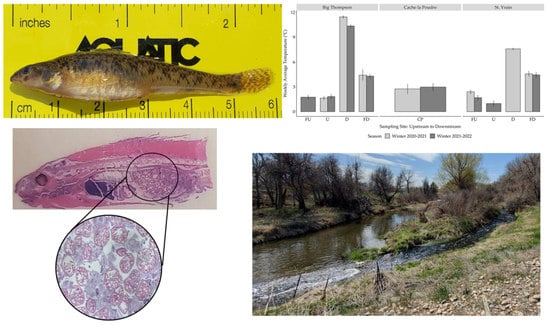
Elevated Winter Stream Temperatures below Wastewater Treatment Plants Shift Reproductive Development of Female Johnny Darter Etheostoma nigrum: A Field and Histologic Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Fish Collection
2.3. Temperature Monitoring
2.4. Water Quality
2.5. Histological Analysis
3. Results
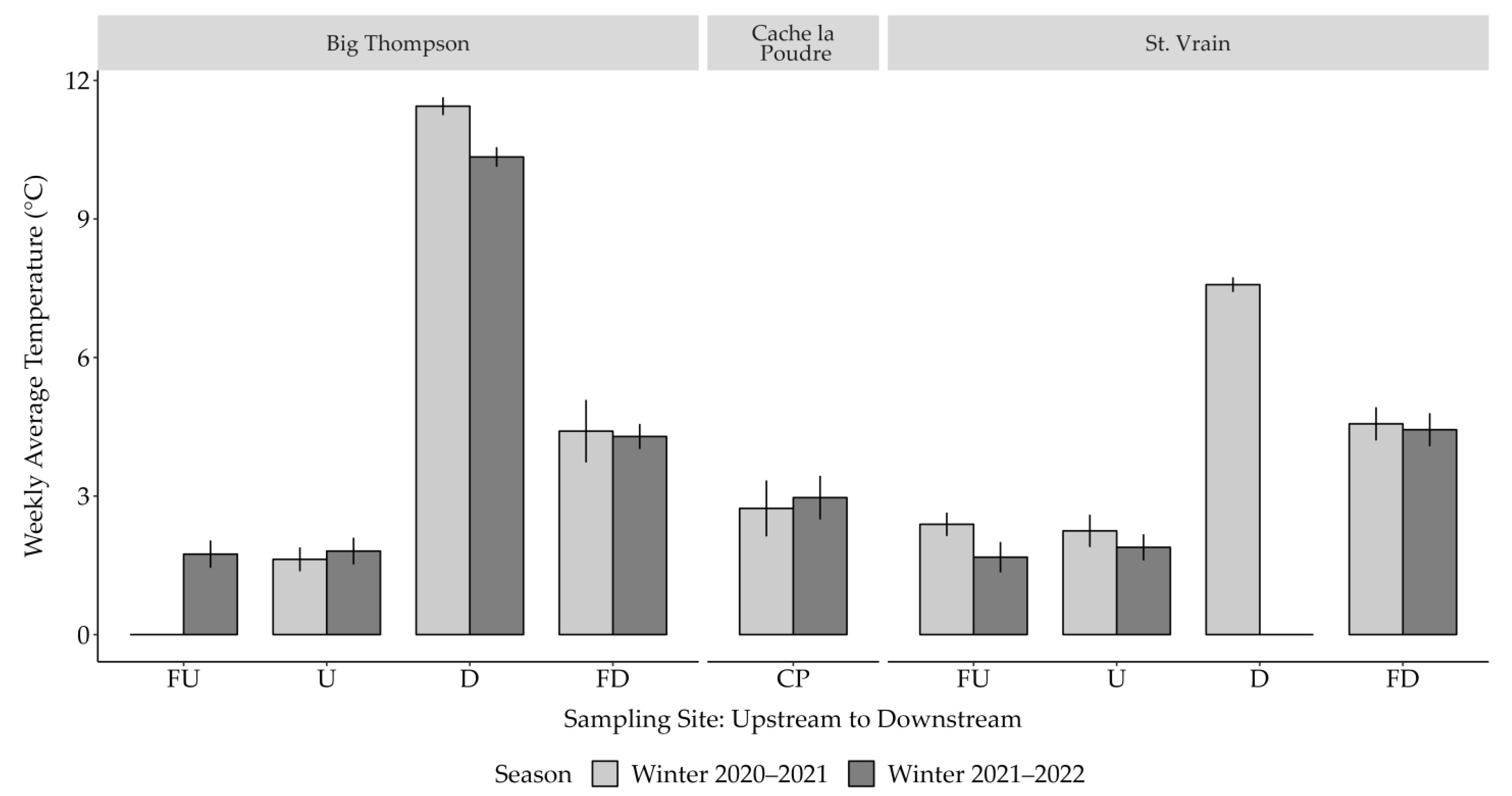
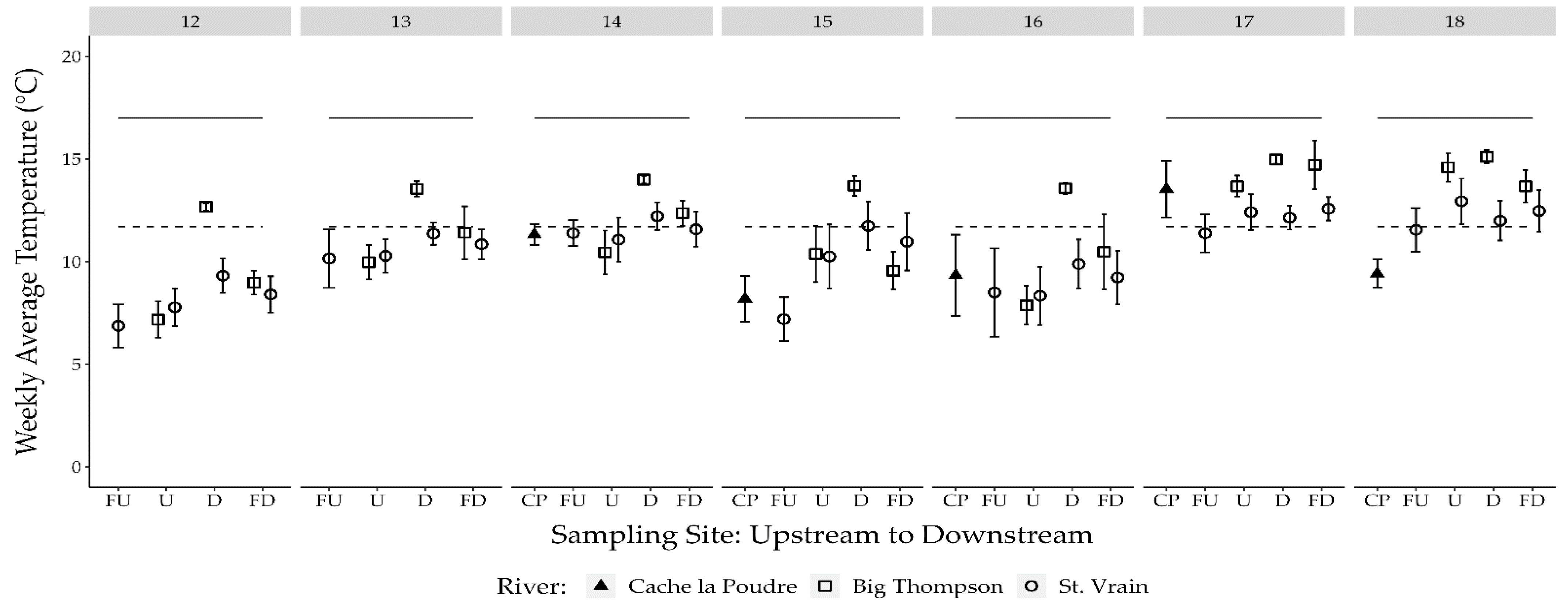
3.1. Temperature
3.2. Water Quality
3.2.1. PPCPs and NNPs
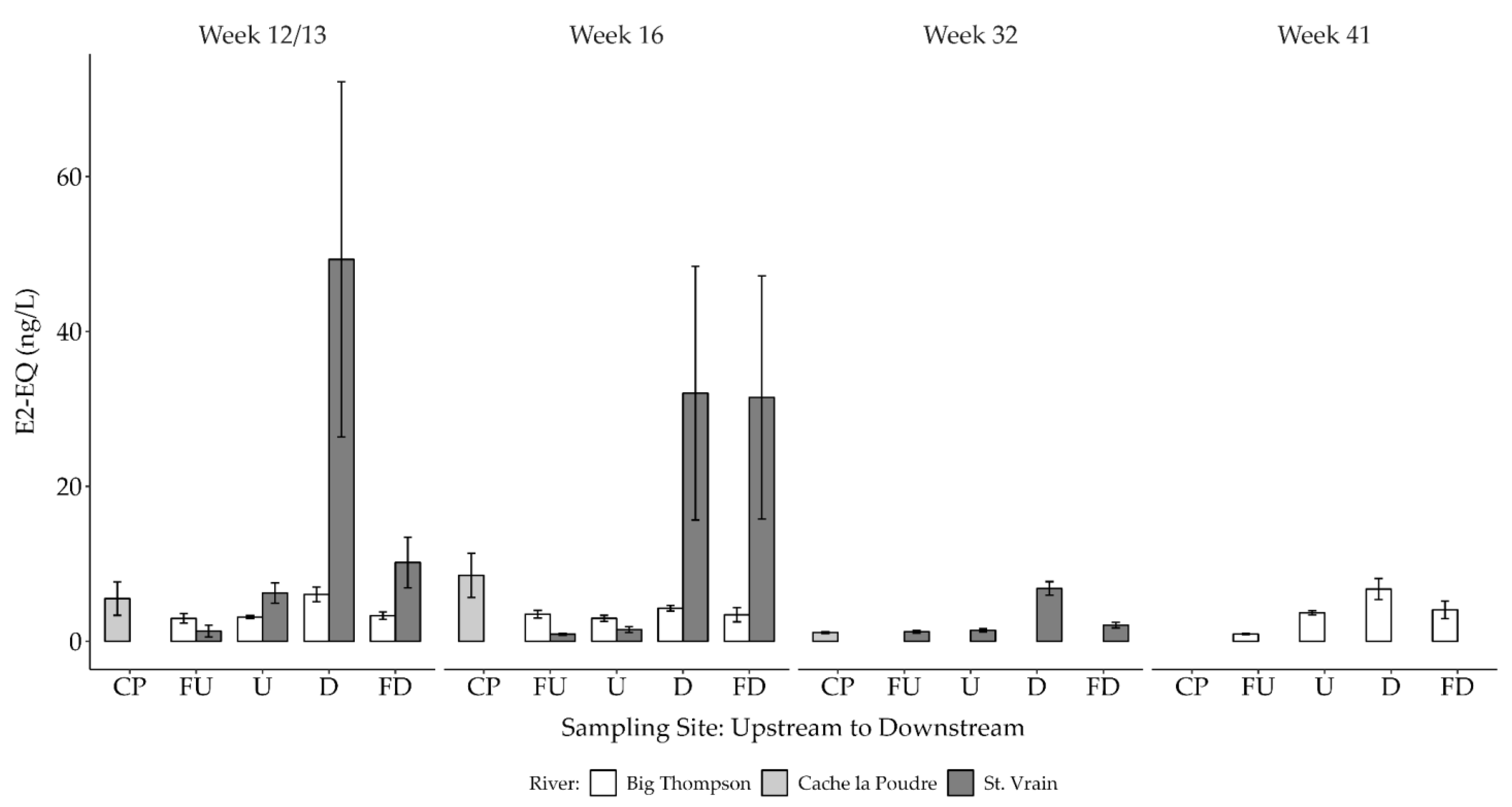
3.2.2. Estrogenicity
3.2.3. Etheostoma nigrum Vtg
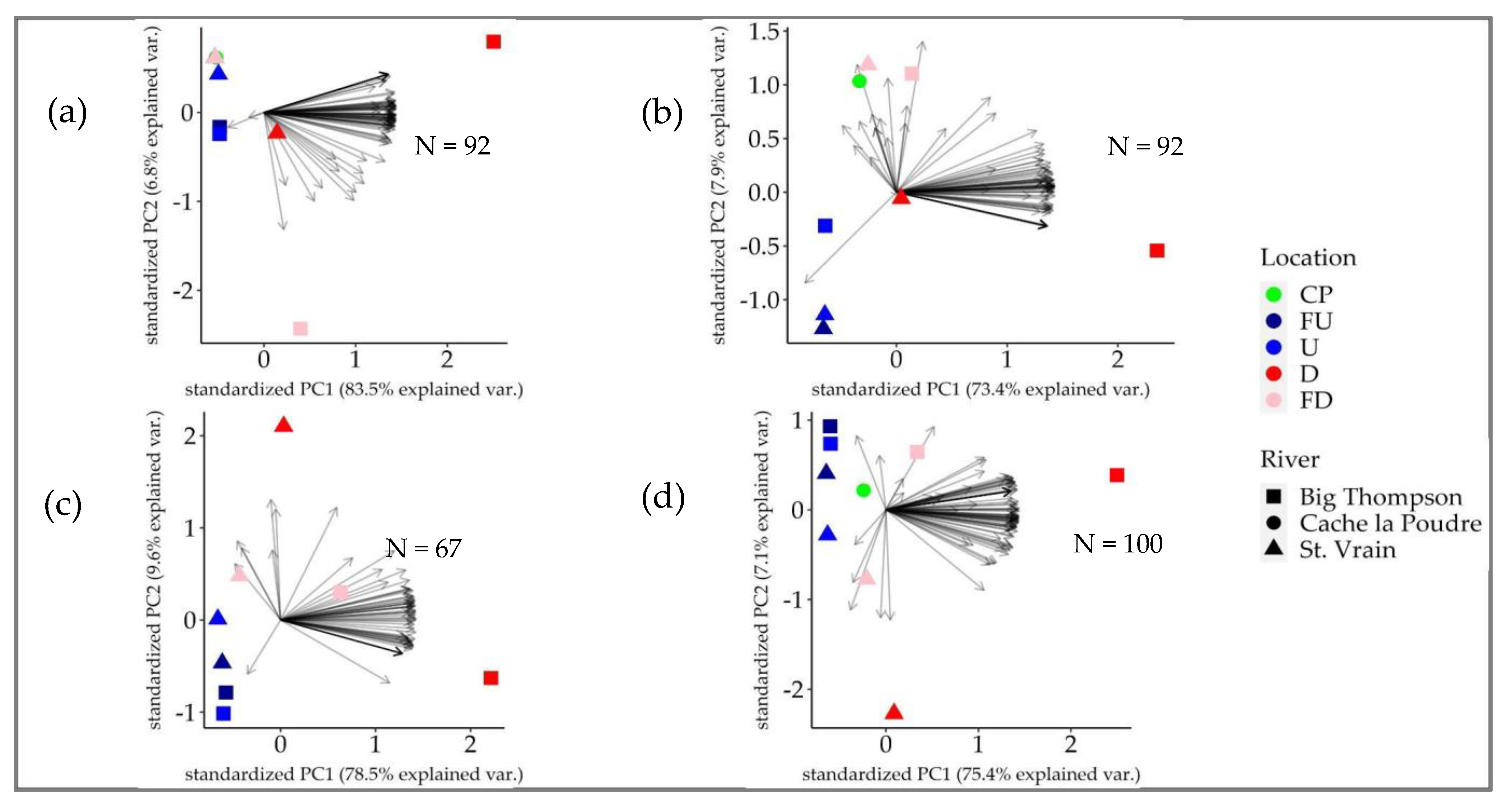
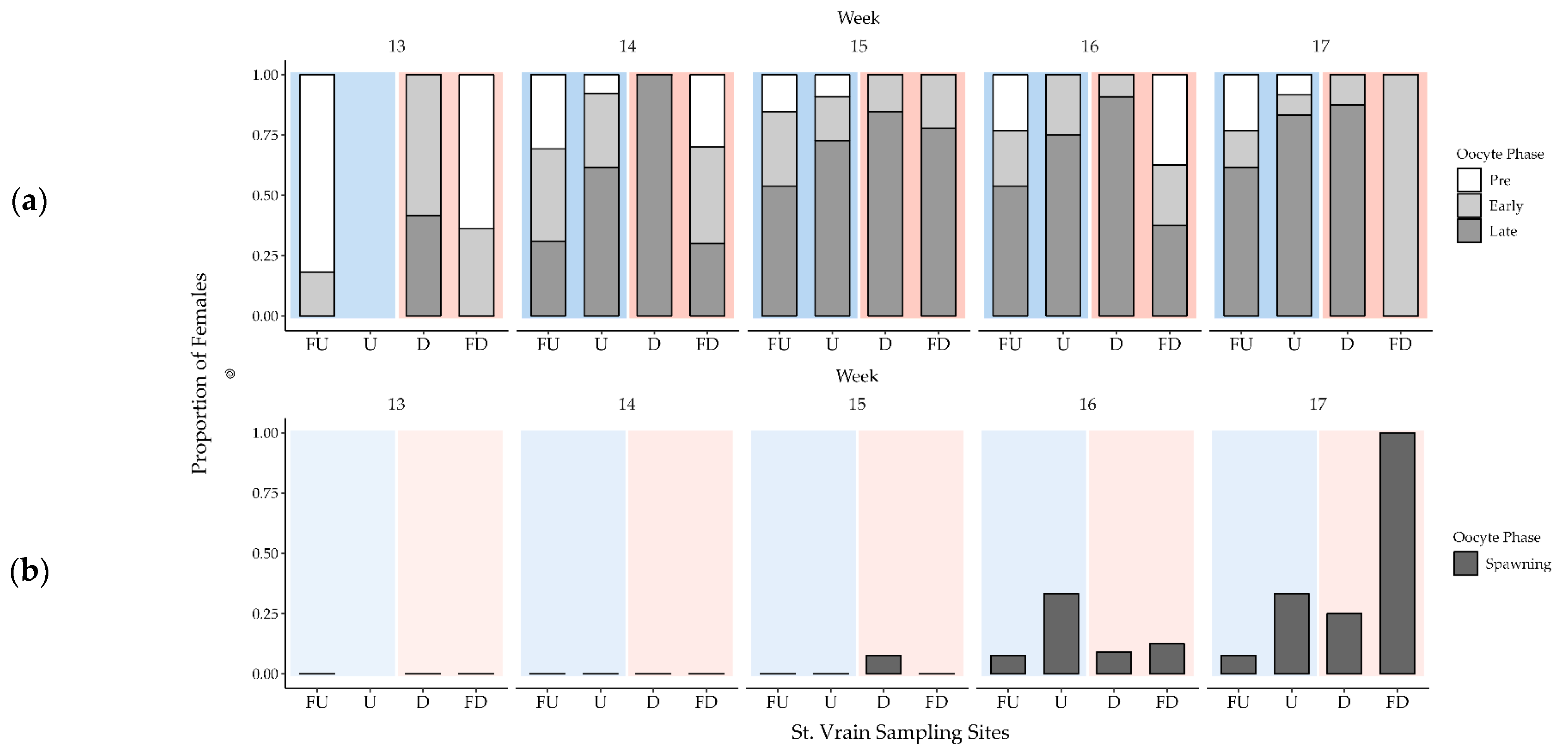
3.3. Female Gonad Histology
3.4. Male Gonad Histology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brett, J.R. Some principles in the thermal requirements of fishes. Q. Rev. Biol. 1956, 31, 75–87. [Google Scholar] [CrossRef]
- Bestgen, K.R.; Williams, M.A. Effects of fluctuating and constant temperatures on early development and survival of Colorado Squawfish. Trans. Am. Fish. Soc. 1994, 123, 574–579. [Google Scholar] [CrossRef]
- Hester, E.T.; Doyle, M.W. Human impacts to the river temperature and their effects on biological processes: A quantitative synthesis. J. Am. Water. Resour. Assoc. 2011, 47, 571–587. [Google Scholar] [CrossRef]
