Study of the Effect of Dietary Agavin Supplementation in Blood Parameters and Antioxidant Enzymes of Juvenile Nile Tilapia (Oreochromis niloticus) under Stress Conditions
Abstract
1. Introduction
2. Materials and Methods
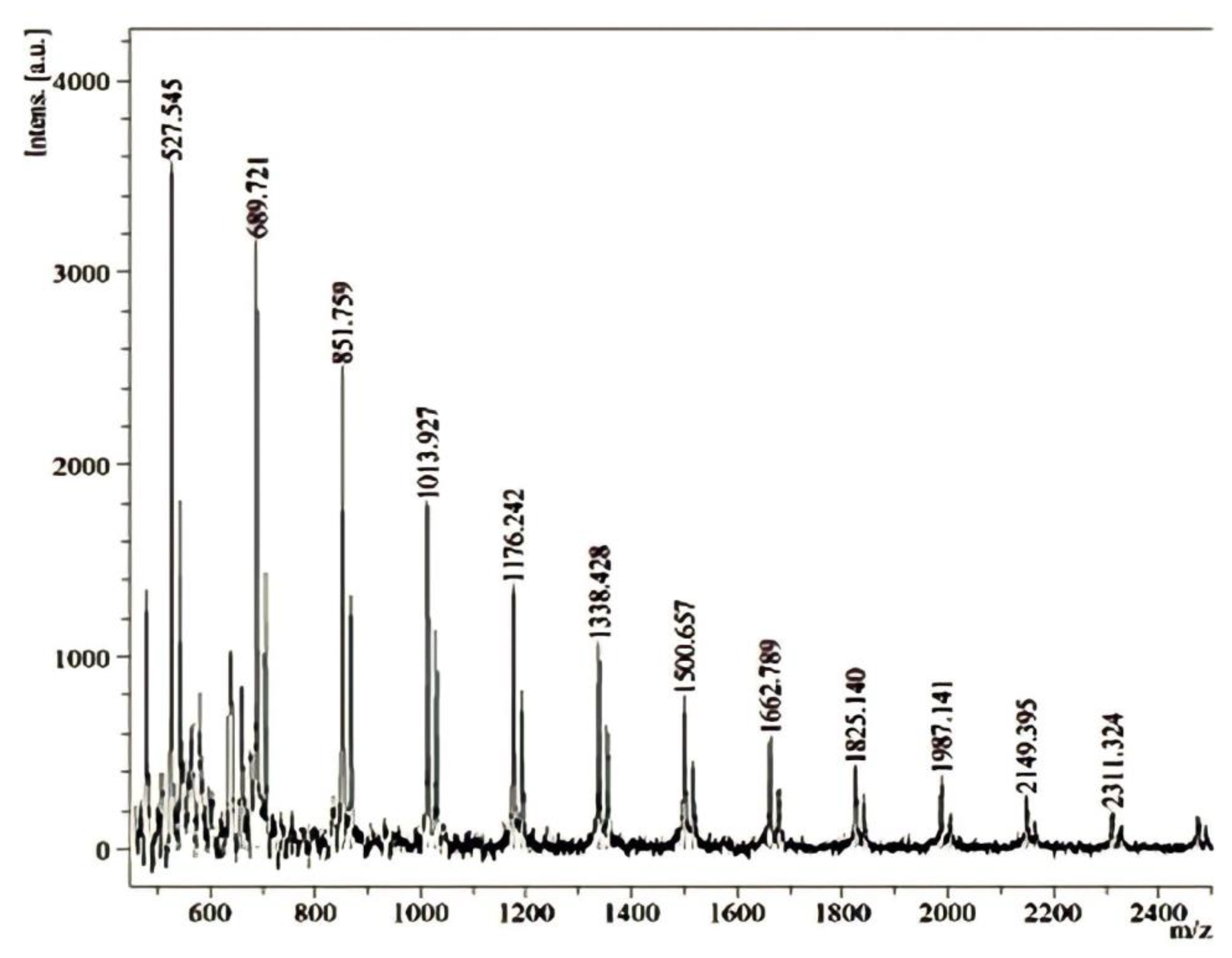
2.1. Agavin Analyses by MALDI-TOF-MS
2.2. Experimental Diets
2.3. Fish and Experimental Conditions
2.4. Growth Performance and Body Composition
2.5. Sample Collection
2.6. Body Composition
2.7. Blood Metabolites
2.8. Antioxidant Analyses
2.8.1. Superoxide Dismutase Activity
2.8.2. Catalase Activity
2.8.3. Lipid Peroxidation: Malondialdehyde Content Determination
