Abstract
Sexual maturation of post-smolts is a concern for Atlantic salmon producers, and its occurrence is dependent upon factors such as water temperature and energy availability, among others. The present study was designed to investigate the effect of different temperatures and feeding regimes on testis development and local regulation of spermatogenesis in salmon post-smolts. A 3 × 2 factorial design was used, with three temperatures (8 °C, 12.5 °C, and 18 °C) and two feed regimes (100% and 67% ration). Salmon (1800 parr, initial mean weight 23.1 ± 7.2 g) were reared from 28 October 2018 to 30 May 2019 in a freshwater flow-through system under continuous light (LD24:0), except for a 5-week winter signal (LD12:12) introduced on 4 February 2019. Testis histology, transcription of follicle-stimulating hormone receptor (fshr) and luteinizing hormone receptor (lhr), and transcription of testis proteins involved in spermatogenesis regulation such as gonadal-soma-derived factors 1 (gsdf1) and 2 (gsdf2), anti-Müllerian hormone (amh), and insulin-like growth factor-3 (igf3), were analyzed. Results showed that high temperature alone (18 °C), irrespective of the feed regime, promoted early presence of type B spermatogonia and reduced transcript levels of the proliferation-inhibitory factor amh in males still considered immature, of groups 18–100% and 18–67% that later matured in high proportion (~100%). This effect was also present to some degree in the group 12.5–100% (40% maturation), and absent in 12.5–67%, 8–100%, and 8–67% (groups with little or no maturation). Later, at onset of rapid testis growth, high temperature was linked to a pronounced downregulation of amh and of the self-renewal factors gsdf1 and gsdf2, as well as to a pronounced upregulation of the proliferation-stimulating factor igf3. Overall, the present findings demonstrate that rearing salmon at high temperatures can stimulate an early activation of the brain–pituitary–gonad axis before actual onset of rapid testis growth, enhancing and accelerating the mechanisms that control initiation and progression of maturation, while the feed regime has a minor impact. This poses a challenge for current salmon aquaculture practices that use sustained high temperature to maximize growth, since these practices increase the risk of early post-smolt maturation.
1. Introduction
Early maturation of male Atlantic salmon post-smolts, or “jacking”, is a growing issue in aquaculture due to its increasing presence under intensive rearing conditions [1,2,3]. Other male early maturing phenotypes such as mature parr or grilse have concerned salmon producers for many years [3,4,5,6,7], while jacking is a more recent phenomenon seemingly linked to the intensification of rearing conditions during the pre- and post-smolt period [1,8,9,10,11]. Production challenges linked to early maturation include a reduction in growth rates [7,12,13], immunity impairment [13,14,15], and issues with hypo-osmoregulatory abilities [16,17,18,19], which altogether can impact fish welfare and cause economic losses [1,13].
Sexual maturation is influenced by external and internal factors, including photoperiod changes, water temperature, access to feed, growth rate, energy, body size, or genetics, among others [3,20,21]. These factors are all manipulated under intensive aquaculture conditions, which can induce an earlier engagement in sexual maturation. For example, the use of high temperatures [22,23,24], a constant photoperiod [10], and high access to energy [6,24] accelerate the salmon growth rate and development, advancing the time at which physiology of males is ready for maturation. High temperature also seems to induce an early activation of the reproductive axis [9,10,11,25,26]. On the other hand, a change in the photoperiod, specifically the increasing day length after a winter period, acts as an environmental signal that entrains the initiation of key developmental processes, such as sexual maturation [9,13,21,27,28] and smoltification [29,30,31,32]. In intensive aquaculture facilities, manipulation of these factors (use of high temperatures, photoperiod manipulation, continuous supply of high-energy feed, fast growth, large post-smolt size) can thus stimulate an early commitment to sexual maturation in male salmon.
Onset of maturation starts with the activation of the BPG axis upon the adequate combination of internal and external factors. This information is integrated in specific areas of the brain, such as the KISS area of the hypothalamus [33,34], that stimulate the pituitary to produce the gonadotropins follicle-stimulating hormone (Fsh) and luteinizing hormone (Lh) [35,36,37]. Gonadotropins bind to their receptors in the testes (Fshr and Lhr), inducing steroidogenesis in Leydig cells, and together with steroids such as 11-Ketotestosterone (11-KT), regulating the different phases of testis maturation or spermatogenesis [13,37]. Spermatogenesis is a complex process by which diploid germ cells or spermatogonia proliferate, differentiate, and end up producing mature haploid spermatozoa [37,38]. The proliferation and differentiation of germ cells is regulated, among others, by a series of growth factors produced by somatic Sertoli cells [36,39,40]. These cells are in close contact with germ cells, providing nutritional, structural, and regulatory support [37,41]. The regulatory growth factors secreted by Sertoli cells include members of the TGF-β superfamily such as anti-Müllerian hormone (Amh) and gonadal-soma-derived factors 1 (Gsdf1) and 2 (Gsdf2), and of the IGF pathway such as insulin-like growth factor-3 (Igf3) [37,42,43,44].
Each of these Sertoli cell growth factors plays a specific role during spermatogenesis. Thus, Amh impairs germ cell proliferation and differentiation when the fish is immature, permitting only germ cell self-renewal and acting as negative feedback for steroidogenesis [36,40,44,45,46,47]. In immature fish, Gsdf1 and Gsdf2 also participate in the regulation of germ cell self-renewal and in the initial mitotic proliferation of type A undifferentiated spermatogonia [42,48,49,50]. At the onset of maturation, increased Fsh stimulation from the pituitary induces steroidogenesis in Leydig cells and a decrease in Sertoli cell production of Amh, Gsdf1, and Gsdf2, thus allowing commitment to spermatogonial proliferation [36,39]. Further Fsh and 11-KT stimulation after the reduction in Amh triggers the production of the proliferation-inducing Igf3. This factor, found only in fish [40,51,52], accelerates spermatogenesis until entry into meiosis [36,40,45]. From here, the meiotic phase is characterized mainly by increases in production of the gonadotropin Lh and progestins [13,35,37], which regulate the final stages of spermatogenesis until the release of mature spermatozoa.
Sustained high temperature and continuous access to feed can act as stimulatory conditions for an early activation of the BPG axis in male salmon [11], but knowledge on the physiological changes induced in the testes by these conditions that lead to spermatogenesis is limited. In a previous study that focused on gonadotropin and steroid regulation of maturation at different temperatures and feed regimes, it was concluded that high temperature caused an early activation of the BPG axis and led to a high proportion of early maturing males irrespective of the feed regime [11]. However, the effects of these conditions on regulation of spermatogenesis were not analyzed. High temperature is believed to accelerate spermatogenesis, although studies on testis development at different temperatures have been performed mostly on warm water species such as Nile tilapia Oreochromis niloticus [53,54] or Astyanax altiparanae [55]. Thus, further research on changes occurring during spermatogenesis in response to different temperatures in temperate species such as Atlantic salmon is needed. Similarly, little is known on how different feed regimes may alter testis regulation of spermatogenesis. Generally, previous studies have found that using feed restrictions ranging between 43% and 67% of a 100% ration decreases the proportion of males maturing early [3,11,39,56]. However, the changes induced on endocrine mechanisms regulating spermatogenesis have not been consistent. While some of the studies found that restricted feeding reduced transcript levels of maturation-promoting factors such as pituitary fshb [11,56], others have reported an unexpected compensatory response in plasma 11-KT concentration, pituitary fshb, and in testis amh and igf3 transcript levels in salmon that matured under restricted feeding [39]. Thus, these authors concluded that salmon maturing under caloric restriction may require a greater upregulation or downregulation of factors promoting (11-KT, fshb, and igf3) or inhibiting (amh) sexual maturation, respectively.
The present study was designed to understand how the combination of different temperatures and feed regimes affect the regulatory mechanisms in testis that control the progression of spermatogenesis in salmon, resulting in different proportions of male post-smolts maturing early. For that purpose, we assessed the transcriptional response of various testis genes in immature and early maturing post-smolts reared under different conditions of temperature and access to feed. This was performed using testis samples collected during the experiment described in [11].
2. Materials and Methods
2.1. Experimental Setup
The experimental setup of this study has been previously described in [11]. Briefly, it consisted of a 3 × 2 factorial design with 3 temperatures (8 °C, 12.5 °C, and 18 °C) and 2 feed regimes (100% ration and a 67% ration). The 67% feed regime was provided by fasting every third day in an attempt to minimize the establishment of dominance hierarchies or aggression. Experimental conditions produced six experimental groups (18–100%, 18–67%, 12.5–100%, 12.5–67%, 8–100%, 8–67%) that were reared in duplicate from 27 September 2018 to 30 May 2019 in freshwater (see Figure 1 in [11]). All groups were kept under constant light (LD24:0), except for a five-week LD12:12 winter signal starting 4 February 2019.
