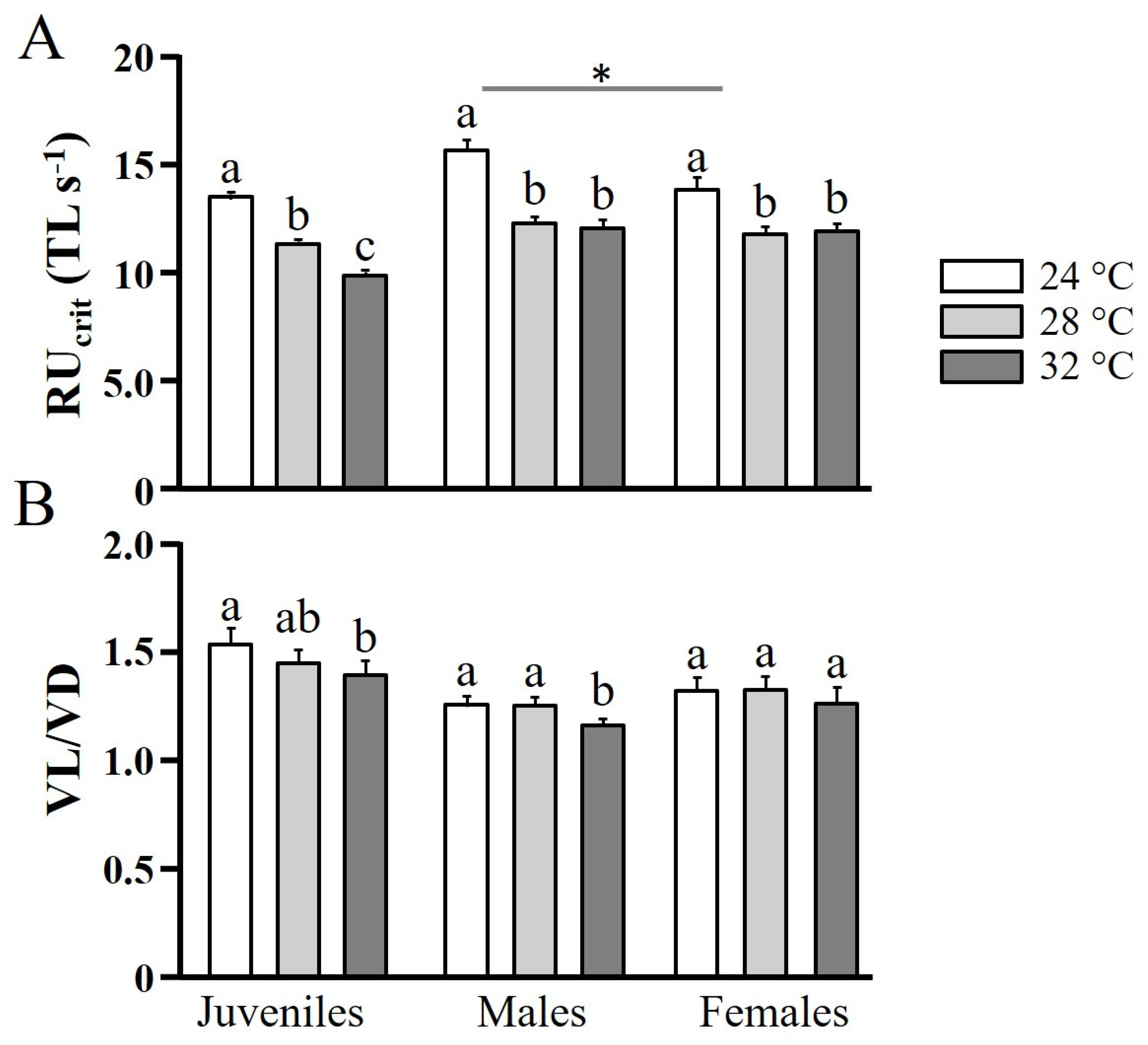
2.2. Swimming Performance
2.3. Heart Morphology
2.4. Data Analysis
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frölicher, T.L.; Fischer, E.M.; Gruber, N. Marine heatwaves under global warming. Nature 2018, 560, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Fordyce, J.A. The evolutionary consequences of ecological interactions mediated through phenotypic plasticity. J. Exp. Biol. 2006, 209, 2377–2383. [Google Scholar] [CrossRef] [PubMed]
- Crozier, L.G.; Hutchings, J.A. Plastic and evolutionary responses to climate change in fish. Evol. Appl. 2014, 7, 68–87. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.A.; Lee, H.-T.; Macqueen, D.J.; Paranthaman, K.; Kawashima, C.; Anwar, A.; Kinghorn, J.R.; Dalmay, T. Embryonic temperature affects muscle fibre recruitment in adult zebrafish: Genome-wide changes in gene and microRNA expression associated with the transition from hyperplastic to hypertrophic growth phenotypes. J. Exp. Biol. 2009, 212, 1781–1793. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.R.; Johnston, I.A. Temperature during embryonic development has persistent effects on thermal acclimation capacity in zebrafish. Proc. Natl. Acad. Sci. USA 2012, 109, 14247–14252. [Google Scholar] [CrossRef] [PubMed]
- Abozaid, H.; Wessels, G.S. Hörstgen-Schwark, Effect of rearing temperatures during embryonic development on the phenotypic sex in zebrafish (Danio rerio). Sex. Dev. Genet. Mol. Biol. Evol. Endocrinol. Embryol. Pathol. Sex Determ. Differ. 2011, 5, 259–265. [Google Scholar]
- Claireaux, G.; McKenzie, D.J.; Genge, A.G.; Chatelier, A.; Aubin, J.; Farrell, A.P. Linking swimming performance, cardiac pumping ability and cardiac anatomy in rainbow trout. J. Exp. Biol. 2005, 208, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Brett, J.R. The Respiratory Metabolism and Swimming Performance of Young Sockeye Salmon. J. Fish. Board Can. 1964, 21, 1183–1226. [Google Scholar] [CrossRef]
- Koumoundouros, G.; Ashton, C.; Sfakianakis, D.G.; Divanach, P.; Kentouri, M.; Anthwal, N.; Stickland, N.C. Thermally induced phenotypic plasticity of swimming performance in European sea bass Dicentrarchus labrax juveniles. J. Fish Biol. 2009, 74, 1309–1322. [Google Scholar] [CrossRef] [PubMed]