2.8.4. Protein Concentration
2.9. Statistical Analyses
3. Results
3.1. Agavin Analyses by MALDI-TOF-MS
3.2. Growth Performance and Body Composition
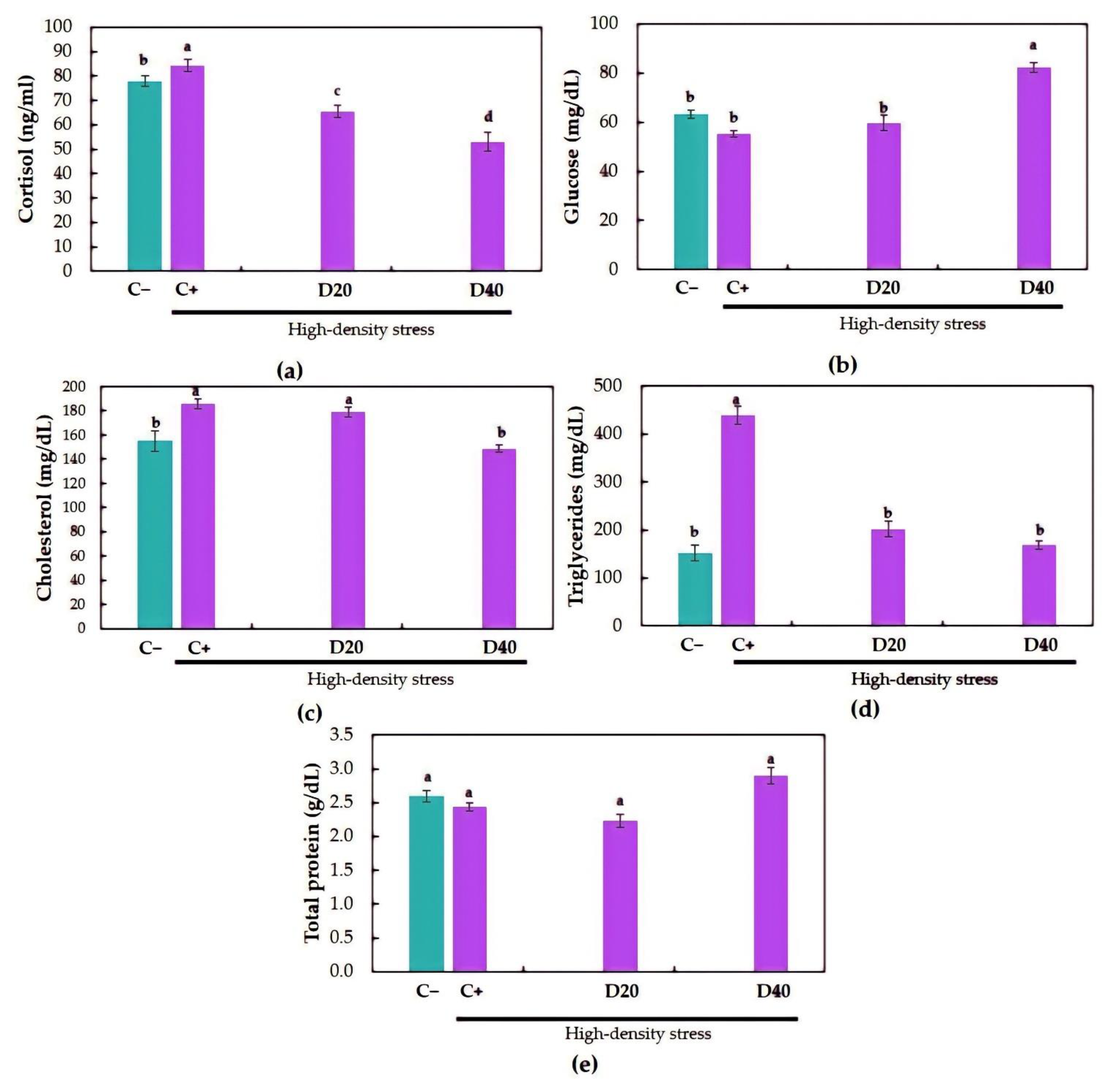
3.3. Blood Metabolites
3.4. Hepatic Antioxidant Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CONAPESCA. Comisión Nacional de Acuacultura y Pesca. 2017. Available online: https://www.gob.mx/conapesca/documentos/bibliografia-pesquera-y-acuicola. (accessed on 3 August 2021).
- Food and Agricultural Organization (FAO). Available online: http://www.fao.org/fishery/culturedspecies/Oreochromis_niloticus/en (accessed on 21 July 2021).