2.2. Samplings
The sampling procedure is described in detail in [11]. Eight samplings were performed during the experiment. In each of them, n ≥ 12 males were collected per experimental group to assess the proportion of males maturing early. Fish were sacrificed with an overdose of benzocaine by bath (Benzoak vet.® 20%, ACD Pharma AS, Norway) higher than 50 mg/L, weighed to the nearest 0.1 g, and dissected to determine the sex and degree of maturation. Only males were kept for further analysis. Testes were removed and weighed to the nearest 0.001 g. One testis was kept in RNAlater® for 24 h, at 4 °C, and stored at −80 °C until analysis. The other testis was placed in paraformaldehyde (4%) for fixation and subsequent histology analysis. A subsample of n = 3 individuals per experimental group and sampling was used for histology (n = 144). A subsample of 432 individuals from all samplings and treatments was used for gene transcription analysis.
2.3. Lab Analyses
2.3.1. Testis Histology
Histology was performed on 144 fixed testis samples (n = 3 samples per experimental group and sampling). Fixed testes were processed in a Thermo Scientific Excelsior tissue processor (Thermo Scientific, Boston, MA, USA) and embedded in paraffin Histowax (Histolab, Askim, Sweden) using a Tissue–Tek, TEC 5 (Sakura, Alphen aan den Rijn, The Netherlands) embedding center. Embedded tissue was sectioned at 1.5–2 µm using a Leica RM 2255 Microtome (Leica microsystems, Buffalo Grove, IL, USA), and sections were mounted on glass slides and stained with hematoxylin–eosin (HE). Stained slides were scanned in an Aperio ScanScope® AT Turbo slide scanner and read using Aperio ImageScope® (Leica microsystems) (magnification: 10×). Each slide was scored from 1 to 6 depending on testis developmental stage, following a method modified from [8].
2.3.2. Gene Transcription Analyses in Testis
Real-time quantitative PCR (RT-qPCR) was used to analyze transcription of testis genes fshr, lhr, gdsf1, gsdf2, amh, and igf3. Total RNA from large testis samples (n = 276) was extracted using the QIAsymphony SP automated nucleic acid extraction robot (Qiagen, Hilde, Germany) with the QIAsymphony RNA kit (Qiagen), following the manufacturer’s protocol. A total of 25 mg of tissue was homogenized with RLT plus lysis buffer (Qiagen) in a Precellys 24 Tissue Homogenizer (Bertin technologies, Versailles, France). Total RNA from small testis samples (n = 156) was extracted following a standard protocol [57] with TRI reagent (Invitrogen, Carlsbad, CA, USA) to ensure sufficient RNA yield. The total RNA concentration (ng·µL−1) was measured with the Qubit RNA BR assay kit (ThermoFisher Scientific, MA, USA) in a Qubit 3.0 Fluorometer (ThermoFisher Scientific). Total RNA purity was measured using the NanoDrop One microvolume UV-Vis spectrophotometer (ThermoFisher Scientific). The ratio of absorbance for samples was ~2 for 260/280 nm (A260/280) and 260/230 nm (A260/230), indicating sufficient purity. Purified RNA was thereafter stored in a −80 °C freezer until cDNA synthesis.
Total RNA (300 ng) was reverse-transcribed to cDNA using SuperscriptTM III Reverse Transcriptase and Oligo(dT)20 Primer (InvitrogenTM, ThermoFisher Scientific, USA) according to the manufacturer’s instructions. Gene transcription was analyzed in a Bio-Rad CFX96 Touch Real-Time PCR system (Bio-Rad Laboratories, Hercules, CA, USA) using iTaq™ Universal SYBR® Green Supermix (Bio-Rad, USA) in a total reaction volume of 12.5 μL per well, including 2.5 μL of 1:60 diluted cDNA and 0.25 μM of each primer. All pipetting was performed using the Hamilton Microlab STARlet pipetting robot (Hamilton, NV, USA). The RT-qPCR protocol consisted of 3 min at 95 °C followed by 35 cycles at 95 °C for 15 s and 60 °C for 1 min, including a melting curve analysis section at the end. Samples were run in duplicate with the oligos listed in Table 1. Duplicates with a CV > 1.5% were eliminated in genes displaying a higher expression (gsdf1, gsdf2, and amh), while this CV threshold was increased to 2.5% in genes with a lower expression (fshr, lhr, and igf3). All oligos were validated for efficiency and optimal dilution by running two-fold dilution series using a pooled cDNA sample that included fish from all treatments and in all stages of maturation. The efficiency (E) was estimated with the formula: E = 10(−1/slope), obtaining the slope from the plot of the log cDNA concentration of the pool versus the threshold cycle (Ct). After RT-qPCR, the relative transcription of the genes was calculated with the efficiency-corrected method using ef1a as the reference gene [58]. The specificity of each primer pair was corroborated by sequencing. For each primer pair, PCR was carried out in a 25 μL volume, including 0.25 mM of dNTPs, 0.2 μM of each primer, and 1.25 U/50 μL PCR of Taq DNA polymerase (New England Biolabs, Ispwich, MA, USA) and 1 μL of PCR product as a template. PCR was conducted on a C1000TM Thermocycler (Bio-Rad, Hercules, CA, USA) with an initial denaturation at 95 °C for 30 s, 35 cycles of 95 °C for 15 s, 60 °C for 20 s, 68 °C for 20 s, and a final extension step of 5 min at 68 °C.

Table 1.
Oligo sequences used for RT-qPCR during gene transcription analysis on testis samples. F = forward primer, R = reverse primer.
PCR products were directly sequenced with the forward and the reverse primer in separate reaction mixtures using the BigDye terminator 3.1 in an Applied Biosystem 3730XL capillary sequencer (Applied Biosystems, Foster City, CA, USA) at the University of Bergen sequencing facility. The obtained sequences were manually edited and cleaned using FinchTV (Geospiza, Seattle, WA, USA) and compared with original deposited sequences in the GeneBank by alignment in MEGA5.2 [61]
2.4. Statistical Analyses
The gonadosomatic index (GSI) was calculated by Pino Martinez et al. in [11] as: GSI (%) = Gonad weight*100/Body weight. A GSI > 0.06% was used as the threshold to identify maturing males and to determine the proportion of maturation per group and sampling (see Figure 4 in [11]). Differences in mean GSI between testis developmental stages were assessed with a Kruskal–Wallis test, and pairwise comparisons were performed using a Wilcoxon rank sum test with continuity correction. An ordinal logistic regression was fitted between median “testis developmental stage” (response) and “temperature”, “feed regime”, “time”, and the interaction between temperature and feed regime (predictors). Linear mixed effects models (LME) were fitted between gene transcription data and the predictors “temperature”, “feed regime”, “time”, and their two-way interactions as fixed effects, and “tank” as a random effect, to estimate the random variance caused by tank replicates (see Table 2). Prior to fitting LMEs, the distribution and existence of outliers in the response was checked with the Shapiro–Wilks test and boxplots, respectively. After fitting the models, residuals plots were checked to assess normality (q-q plots), linearity (residuals versus fitted plots), homogeneity of variance (scale–location plots), and influential outliers (Cook’s distance). Homogeneity of variance was also checked with Levene’s test. If data did not meet the model assumptions, the response variable was log-, square root-, or inverse-transformed (Table 2), the model was repeated, and the assumptions were re-checked. Tukey’s HSD post-hoc tests were run to find significant differences in the response variable between pairwise groups at each sampling, and within experimental groups overtime. Plots of gene transcription data over time for each treatment display mean ± standard error. A significance level of α = 0.05 was always used. All statistical analyses were performed in R and Rstudio, using the packages “car” [62], “ggplot2” [63], “ggpubr” [64], “Rmisc” [65], “emmeans” [66], “nlme” [67], and “ordinal” [68].

Table 2.
Model used, percentage of total model variance identified as random variance caused by tank replicates, and transformations of the response variable applied. All models were fitted versus temperature, photoperiod, time, and their resulting two-way interactions.
3. Results
3.1. Testis Histology and Developmental Stage
After histological examination, testes were classified in one of six developmental stages following a system adapted from Fjelldal et al. in 2018 [8]. Figure 1 displays examples of the six developmental stages and provides a brief description of each. Figure 2 displays the relationship between these six stages and the GSI observed. GSI increased significantly with testis developmental stage (p < 0.001). GSI was the lowest in stage 1, increased in stage 2, and remained similar in stage 3 (although with some variability). Afterwards, GSI significantly increased with each of the remaining developmental stages (Wilcoxon rank sum tests with continuity correction, all p < 0.05).
