Effects of Short-Term Salinity Stress on Ions, Free Amino Acids, Na+/K+-ATPase Activity, and Gill Histology in the Threatened Freshwater Shellfish Solenaia oleivora
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Mussels and Rearing Conditions
2.2. Salinity Challenge and Sampling
2.3. Measurement of Hemolymph Osmolality
2.4. Detection of Ion Concentrations and Enzyme Activity
2.5. Detection of FAA Content in Hemolymph
2.6. Gill Histology
2.7. Data Analysis
3. Results
3.1. Effects of Salinity Stress on Hemolymph Osmolality and Ion Concentrations
3.2. Effects of Salinity Stress on NKA Activity
3.3. Effects of Salinity Stress on FAA Contents
3.4. Effects of Salinity Stress on Gill Histomorphology
3.5. PCA Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, X.; Zhang, X.; Tian, L.; Zhang, P. Effect of salinity stress on hemolymph osmolality and gill Na+/K+-ATPase activity of juvenile ark shell (Anadara broughtonii). South China Fish. Sci. 2015, 11, 12–19. [Google Scholar]
- Hossain, F.; Islam, S.M.M.; Islam, M.S.; Shahjahan, M. Behavioral and histo-pathological indices of striped catfish (Pangasionodon hypophthalmus) exposed to different salinities. Aquac. Rep. 2022, 23, 101038. [Google Scholar] [CrossRef]
- Wang, T.; Li, Q. Effects of Salinity and Temperature on Growth and Survival of Juvenile Iwagaki Oyster Crassostrea nippona. J. Ocean. Univ. China 2018, 17, 941–946. [Google Scholar] [CrossRef]
- Bertrand, C.; Devin, S.; Mouneyrac, C.; Giambérini, L. Eco-physiological responses to salinity changes across the freshwater-marine continuum on two euryhaline bivalves: Corbicula fluminea and Scrobicularia plana. Ecol. Indic. 2017, 74, 334–342. [Google Scholar] [CrossRef]
- Velasco, J.; Gutierrez-Canovas, C.; Botella-Cruz, M.; Sanchez-Fernandez, D.; Arribas, P.; Carbonell, J.A.; Millan, A.; Pallares, S. Effects of salinity changes on aquatic organisms in a multiple stressor context. Philos. Trans. R. Soc. B-Biol. Sci. 2019, 374, 20180011. [Google Scholar] [CrossRef]