- Farmer, T.M.; Marschall, E.A.; Dabrowski, K.; Ludsin, S.A. Short winters threaten temperate fish populations. Nat. Commun. 2015, 6, 7724. [Google Scholar] [CrossRef] [PubMed]
- Firkus, T.; Rahel, F.J.; Bergman, H.L.; Cherrington, B.D. Warmed winter water temperatures alter reproduction in two fish species. Environ. Manag. 2018, 61, 291–303. [Google Scholar] [CrossRef]
- Jones, B.R.; Hokanson, K.E.F.; McCormick, J.H. Winter temperature requirements for maturation and spawning of yellow perch Perca flavescens (Mitchill). In Biological Balance and Thermal Modification; Marios, M., Ed.; Pergamon Press: Oxford, UK, 1972; Volume 3, pp. 189–192. [Google Scholar]
- De Vlaming, V.L. Effects of photoperiod and temperature on gonadal activity in the cyprinid teleost Notemigonus crysoleucas. Biol. Bull. 1975, 148, 402–415. [Google Scholar] [CrossRef]
- McCormick, J.H.; Jones, B.R.; Hokanson, K.E. White sucker (Catostomus commersoni) embryo development, and early growth and survival at different temperatures. J. Fish. Board Can. 1977, 34, 1019–1025. [Google Scholar] [CrossRef]
- Propst, D.L.; Carlson, C.A. Life History Notes and Distribution of the Etheostoma nigrum (Percidae), in Colorado. Southwest Nat. 1989, 34, 250–259. [Google Scholar] [CrossRef]