- Jun, Q.; Hong, Y.; Hui, W.; Didlyn, K.M.; Jie, H.; Pao, X. Physiological responses and HSP70 mRNA expression in GIFT tilapia juveniles, Oreochromis niloticus under short-term crowding. Aquac. Res. 2015, 46, 335–345. [Google Scholar] [CrossRef]
- Hoyt, L.T.; Zeiders, K.H.; Ehrlich, K.B.; Adam, E.K. Positive upshots of cortisol in everyday life. Emotion 2016, 16, 431–435. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Metwally, A.E.-S.; El-Sharawy, M.E.; Atta, A.M.; Elbialy, Z.I.; Abdel-Latif, H.M.R.; Paray, B.A. The role of β-glucan in the growth, intestinal morphometry, and immune-related gene and heat shock protein expressions of Nile tilapia (Oreochromis niloticus) under different stocking densities. Aquaculture 2020, 523, 735205. [Google Scholar] [CrossRef]
- Dediu, L.; Docan, A.; Crețu, M.; Grecu, I.; Mogodan, A.; Maereanu, M.; Oprea, L. Effects of Stocking density on growth performance and stress responses of bester and bester ♀ × beluga ♂ juveniles in recirculating aquaculture systems. Animals 2021, 11, 2292. [Google Scholar] [CrossRef]
- Birnie-Gauvin, K.; Costantini, D.; Cooke, S.J.; Willmore, W.G. A comparative and evolutionary approach to oxidative stress in fish: A review. Fish Fish. 2017, 18, 928–942. [Google Scholar] [CrossRef]
- Cedeño-Pinos, C.; Martínez-Tomé, M.; Murcia, M.A.; Jordán, M.J.; Bañón, S. Assessment of Rosemary (Rosmarinus officinalis L.) Extract as Antioxidant in Jelly Candies Made with Fructan Fibres and Stevia. Antioxidants 2020, 9, 1289. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Lizárraga-Velázquez, C.E.; Hernández, C.; González-Aguilar, G.A.; Heredia, J.B. Effect of hydrophilic and lipophilic antioxidants from mango peel (Mangifera indica L. cv. Ataulfo) on lipid peroxidation in fish oil. CyTA J. Food 2018, 16, 1095–1101. [Google Scholar] [CrossRef]
- Galeana-López, J.A.; Hernández, C.; Leyva-López, N.; Lizárraga-Velázquez, C.E.; Sánchez-Gutiérrez, E.Y.; Basilio Heredia, J. Corn husk extracts as an antioxidant additive in diets for Nile tilapia (Oreochromis niloticus) fingerlings: Effect on growth performance, feed intake and toxicity. Biotecnia 2020, 22, 147–154. [Google Scholar] [CrossRef]
- Lopez, M.G.; Mancilla-Margalli, N.A.; Mendoza-Diaz, G. Molecular Structures of Fructans from Agave tequilana Weber var. azul. J. Agric. Food Chem. 2003, 51, 7835–7840. [Google Scholar] [CrossRef]
- García-Curbelo, Y.; Bocourt, R.; Savón, L.L.; García-Vieyra, M.I.; López, M.G. Prebiotic effect of Agave fourcroydes fructans: An animal model. Food Funct. 2015, 6, 3177–3182. [Google Scholar] [CrossRef] [PubMed]
- López-Romero, J.C.; Ayala-Zavala, J.F.; González-Aguilar, G.A.; Peña-Ramos, E.A.; González-Ríos, H. Biological activities ofAgaveby-products and their possible applications in food and pharmaceuticals. J. Sci. Food Agric. 2018, 98, 2461–2474. [Google Scholar] [CrossRef] [PubMed]
- Consejo Regulador de Tequila (CRT). Available online: https://www.crt.org.mx/index.php/es/ (accessed on 3 December 2021).
- Cámara Nacional de la Industria Tequilera (CNIT). Available online: https://www.tequileros.org/camara (accessed on 2 December 2021).