- Beatty, S.J.; Morgan, D.L.; Rashnavadi, M.; Lymbery, A.J. Salinity tolerances of endemic freshwater fishes of south-western Australia: Implications for conservation in a biodiversity hotspot. Mar. Freshw. Res. 2011, 62, 91–100. [Google Scholar] [CrossRef]
- Galkanda-Arachchige, H.S.C.; Davis, R.P.; Nazeer, S.; Ibarra-Castro, L.; Davis, D.A. Effect of salinity on growth, survival, and serum osmolality of red snapper, Lutjanus campechanus. Fish Physiol. Biochem. 2021, 47, 1687–1696. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Y.; Zhang, L.; Zhuang, P.; Liu, J. Survival, growth, food conversion efficiency and plasma osmolality of juvenile Siganus guttatus (Bloch, 1787): Experimental analyses of salinity effects. Fish Physiol. Biochem. 2013, 39, 1025–1030. [Google Scholar] [CrossRef]
- Berger, V.J.; Kharazova, A.D. Mechanisms of salinity adaptations in marine molluscs. In Interactions and Adaptation Strategies of Marine Organisms; Springer: Dordrecht, The Netherlands, 1997. [Google Scholar]
- Luzio, A.; Parra, S.; Costa, B.; Santos, D.; Alvaro, A.R.; Monteiro, S.M. Copper impair autophagy on zebrafish (Danio rerio) gill epithelium. Environ. Toxicol. Pharmacol. 2021, 86, 103674. [Google Scholar] [CrossRef] [PubMed]
- Laverty, G.; Skadhauge, E. Adaptation of teleosts to very high salinity. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 163, 1–6. [Google Scholar] [CrossRef]
- Cao, W.; Bi, S.; Chi, C.; Dong, Y.; Xia, S.; Liu, Z.; Zhou, L.; Sun, X.; Geng, Y.; Wu, B. Effects of High Salinity Stress on the Survival, Gill Tissue, Enzyme Activity and Free Amino Acid Content in Razor Clam Sinonovacula constricta. Front. Mar. Sci. 2022, 9, 839614. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, W.; Li, J.; Huang, X.; Duan, L.; Zhan, Y. Effects of acute salinity stress on gill structure and four enzyme activities in Saxidomus purpurata. J. Agric. Sci. Technol. 2016, 18, 178–186. [Google Scholar]
