Abstract
The giant mottled eel, Anguilla marmorata, is at high risk of extinction due to overfishing of glass eels and elvers to provide stock for eel farming. In Vietnam, information on the genetic diversity and population structure of this species, which is necessary for resource management, is limited. In order to address this paucity of information, sequencing of the entire mitochondrial control region (mtDNA) was carried out for 176 individuals collected from central Vietnam. The sequences were investigated using various genetic, phylogenetic and population analyses. A total of 165 distinct haplotypes were identified. The percentage of variation within and among populations was 99.26% and 0.74%, respectively. The fixation index was low (0.007) and not significant (p = 0.096). Therefore, panmixia and a lack of significant population genetic structuring seem likely for A. marmorata in central Vietnam. Most sampled eels were genetically similar to eels found in North Pacific populations (Japan, Taiwan and the Philippines), except for one sample from Quang Tri and two samples from Binh Dinh, which had high values of genetic identity (97% to 99%) with South Pacific populations (Tahiti, Fiji, New Caledonia and Papua New Guinea). Taken together, we suggest that A. marmorata from the North and South Pacific populations co-exist in central Vietnam.
1. Introduction
The giant mottled eel, Anguilla marmorata Quoy & Gaimard, 1824, has the widest known geographic distribution among the nineteen species/subspecies of the genus Anguilla (Family Anguillidae) [1,2]. Unlike many anguillid species, A. marmorata is believed to have at least four distinct spawning grounds, multiple population structure [3,4] and spawning throughout much of the year [5,6,7,8,9,10].
Due to the over-exploitation of temperate anguillid eel species (i.e., A. anguilla and A. japonica) coupled with the drastic decline in juvenile recruitment in Europe and East Asia, A. marmorata and many other tropical eel species in Southeast Asia have recently become targets to replace the temperate eels as species for human consumption [11,12,13,14]. Therefore, it is conceivable that tropical eel species will soon face an increased threat of extinction, as seen in their temperate congeners, if proper management and conservation policies are not implemented. In order to develop appropriate policies, data on genetic diversity and population structure of wild eel stocks are urgently needed [15]. Indeed, the genetic population diversity of tropical anguillid eels has received intense research attention in recent years, with several studies published on A. marmorata [3,4,16,17,18], A. bicolor [19,20], A. bicolor bicolor [21], A. megastoma [22] and A. bicolor pacifica [15]. Mitochondrial sequences, nuclear microsatellites and amplified fragment-length polymorphism have been used to determine the population genetic structure of A. marmorata [3,4]. These methods revealed the existence of five populations, as determined on the basis of genetic differentiation indices, in the North Pacific Ocean, South Pacific Ocean, Southwestern Indian Ocean, Sumatra and Guam [18].
Irrespective of the results obtained from these studies, future investigation into genetic diversity and population structure of tropical anguillid species in specific locations such as Vietnam is still required to gain insight into their migratory ecology and speciation mechanisms [23].
Vietnam is known as one of the richest regions in the world in terms of marine biodiversity [24]. There are at least three freshwater eel species (A. marmorata, A. japonica and A. bicolor pacifica) found in the central parts of Vietnam [25]. Of these species, A. marmorata predominates and is consequently cultured widely throughout Vietnam, but farms depend entirely on wild-caught seed to stock tanks [26]. There are currently no specific policies for active management or conservation of anguillid eels in Vietnam, mainly due to budget shortages [26]. In the context of global conservation and management of anguillid eels, broad cooperation between countries is required [14], and information about genetic diversity and population structure within a species is necessary for sustainable fisheries management [27]. However, there is a severe lack of data on the A. marmorata population in Vietnam.
This study provides the first definitive data on population structure of freshwater eels in Vietnam, for use in eel conservation and management of aquaculture on a regional and/or global scale. By analyzing the sequences of the mitochondrial DNA control region (mtCR) for 176 individuals caught in locations throughout central Vietnam, we determined the population structure of A. marmorata in the area. Additionally, this study investigated the relationship of the Vietnam population to other A. marmorata populations around the world.