- Ortiz, L.; Rebolé, A.; Velasco, S.; Rodríguez, M.; Treviño, J.; Tejedor, J.; Alzueta, C. Effects of inulin and fructooligosaccharides on growth performance, body chemical composition and intestinal microbiota of farmed rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2013, 19, 475–482. [Google Scholar] [CrossRef]
- Fuentes-Quesada, J.P.; Cornejo-Granados, F.; Mata-Sotres, J.A.; Ochoa-Romo, J.P.; Rombenso, A.N.; Guerrero-Rentería, Y.; Lazo, J.P.; Pohlenz, C.; Ochoa-Leyva, A.; Viana, M.T. Prebiotic agavin in juvenile totoaba, Totoaba macdonaldi diets, to relieve soybean meal-induced enteritis: Growth performance, gut histology and microbiota. Aquac. Nutr. 2020, 26, 2115–2134. [Google Scholar] [CrossRef]
- Franco-Robles, E.; López, M.G. Implication of Fructans in Health: Immunomodulatory and Antioxidant Mechanisms. Sci. World J. 2015, 2015, 289267. [Google Scholar] [CrossRef] [PubMed]
- Franco-Robles, E.; López, M.G. Agavins Increase Neurotrophic Factors and Decrease Oxidative Stress in the Brains of High-Fat Diet-Induced Obese Mice. Molecules 2016, 21, 998. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, J.; Yan, M.; Tang, S.; Wang, X.; Qin, J.G.; Chen, L.; Li, E. Inulin alleviates hypersaline-stress induced oxidative stress and dysbiosis of gut microbiota in Nile tilapia (Oreochromis niloticus). Aquaculture 2020, 529, 735681. [Google Scholar] [CrossRef]
- Van den Ende, W.; Peshev, D.; De Gara, L. Disease prevention by natural antioxidants and prebiotics acting as ROS scavengers in the gastrointestinal tract. Trends Food Sci. Technol. 2011, 22, 689–697. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.G.; Mateos, R.; Ortiz-Basurto, R.I.; Largo, C.; Serrano, J.; Granado-Serrano, A.B.; Sarriá, B.; Bravo, L.; Tabernero, M. Effects of consuming diets containing Agave tequilana dietary fibre and jamaica calyces on body weight gain and redox status in hypercholesterolemic rats. Food Chem. 2014, 148, 54–59. [Google Scholar] [CrossRef]
- Campos-Valdez, A.R.; Casas-Godoy, L.; Sandoval, G.; Hernández, L.; Sassaki, G.L.; de Menezes, L.R.A.; Campos-Terán, J.; Reyes-Duarte, D.; Arrizon, J. Regioselective synthesis of 6″-O-lauroyl-1-kestose and 6‴-O-lauroylnystose by sequential enzymatic reactions of transfructosylation and acylation. Biocatal. Biotransformation 2022, 40, 133–143. [Google Scholar] [CrossRef]
- Ulloa-Valdez, A. Evaluación de la Especie, Salinidad y Dieta Como Factores que Afectan el Rendimiento Productivo a Través del Tiempo y la Salud Intestinal de Juveniles de Tilapia (Oreochromis spp.). Master´s Thesis, Centro de Investigación en Alimentación y Desarrollo (CIAD), Culiácan Rosales, Mexico, 2020. [Google Scholar]
- Mirghaed, A.T.; Hoseini, S.M.; Ghelichpour, M. Effects of dietary 1,8-cineole supplementation on physiological, immunological and antioxidant responses to crowding stress in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2018, 81, 182–188. [Google Scholar] [CrossRef]
- Leary, S.; Ridge, H.; Underwood, W.; Anthony, R.; Cartner, S.; Grandin, T.; Collins, F.; Greenacre, C.; Gwaltney-Brant, S.; Network, I.; et al. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition*; Spandidos: Schaumburg, IL, USA, 2020. [Google Scholar]
- Torrecillas, S.; Terova, G.; Makol, A.; Serradell, A.; Valdenegro-Vega, V.; Izquierdo, M.; Acosta, F.; Montero, D. Dietary Phytogenics and Galactomannan Oligosaccharides in Low Fish Meal and Fish Oil-Based Diets for European Sea Bass (Dicentrarchus labrax) Juveniles: Effects on Gill Structure and Health and Implications on Oxidative Stress Status. Front. Immunol. 2021, 12, 663106. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 2011. [Google Scholar]
- Del Rio-Zaragoza, O.; Fajer-Ávila, E.; Almazán-Rueda, P.; de la Parra, M.A. Hematological characteristics of the spotted rose snapper Lutjanus guttatus (Steindachner, 1869) healthy and naturally infected by dactylogyrid monogeneans. Cell Tissue Res. 2011, 43, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice-Hall international editions: Upper Saddle River, NJ, USA, 1999; Volume 929. [Google Scholar]
- M’Balaka, M.; Kassam, D.; Rusuwa, B. The effect of stocking density on the growth and survival of improved and unimproved strains of Oreochromis shiranus. Egypt. J. Aquat. Res. 2012, 38, 205–211. [Google Scholar] [CrossRef]
- Soaudy, M.R.; Mohammady, E.Y.; Elashry, M.A.; Ali, M.M.; Ahmed, N.M.; Hegab, M.H.; El-Garhy, H.A.; El-Haroun, E.R.; Hassaan, M.S. Possibility mitigation of cold stress in Nile tilapia under biofloc system by dietary propylene glycol: Performance feeding status, immune, physiological responses and transcriptional response of delta-9-desaturase gene. Aquaculture 2021, 538, 736519. [Google Scholar] [CrossRef]