- Sun, J.; Chen, M.; Fu, Z.; Yang, J.; Zhou, S.; Yu, G.; Zhou, W.; Ma, Z. A Comparative Study on Low and High Salinity Tolerance of Two Strains of Pinctada fucata. Front. Mar. Sci. 2021, 8, 704907. [Google Scholar] [CrossRef]
- Gao, X.; Li, Y.; Li, X.; Wu, F.; Song, C.; Liu, Y. The response and osmotic pressure regulation mechanism of Haliotis discus hannai (Mollusca, Gastropoda) to sudden salinity changes. Hydrobiologia 2017, 795, 181–198. [Google Scholar] [CrossRef]
- Lin, C.-H.; Yeh, P.-L.; Lee, T.-H. Ionic and Amino Acid Regulation in Hard clam (Meretrix Iusoria) in Response to Salinity Challenges. Front. Physiol. 2016, 7, 368. [Google Scholar] [CrossRef]
- Zurburg, W.; De Zwaan, A. The role of amino acids in anaerobiosis and osmoregulation in bivalves. J. Exp. Zool. 1981, 215, 315–325. [Google Scholar] [CrossRef]
- Deaton, L.E.; Hilbish, T.J.; Koehn, R.K. Hyperosmotic volume regulation in the tissues of the mussel Mytilus edulis. Comp. Biochem. Physiol. Part A Physiol. 1985, 80, 571–574. [Google Scholar] [CrossRef]
- Saintsing, D.G.; Towle, D.W. Na++K+-ATPase in the osmoregulating clam Rangia cuneata. J. Exp. Zool. 1978, 206, 435–442. [Google Scholar] [CrossRef]
- Evans, A.N.; Lambert, F.N. Na+/K+-ATPase α1 mRNA expression in the gill and rectal gland of the Atlantic stingray, Dasyatis sabina, following acclimation to increased salinity. BMC Res. Notes 2015, 8, 219. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, M. Effect of salinity on activities of Na+/K+-ATP enzymes in rock scallop Crassadoma gigantea. Technol. Wind. 2019, 7, 241–243+245. [Google Scholar]
- Xu, Q.; Liu, J.; He, L. Flesh content and nutrition component of Solenaia oleivora. Freshw. Fish. 2003, 33, 28–29. [Google Scholar]
- Yang, X.; Li, H.; Song, L. Gonadal develpment and growth of freshwater mussel Solenaia oleivora. Freshw. Fish. 2011, 27, 580–582. [Google Scholar]