2. Materials and Methods
2.1. Sampling Location
Sampling was conducted across four provinces of central Vietnam in 2020 (Figure 1). A total of 176 individuals of A. marmorata, ranging in total length from 44 to 265 mm, were collected by electrofishing and trawl netting from the following provinces: Quang Tri (QT), Quang Ngai (QN), Binh Dinh (BD) and Phu Yen (PY) (Table 1). The samples collected from QT (October 2020) and QN (May 2020) were elvers (n = 30 for each location). The BD and PY samples were glass eels that were collected at two different time points, reflecting their recruitment season (March 2020: BD1 (n = 29), PY1 (n = 28); December 2020: BD2 (n = 31), PY2 (n = 28)).
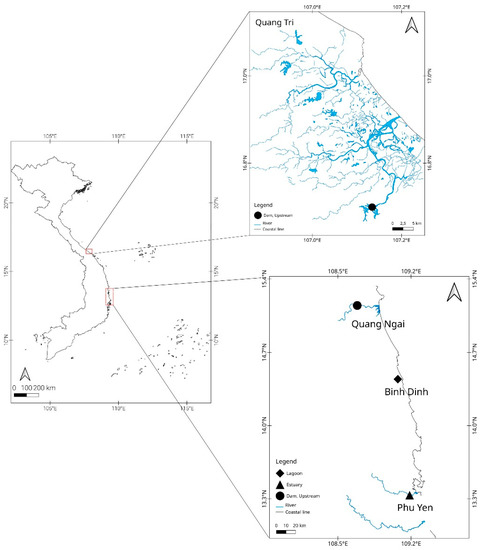
Figure 1.
Sampling localities of eels in Vietnam.

Table 1.
Summary of the collection sites and sizes of Anguilla marmorata that were used for mtDNA analyses.
2.2. Sampling Protocols
The sampling protocols were as described by Nguyen et al. [25]. Briefly, eels were anesthetized with Aqui-S (0.01 mL/L) before fin clips (approx. 5 mm2) were taken, preserved in 70% ethanol and stored at 4 °C until DNA extraction. Revived eels were returned to the wild. All animal manipulations were approved by the Animal Ethics Committee of Hue University, Vietnam (permit No. HUVN0015/QDDHH).
2.3. DNA Extraction and Amplification
Genomic DNA was extracted from fin clips using a modified CTAB protocol [28]. A DNA fragment (1.0–1.2 kb) containing the entire A. marmorata mtCR was amplified via PCR using the following external primer pair: FW: 5′–TTTGTAATCCGAAGATTGAAG-3′ and RV: 5′–CAGAACTGATGTTAAAGTCAG-3′ [4]. A nested primer pair (FW: CATTTGGTTCCTATTTCAGG and RV: CCGTGAATTAATGCTCGGC) [4], was used for confirmation sequencing. The PCR reaction was performed in a total volume of 50 μL including 10 μL of buffer, 0.2 μL MyTaq HS Polymerase (Bioline, Luckenwalde, Germany), 0.4 μM of each primer and 5 ng of the template DNA. The PCR amplification was carried out using an Eppendorf EpGradient S PCR machine under the following conditions: initial denaturation at 95 °C for two min, followed by 35 cycles of 40 s at 95 °C for denaturation, annealing at 52 °C for 40 s and extension at 72 °C for 90 s. A total of 5 µL of PCR product was run out on a 1% agarose gel and visualized using 6XGelRed® stain (Biotium, Fremont, CA, USA). Sequencing reactions were performed at 1st Base (Kualar Lumpur, Malaysia) using an ABI Prism 3700 DNA Analyser (Applied Biosystems, Waltham, WA, USA).
2.4. Sequencing Analysis
MtCR sequences of 176 eel samples were confirmed and assembled using Sequences v.4.8 (Gen Codes) and subsequently aligned using BioEdit v.7.0 [29]. The haplotype number (Nh), haplotype diversity (h), nucleotide diversity (π), number of polymorphic sites (S), number of mutations (η), average number of nucleotides differences (k), Tajima’s D and Fu’s Fs were calculated using DnaSP v6.12 [30]. Population expansion patterns in the Vietnam eel populations were tested by estimating Fu’s Fs [31] and Tajima’s D [32], as well as their significance. Fu’s Fs statistic is based on the distribution of haplotypes, while Tajima’s D is based on the allele frequency when comparing pairwise differences between sequences [33].