- Mancilla-Margalli, N.A.; Pez, Ä. Water-soluble carbohydrates and fructan structure patterns from Agave and Dasylirion species. J. Agric. Food Chem. 2006, 54, 7832–7839. [Google Scholar] [CrossRef]
- Arrizon, J.; Morel, S.; Gschaedler, A.; Monsan, P. Comparison of the water-soluble carbohydrate composition and fructan structures of Agave tequilana plants of different ages. Food Chem. 2010, 122, 123–130. [Google Scholar] [CrossRef]
- Huazano-García, A.; López, M.G. Agavins reverse the metabolic disorders in overweight mice through the increment of short chain fatty acids and hormones. Food Funct. 2015, 6, 3720–3727. [Google Scholar] [CrossRef] [PubMed]
- Tiengtam, N.; Paengkoum, P.; Sirivoharn, S.; Phonsiri, K.; Boonanuntanasarn, S. The effects of dietary inulin and Jerusalem artichoke (Helianthus tuberosus) tuber on the growth performance, haematological, blood chemical and immune parameters of Nile tilapia (Oreochromis niloticus) fingerlings. Aquac. Res. 2017, 48, 5280–5288. [Google Scholar] [CrossRef]
- Raffic Ali, S.S.; Ambasankar, K.; Nandakumar, S.; Praveena, P.E.; Syamadayal, J. Effect of dietary prebiotic inulin on growth, body composition and gut microbiota of Asian seabass (Lates calcarifer). Anim. Feed Sci. Technol. 2016, 217, 87–94. [Google Scholar] [CrossRef]
- Baltzegar, D.A.; Reading, B.J.; Douros, J.D.; Borski, R.J. Role for leptin in promoting glucose mobilization during acute hyperosmotic stress in teleost fishes. J. Endocrinol. 2014, 220, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Erikson, U.; Gansel, L.; Frank, K.; Svendsen, E.; Digre, H. Crowding of Atlantic salmon in net-pen before slaughter. Aquaculture 2016, 465, 395–400. [Google Scholar] [CrossRef]
- Kumari, S.; Harikrishna, V.; Surasani, V.K.R.; Balange, A.K.; Babitha Rani, A.M. Growth, biochemical indices and carcass quality of red tilapia reared in zero water discharge based biofloc system in various salinities using inland saline ground water. Aquaculture 2021, 540, 736730. [Google Scholar] [CrossRef]
- Sadoul, B.; Geffroy, B. Measuring cortisol, the major stress hormone in fishes. J. Fish Biol. 2019, 94, 540–555. [Google Scholar] [CrossRef]
- Bochem, A.E.; Holleboom, A.G.; Romijn, J.A.; Hoekstra, M.; Dallinga-Thie, G.M.; Motazacker, M.M.; Hovingh, G.K.; Kuivenhoven, J.A.; Stroes, E.S.G. High density lipoprotein as a source of cholesterol for adrenal steroidogenesis: A study in individuals with low plasma HDL-C. J. Lipid Res. 2013, 54, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Conde-Sieira, M.; Muñoz, J.L.P.; López-Patiño, M.A.; Gesto, M.; Soengas, J.L.; Míguez, J.M. Oral administration of melatonin counteracts several of the effects of chronic stress in rainbow trout. Domest. Anim. Endocrinol. 2014, 46, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.M.; Rodriguez-Barreto, D.; Martin, S.A.M.; Van Oosterhout, C.; Orozco-Terwengel, P.; Cable, J.; Hamilton, A.; De Leaniz, C.G.; Consuegra, S. Contrasting effects of acute and chronic stress on the transcriptome, epigenome, and immune response of Atlantic salmon. Epigenetics 2018, 13, 1191–1207. [Google Scholar] [CrossRef]
- dos Reis Goes, E.S.; Goes, M.D.; De Castro, P.L.; De Lara, J.A.F.; Vital, A.C.; Ribeiro, R.P. Imbalance of the redox system and quality of tilapia fillets subjected to pre-slaughter stress. PLoS ONE 2019, 14, e0210742. [Google Scholar] [CrossRef]
- Naderi, M.; Keyvanshokooh, S.; Ghaedi, A.; Salati, A.P. Effect of acute crowding stress on rainbow trout (Oncorhynchus mykiss): A proteomics study. Aquaculture 2018, 495, 106–114. [Google Scholar] [CrossRef]
- Yaghobi, M.; Heyrati, F.P.; Dorafshan, S.; Mahmoudi, N. Serum biochemical changes and acute stress responses of the endangered iridescent catfish (Pangasianodon hypophthalmus) supplied with dietary nucleotide. JAST 2015, 17, 1161–1170. [Google Scholar]
- David, M.; Shivakumar, R.; Mushigeri, S.B.; Kuri, R.C. Blood glucose and glycogen levels as indicators of stress in the freshwater fish, Labeo rohita under fenvalerate intoxication. J. Ecotoxicol. Environ. Monit. 2005, 15, 01–06. [Google Scholar]
- Martínez-Porchas, M.; Martinez-Cordova, L.R.; Ramos-Enriquez, R. Cortisol and glucose: Reliable indicators of fish stress?—Universidad de Sonora. Biotecnol. Sustentabilidad Acuícolas Univ. Sonora 2009, 4, 158–178. Available online: https://investigadores.unison.mx/en/publications/cortisol-and-glucose-reliable-indicators-of-fish-stress (accessed on 1 November 2022).