- Wen, H.; Sun, G.; Ding, T.; Xu, P.; Wang, L.; Zhang, C.; Cui, L.; Jin, W.; Hua, D.; Gu, R.; et al. Reproductive type and gonad development in the threatened freshwater mussel Solenaia oleivora (Heude) from the Huaihe River. J. Fish. Sci. China 2020, 27, 1156–1166. [Google Scholar]
- Wang, Y.; Zhang, G.; Wei, K.; Gardner, J. Reproductive traits of the threatened freshwater mussel Solenaia oleivora (Bivalvia: Unionidae) from the middle Yangtze River. J. Molluscan Stud. 2015, 81, 522–526. [Google Scholar] [CrossRef][Green Version]
- Zhang, G.; Zhang, W.; Fang, A.; Yang, S. Path analysis of quantitative traits of shellfish Solenaia oleivora population in Ganjiang River. Fish. Sci. 2020, 39, 271–276. [Google Scholar]
- Zhang, T.; Yao, J.; Xu, D.; Ma, X.; Jin, W.; Lv, G.; Gu, R.; Wen, H.; Zhou, Y. Gill physiological and transcriptomic response of the threatened freshwater mussel Solenaia oleivora to salinity shift. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 40, 100913. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lim, S.R.; Ra, C.S.; Kim, J.D. Effects of Dietary Garlic Extracts on Whole Body Amino Acid and Fatty Acid Composition, Muscle Free Amino Acid Profiles and Blood Plasma Changes in Juvenile Sterlet Sturgeon, Acipenser ruthenus. Asian-Australas. J. Anim. Sci. 2012, 25, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Kültz, D. Physiological mechanisms used by fish to cope with salinity stress. J. Exp. Biol. 2015, 218 Pt 12, 1907–1914. [Google Scholar] [CrossRef]
- Song, J.A.; Choi, Y.; Choi, C.Y. Effects of salinity changes on the osmoregulatory and stress responses in the bay scallop Argopecten irradians. Fish. Sci. 2022, 88, 275–283. [Google Scholar] [CrossRef]
- Ruiz, J.L.; Souza, M.M. Osmotic stress and muscle tissue volume response of a freshwater bivalve. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 399–406. [Google Scholar] [CrossRef]