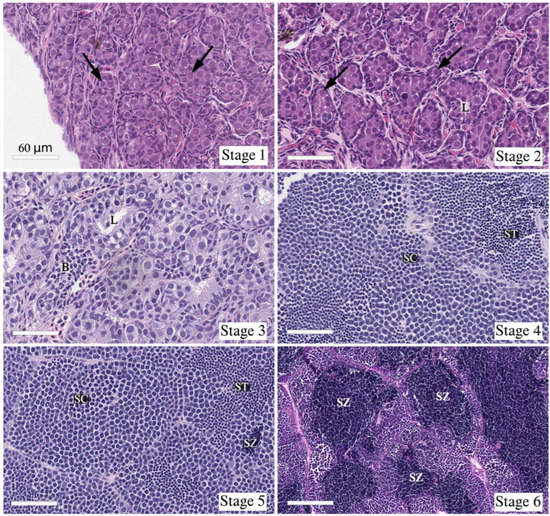
Figure 1.
Examples of testis morphology observed after histology inspection, illustrating the six testis developmental stages. All images are in ×40 magnification. The clear rectangle at the bottom-left corner of the six images represents a length of 60 μm. Developmental stages are characterized by: Stage 1—Type A spermatogonia as furthest developed germ cell type (black arrow); Stage 2—Type A spermatogonia (black arrow) as furthest developed germ cell type and clear formation of tubule luminae (L); Stage 3—Type A and B spermatogonia (B) as furthest developed germ cell type and clear formation of tubule luminae (L), but the majority of germ cells are type A spermatogonia; Stage 4—Spermatocytes (SC) and spermatids (ST) as the furthest developed germ cell types, no presence of spermatozoa; Stage 5—Spermatozoa (SZ) as the furthest developed germ cell type in some tubules, but not dominating; Stage 6—Large numbers of spermatozoa (SZ) in most tubules.
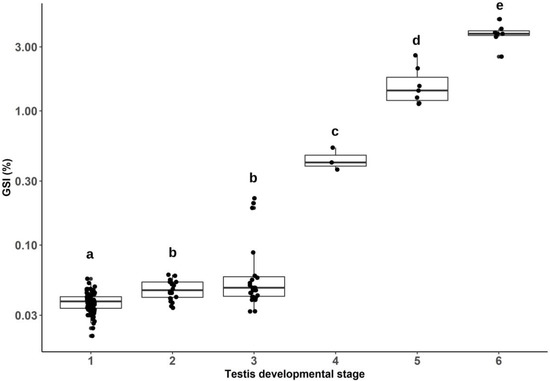
Figure 2.
Boxplots of the gonadosomatic index (GSI) for each testis developmental stage. Each of the boxplots includes a scatter plot to display the number of samples included and their GSI values. Significant differences in GSI between developmental stages were assessed with a Kruskal–Wallis test, followed by pairwise comparisons with a Wilcoxon rank sum test with continuity correction. Different letters indicate significant differences in mean GSI (p < 0.05).
Figure 3 displays the median testis developmental stage over time for each temperature (n = 6 fish per group and sampling). Since feed ration was not significant in the model, data from both feed regimes within each temperature were pooled in the graph to improve visualization. Output from the ordinal logistic regression model (Table 3) revealed that the testis developmental stage was significantly dependent only on temperature (p < 0.001) and time (p < 0.001). The study of the odds ratios displayed that temperature had a large impact on the developmental stage at which testis samples were found. Thus, testes at 18 °C were 2375 times more likely to be at a more advanced developmental stage than testes at 8 °C for a given feed regime and sampling (model output in Table 3).
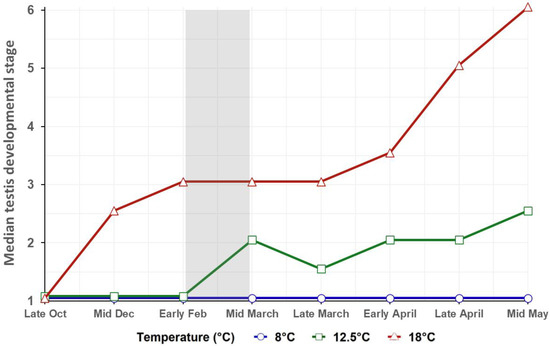
Figure 3.
Median testis developmental stage at each sampling and temperature based upon testis histological image analysis. N in each sampling point is 6 testis samples per temperature group. Scores were based upon [8], depending on the furthest developed germ cell types observed (see caption in Figure 1). Feed regime is not displayed for better visualization and because an ordinal logistic regression revealed that the feed regime had no significant effect on testis developmental stage. The grey-shaded area shows the duration of the LD12:12 winter signal.

Table 3.
Model coefficients, estimates, p-values, odd ratios, and significance of model terms of the ordinal logistic regression with the formula presented below. Asterisks in the last row indicate the significance of the model terms, and are displayed as follows: (*) p-value < 0.05, (**) p-value < 0.01, and (***) p-value < 0.001.
3.2. Gene Transcription of fshr and lhr
Testis fshr transcript levels (Figure 4A) were significantly dependent upon temperature (p < 0.001), time (p < 0.001), and the interaction temperature × time (p < 0.01). Within each feed regime, fshr transcription was generally higher at 8 °C than at higher temperatures, before the winter signal under the 67% ration, and after the winter signal under the 100% ration. Between feed regimes, the only significant difference in fshr transcription was observed between 8–67% and 8–100% in late October (p < 0.01). Over time, significant decreases in fshr transcript levels occurred in all groups, but especially after the winter signal at 18 °C. Decreases commenced earlier in the two 8 °C groups since the baseline transcription of fshr before the winter signal was higher.
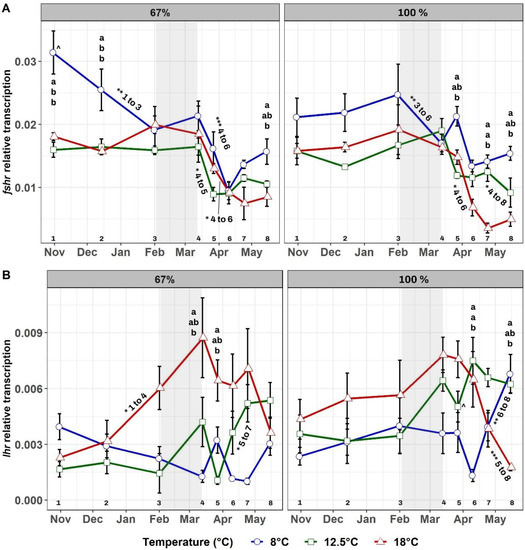
Figure 4.
Mean testis fshr (A) and lhr (B) transcription over time in the six experimental groups. Feeding regimes are displayed separately, with the 67% groups shown on the left and the 100% ration groups on the right. The grey-shaded area shows the duration of the LD12:12 winter signal. Letters “a” and “b” indicate significant differences (p < 0.05) between groups within a feeding regime for a given time. Signs “^” indicate significant differences (p < 0.05) between groups reared at the same temperature but at different feeding regimes at a given time, and they are located next to the largest value of the pair. Numbers from 1 to 8 at the bottom of the graphs represent the sampling number and aid with an explanation of significant differences over time within each group. These are displayed with asterisks as follows: (*) p-value < 0.05, (**) p-value < 0.01, and (***) p-value < 0.001. Asterisks are located next to the corresponding line and are followed by the sampling numbers between which such significant difference occurred.
Testis lhr transcript levels (Figure 4B) were significantly dependent on temperature (p < 0.01), feed regime (p < 0.05), time (p < 0.01), and the interaction temperature × time (p < 0.01). Within each feed regime, lhr transcription was generally higher at 18 °C before and during the winter signal, but not after. Between feed regimes, the only significant difference occurred in late March between 12.5–100% and 12.5–67% (p < 0.01). Over time, a pattern of increase in lhr transcription was observed in all groups, but its occurrence was delayed in time at lower temperatures (see Figure 4B). However, only the 18 °C groups showed a sudden decrease in lhr transcription after the winter signal, which was only significant in fish fed the 100% ration.
3.3. Gene Transcription of gdsf1 and gsdf2
Testis gdsf1 transcript levels (Figure 5A) were significantly dependent upon temperature (p < 0.001), time (p < 0.001), and the interaction temperature × time (p < 0.001). Within each feed regime, differences in gsdf1 transcription between temperature groups occurred mostly after the winter signal, with significantly lower transcript levels of this gene at 18 °C, especially in the 100% regime. Some minor differences were observed between groups at the same temperature but at different feed regimes (8–100% and 8–67% in December, 12.5–100% and 12.5–67% in late March, and 18–100% and 18–67% in mid-May), however these did not alter the similar trends in transcription observed at each feed regime. Over time, all groups displayed a decrease in gsdf1 transcription, but this was more pronounced and significant primarily in the two 18 °C groups, as well as in the two 12.5 °C groups, all after the winter signal.