- Clarkson, R.W.; Childs, M.R. Temperature effects of hypolimnial–release dams on early life stages of Colorado River basin big–river fishes. Copeia 2000, 2000, 402–412. [Google Scholar] [CrossRef]
- Pankhurst, N.W.; Porter, M.J.R. Cold and dark or warm and light: Variations on the theme of environmental control of reproduction. Fish Physiol. Biochem. 2003, 28, 385–389. [Google Scholar] [CrossRef]
- Pankhurst, N.W.; Munday, P.L. Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 2011, 62, 1015–1026. [Google Scholar] [CrossRef]
- Starzynski, D.; Lauer, T.E. How temperature affects timing and duration of yellow perch spawning in the Indian waters of Lake Michigan. J. Freshw. Ecol. 2015, 30, 445–453. [Google Scholar] [CrossRef]
- Fraser, G.S.; Bestgen, K.R.; Winkelman, D.L.; Thompson, K.G. Temperature—not flow—Predicts native fish reproduction with implications for climate change. Trans. Am. Fish. Soc. 2019, 148, 509–527. [Google Scholar] [CrossRef]
- White, D.P.; Colombo, R.E.; Wahl, D.H. Persistently warmer temperature lead to life history changes in bluegill sunfish (Lepomis macrochirus). Envirn. Bio. Fish. 2020, 103, 1165–1177. [Google Scholar] [CrossRef]
- Burton, T.M.; Likens, G.E. Effect of strip–cutting on stream temperatures in Hubbard Brook Experimental Forest, New Hampshire. BioScience 1973, 23, 433–435. [Google Scholar] [CrossRef]