In order to examine the genetic relationships of the A. marmorata population in Vietnam in relation to other populations around the world (Japan (JP), Taiwan (TW), Philippines (PH), Sulawesi (SL), Tahiti (TH), Fiji (FJ), New Caledonia (NC), Papua New Guinea (PNG), Sumatra (SM), Réunion (RU), Madagascar (MD), Ambon (AB) and Guam (GU)), the 176 samples were combined with 267 mtCR sequences from the DDBJ, EMBL and Genbank databases identified by Minegishi et al. [4] (Table 2). Arlequin 3.5.2. [34] was employed to conduct an analysis of molecular variance (AMOVA) in order to obtain the genetic differentiation indices (FST) and genetic variation partitioning within and among populations. The permutation tests for those significances were conducted using 10,000 permutations. Mega 10.1.7 software was used to align the sequences by the MUSCLE algorithm [35], calculate genetic distances and genetic diversity, and to construct a neighbor-joining tree. The confidence level of the phylogenetic tree was tested with 1000 replications [36].

Table 2.
Samples used for statistical analysis of Anguilla marmorata mtDNA.
3. Results
3.1. Genetic Diversity
Analysis of 176 individuals collected from central Vietnam detected a total of 165 distinct haplotypes and 306 polymorphic sites. The π value was 0.026 ± 0.002, and the hd was 0.999 ± 0.001 (Table 3). Across all sampling sites, the lowest and highest hd and π values were found in samples from Binh Dinh. In particular, the samples collected from Binh Dinh in March 2020 (BD1) showed the highest hd (1.000 ± 0009) and π (0.035 ± 0.004), while the lowest values for hd (0.096 ± 0.009) and π (0.019 ± 0.003) were detected in samples collected from Binh Dinh in December 2020 (BD2) (Table 3).

Table 3.
Genetic diversity of A. marmorata populations in Vietnam based on mtCR sequence.
3.2. Population Genetic Structure
The percentages of variation within and among populations were 99.26% and 0.74%, respectively. The fixation index (FST) was low (0.007) and not significant (p = 0.096) (Table 4). Pairwise FST values ranged from −0.02356 to 0.05235 (Table 5). Significant pairwise FST values were only detected between BD1 and BD2 (FST = 0.04418, p < 0.05) and BD1 and QN (0.05235, p < 0.001) (Table 5). Analysis of molecular variance indicated that most of the genetic variation within A. marmorata (99.26%) was within populations, with only a small (and non-significant) amount of the variation attributed to among-site differences (Table 5). Fu’s Fs and Tajima’s D values were significant and negative in most populations (except for BD1 and QT, Tajima’s D) (Table 6).

Table 4.
Analysis of Molecular Variance (AMOVA) results for A. marmorata populations collected in Vietnam.

Table 5.
FST values (above diagonal) and probability values (below diagonal) among different A. marmorata populations in Vietnam.

Table 6.
Tajima’s D and Fu’s Fs with corresponding probability values in parentheses.
The neighbor-joining tree showed that all individuals of the Vietnam population grouped into one large clade with other populations distributed in the North Pacific, except for one sample from Quang Tri (QT-219) and two samples from Binh Dinh (BD1-007 and BD1-013) (Figure S1). These samples were nested within two different groups. In particular, the two samples collected from BD were grouped with the South Pacific populations (FJ10, TH96109, TH96106, TH96112, AM97523, NC97335, Tahiti-4, Tahiti-5, FJ50 and TH96115), while the sample from Quang Tri (QT-219) was nested in the clade with Taiwan-24, Taiwan-43 and other individuals from South Pacific populations (PNG96308, Tahiti-3, TH96111, FJ52, AM97524, TH96114, FJ23 and FJ3).
This result was supported by genetic identity between samples (QT-219, BD1-007 and BD1-013) with other A. marmorata populations (Table 7), being most closely related to the individuals collected from PNG, NC, FJ and TH, with high values of genetic identity ranging from 97% to 99%. Meanwhile, the genetic identity values between these three samples and the VN, PH, TW and JP populations were only 94–95%.

Table 7.
Genetic identity (%) between QT-219, BD1-007 and BD1-013, and other A. marmorata populations.