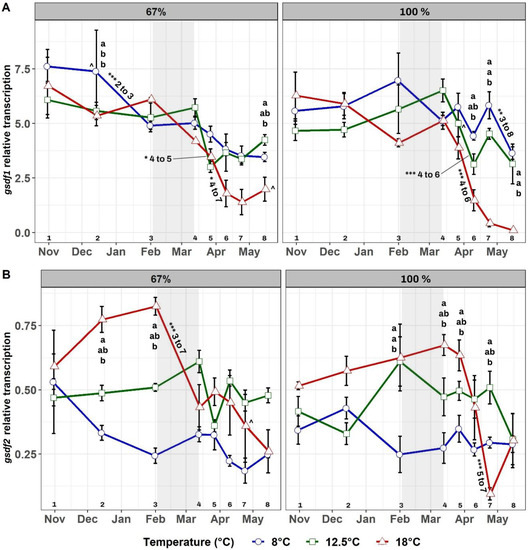
Figure 5.
Mean testis gsdf1 (A) and gsdf2 (B) transcription over time in the six experimental groups. Feeding regimes are displayed separately, with the 67% groups shown on the left and the 100% ration groups on the right. The grey-shaded area shows the duration of the LD12:12 winter signal. Letters “a” and “b” indicate significant differences (p < 0.05) between groups within a feeding regime for a given time. Signs “^” indicate significant differences (p < 0.05) between groups reared at the same temperature but at different feeding regimes at a given time, and they are located next to the largest value of the pair. Numbers from 1 to 8 at the bottom of the graphs represent the sampling number and aid with an explanation of significant differences over time within each group. These are displayed with asterisks as follows: (*) p-value < 0.05, (**) p-value < 0.01, and (***) p-value < 0.001. Asterisks are located next to the corresponding line and are followed by the sampling numbers between which such significant difference occurred.
Testis gdsf2 transcript levels (Figure 5B) were significantly dependent on temperature (p < 0.001), time (p < 0.001), and the interaction temperature × time (p < 0.001). Within each feed regime, gsdf2 transcription was generally higher at 18 °C before the winter signal (especially in the 67% ration group), decreasing remarkably in both groups after the winter signal. Differences between groups at the same temperature but at different feed regimes were observed only in late April between 18–100% and 18–67%. Over time, transcription of this gene remained stable in groups at 8 and 12.5 °C, while both groups at 18 °C displayed a pronounced decline in transcript levels after the winter signal.
3.4. Gene Transcription of amh and igf3
Testis amh transcript levels (Figure 6A) were significantly dependent upon temperature (p < 0.001), feed regime (p < 0.05), time (p < 0.001), and the interactions temperature × feed regime (p < 0.01) and temperature × time (p < 0.01). In each feed regime, amh transcript levels were highest at 8 °C and lowest at 18 °C throughout the experiment, with these differences being already present during the early period pre-winter signal but emphasized after the winter signal. Differences between groups at the same temperature but at different feed regimes were observed only in late January and mid-May between 8–100% and 8–67%, and in mid-May between 18–100% and 18–67%. Over time, the most pronounced decreases in amh transcription occurred in both 18 °C groups after the winter signal, as well as in 12.5–100% during the same period but with higher variability. Significant decreases were also observed in the rest of the groups over time (12.5–67%, 8–100%, 8–67%), but these occurred before the winter signal and commenced from higher baseline levels of transcription than in the previous three groups.
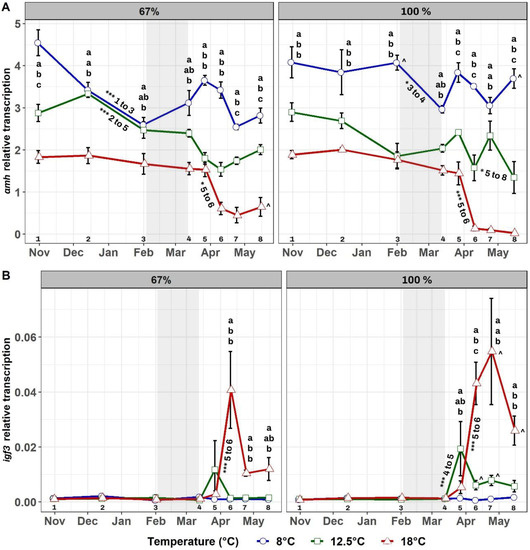
Figure 6.
Mean testis amh (A) and igf3 (B) transcription over time in the six experimental groups. Feeding regimes are displayed separately, with the 67% groups shown on the left and the 100% ration groups on the right. The grey-shaded area shows the duration of the LD12:12 winter signal. Letters “a”, “b”, and “c” indicate significant differences (p < 0.05) between groups within a feeding regime for a given time. Signs “^” indicate significant differences (p < 0.05) between groups reared at the same temperature but at different feeding regimes at a given time, and they are located next to the largest value of the pair. Numbers from 1 to 8 at the bottom of the graphs represent the sampling number and aid with an explanation of significant differences over time within each group. These are displayed with asterisks as follows: (*) p-value < 0.05 and (***) p-value < 0.001. Asterisks are located next to the corresponding line and are followed by the sampling numbers between which such significant difference occurred.
Testis igf3 transcript levels (Figure 6B) were significantly dependent upon temperature (p < 0.001), feed regime (p < 0.001), time (p < 0.001), and the interactions temperature × time (p < 0.001) and feed regime × time (p < 0.001). In each feed regime, igf3 transcription was similar before the winter signal at all temperatures, but afterwards higher levels were observed in both 18 °C groups than in the rest. Significant differences between groups at the same temperature but at different feed regimes were present in middle and late April between 12.5–100% and 12.5–67%, and in late April and mid-May between 18–100% and 18–67%. Over time, pronounced increases in igf3 transcript levels occurred only in the two 18 °C groups after the winter signal, but a less intense increase in transcription of this gene was also present in 12.5–100% during the same period. No significant changes were observed over time in the remaining three groups (12.5–67%, 8–100%, 8–67%).
4. Discussion
Water temperature and energy availability are some of the factors that can influence the occurrence of male post-smolt maturation, a phenomenon undesirable in aquaculture facilities due to its negative implications on fish growth and welfare. In a previous study of ours [11], high temperature (18 °C) led to an early stimulation of the BPG axis in Atlantic salmon and acted as the main factor determining early commitment to sexual maturation as post-smolt. In contrast, the feed ration only had a temperature-dependent modulatory effect. Findings in the present study, which is part of the same experimental setup, complement and support those conclusions. We have now observed clear evidence of early proliferation and differentiation of germ cells in testes of salmon reared at 18 °C, followed by a synchronized and coordinated transcriptional response of stimulatory and inhibitory Sertoli cell growth factors and gonadotropin receptors that, together with gonadotropins and sex steroids, orchestrate the regulation of spermatogenesis.
4.1. High Temperature Stimulated the Early Presence of Type B Spermatogonia
In our previous study [11], males at 18 °C in both feed regimes displayed comparatively higher GSI and pituitary fshb transcription in December–January, when they were still immature according to the GSI threshold used. This evidenced a certain degree of activation in the BPG axis of immature males in response to high temperature and is consistent with present findings. We now observed that water temperature was the main determinant for the testis developmental stage of salmon, with individuals at 18 °C being much more likely to have developed testes to a more advanced stage than salmon reared at 12.5 or 8 °C, and the feeding regime had little effect. Indeed, testis developmental stage of salmon at 18 °C in December–February was between stages 2 and 3. These stages are characterized by the presence of type B spermatogonia as the furthest developed germ cell type [8], or even spermatocytes [69]. According to the review by Schulz et al. in 2010 [37], reaching early type B spermatogonia usually entails irreversible commitment to maturation, and from here spermatogonia divide more rapidly until reaching meiosis. Similarly, in [26], Melo et al. stated that a lower number of type A undifferentiated spermatogonia and an increased number of type A differentiated, as well as the presence of type B spermatogonia, were indicative of being recruited into sexual maturation. Consequently, in our study, the presence of type B spermatogonia at 18 °C between December and February implies that spermatogonial proliferation and differentiation had to some extent commenced and progressed. This suggests that the majority of males at 18 °C were experiencing preparatory changes for testis maturation before its actual onset, and these early changes probably resulted in the high proportion of maturing males (~100%) at the end of the experiment (see Figure 4 in [11]). The lower testis developmental stages observed over time at 12.5 and 8 °C are fully consistent with the claimed primary stimulatory role of temperature on proliferation and differentiation of germ cells during spermatogenesis. Furthermore, this conclusion is aligned with previous studies on environmental control of early maturation, many of which reported a stimulatory effect of a high water temperature on the activation of gonadotropin and sex steroid production, that later resulted in a high percentage of individuals maturing early as post-smolts [9,10,11,26].