- Nelson, K.; Palmer, M.A. Predicting stream temperature under urbanization and climate change: Implications for stream biota. J. Am. Water Resour. Assoc. 2007, 43, 440–452. [Google Scholar] [CrossRef]
- Webb, B.W.; Nobilis, F. Long–term changes in river temperature and the influence of climatic and hydrologic factors. Hydrol. Sci. J. 2007, 52, 74–85. [Google Scholar] [CrossRef]
- Kinouchi, T.; Yagi, H.; Miyamoto, M. Increase in stream temperature related to anthropogenic heat input from urban wastewater. J. Hydrol. 2007, 335, 78–88. [Google Scholar] [CrossRef]
- Graham, J.L.; Stone, M.L.; Rasmussen, T.J.; Foster, G.M.; Poulton, B.C.; Paxson, C.R.; Harris, T.D. Effects of Wastewater Effluent Discharge and Treatment Facility Upgrades on Environmental and Biological Conditions of Indian Creek, Johnson County, Kansas, June 2004 through June 2013. U.S. Geological Survey Scientific Investigations Report 2014–5187. 2014. Available online: https://doi.org/10.3133/sir20145187 (accessed on 28 November 2022).
- Tuncay, E. The Effect of Wastewater Treatment Plant Effluent on Water Temperature, Macroinvertebrate Community, and Functional Feeding Groups Structure in the Lower Rouge River, Michigan. Master’s Thesis, University of Michigan, Dearborn, MI, USA, 2016. [Google Scholar]
- Lewis, W.M.; McCutchan, J.H. Regulatory temperature compliance for the South Platte River downstream of the Metro District R. W. Hite Treatment Facility (RWHTF). Metro Wastewater Reclamation District Report 326. 2012. [Google Scholar]