- Jentoft, S.; Aastveit, A.H.; Torjesen, P.A.; Andersen. Effects of stress on growth, cortisol and glucose levels in non-domesticated Eurasian perch (Perca fluviatilis) and domesticated rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part A 2005, 141, 353–358. [Google Scholar] [CrossRef]
- Dalile, B.; Vervliet, B.; Bergonzelli, G.; Verbeke, K.; Van Oudenhove, L. Colon-delivered short-chain fatty acids attenuate the cortisol response to psychosocial stress in healthy men: A randomized, placebo-controlled trial. Neuropsychopharmacology 2020, 45, 2257–2266. [Google Scholar] [CrossRef]
- Chu, B.; Marwaha, K.; Sanvictores, T.; Ayers, D. Physiology, Stress Reaction. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Lepczyński, A.; Herosimczyk, A.; Ożgo, M.; Marynowska, M.; Pawlikowska, M.; Barszcz, M.; Taciak, M.; Skomiał, J. Dietary chicory root and chicory inulin trigger changes in energetic metabolism, stress prevention and cytoskeletal proteins in the liver of growing pigs—A proteomic study. J. Anim. Physiol. Anim. Nutr. 2017, 101, e225–e236. [Google Scholar] [CrossRef]
- Birmani, M.W.; Nawab, A.; Ghani, M.W.; Li, G.; Xiao, M.; An, L. A Review: Role of Inulin in Animal Nutrition. Food Sci. Technol. Res. 2019, 6, 18–27. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Clarendon Press: Oxford, UK, 2015. [Google Scholar]
- Urías-Silvas, J.E.; Cani, P.D.; Delmée, E.; Neyrinck, A.; López, M.G.; Delzenne, N.M. Physiological effects of dietary fructans extracted from Agave tequilanaGto. and Dasylirion spp. Br. J. Nutr. 2008, 99, 254–261. [Google Scholar] [CrossRef]
- De Lucas Rodrigues Bittencourt, N.; Molinari, L.M.; De Oliveira Scoaris, D.; Pedroso, R.B.; Nakamura, C.V.; Ueda-Nakamura, T.; De Abreu Filho, B.A.; Dias Filho, B.P. Haematological and biochemical values for Nile tilapia Oreochromis niloticus cultured in semi-intensive system. Acta Sci. Biol. Sci. 2003, 25, 385–389. [Google Scholar]
- Aketch, B.O.; Ang’Ienda, P.O.; Radull, J.O.; Waindi, E.N. Effect of stocking density on the expression of glucose transporter protein 1 and other physiological factors in the Lake Victoria Nile tilapia, Oreochromis niloticus (L.). Int. Aquat. Res. 2014, 6, 69. [Google Scholar] [CrossRef][Green Version]
- Biller, J.D.; Takahashi, L.S. Oxidative stress and fish immune system: Phagocytosis and leukocyte respiratory burst activity. An. Acad. Bras. Cienc. 2018, 90, 3403–3414. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Lizárraga-Velázquez, C.E.; Hernández, C.; Sánchez-Gutiérrez, E.Y. Exploitation of Agro-Industrial Waste as Potential Source of Bioactive Compounds for Aquaculture. Foods 2020, 9, 843. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, J.; Zhang, Z.; Xu, J.; Xie, Z.; Slavin, M.; Gao, X. In vitro and in vivo antioxidant activity of a fructan from the roots of Arctium lappa L. Int. J. Biol. Macromol. 2014, 65, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.-F.; Zhang, Y.-Y.; Zhu, Z.-K.; Fu, Y.-P.; Paulsen, B.S.; Huang, C.; Feng, B.; Li, L.-X.; Chen, X.-F.; Jia, R.-Y.; et al. Characterization of inulin-type fructans from two species of Radix Codonopsis and their oxidative defense activation and prebiotic activities. J. Sci. Food Agric. 2021, 101, 2491–2499. [Google Scholar] [CrossRef]