The mechanisms by which a high water temperature could induce the advancement in spermatogonial development are not clear. As ectothermic organisms, fish experience remarkable physiological, endocrine, and metabolic changes when exposed to different water temperatures, and this includes adaptations in the reproductive axis [13,22]. For example, the duration of spermatogenesis in fish depends on the water temperature, with high temperatures within the normal thermal tolerance range of the species accelerating the process and reducing its duration [53,54,55]. In Atlantic salmon, initiation of spermatogenesis is concomitant with increases in the water temperature and photoperiod occurring in the spring/summer [28], and thus it is likely that exposure to a sustained high temperature in our study stimulated entering the first phase of spermatogenesis. This phase is characterized by a rapid mitotic proliferation of germ and Sertoli cells [37] through a fixed number of mitotic divisions (6–8 in Salmoniformes according to [53]), before formation of the spermatocytes that enter meiosis. The speed of this mitotic proliferation as well as the following meiotic phase has been found dependent upon temperature in tilapia Oreochromis niloticus [54] and in the tropical fish Astyanax altiparanae [55]. Similarly, our study also documents an advanced rate of spermatogenesis in response to high temperature but in a temperate species. These results have clear implications for salmon aquaculture. In modern rearing facilities where water temperature is artificially maintained very high over time, the resulting environment can represent a strong stimulation to the early enhancement of the initiation and development of the proliferative phase.
The presence of relatively advanced germ cells such as type B spermatogonia in testis of individuals that had GSI typical of immature fish needs to be justified. At the onset of spermatogonial proliferation, the cyst volume increases due to germ and Sertoli cells’ mitotic divisions, resulting in the observed increase of GSI as fish mature. However, this increase can be initially slow due to the gradual decrease in germ cell volume as they divide [54]. This entails that the first 2–3 mitotic divisions at the onset of spermatogenesis could be associated only to a small increase in testis volume and GSI (as shown in Figure 2 in [54] for tilapia). Our results are consistent with this hypothesis, with testis presenting type B spermatogonia only displaying a slight but significant increase in GSI (see Figure 5C in [11]). This means that sustained exposure to a high temperature probably stimulated a switch from basic germ cell self-renewal processes to early differentiation into type A differentiated and type B, even in fish that displayed low GSI and therefore were still considered as immature.
4.2. Temperature-Dependent Regulation of Testis Development before and during Maturation
The presence of type B spermatogonia in December–January in immature salmon at 18 °C cannot be explained by changes in transcription of gonadotropin receptors fshr and lhr, or in factors gsdf1 and igf3. This is because, generally, transcription of these four genes during that early period was similar in all treatments. However, this early presence of type B spermatogonia was linked to significantly lower transcript levels of testis amh and to higher levels of gsdf2 at 18 °C. Thus, it is likely that the lower baseline transcription of amh and higher transcription of gsdf2 in response to high temperature contributed to the early presence of type B spermatogonia in December–January in salmon that were still immature. Since Amh acts as an inhibiting factor for steroidogenesis [46,47] and spermatogonial proliferation [36,39,40,45], the lower transcription of amh probably removed limitations to allow a degree of early germ cell proliferation and differentiation, while higher levels of gsdf2 may have contributed to an initial early differentiation of type A differentiated spermatogonia, thus preparing the testes for further development. Most likely, these transcriptional changes inducing early testis development highly contributed to the large proportion of maturing males at 18 °C by the end of the experiment.
Afterwards, all major transcriptional changes took place in March after the winter signal, simultaneous to the increase in the most relevant indicators of initiation of sexual maturation such as pituitary fshb transcription, plasma 11-KT, and GSI (see [11]). This suggests that it was the switch in photoperiod from a short to a long day which acted as zeitgeber that synchronized the initiation of sexual maturation [9,27], as also suggested in [11]. The patterns of gene transcription observed are consistent with the current knowledge on the reproductive physiology of teleosts, which considers Fsh the hormone that, upon neuroendocrine control [13,33,34], initiates and orchestrates spermatogenesis [36,37,39,40,45]. The receptor fshr is often clearly expressed in immature testes before a rise in Fsh at the onset of maturation, thus being ready to respond to its ligand [36,37]. Accordingly, in our study we found fshr clearly and similarly transcribed in all groups before the winter signal, and it only decreased after returning to constant light when fshb increased in most groups (see [11]). This suggests that changes in fshr transcription are dependent upon interactions with the ligand (Fsh) rather than on environmental conditions, thus explaining the decrease in fshr observed in most groups as fshb increased. Before this interaction occurs (immature fish), amh is upregulated in Sertoli cells to inhibit steroidogenesis in Leydig cells [46,47] and impair further germ cell proliferation [36,39,40,45]. Simultaneously, gsdf1 and gsdf2 in Sertoli cells of immature fish are considered to participate only in the self-renewal of germ cell and type A undifferentiated spermatogonia [48,50,70], but not further. According to this, in immature salmon, a higher transcription of amh, gsdf1, and gsdf2 can be expected, while in salmon initiating maturation, transcription of these genes is expected to decrease. The transcriptional patterns of these genes observed in our maturing groups in March are fully aligned with this hypothesis.
At the onset of maturation, Fsh stimulation induces steroidogenesis in Leydig cells, resulting in an increase in plasma 11-KT concentration, and also a downregulation of amh in Sertoli cells permitting the initiation of spermatogonial proliferation and differentiation [36,37]. As mitotic proliferation is initiated, self-renewal of germ cell and type A undifferentiated spermatogonia is no longer necessary and thus gsdf1 and gsdf2 are also downregulated in Sertoli cells [48,50,70]. This is consistent with the increases we observed in pituitary fshb transcription and plasma 11-KT concentration [11], simultaneous to the downregulation of testis fshr, amh, gsdf1, and gsdf2, all in the maturing groups at 18 °C after the winter signal. Progression of spermatogenesis is highly stimulated by an Fsh-mediated upregulation of igf3 in Sertoli cells, a factor found only in fish that further accelerates germ cell proliferation until reaching meiosis [36,39,40,45]. Consequently, an upregulation of this growth factor can be expected in sexually maturing individuals. Indeed, an upregulation of igf3 was observed in the groups containing early maturing males in April, simultaneous to the rapid onset of spermatogenesis and to the downregulation of amh, gsdf1, and gsdf2. This adds further evidence to the enhancing effect of high temperature on the speed and intensity of transcriptional changes occurring in Sertoli cells that induce early maturation. However, despite the clear stimulatory role of Igf3 on testis development, this hormone could not contribute to the early presence of type B spermatogonia at 18 °C from December to the onset of maturation in late Mach. This is because during that period, igf3 transcription was similar in all groups, further evidencing that the early presence of type B spermatogonia must have been linked to the downregulation of amh and to some extent to the increased levels of gsdf2 transcription.
In summary, our results for both maturing and non-maturing groups are very consistent with the current knowledge on endocrine mechanisms regulating spermatogenesis, and with the final proportions of maturation reported in each experimental group in our first study [11]. The coordinated orchestration of these regulatory processes allows germ cell proliferation and differentiation over time, promoting the advance through the different stages of development until final spermiogenesis and the release of mature spermatozoa [37,38]. The main implication of our findings is that the intensity of transcriptional changes in all genes seems primarily affected by temperature, with high temperature inducing large and intense changes in contrast to low temperature. The consequence is that rearing salmon at a high temperature can dramatically enhance mechanisms promoting sexual development and early maturation.
4.3. Possible Roles of gsdf1 and gsdf2
Gonadal-soma-derived factor (Gsdf) is expressed in Sertoli cells of many teleosts [48,50,70,71] in the form of two paralogs, gsdf1 and gsdf2 [48,72]. Gsdf regulates germ cell self-renewal upon E2 stimulation [33] and proliferation of type A undifferentiated spermatogonia [50,73], however it has also been found to inhibit that proliferation [71]. In our study, transcription of the two paralogs displayed slight differences, suggesting the possibility of some functional divergence. Both gsdf1 and gsdf2 displayed a clear downregulation at the onset of maturation, suggesting that in Atlantic salmon, neither of them plays a crucial role in germ cell proliferation beyond type A differentiated spermatogonia. This is because this downregulation occurred simultaneous to that of amh when spermatogenesis was initiated, which entails that gsdf1 and gsdf2 are not necessary to support further progression of testis development. This hypothesis is in line with previous studies that have reported a decrease in gsdf expression as spermatogenesis progresses. For example, gsdf in medaka (Oryzias latipes) was progressively downregulated in Sertoli cells that were in contact with meiotic germ cells, and was hardly detected in Sertoli cells next to spermatids or spermatozoa [70]. Similarly, in European seabass (Dicentrarchus labrax), the onset of puberty was negatively correlated with gsdf1 expression [48]. Both studies concluded that at the onset of spermatogenesis, type A undifferentiated spermatogonia stop the self-renewal process to progress towards meiosis, and consequently gsdf1 is no longer necessary and its transcription decreases. Finally, a clear decrease in gsdf1 and gsdf2 expression has been observed in rainbow trout (Oncorhynchus mykiss) testes at developmental stage II compared to stage I testes [72]. However, when fish are still immature, stimulation of proliferation of type A undifferentiated spermatogonia induced by upregulation of gsdf (1 and/or 2) may influence whether they are later recruited into sexual maturation or not. For example, seabass forced to swim has been found to display an extreme downregulation of gsdf1 and later significantly lower GSI, while resting seabass has showed significantly higher transcript levels of gsdf1 and subsequent high GSI [73]. In our study, we observed that gsdf1 transcription was very similar across all groups before the winter signal, and thus it most likely did not play a relevant stimulatory role in maturation. However, this was not the case of gsdf2, whose transcript levels before the onset of maturation showed some higher values at 18 °C in the groups that matured later. Accordingly, we could speculate that gsdf2 contributed more to the early signs of testis development observed in December than gsdf1 by stimulating the processes previously mentioned, finally resulting in a large proportion of males entering maturation. However, a more definitive conclusion in this regard requires further investigation.