- Hosoi, M.; Yoshinaga, Y.; Toyohara, M.; Shiota, F.; Toyohara, H. Freshwater bivalve Corbicula sandai uses free amino acids as osmolytes under hyperosmotic condition. Fish. Sci. 2008, 74, 1339–1341. [Google Scholar] [CrossRef]
- Cheng, W.; Yeh, S.-P.; Wang, C.-S.; Chen, J.-C. Osmotic and ionic changes in Taiwan abalone Haliotis diversicolor supertexta at different salinity levels. Aquaculture 2002, 203, 349–357. [Google Scholar] [CrossRef]
- Natochin, Y.V.; Berger, V.Y.; Khlebovich, V.V.; Lavrova, E.A.; Michailova, O.Y. The participation of electrolytes in adaptation mechanisms of intertidal molluscs’ cells to altered salinity. Comp. Biochem. Physiol. Part A Physiol. 1979, 63, 115–119. [Google Scholar] [CrossRef]
- Kulac, B.; Atli, G.; Canli, M. Response of ATPases in the osmoregulatory tissues of freshwater fish Oreochromis niloticus exposed to copper in increased salinity. Fish Physiol. Biochem. 2013, 39, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Ge, X.; Wang, J.; Wang, A.; Lv, F.; Wang, Q. Effects of salinity on Na+/K+-ATPase activity, the osmolality of pericardial cavity fluid and peritoneal fluid and ion comtent in Onchidium struma. J. Fish. Sci. China 2013, 37, 851–857. [Google Scholar] [CrossRef]
- Yancey, P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005, 208 Pt 15, 2819–2830. [Google Scholar] [CrossRef]
- Hosoi, M.; Kubota, S.; Toyohara, M.; Toyohara, H.; Hayashi, I. Effect of salinity change on free amino acid content in Pacific oyster. Fish. Sci. 2003, 69, 395–400. [Google Scholar] [CrossRef]
- Jordan, P.J.; Deaton, L.E. Osmotic regulation and salinity tolerance in the freshwater snail Pomacea bridgesi and the freshwater clam Lampsilis teres. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 1999, 122, 199–205. [Google Scholar] [CrossRef]
- Gainey, J.L.F. The Response of the Corbiculidae (Mollusca: Bivalvia) to Osmotic Stress: The Organismal Response. Physiol. Zool. 1978, 51, 68–78. [Google Scholar] [CrossRef]