- Zhang, C.-N.; Tian, H.-Y.; Li, X.-F.; Zhu, J.; Cai, D.-S.; Xu, C.; Wang, F.; Zhang, D.-D.; Liu, W.-B. The effects of fructooligosaccharide on the immune response, antioxidant capability and HSP70 and HSP90 expressions in blunt snout bream (Megalobrama amblycephala Yih) under high heat stress. Aquaculture 2014, 433, 458–466. [Google Scholar] [CrossRef]
- Corrêa-Ferreira, M.L.; Verdan, M.H.; dos Reis Lívero, F.A.; Galuppo, L.F.; Telles, J.E.Q.; Alves Stefanello, M.É.; Acco, A.; de Oliveira Petkowicz, C.L. Inulin-type fructan and infusion of Artemisia vulgaris protect the liver against carbon tetrachloride-induced liver injury. Phytomedicine 2017, 24, 68–76. [Google Scholar] [CrossRef]
- Sun, B.; Jia, Y.; Yang, S.; Zhao, N.; Hu, Y.; Hong, J.; Gao, S.; Zhao, R. Sodium butyrate protects against high-fat diet-induced oxidative stress in rat liver by promoting expression of nuclear factor E2-related factor 2. Br. J. Nutr. 2019, 122, 400–410. [Google Scholar] [CrossRef]
- García-Curbelo, Y.; Ayala, L.; Bocourt, R.; Albelo, N.; Nuñez, O.; Rodríguez, Y.; López, M.G. Agavins as prebiotic. Their influence on lipid metabolism of pigs. Cuba. J. Agric. Sci. 2018, 52, 395–400. [Google Scholar]

| Ingredient (g/100 g) | D0 | D20 * | D40 * |
|---|---|---|---|
| Fish meal a | 32.60 | 32.60 | 32.60 |
| Soybean meal b | 33.00 | 33.00 | 33.00 |
| Polished rice meal b | 4.00 | 4.00 | 4.00 |
| Sorghum meal b | 3.00 | 3.00 | 3.00 |
| Meat and bone meal b | 7.00 | 7.00 | 7.00 |
| Soybean lecithin b | 0.65 | 0.65 | 0.65 |
| Corn meal b | 8.40 | 6.20 | 4.00 |
| Krill meal b | 2.00 | 2.00 | 2.00 |
| Wheat meal c | 5.00 | 5.00 | 5.00 |
| Calcium phosphate dibasic d | 0.20 | 0.20 | 0.20 |
| Hydrolyzed fish e | 3.00 | 3.00 | 3.00 |
| Vitamin and mineral mix f | 1.00 | 1.00 | 1.00 |
| Fish and vegetable oil g | 0.20 | 0.20 | 0.20 |
| Agavin (fructan) h | 0.0 | 2 | 4 |
| Proximal composition | |||
| Dry matter | 93.41 | 93.07 | 93.79 |
| Crude protein | 35.90 | 35.81 | 35.63 |
| Crude lipid | 6.98 | 7.01 | 6.90 |
| Ash | 18.96 | 18.79 | 18.49 |
| Nitrogen-free extract (NFE) | 31.57 | 31.46 | 32.77 |
| C− | C+ | D20 | D40 | |
|---|---|---|---|---|
| Initial body weight (g) | 1.04 ± 0.01 | 1.04 ± 0.01 | 1.03 ± 0.01 | 1.03 ± 0.01 |
| Final body weight (g) | 39.0 ± 9.4 | 40.7 ± 7.3 | 39.9 ± 7.6 | 38.3 ± 9.6 |
| Weight gain (g) | 37.9 ± 4.1 | 39.5 ± 2.9 | 38.9 ± 1.6 | 37.5 ± 2.5 |
| SGR (%/day) | 4.49± 0.1 | 4.48 ±0.1 | 4.51 ± 0.05 | 4.50 ± 0.1 |
| IFC | 21.2 ± 2.7 | 22.8 ± 2.1 | 23.3 ± 1.1 | 23.0 ± 2.3 |
| FCR | 1.17 ± 0.07 | 1.18 ± 0.06 | 1.14 ± 0.05 | 1.18 ± 0.04 |
| Survival (%) | 100 ± 0.0 | 98 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 |
| Body composition | ||||
| Moisture (%) | 76.4 ± 0.1 | 76.5 ± 0.1 | 77.1 ± 0.5 | 75.1 ± 0.4 |
| Crude protein (%) | 12.7 ± 0.8 | 13.1 ± 0.2 | 12.5 ± 0.9 | 13.8 ± 0.3 |
| Crude lipids (%) | 5.1 ± 0.2 | 5.1 ± 0.1 | 4.9 ± 0.1 | 5.1 ± 0.2 |
| Ash (%) | 1.4 ± 0.6 | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.7 ± 0.1 |
| C− | C+ | D20 | D40 | |
|---|---|---|---|---|