4.4. Feed Ration Had a Minor Effect on the Regulation of Spermatogenesis
In our previous study, we concluded that feed regime had a minor impact on early maturation at 18 °C, but a larger impact at 12.5 °C that, however, was not sufficient to impair its initiation in all individuals fed the 67% ration [11]. The results from the current study, which found very minor effects of feed regime on testis developmental stage and transcription of fshr, lhr, gsdf1, and gsdf2, are aligned with the conclusions in the previous study. Moreover, the absence of ration-induced effects was also obvious in the two groups at 12.5 °C, which had, however, shown clear differences in the proportion of maturing males, GSI, plasma 11-KT, and pituitary fshb transcription [11]. This suggests that the regulation of gonadotropin receptors, and of the two gonadal-soma-derived factors involved in spermatogonial self-renewal, may not be highly dependent on nutritional or energy status and rather more responsive to environmental conditions, in connection with Fsh production. Similarly, the overall patterns of amh transcription, despite being significantly affected by feed regime according to our statistical model, did not vary remarkably between 67% and 100% rations at the same temperature (at 18 °C, or at 12.5 °C). Consequently, this suggests that the caloric restriction was not a major factor altering the regulation of Amh in Sertoli cells, which was mainly determined by the temperature. However, the feed regime did have a relevant impact on testis transcription of igf3 at 18 and 12.5 °C. Transcription of igf3 in each of these groups was similar to the patterns observed in pituitary transcription of fshb (Figure 6B in [11]), which links Igf3 production to an increase in Fsh at the onset of maturation. This is supported by previous studies that consider Fsh the main hormone orchestrating steroidogenesis, initiation of maturation, and the regulation of inhibitory (Amh) and stimulatory (Igf3) factors for spermatogenesis in teleosts [36,39,40,45]. Although Igf3 is considered a potent stimulatory factor during spermatogenesis, our results suggest that the “decision” or initial recruitment of salmon into maturation and its further progression is more dependent upon removing the inhibition of germ cell proliferation imposed by Amh. This was inferred after observing similar high proportions of maturing males under 67% and 100% regimes at 18 °C, as well as similar reduced amh transcription in both groups, but lower transcription of igf3 under calory restriction. It seems that a reduction in amh transcription alone is sufficient to allow spermatogenesis to commence, however an increased production of this hormone further stimulates and accelerates the process.
The lower igf3 transcription under the 67% ration at both 18 and 12.5 °C suggests that a link exists between the growth axis or the caloric availability, and the production of Igf3 in Sertoli cells. This lower transcription of igf3 under restricted feeding was also highly correlated with plasma 11-KT concentrations (Figure 6A in [11]), whose levels were also dependent on the ration. This may suggest a link between energy levels, Igf3, and sex steroid production. According to studies in Nile tilapia [74,75], Igf3 is involved in the expression of testis genes that regulate steroidogenesis. Since the production of both Igf3 and sex steroids seems affected by energy levels, the use of a relevant restricted feeding may impair fish to produce the same levels of Igf3 and sex steroids as if they were fed a full ration. This resulting lower igf3 transcription and 11-KT production may lead to a reduced number of males initiating maturation, as we indeed found in our study. However, other studies seem in conflict with this hypothesis. For example, a clear upregulation of igf3 and higher levels of 11-KT were observed in maturing Atlantic salmon subjected to a large feed restriction (43% of a complete ration), in contrast to those maturing under the 100% ration [39]. As a result, these authors concluded that maturing Atlantic salmon under restricted feeding were displaying a compensatory larger activation in the BPG axis. Considering the reduced availability in nutritional resources, restrictively fed fish needed a greater activation of stimulatory factors for sexual maturation such as Igf3 and 11-KT to complete maturation. Further research is needed to further clarify the mechanisms by which caloric restrictions modulate the decision to mature, and how it affects the production of stimulatory factors such as Igf3 and 11-KT, which are most likely dependent upon energy availability.
5. Conclusions
Rearing salmon at a high water temperature resulted in an early proliferation and differentiation of germ cells. This early effect was noticeable in the groups 18–100% and 18–67% before the actual onset of sexual maturation and was characterized by the presence of type B spermatogonia and reduced transcript levels of the inhibitory factor amh. Clear changes in the transcription of all factors were later linked to the onset of maturation (defined by increases in fshb transcription, 11-KT levels, and GSI) after the winter signal, especially in the two groups at 18 °C. The present findings revealed the importance of a high water temperature to induce early stimulatory changes in the BPG axis that promote and accelerate the process of spermatogenesis. In contrast, the feed regime had a lower effect on testis development and on the transcriptional changes of the genes analyzed. The potential detrimental effects of the restricted feeding on spermatogenesis were not sufficient to completely suppress early maturation in all individuals, thus casting doubts on the possibility to use a feed restriction to control early maturation in aquaculture settings without significantly affecting the growth.
Author Contributions
Conceptualization, E.P.M., P.B., A.K.D.I., and S.O.H.; formal analysis, E.P.M., M.F.B., P.B., M.K., and C.P.; funding acquisition, A.K.D.I. and S.O.H.; methodology, E.P.M., P.B., and S.O.H.; project administration, A.K.D.I. and S.O.H.; resources, P.B., M.K., C.P., and S.O.H.; supervision, P.B. and S.O.H.; validation, P.B. and C.P.; visualization, E.P.M., M.F.B., and M.K.; writing—original draft, E.P.M. and M.F.B.; writing—review and editing, E.P.M., M.F.B., P.B., M.K., C.P., A.K.D.I., and S.O.H. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the Research Council of Norway through projects SAFT (project number 286597), FREMAD (project number 309288), and KABIS (Kapasitetsløft for bærekraftig og innovativ sjømatproduksjon, project number 280782).
Institutional Review Board Statement
The animal study protocol was approved by the local representative of Animal Welfare at the Department of Biological Sciences, University of Bergen, Norway (FOTS application ID7183), and samplings were performed as established by the Norwegian Animal Research Authority.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author, upon reasonable request.