- Baum, C.M. Temperature and Winter Duration Requirements for Reproductive Success in Johnny Darter Etheostoma nigrum in the South Platte River Basin, Colorado. Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2021. [Google Scholar]
- U.S. Census Bureau. COLORADO: 2020 Census. 2020. Available online: https://www.census.gov/library/stories/state–by–state/colorado–population–change–between–census–decade.html (accessed on 1 September 2022).
- CDPHE. Regulation No. 38-Classifications and Numeric Standards for South Platte River Basin, Laramie River Basin, Republican River Basin, Smoky Hill River Basin; Colorado Department of Public Health and the Environment: Denver, CO, USA, 2020. [Google Scholar]
- Woodling, J.D. Colorado’s Little Fish: A Guide to the Minnows and Other Lesser Known Fishes in the State of Colorado; Colorado Division of Wildlife: Denver, CO, USA, 1985. [Google Scholar]
- Zuellig, R.E.; Sprague, L.A.; Collins, J.A.; Cox, O.N. Aquatic Communities and Selected Water Chemistry in St. Vrain Creek Near the City of Longmont, Colorado, Wastewater–Treatment Plant, 2005 and 2006: U.S. Geological Survey Data Series 253. 2007. Available online: https://doi.org/10.3133/ds253 (accessed on 28 November 2022).