- Adriaens, D.; Sochacka-Marlowe, A.; Kegel, B.; Denis, V.; Hoorebeke, L. Soft tissue discrimination with contrast agents using micro-CT scanning. Belg. J. Zool. 2014, 144, 20–40. [Google Scholar]
- Hicken, C.E.; Linbo, T.L.; Baldwin, D.H.; Willis, M.L.; Myers, M.S.; Holland, L.; Larsen, M.; Stekoll, M.S.; Rice, S.D.; Collier, T.K.; et al. Sublethal exposure to crude oil during embryonic development alters cardiac morphology and reduces aerobic capacity in adult fish. Proc. Natl. Acad. Sci. USA 2011, 108, 7086–7090. [Google Scholar] [CrossRef] [PubMed]
- Glickman, N.S.; Yelon, D. Cardiac development in zebrafish: Coordination of form and function. Semin. Cell Dev. Biol. 2002, 13, 507–513. [Google Scholar] [CrossRef]
- Barrionuevo, W.R.; Burggren, W.W. O2 consumption and heart rate in developing zebrafish (Danio rerio): Influence of temperature and ambient O2. Am. J. Physiol. 1999, 276, R505–R513. [Google Scholar] [PubMed]
- Dimitriadi, A.; Beis, D.; Arvanitidis, C.; Adriaens, D.; Koumoundouros, G. Koumoundouros, Developmental temperature has persistent, sexually dimorphic effects on zebrafish cardiac anatomy. Sci. Rep. 2018, 8, 8125. [Google Scholar] [CrossRef] [PubMed]
- Hobday, A.J.; Alexander, L.V.; Perkins, S.E.; Smale, D.A.; Straub, S.C.; Oliver, E.C.J.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Feng, M.; et al. A hierarchical approach to defining marine heatwaves. Prog. Oceanogr. 2016, 141, 227–238. [Google Scholar] [CrossRef]
- Plaut, I. Critical swimming speed: Its ecological relevance. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 131, 41–50. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitriadi, A.; Koumoundouros, G. Elevated Embryonic Temperature Has Persistent Adverse Effects on Zebrafish Swimming Capacity. Fishes 2022, 7, 373. https://doi.org/10.3390/fishes7060373
Dimitriadi A, Koumoundouros G. Elevated Embryonic Temperature Has Persistent Adverse Effects on Zebrafish Swimming Capacity. Fishes. 2022; 7(6):373. https://doi.org/10.3390/fishes7060373
Chicago/Turabian StyleDimitriadi, Anastasia, and George Koumoundouros. 2022. "Elevated Embryonic Temperature Has Persistent Adverse Effects on Zebrafish Swimming Capacity" Fishes 7, no. 6: 373. https://doi.org/10.3390/fishes7060373
APA StyleDimitriadi, A., & Koumoundouros, G. (2022). Elevated Embryonic Temperature Has Persistent Adverse Effects on Zebrafish Swimming Capacity. Fishes, 7(6), 373. https://doi.org/10.3390/fishes7060373