Acknowledgments
The authors wish to thank Geir Magne Knutsen, Inger Lise Breivik, and staff from Bremnes Seashore AS for their continuous support of this research. The authors also acknowledge Tom Ole Nilsen and members of NORCE’s Integrative Fish Biology (IFB) research group Valentina Tronci and Naouel Gharbi, who contributed to samplings, laboratory work, and logistics. In addition, the authors thank Helene Wisløff for formatting the histology images.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fraser, T.W.; Fjelldal, P.G.; Schulz, R.W.; Norberg, B.; Hansen, T.J. Termination of puberty in out-of-season male Atlantic salmon smolts. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 232, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Fjelldal, P.G.; Hansen, T.J.; Wargelius, A.; Ayllon, F.; Glover, K.A.; Schulz, R.W.; Fraser, T.W.K. Development of supermale and all-male Atlantic salmon to research the vgll3 allele—Puberty link. BMC Genet. 2020, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Good, C.; Davidson, J. A Review of Factors Influencing Maturation of Atlantic Salmon, Salmo salar, with Focus on Water Recirculation Aquaculture System Environments. J. World Aquac. Soc. 2016, 47, 605–632. [Google Scholar] [CrossRef]
- Berrill, I.; Porter, M.; Smart, A.; Mitchell, D.; Bromage, N. Photoperiodic effects on precocious maturation, growth and smoltification in Atlantic salmon, Salmo salar. Aquaculture 2003, 222, 239–252. [Google Scholar] [CrossRef]
- Skilbrei, O.T.; Heino, M. Reduced daylength stimulates size-dependent precocious maturity in 0+ male Atlantic salmon parr. Aquaculture 2011, 311, 168–174. [Google Scholar] [CrossRef]
- Rowe, D.K.; Thorpe, J.E.; Shanks, A.M. Role of Fat Stores in the Maturation of Male Atlantic Salmon (Salmo salar) Parr. Can. J. Fish. Aquat. Sci. 1991, 48, 405–413. [Google Scholar] [CrossRef]
- McClure, C.A.; Hammell, K.L.; Moore, M.; Dohoo, I.R.; Burnley, H. Risk factors for early sexual maturation in Atlantic salmon in seawater farms in New Brunswick and Nova Scotia, Canada. Aquaculture 2007, 272, 370–379. [Google Scholar] [CrossRef]
- Fjelldal, P.G.; Schulz, R.; Nilsen, T.O.; Andersson, E.; Norberg, B.; Hansen, T.J. Sexual maturation and smoltification in domesticated Atlantic salmon (Salmo salar L.)—Is there a developmental conflict? Physiol. Rep. 2018, 6, 1–18. [Google Scholar] [CrossRef]
- Fjelldal, P.G.; Hansen, T.; Huang, T.-S. Continuous light and elevated temperature can trigger maturation both during and immediately after smoltification in male Atlantic salmon (Salmo salar). Aquaculture 2011, 321, 93–100. [Google Scholar] [CrossRef]
- Imsland, A.K.; Handeland, S.O.; Stefansson, S.O. Photoperiod and temperature effects on growth and maturation of pre- and post-smolt Atlantic salmon. Aquac. Int. 2014, 22, 1331–1345. [Google Scholar] [CrossRef]
- Pino Martinez, E.; Balseiro, P.; Stefansson, S.O.; Kaneko, N.; Norberg, B.; Fleming, M.S.; Imsland, A.K.; Handeland, S.O. Interaction of temperature and feed ration on male postsmolt maturation of Atlantic salmon (Salmo salar L.). Aquaculture 2023, 562, 738877. [Google Scholar] [CrossRef]
- Thorpe, J. Maturation responses of salmonids to changing developmental opportunities. Mar. Ecol. Prog. Ser. 2007, 335, 285–288. [Google Scholar] [CrossRef]
- Taranger, G.L.; Carrillo, M.; Schulz, R.W.; Fontaine, P.; Zanuy, S.; Felip, A.; Weltzien, F.-A.; Dufour, S.; Karlsen, Ø.; Norberg, B.; et al. Control of puberty in farmed fish. Gen. Comp. Endocrinol. 2010, 165, 483–515. [Google Scholar] [CrossRef]
- Hou, Y.; Suzuki, Y.; Aida, K. Changes in Immunoglobulin Producing Cells in Response to Gonadal Maturation in Rainbow Trout. Fish. Sci. 1999, 65, 844–849. [Google Scholar] [CrossRef]
- Harris, J.; Bird, D.J. Modulation of the fish immune system by hormones. Veter Immunol. Immunopathol. 2000, 77, 163–176. [Google Scholar] [CrossRef]
- Lundqvist, H.; Borg, B.; Berglund, I. Androgens impair seawater adaptability in smolting Baltic salmon (Salmo salar). Can. J. Zool. 1989, 67, 1733–1736. [Google Scholar] [CrossRef]
- McCormick, S.D.; O’Dea, M.F.; Moeckel, A.M.; Lerner, D.T.; Björnsson, B.T. Endocrine disruption of parr-smolt transformation and seawater tolerance of Atlantic salmon by 4-nonylphenol and 17β-estradiol. Gen. Comp. Endocrinol. 2005, 142, 280–288. [Google Scholar] [CrossRef]
- Schulz, R.W.; Andersson, E.; Taranger, G.L. Photoperiod manipulation can stimulate or inhibit pubertal testis maturation in Atlantic salmon (Salmo salar). Anim. Reprod. 2006, 31, 121–126. [Google Scholar]
- Shrimpton, J.M. Seawater to Freshwater Transitions in Diadromous Fishes. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 327–393. [Google Scholar] [CrossRef]
- Good, C. The Impact of Water Exchange Rate and Treatment Processes on Water-Borne Hormones in Recirculation Aquaculture Systems Containing Sexually Maturing Atlantic Salmon Salmo salar. J. Aquac. Res. Dev. 2014, 05, 260. [Google Scholar] [CrossRef]
- Bromage, N.; Porter, M.; Randall, C. The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture 2001, 197, 63–98. [Google Scholar] [CrossRef]
- Pankhurst, N.W.; King, H.R. Temperature and salmonid reproduction: Implications for aquaculture. J. Fish Biol. 2010, 76, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Handeland, S.O.; Imsland, A.K.; Stefansson, S.O. The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture 2008, 283, 36–42. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N.; Finstad, A.G. Effects of temperature and food quality on age and size at maturity in ectotherms: An experimental test with Atlantic salmon. J. Anim. Ecol. 2012, 82, 201–210. [Google Scholar] [CrossRef]
- Adams, C.E.; Thorpe, J.E. Photoperiod and temperature effects on early development and reproductive investment in Atlantic salmon (Salmo salar L.). Aquaculture 1989, 79, 403–409. [Google Scholar] [CrossRef]
- Melo, M.C.; Andersson, E.; Fjelldal, P.G.; Bogerd, J.; França, L.R.; Taranger, G.L.; Schulz, R. Salinity and photoperiod modulate pubertal development in Atlantic salmon (Salmo salar). J. Endocrinol. 2013, 220, 319–332. [Google Scholar] [CrossRef]
- Fraser, T.W.; Hansen, T.J.; Norberg, B.; Nilsen, T.O.; Schulz, R.W.; Fjelldal, P.G. Atlantic salmon male post-smolt maturation can be reduced by using a 3-hour scotophase to induce smoltification. Aquaculture 2022, 562, 738772. [Google Scholar] [CrossRef]
- Thorpe, J.E. Reproductive strategies in Atlantic salmon, Salmo salar L. Aquac. Res. 1994, 25, 77–87. [Google Scholar] [CrossRef]
- McCormick, S.D. Smolt Physiology and Endocrinology, Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 199–251. [Google Scholar] [CrossRef]
- McCormick, S.D.; Sheridan, M.; Eilerlson, C.; Carey, J.B.; O’Dea, M. Increased daylength stimulates plasma growth hormone and gill Na+, K+-ATPase in Atlantic salmon (Salmo salar). J. Comp. Physiol. B 1995, 165, 245–254. [Google Scholar] [CrossRef]
- Stefansson, S.O.; Björnsson, B.T.; Ebbesson, L.O.; McCormick, S.D. Smoltification. In Fish Larval Physiology; Finn, R.N., Kapoor, B.G., Eds.; Science Publishers: Enfield, NH, USA, 2008; pp. 639–681. [Google Scholar] [CrossRef]
- Zydlewski, J.; Wilkie, M.P. Freshwater to Seawater Transitions in Migratory Fishes, Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Yaron, Z.; Levavi-Sivan, B.; Yaron, Z.; Levavi-Sivan, B. Hormonal control of reproduction and growth | Endocrine Regulation of Fish Reproduction, Encyclopedia of Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Zohar, Y.; Muñoz-Cueto, J.A.; Elizur, A.; Kah, O. Neuroendocrinology of reproduction in teleost fish. Gen. Comp. Endocrinol. 2010, 165, 438–455. [Google Scholar] [CrossRef]
- Maugars, G.; Schmitz, M. Expression of gonadotropin and gonadotropin receptor genes during early sexual maturation in male Atlantic salmon parr. Mol. Reprod. Dev. 2008, 75, 403–413. [Google Scholar] [CrossRef]
- Schulz, R.W.; Taranger, G.L.; Bogerd, J.; Nijenhuis, W.; Norberg, B.; Male, R.; Andersson, E. Entry into puberty is reflected in changes in hormone production but not in testicular receptor expression in Atlantic salmon (Salmo salar). Reprod. Biol. Endocrinol. 2019, 17, 1–16. [Google Scholar] [CrossRef]