- Page, L.M.; Burr, B.M. Peterson Field Guide to Freshwater Fishes of North America and Mexico, 2nd ed.; Houghton Mifflin Harcourt: New York, NY, USA, 1991. [Google Scholar]
- Becker, G.C. Fishes of Wisconsin; University of Wisconsin Press: Madison, WI, USA, 1983; Available online: http://digital.library.wisc.edu/1711.dl/EcoNatRes.FishesWI (accessed on 14 April 2022).
- Propst, D.L. Warm Water Fishes of the Platte River Basin, Colorado; Distribution, Ecology, and Community Dynamics. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 1982. [Google Scholar]
- Overturf, M.D.; Anderson, J.C.; Pandelides, Z.; Beyger, L.; Holdway, D. Pharmaceuticals and personal care products: A critical review of the impacts on fish reproduction. Crit. Rev. Toxicol. 2015, 45, 469–491. [Google Scholar] [CrossRef]
- Schäfers, C.; Teigeler, M.; Wenzel, A.; Maack, G.; Fenske, M.; Segner, H. Concentration- and Time-dependent Effects of the Synthetic Estrogen, 17α- ethinylestradiol, on Reproductive Capabilities of the Zebrafish, Danio rerio. J. Toxicol. Environ. Health Part A 2007, 70, 768–779. [Google Scholar] [CrossRef]
- Länge, R.; Hutchinson, T.H.; Croudace, C.P.; Siemund, F.; Schweinfurth, H.; Hampe, P.; Panter, G.H.; Sumpter, J.P. Effects of the synthetic estrogen 17α- ethinylestradiol on the life-cycle of the Fathead Minnow (Pimephales promelas). Environ. Tox. Chem. 2001, 20, 1216–1227. [Google Scholar] [CrossRef]
- Brain, J.V.; Harris, C.A.; Scholze, M.; Kortenkamp, A.; Booy, P.; Lamoree, M.; Pojana, G.; Jonkers, N.; Marcomini, A.; Sumpter, J.P. Evidence of estrogenic mixture effects on the reproductive performance of fish. Environ. Sci. Technol. 2007, 41, 337–344. [Google Scholar] [CrossRef]
- Corriero, A.; Zupa, R.; Mylonas, C.C.; Letizia, P. Atresia of ovarian follicles in fishes, and implications and used in aquaculture and fisheries. J. Fish Dis. 2021, 44, 1271–1291. [Google Scholar] [CrossRef] [PubMed]
- Woodling, J.D.; Lopez, E.M.; Maldonado, T.A.; Norris, D.O.; Vajda, A.M. Intersex and other reproductive disruption of fish in wastewater effluent dominated Colorado Streams. Comp. Biochem. Phys. Part C 2006, 144, 10–99. [Google Scholar] [CrossRef]
- Adams, C.M. Colorado Cooperative Fish and Wildlife Research Unit; Colorado State University: Fort Collins, CO, USA, 2020; Unpublished data. [Google Scholar]
- USGS. Big Thompson River. Geographic Names Phase I Data Compilation (1976–1981), United States Geological Survey, Feature ID: 205019. 1981. Available online: https://edits.nationalmap.gov/apps/gaz-domestic/public/summary/205019 (accessed on 5 May 2022).
- USGS. Saint Vrain Creek. Geographical Names Phase I Data Compilation (1976–1981), United States Geological Survey, Feature ID: 205012. 1981. Available online: https://edits.nationalmap.gov/apps/gaz-domestic/public/summary/205012 (accessed on 5 May 2022).
- USGS. Cache la Poudre River. Geographic Names Post Phase I Board/Staff Revisions, United States Geological Survey, Feature ID: 205018. 2000. Available online: https://edits.nationalmap.gov/apps/gaz–domestic/public/summary/205018 (accessed on 5 May 2022).
- Eisenhour, D.; Washburn, B.A. Long–distance movements of six darters (Teleostei: Percidae) in the Red River, Kentucky. J. Ky. Acad. Sci. 2016, 77, 19–24. [Google Scholar] [CrossRef]
- Smith, R.K.; Fausch, K.D. Thermal tolerance and vegetation preference of Arkansas Darter and Johnny Darter from Colorado plains streams. Trans. Am. Fish Soc. 1997, 126, 676–686. [Google Scholar] [CrossRef]
- Kassambara, A. rstatix: Pipe–Friendly Framework for Basic Statistical Tests. R Package Version 0.7.0. 2021. Available online: https://CRAN.R–project.org/package=rstatix (accessed on 15 March 2022).