- Matsushima, O.; Hayashi, Y.; Katayama, H.; Yamada, K.; Kado, Y. Effect of metabolic inhibitors on hyperosmotically induced free amino acid accumulation in the isolated foot muscle of the brackish-water bivalve Corbicula japonica. Comp. Biochem. Physiol. Part A Physiol. 1984, 79, 685–690. [Google Scholar] [CrossRef]
- DiMaggio, M.A.; Ohs, C.L.; Petty, B.D. Salinity tolerance of the Seminole killifish, Fundulus seminolis, a candidate species for marine baitfish aquaculture. Aquaculture 2009, 293, 74–80. [Google Scholar] [CrossRef]
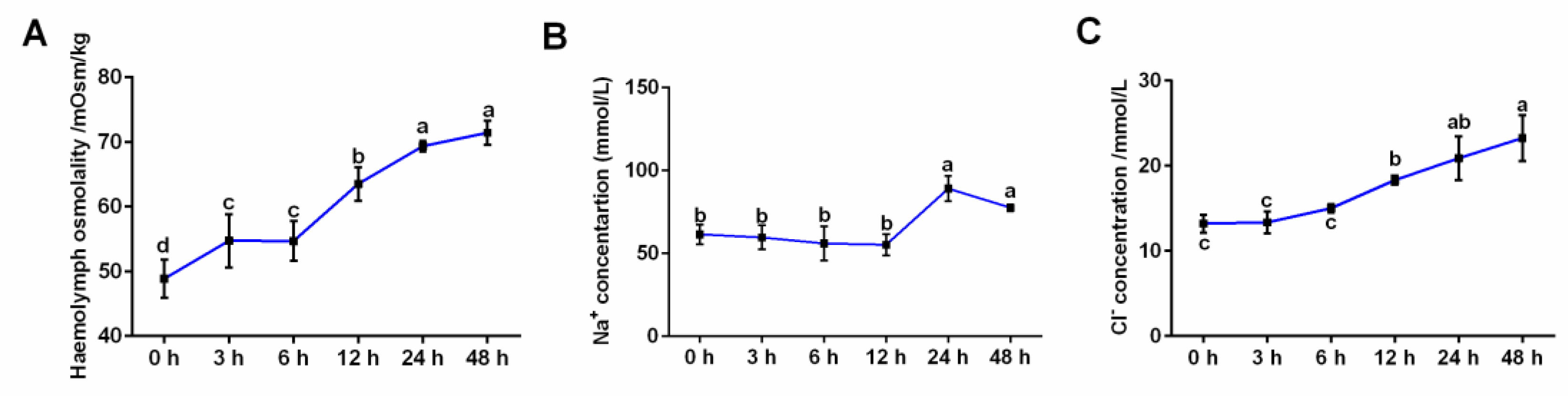
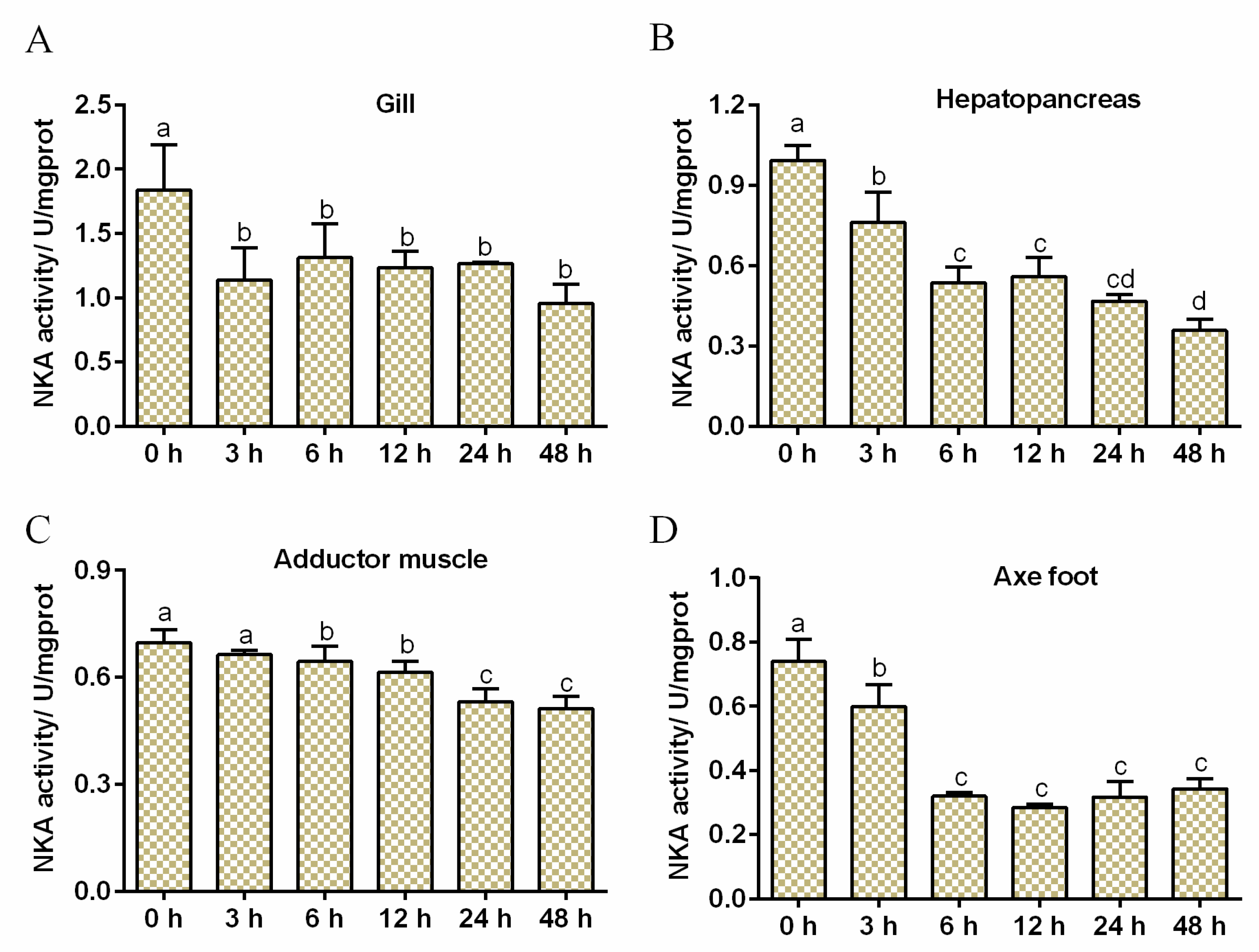
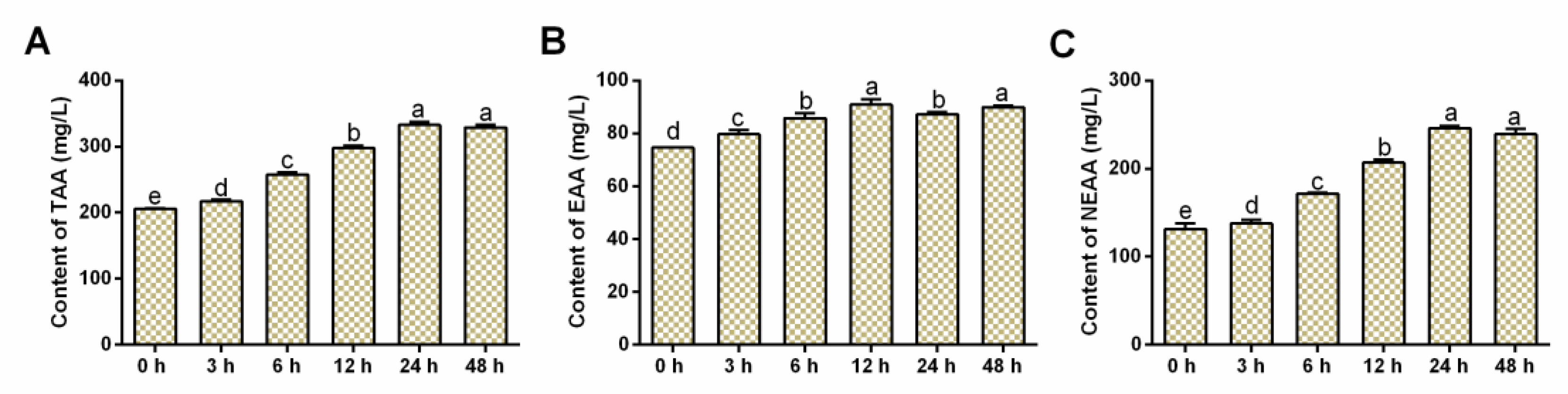

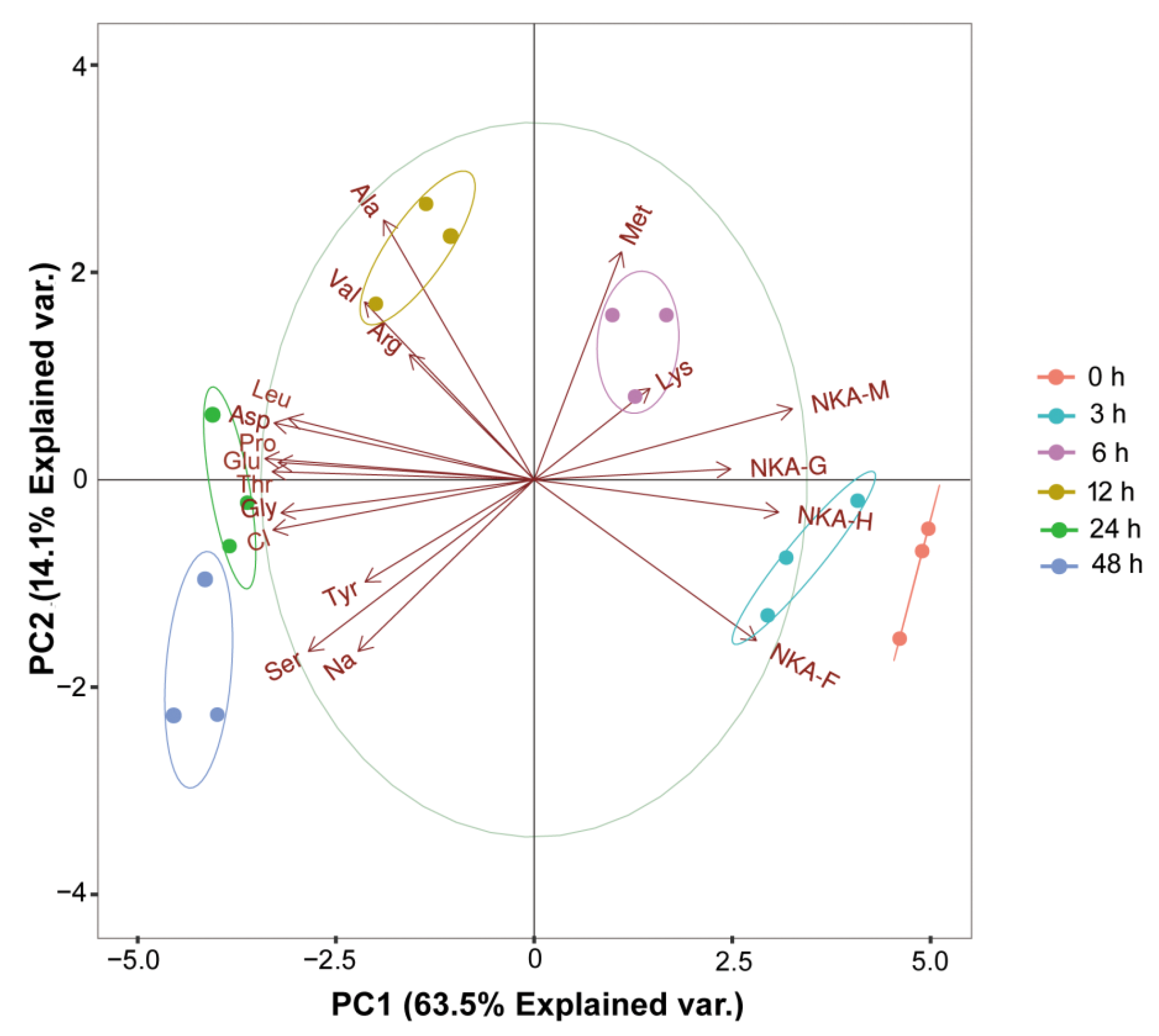
| Group | 0 h | 3 h | 6 h | 12 h | 24 h | 48 h |
|---|---|---|---|---|---|---|
| Aspartate | 13.47 ± 0.83 d | 13.23 ± 0.93 d | 16.77 ± 1.27 c | 23.55 ± 0.87 b | 25.66 ± 1.38 a | 24.06 ± 1.15 a,b |
| Threonine | 25.10 ± 1.99 c | 26.41 ± 2.07 b,c | 30.45 ± 2.53 b | 41.82 ± 2.37 a | 44.29 ± 2.30 a | 46.12 ± 3.35 a |
| Serine | 10.14 ± 1.33 b | 12.12 ± 1.48 b | 9.76 ± 0.93 b | 11.99 ± 0.99 b | 20.25 ± 2.06 a | 22.21 ± 1.76 a |
| Glutamine | 42.23 ± 2.34 e | 39.06 ± 2.69 e | 52.15 ± 2.93 d | 58.40 ± 2.66 c | 72.27 ± 4.43 a | 65.84 ± 2.94 b |
| Glycine | 14.87 ± 1.73 d | 18.98 ± 1.75 c | 24.55 ± 1.92 b | 23.48 ± 1.63 b | 30.12 ± 2.01 a | 31.81 ± 1.70 a |
| Alanine | 11.56 ± 1.37 c | 11.17 ± 1.19 c | 13.59 ± 1.09 b,c | 17.77 ± 1.16 a | 15.55 ± 1.68 a,b | 13.02 ± 1.60 b,c |
| Cysteine | 1.34 ± 0.26 | 0.99 ± 0.17 | 1.06 ± 0.21 | 1.43 ± 0.15 | 1.24 ± 0.17 | 1.33 ± 0.23 |
| Proline | 12.47 ± 0.41 e | 15.98 ± 0.95 d | 23.39 ± 1.38 c | 29.00 ± 2.77 b | 36.38 ± 1.61 a | 34.69 ± 2.29 a |
| Valine | 7.89 ± 1.08 c | 9.93 ± 1.05 b,c | 11.73 ± 0.89 a,b | 13.51 ± 0.96 a | 11.63 ± 1.38 a,b | 11.65 ± 1.83 a,b |
| Methionine | 6.78 ± 0.70 a,b | 5.67 ± 0.53 b,c | 7.27 ± 0.77 a | 6.51 ± 0.58 a,b | 6.21 ± 0.59 a,b,c | 5.53 ± 0.59 c |