3.3. The Genetic Relationship between the A. marmorata Population in Vietnam and Other Populations
In the pairwise FST comparisons between the A. marmorata population in Vietnam and the other 13 populations surveyed in Minegishi et al. [4], the FST values ranged from −0.0141 to 0.6065 (Table 8). The FST values between Vietnam and other localities in the North Pacific (Japan, Taiwan and the Philippines) were very low (−0.0068 to 0.0083, p > 0.05) while these values between Vietnam and the remaining ten localities from the other areas were relatively high (0.24543 to 0.6065, p < 0.05). A similar pattern was observed for genetic identity. In particular, the values of genetic identity between Vietnam, the Philippines, Taiwan and Japan were relatively high (97.32%, 96.94% and 97.32%, respectively). Meanwhile, this value ranged from 93–95% between Vietnam and the remaining localities in the study by Minegishi et al. [4].

Table 8.
FST, probability values and genetic identity between the A. marmorata population in Vietnam and other populations around the world.
4. Discussion
Among freshwater eel species in Vietnam, A. marmorata is the most economically important [26], which is reflected by the fact that 95% of Vietnamese eel farms focus on the giant mottled eel [37]. The inability to reliably propagate eels in captivity sees current farming practices relying solely on fattening of wild-caught glass eels. This over-exploitation of wild eel stocks has contributed heavily to declining populations and has left the species under threat. Information on the genetic status of A. marmorata is necessary for effective resource management, but is currently limited. The present study examined the population structure of A. marmorata in Vietnam and the relationship of this population to other previously researched populations around the globe.
Analysis of the complete mtCR sequence found high genetic diversity within the A. marmorata population in central Vietnam. This was indicated by 165 distinct haplotypes and 306 polymorphic sites detected from 176 samples. Comparable variation has been reported in similar studies, which utilized the mtCR as a molecular marker to evaluate genetic population diversity of the giant mottled eel. For example, Ishikawa et al. [3] identified 151 mt DNA haplotypes from 162 A. marmorata individuals, collected across ten localities (three in Japan, four in Indonesia and one each in Fiji, Tahiti and Madagascar). More recently, Minegishi et al. [4] detected 267 separate haplotypes in a total of 290 individuals sampled from 13 different regions within the Pacific and Indian Oceans. Irrespective of rapid molecular evolution, the control region of the mtDNA is accepted as a suitable molecular marker for investigating intraspecific population relationships, due to its high mutation rate in comparison with other mtDNA regions [38,39,40]. Therefore, the high value of haplotype diversity (hd = 0.999 ± 0.001) found in this study is reasonable. With regard to demographic estimators, the A. marmorata population in Vietnam exhibited significant and negative Tajima’s D and Fu’s Fs values, indicating an excess of rare haplotypes and rapid population growth.
According to Frankham et al. [41], an FST value > 0.15 implies significant genetic differentiation among sampling groups. In the present study, the FST value was very low (FST = 0.007) and non-significant (p = 0.096). Moreover, there were only two pairs (BD1 and BD2; BD1 and QN) showing significant differences in the pairwise comparisons of FST values (see Figures S2 and S3 for haplotype networks between these populations). Panmixia and a lack of significant population genetic structuring seem likely for A. marmorata across our sampling sites. This assumption was supported by findings from Minegishi et al. [4], in which the North Pacific population was considered to be fully panmictic.
Mitochondrial DNA markers are widely accepted in evolutionary and population genetics studies [40]. However, the employment of an mtDNA marker in the present study inevitably comes with limitations. In particular, mtDNA has a high evolutionary rate and an effective population size, approximately one-quarter that of nuclear markers. This can lead to an underestimation of genetic diversity, unwanted biases in the genealogical analysis due to missing links in mitochondrial haplotypes and the limited detection of remote population processes [42]. These weaknesses may have contributed to the identification of significant differences in the pairwise comparisons of FST values in only two pairs: BD1 and BD2; and BD1 and QN. Moreover, mtDNA is maternally inherited (i.e., lacks recombination) and inherently reflects only the matrilineal history instead of that of the overall population or species [42]. Due to this restriction, it can be beneficial to combine multiple molecular markers, such as microsatellites, which can provide data on gene flow in the present day or in recent generations [4]; this is something that should be incorporated into future studies in order to obtain a comprehensive understanding (i.e., parentage, relatedness and inbreeding depression) of the population genetic structure of A. marmorata in Vietnam.