- Schenker, N.; Gentleman, J.F. Judging the significance of differences by examining the overlap between confidence intervals. Am. Stat. 2001, 55, 182–186. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency, EPA Region 8 Laboratory. Determination of Pharmaceuticals and Personal Care Products (PPCPs) in Water by Direct Aqueous Injection UHPLC and LC/MS/MS; Technical Standard Operating Procedure: Lakewood, CO, USA, 2019. [Google Scholar]
- U.S. Environmental Protection Agency, EPA Region 8 Laboratory. Automated Determination of Nitrate-Nitrogen, Nitrite-Nitrogen, and Orthophosphate-Phosphorus Using the Lachat QC 8500 Flow Injection Autoanalyzer; Technical Standard Operating Procedure: Lakewood, CO, USA, 2019. [Google Scholar]
- Johnson, R.; Wolf, J.; Braunbreck, T.; OECD Guidance Document for the Diagnosis of Endocrine–Related Histopathology of Fish Gonads. OECD Environment, Health, and Safety Publications, Series on Testing and Assessment No. 123. 2009. Available online: http://www.oecd.org/chemicalsafety/testing/42135693.doc (accessed on 8 August 2020).
- U.S. Environmental Protection Agency. Histopathology Guidelines for the Fathead Minnow (Pimephales promelas) 21–Day Reproduction Assay. Duluth, MN, USA; 2006. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/P100N3BN.PDF?Dockey=P100N3BN.PDF (accessed on 28 November 2022).
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the Next Generation of Scientific Image Data. BMC Bioinformatics, 18. 2017. Available online: https://imagej.nih.gov/ij/ (accessed on 7 June 2020).
- Óskarsson, G.J.; Kjesbu, O.S.; Slotte, A. Predictions of realized fecundity and spawning time in Norwegian spring–spawning herring (Clupea harengus). J. Sea Res. 2002, 48, 59–79. [Google Scholar] [CrossRef]
- Leino, R.L.; Jensen, K.M.; Ankley, G.T. Gonadal histology and characteristics histopathology associated with endocrine disruption in adult Fathead Minnow (Pimephales promelas). Environ. Toxicol. Pharmacol. 2005, 19, 85–98. [Google Scholar] [CrossRef]
- Borg, B.; Veen, T.V. Seasonal effects of photoperiod and temperature on the ovary of Three–Spined Stickleback, Gasterosteus aculeatus L. Can. J. Zool. 1982, 60, 3387–3393. [Google Scholar] [CrossRef]
- Blazer, V. Histopathological assessment of gonadal tissue in wild fishes. Fish Physiol. Biochem. 2002, 26, 85–101. [Google Scholar] [CrossRef]
- Adams, C.M. Colorado Cooperative Fish and Wildlife Research Unit; Colorado State University: Fort Collins, CO, USA, 2022; Unpublished data. [Google Scholar]
- Adams, C.M.; Winkelman, D.L.; Fitzpatrick, R.F. Impact of Wastewater Treatment Plant Effluent on the Winter Thermal Regime of Two Urban South Platte Tributaries; Colorado Parks and Wildlife: Fort Collins, CO, USA, 2022. [Google Scholar]
- Stallsmith, B.; Bedingfield, R. Reproductive development in the Black Darter (Etheostoma duryi). J. Alab. Aca. Sci. 2015, 86, 15–28. [Google Scholar]
- Bowerman, T.E.; Pinson-Dumm, A.; Peery, C.A.; Caudill, C.C. Reproductive energy expenditure and changes in body morphology for a population of Chinook salmon Oncorhynchus tshawytscha with a long distance migration. J. Fish Biol. 2017, 90, 1960–1979. [Google Scholar] [CrossRef] [PubMed]
- Encina, L.; Granado-Lorencio, C. Seasonal changes in condition, nutrition, gonad maturation and energy content in barrel, Barbus sclateri, inhabiting a fluctuating river. Environ. Biol. Fish. 1997, 50, 75–84. [Google Scholar] [CrossRef]
- Bunnel, D.B.; Thomas, S.E.; Stein, R.A. Prey Resources before spawning influence gonadal investment of female, but not male, White Crappie. J. Fish Biol. 2007, 70, 1838–1854. [Google Scholar] [CrossRef]
- Maack, G.; Segner, H. Life-stage-dependent sensitivity of zebrafish (Danio rerio) to estrogen exposure. Comp. Biochem. Phys. Part C 2004, 139, 47–55. [Google Scholar] [CrossRef]
- Segner, H.; Caroll, K.; Fenske, M.; Janssen, C.R.; Maack, G.; Pascoe, D.; Schafers, C.; Vandenbergh, G.F.; Watts, M.; Wensel, A. Identification of endocrine–disrupting effects in aquatic vertebrates and invertebrates: Report from the European IDEA project. Ecotox. Environ. Saf. 2003, 54, 302–314. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, B.R. Turbulence, larval fish ecology and fisheries recruitment: A review of field studies. Oceanol. Acta 2000, 23, 357–375. [Google Scholar] [CrossRef]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 30–320. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information (NCBI) [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information [1988]. 2022. Available online: https://www.ncbi.nlm.nih.gov/protein (accessed on 29 April 2022).
- The UniProt Consortium. UniProt: The Universal Protein Knowledgebase in 2021. Nucleic Acids Res. 2020, 49, D480–D489. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (NCBI). Gene [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information [1988]. Gene ID: 116695776, Vtg3 Vitellogenin 3, Phosvitinless [Etheostoma spectabile (Orangethroat Darter)]. 2021. Available online: https://www.ncbi.nlm.nih.gov/gene/116695776 (accessed on 29 April 2022).