- Schulz, R.W.; de França, L.R.; Lareyre, J.J.; LeGac, F.; Chiarini-Garcia, H.; Nobrega, R.H.; Miura, T. Spermatogenesis in fish. Gen. Comp. Endocrinol. 2010, 165, 390–411. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.W.; Menting, S.; Bogerd, J.; França, L.R.; Vilela, D.A.; Godinho, H.P.; Schulz, R. Sertoli Cell Proliferation in the Adult Testis—Evidence from Two Fish Species Belonging to Different Orders1. Biol. Reprod. 2005, 73, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Crespo, D.; Bogerd, J.; Sambroni, E.; LeGac, F.; Andersson, E.; Edvardsen, R.B.; Bergman, E.J.; Björnsson, B.T.; Taranger, G.L.; Schulz, R.W. The initiation of puberty in Atlantic salmon brings about large changes in testicular gene expression that are modulated by the energy status. BMC Genom. 2019, 20, 475. [Google Scholar] [CrossRef]
- Nóbrega, R.H.; Morais, R.D.V.D.S.; Crespo, D.; de Waal, P.P.; de França, L.R.; Schulz, R.W.; Bogerd, J. Fsh Stimulates Spermatogonial Proliferation and Differentiation in Zebrafish via Igf3. Endocrinology 2015, 156, 3804–3817. [Google Scholar] [CrossRef]
- Schulz, R.W.; Nobrega, R.H. Anatomy and Histology of Fish Testis. In Encyclopedia of Fish Physiology: From Genome to Environment; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Sambroni, E.; Rolland, A.D.; Lareyre, J.-J.; Le Gac, F. Fsh and Lh have common and distinct effects on gene expression in rainbow trout testis. J. Mol. Endocrinol. 2013, 50, 1–18. [Google Scholar] [CrossRef]
- Zheng, S.; Long, J.; Liu, Z.; Tao, W.; Wang, D. Identification and Evolution of TGF-β Signaling Pathway Members in Twenty-Four Animal Species and Expression in Tilapia. Int. J. Mol. Sci. 2018, 19, 1154. [Google Scholar] [CrossRef]
- Miura, T.; Miura, C.; Konda, Y.; Yamauchi, K. Spermatogenesis-preventing substance in Japanese eel. Development 2002, 129, 2689–2697. [Google Scholar] [CrossRef]
- Crespo, D.; Assis, L.H.; Furmanek, T.; Bogerd, J.; Schulz, R.W. Expression profiling identifies Sertoli and Leydig cell genes as Fsh targets in adult zebrafish testis. Mol. Cell. Endocrinol. 2016, 437, 237–251. [Google Scholar] [CrossRef]
- Pfennig, F.; Standke, A.; Gutzeit, H.O. The role of Amh signaling in teleost fish—Multiple functions not restricted to the gonads. Gen. Comp. Endocrinol. 2015, 223, 87–107. [Google Scholar] [CrossRef]
- Skaar, K.S.; Nóbrega, R.H.; Magaraki, A.; Olsen, L.C.; Schulz, R.W.; Male, R. Proteolytically Activated, Recombinant Anti-Müllerian Hormone Inhibits Androgen Secretion, Proliferation, and Differentiation of Spermatogonia in Adult Zebrafish Testis Organ Cultures. Endocrinology 2011, 152, 3527–3540. [Google Scholar] [CrossRef] [PubMed]
- Crespo, B.; Gómez, A.; Mazón, M.J.; Carrillo, M.; Zanuy, S. Isolation and characterization of Ff1 and Gsdf family genes in European sea bass and identification of early gonadal markers of precocious puberty in males. Gen. Comp. Endocrinol. 2013, 191, 155–167. [Google Scholar] [CrossRef]
- Kleppe, L.; Edvardsen, R.B.; Furmanek, T.; Andersson, E.; Skaftnesmo, K.O.; Segafredo, F.T.; Wargelius, A. Transcriptomic analysis of dead end knockout testis reveals germ cell and gonadal somatic factors in Atlantic salmon. BMC Genom. 2020, 21, 99. [Google Scholar] [CrossRef] [PubMed]
- Sawatari, E.; Shikina, S.; Takeuchi, T.; Yoshizaki, G. A novel transforming growth factor-β superfamily member expressed in gonadal somatic cells enhances primordial germ cell and spermatogonial proliferation in rainbow trout (Oncorhynchus mykiss). Dev. Biol. 2007, 301, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Sambroni, E.; Lareyre, J.-J.; Le Gac, F. Fsh Controls Gene Expression in Fish both Independently of and through Steroid Mediation. PLoS ONE 2013, 8, e76684. [Google Scholar] [CrossRef]
- Wang, D.-S.; Jiao, B.; Hu, C.; Huang, X.; Liu, Z.; Cheng, C.H. Discovery of a gonad-specific IGF subtype in teleost. Biochem. Biophys. Res. Commun. 2008, 367, 336–341. [Google Scholar] [CrossRef]
- Nóbrega, R.H.; Batlouni, S.R.; França, L.R. An overview of functional and stereological evaluation of spermatogenesis and germ cell transplantation in fish. Fish Physiol. Biochem. 2009, 35, 197–206. [Google Scholar] [CrossRef]
- Vilela, D.A.R.; Silva, S.G.B.; Peixoto, M.T.D.; Godinho, H.P.; França, L.R. Spermatogenesis in teleost: Insights from the Nile tilapia (Oreochromis niloticus) model. Fish Physiol. Biochem. 2003, 28, 187–190. [Google Scholar] [CrossRef]
- Postingel Quirino, P.; Rodrigues, M.D.S.; Cabral, E.M.D.S.; de Siqueira-Silva, D.H.; Mori, R.H.; Butzge, A.J.; Nóbrega, R.H.; Ninhaus-Silveira, A.; Veríssimo-Silveira, R. The influence of increased water temperature on the duration of spermatogenesis in a neotropical fish, Astyanax altiparanae (Characiformes, Characidae). Fish Physiol. Biochem. 2020, 47, 747–755. [Google Scholar] [CrossRef]
- Trombley, S.; Mustafa, A.; Schmitz, M. Regulation of the seasonal leptin and leptin receptor expression profile during early sexual maturation and feed restriction in male Atlantic salmon, Salmo salar L., parr. Gen. Comp. Endocrinol. 2014, 204, 60–70. [Google Scholar] [CrossRef]
- Chomczynski, P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 1993, 15, 532–534, 536–537. [Google Scholar]
- Pfaffl, M. Development and Validation of an Externally Standardised Quantitative Insulin-like Growth Factor-1 RT-PCR Using LightCycler SYBR Green I Technology. In Rapid Cycle Real-Time PCR; Springer: Berlin/Heidelberg, Germany, 2001; pp. 281–291. [Google Scholar] [CrossRef]
- Middleton, M.A.; Larsen, D.A.; Dickey, J.T.; Swanson, P. Evaluation of endocrine and transcriptomic markers of male maturation in winter-run steelhead trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 2019, 281, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Olsvik, P.A.; Lie, K.K.; Jordal, A.E.O.; Nilsen, T.O.; Hordvik, I. Evaluation of potential reference genes in real-time RT-PCR studies of Atlantic salmon. BMC Mol. Biol. 2005, 6, 1–9. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Kassambara, A. ggpubr: “ggplot2” based publication ready plots. In R Package Version 0.2; CRAN, 2017. [Google Scholar]
- Hope, R.M. Rmisc: Ryan miscellaneous. In R Package version 1.5; CRAN, 2013. [Google Scholar]
- Lenth, R.; Singmann, H.; Love, J. Emmeans: Estimated marginal means, aka least-squares means. R Packag. Version 2018, 1, 3. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Heisterkamp, S.; Van Willigen, B.; Maintainer, R. Package ‘nlme.’ Linear nonlinear Mix. Eff. Model, Version 3. 2017.
- Christensen, R.H.B. “ordinal”—Regression Models for Ordinal Data. R Package Version. Comput. Softw. 2019, 10–12. [Google Scholar]
- Ciani, E.; von Krogh, K.; Nourizadeh-Lillabadi, R.; Mayer, I.; Fontaine, R.; Weltzien, F.-A. Sexual maturation in Atlantic salmon male parr may be triggered both in late summer and early spring under standard farming conditions. Aquaculture 2021, 544, 737086. [Google Scholar] [CrossRef]
- Gautier, A.; Le Gac, F.; Lareyre, J.-J. The gsdf gene locus harbors evolutionary conserved and clustered genes preferentially expressed in fish previtellogenic oocytes. Gene 2011, 472, 7–17. [Google Scholar] [CrossRef]
- Hsu, C.-W.; Chung, B.-C. Evolution, Expression, and Function of Gonadal Somatic Cell-Derived Factor. Front. Cell Dev. Biol. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Lareyre, J.J.; Ricordel, M.J.; Mahé, S.; Goupil, A.S.; Vizziano, D.; Bobe, J.; Guiguen, Y.; Le Gac, F. Two new TGF beta members are restricted to the gonad and differentially expressed during sex differentiation and gametogenesis in trout. Cybium 2008, 32, 202. [Google Scholar]
- Graziano, M.; Benito, R.; Planas, J.V.; Palstra, A.P. Swimming exercise to control precocious maturation in male seabass (Dicentrarchus labrax). BMC Dev. Biol. 2018, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, F.; Gu, Y.; Wang, T.; Wang, H.; Yang, S.; Sun, Y.; Zhou, L.; Huang, X.; Jiao, B.; et al. Insulin-Like Growth Factor 3 Regulates Expression of Genes Encoding Steroidogenic Enzymes and Key Transcription Factors in the Nile Tilapia Gonad1. Biol. Reprod. 2012, 86, 163. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, X.; Dai, S.; Xiao, H.; Qi, S.; Li, Y.; Zheng, Q.; Jie, M.; Cheng, C.H.K.; Wang, D. Regulation of spermatogenesis and reproductive capacity by Igf3 in tilapia. Cell. Mol. Life Sci. 2020, 77, 4921–4938. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).