| Isoleucine | 6.15 ± 0.33 | 5.97 ± 0.45 | 6.27 ± 0.95 | 5.25 ± 0.86 | 5.33 ± 0.39 | 6.24 ± 0.64 |
| Leucine | 16.85 ± 0.98 c | 16.93 ± 0.78 c | 20.98 ± 0.61 b | 21.62 ± 1.06 b | 24.53 ± 1.39 a | 22.59 ± 1.22 b |
| Tyrosine | 5.19 ± 0.45 c | 5.02 ± 0.53 c | 5.67 ± 0.44 c | 7.21 ± 1.20 b | 5.28 ± 0.20 c | 11.18 ± 0.48 a |
| Phenylalanine | 7.05 ± 0.61 | 7.16 ± 0.68 | 7.60 ± 0.35 | 7.85 ± 0.77 | 7.42 ± 0.59 | 7.07 ± 0.52 |
| Histidine | 3.69 ± 0.34 | 4.78 ± 0.35 | 4.34 ± 0.27 | 4.57 ± 0.88 | 4.01 ± 0.42 | 4.65 ± 0.53 |
| Lysine | 10.24 ± 0.45 c | 13.27 ± 0.84 a | 11.96 ± 0.50 a,b | 11.36 ± 1.07 b,c | 10.16 ± 0.71 c | 10.21 ± 0.69 c |
| Arginine | 10.75 ± 0.52 b | 10.98 ± 0.68 b | 9.93 ± 0.66 b | 12.97 ± 0.90 a | 12.64 ± 0.83 a | 10.84 ± 0.88 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Yao, J.; Xu, D.; Lv, G.; Wen, H. Effects of Short-Term Salinity Stress on Ions, Free Amino Acids, Na+/K+-ATPase Activity, and Gill Histology in the Threatened Freshwater Shellfish Solenaia oleivora. Fishes 2022, 7, 346. https://doi.org/10.3390/fishes7060346
Zhang T, Yao J, Xu D, Lv G, Wen H. Effects of Short-Term Salinity Stress on Ions, Free Amino Acids, Na+/K+-ATPase Activity, and Gill Histology in the Threatened Freshwater Shellfish Solenaia oleivora. Fishes. 2022; 7(6):346. https://doi.org/10.3390/fishes7060346
Chicago/Turabian StyleZhang, Ting, Jingting Yao, Dongpo Xu, Guohua Lv, and Haibo Wen. 2022. "Effects of Short-Term Salinity Stress on Ions, Free Amino Acids, Na+/K+-ATPase Activity, and Gill Histology in the Threatened Freshwater Shellfish Solenaia oleivora" Fishes 7, no. 6: 346. https://doi.org/10.3390/fishes7060346
APA StyleZhang, T., Yao, J., Xu, D., Lv, G., & Wen, H. (2022). Effects of Short-Term Salinity Stress on Ions, Free Amino Acids, Na+/K+-ATPase Activity, and Gill Histology in the Threatened Freshwater Shellfish Solenaia oleivora. Fishes, 7(6), 346. https://doi.org/10.3390/fishes7060346