- Wilson, V.S.; Bobseine, K.; Gray, L.E. Development and characterization of a cell line that stably expresses an estrogen-responsive luciferase reporter for the detection of estrogen receptor agonist and antagonists. Toxicol. Sci. 2004, 81, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Vermeirssen, E.L.M.; Buchinger, S.; Behr, M.; Magdeburg, A.; Oehlmann, J. Deriving bio-equivalents from in vitro bioassays: Assessment of existing uncertainties and strategies to improve accuracy and reporting. Environ. Toxicol. Chem. 2013, 32, 1906–1917. [Google Scholar] [CrossRef] [PubMed]





| River | Week of the Year 2021 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 32 | 41 | |
| Big Thompson | 5 January | 4 February | 23 March | - | 6 April | 13 April | 21 April | 28 April | 6 May | - | 11 October |
| St. Vrain | - | 2 February | - | 29 March | 7 April | 12 April | 19 April | 26 April | - | 9 August | - |
| 30 March | |||||||||||
| Cache la Poudre | 5 January | - | 22 March | - | 6 April | 13 April | 20 April | 28 April | - | 10 August | - |
| Sex | Week | Cache la Poudre | Big Thompson River | St. Vrain Creek | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CP | FU-BT | U-BT | D-BT | FD-BT | FU-SV | U-SV | D-SV | FD-SV | ||
| F | 12 | 15 | 10 | 10 | 10 | 8 | - | - | - | - |
| M | 4 | 8 | 2 | 6 | 12 | - | - | - | - | |
| unk | 2 | 1 | 0 | 2 | 0 | - | - | - | - | |
| F | 13 | - | - | - | - | - | 12 | - | 12 | 11 |
| M | - | - | - | - | - | 8 | - | 8 | 4 | |
| unk | - | - | - | - | - | 0 | - | 0 | 0 | |
| F | 14 | 10 | 6 | 5 | 10 | 10 | 13 | 13 | 11 | 10 |
| M | 8 | 9 | 5 | 9 | 10 | 7 | 7 | 8 | 7 | |
| unk | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 2 | |
| F | 15 | 10 | 13 | 8 | 11 | 15 | 13 | 11 | 13 | 9 |
| M | 7 | 7 | 3 | 9 | 4 | 6 | 9 | 6 | 9 | |
| unk | 3 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 2 | |
| F | 16 | 11 | 15 | 3 | 12 | 6 | 11 | 12 | 11 | 8 |
| M | 6 | 5 | 3 | 8 | 12 | 8 | 7 | 9 | 10 | |
| unk | 2 | 0 | 0 | 0 | 2 | 1 | 1 | - | - | |
| F | 17 | 13 | 2 | 2 | 12 | 7 | 13 | 12 | 8 | 1 |
| M | 5 | 0 | 0 | 7 | 3 | 7 | 8 | 1 | 1 | |
| unk | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| F | 18 | - | 17 | 14 | 11 | 5 | - | - | - | - |
| M | - | 3 | 6 | 9 | 8 | - | - | - | - | |
| unk | - | 0 | 0 | 0 | 0 | - | - | - | - | |
| F | 32 | - | - | - | - | - | 2 | 5 | - | 10 |
| M | - | - | - | - | - | 1 | 0 | - | 0 | |
| unk | - | - | - | - | - | 0 | 1 | - | 10 | |
| F | 41 | - | 5 | 0 | 7 | 0 | - | - | - | - |
| M | - | 3 | 1 | 1 | 1 | - | - | - | - | |
| unk | - | 0 | 0 | 1 | 1 | - | - | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adams, C.M.; Winkelman, D.L.; Schaffer, P.A.; Villeneuve, D.L.; Cavallin, J.E.; Ellman, M.; Santana Rodriguez, K.; Fitzpatrick, R.M. Elevated Winter Stream Temperatures below Wastewater Treatment Plants Shift Reproductive Development of Female Johnny Darter Etheostoma nigrum: A Field and Histologic Approach. Fishes 2022, 7, 361. https://doi.org/10.3390/fishes7060361
Adams CM, Winkelman DL, Schaffer PA, Villeneuve DL, Cavallin JE, Ellman M, Santana Rodriguez K, Fitzpatrick RM. Elevated Winter Stream Temperatures below Wastewater Treatment Plants Shift Reproductive Development of Female Johnny Darter Etheostoma nigrum: A Field and Histologic Approach. Fishes. 2022; 7(6):361. https://doi.org/10.3390/fishes7060361
Chicago/Turabian StyleAdams, Catherine M., Dana L. Winkelman, Paula A. Schaffer, Daniel L. Villeneuve, Jenna E. Cavallin, Michael Ellman, Kelvin Santana Rodriguez, and Ryan M. Fitzpatrick. 2022. "Elevated Winter Stream Temperatures below Wastewater Treatment Plants Shift Reproductive Development of Female Johnny Darter Etheostoma nigrum: A Field and Histologic Approach" Fishes 7, no. 6: 361. https://doi.org/10.3390/fishes7060361
APA StyleAdams, C. M., Winkelman, D. L., Schaffer, P. A., Villeneuve, D. L., Cavallin, J. E., Ellman, M., Santana Rodriguez, K., & Fitzpatrick, R. M. (2022). Elevated Winter Stream Temperatures below Wastewater Treatment Plants Shift Reproductive Development of Female Johnny Darter Etheostoma nigrum: A Field and Histologic Approach. Fishes, 7(6), 361. https://doi.org/10.3390/fishes7060361