| Initial body weight (g) | 39.0 ± 9.4 | 40.7 ± 7.3 | 39.9 ± 7.6 | 38.3 ± 9.6 |
| Final body weight (g) | 235.6 ± 22.8 a | 159.6 ± 27.5 c | 219.8 ± 24.5 ab | 193.2 ± 16.6 bc |
| Weight gain (g) | 196.0 ± 13.7 a | 118.9 ± 14.2 c | 179.9 ± 14.4 ab | 154.9 ± 24.4 b |
| SGR (%/day) | 7.4 ± 0.3 a | 6.1 ± 0.6 b | 6.9 ± 0.5 ab | 6.5 ± 0.7 ab |
| IFC | 147.8 ± 19.3 a | 117.8 ± 15.4 b | 137.0 ± 10.2 ab | 129.8 ± 16.7 ab |
| FCR | 0.94 ± 0.1 | 1.0 ± 0.06 | 1.0 ± 0.09 | 1.0 ± 0.1 |
| K (%) | 1.9 ± 0.1 | 1.8 ± 0.1 | 1.8 ± 0.05 | 1.8 ± 0.1 |
| HSI (%) | 1.5 ± 0.3 | 2.2 ± 0.7 | 2.7 ± 0.7 | 1.9 ± 0.8 |
| VSI (%) | 5.1 ± 0.9 | 5.8 ± 0.9 | 6.2 ± 1.6 | 6.1 ± 1.9 |
| Survival (%) | 97.0 ± 0.0 | 97 ± 0.0 | 94 ± 0.0 | 100 ± 0.0 |
| Body composition | ||||
| Moisture (%) | 74.2 ± 0.4 | 73.8 ± 0.3 | 73.5 ± 0.5 | 74.6 ± 0.4 |
| Crude protein (%) | 14.1 ± 0.6 | 14.4 ± 0.6 | 14.8 ± 0.2 | 14.3 ± 0.8 |
| Crude lipids (%) | 6.2 ± 0.1 | 5.7 ± 0.1 | 5.8 ± 0.1 | 5.3 ± 0.1 |
| Ash (%) | 1.9 ± 0.2 | 2.1 ± 0.1 | 2.0 ± 0.1 | 2.2 ± 0.2 |
| C− | C+ | D20 | D40 | |
|---|---|---|---|---|
| Cortisol (ng/mL) | 45.5± 4.8 | 44.9 ± 2.3 | 45.9 ± 1.6 | 44.0 ± 1.7 |
| Glucose (mg/dL) | 63.0 ± 2.2 | 68.1 ± 2.5 | 64.9 ± 2.9 | 69.6 ± 2.1 |
| Cholesterol (mg/dL) | 143.6 ± 4.7 | 154.5 ± 3.1 | 134.0 ± 7.3 | 156.5 ± 8.4 |
| Triglycerides (mg/dL) | 330.2 ± 8.8 | 283.0 ± 12.5 | 251.0 ± 8.1 | 260.0 ± 14.8 |
| Total protein (g/dL) | 2.8 ± 0.1 | 2.7 ± 0.1 | 2.5 ± 0.1 | 2.7 ± 0.2 |
| C− | C+ | D20 | D40 | |
|---|---|---|---|---|
| CAT | 31.8 ± 5.3 | 27.3 ± 7.1 | 33.9 ± 2.3 | 24.7 ± 1.3 |
| SOD | 7.1 ± 1.4 b | 3.8 ± 0.7 c | 11.2 ± 0.6 a | 3.7 ± 0.1 c |
| MDA | 0.14 ± 0.06 | 0.15 ± 0.12 | 0.08 ± 0.04 | 0.04 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Méndez, L.C.; Lizárraga-Velázquez, C.E.; Sánchez-Gutiérrez, E.Y.; Arrizon, J.; Leyva-López, N.; Hernández, C. Study of the Effect of Dietary Agavin Supplementation in Blood Parameters and Antioxidant Enzymes of Juvenile Nile Tilapia (Oreochromis niloticus) under Stress Conditions. Fishes 2022, 7, 340. https://doi.org/10.3390/fishes7060340
Flores-Méndez LC, Lizárraga-Velázquez CE, Sánchez-Gutiérrez EY, Arrizon J, Leyva-López N, Hernández C. Study of the Effect of Dietary Agavin Supplementation in Blood Parameters and Antioxidant Enzymes of Juvenile Nile Tilapia (Oreochromis niloticus) under Stress Conditions. Fishes. 2022; 7(6):340. https://doi.org/10.3390/fishes7060340
Chicago/Turabian StyleFlores-Méndez, Lizeth Carolina, Cynthia E. Lizárraga-Velázquez, Erika Y. Sánchez-Gutiérrez, Javier Arrizon, Nayely Leyva-López, and Crisantema Hernández. 2022. "Study of the Effect of Dietary Agavin Supplementation in Blood Parameters and Antioxidant Enzymes of Juvenile Nile Tilapia (Oreochromis niloticus) under Stress Conditions" Fishes 7, no. 6: 340. https://doi.org/10.3390/fishes7060340
APA StyleFlores-Méndez, L. C., Lizárraga-Velázquez, C. E., Sánchez-Gutiérrez, E. Y., Arrizon, J., Leyva-López, N., & Hernández, C. (2022). Study of the Effect of Dietary Agavin Supplementation in Blood Parameters and Antioxidant Enzymes of Juvenile Nile Tilapia (Oreochromis niloticus) under Stress Conditions. Fishes, 7(6), 340. https://doi.org/10.3390/fishes7060340