When comparing eels collected in Vietnam to other A. marmorata populations around the world, most were genetically similar to eels found in Japan, Taiwan and the Philippines; all of these eels were assigned to the North Pacific population by Ishikawa et al. [3] and Minegishi et al. [4]. This was supported by high genetic identity (approximately 97%) and low FST values (−0.0068 to 0.0083, p > 0.05) when comparing the Vietnam population to the three aforementioned populations (c.f. Table 8). The findings from our study further reinforce this sentiment, as all individuals from the Vietnam population, except for one sample from Quang Tri and two samples from Binh Dinh (see below), formed a single large clade with other populations from the North Pacific (c.f. the neighbor-joining tree).
Miller et al. [43] and Tsukamoto et al. [44] proposed for the spawning area of A. marmorata to be in the western North Pacific, close to the spawning ground of A. japonica. Closeness of the spawning grounds is reflected, at least in part, in the well-documented sympatry of both species in China [45], Taiwan and southern Japan (e.g., Wakiya et al. [46]). Indeed, we have previously reported the coexistence of A. marmorata and A. japonica in the inland waters of Vietnam [25]. Based on the currents in the South China Sea, we assume that recruitment of A. marmorata along the coast of central Vietnam is influenced by two main currents, the South China Sea Warm Current and the Kuroshio Intrusion Current. Both of these currents originate from the main body of the Kuroshio Current [47,48] which is known to be one of the main currents transporting larval A. marmorata throughout the Pacific [2,23].
Interestingly, we identified three individuals (QT-219, BD1-07 and BD1-013) which were more genetically similar to the South Pacific populations than to the North Pacific populations. Not only did these individuals show high values of genetic identity with A. marmorata populations from Tahiti, Fiji, New Caledonia and Papua New Guinea, but they were also nested within the South Pacific clade of our neighbor-joining tree. Our findings are supported by A. marmorata belonging to South Pacific populations found in areas of Southeast Asia, such as Indonesia (GenBank KU695248, KU692251 and KU692252) and Taiwan (KU885607), as documented in Arai et al. [49].
The spawning area of A. marmorata in the South Pacific remains incompletely defined. However, a recent study has suggested that there could be at least two spawning areas within the New Caledonia to Samoa region, and another further east [50]. Additionally, Schabetsberger et al. [51] proposed that the spawning area of A. marmorata is likely located somewhere near Tuvalu, and the larvae are transported towards Southeast Asia by the South Equatorial Current at a maximum speed of 0.15 ms−1. We note that there are several complex seasonal currents connecting the western region of the South Pacific to the Indian Ocean through Indonesia [50]. This is reinforced by the complex currents depicted by Lee et al. [52] and Pattiaratchi & Siji [53]. According to Ganachaud et al. [54], the North Vanuatu Jet is supplied by waters from the South Equatorial Current flowing north of Fiji. To the northeast of Australia, the North Vanuatu Jet forks with the northern portion flowing directly into the Solomon Sea, the remainder continuing towards the Queensland coast before turning north to join the Gulf of Papua Current [55]. Further, the waters from both the North and South Pacific make their way to the Indian Ocean through the Indonesian Throughflow. Previous studies indicate that the North and South Pacific waters reach the Indonesia Seas through Makassar Strait, with a smaller contribution entering through Lifamatola Passage, the South China Sea and Karimatra Strait [56,57,58,59]. Taken together, it seems very likely that these complex currents could transport A. marmorata from Micronesia to the South China Sea, but the specific current(s) responsible for this transport is (are) presently unknown. According to Dao et al. [60], there was no difference in genetic population structure of the ornate spiny lobster, Panulirus ornatus, across a broad region of the Southeast ranging from Vietnam, Indonesia, Australia and Papua New Guinea, due to its long oceanic larval development phase and wide larval transport capability [61]. Hence, the finding of A. marmorata glass eels originating from the South Pacific populations in the present study is reasonable.
Notably, the South China Sea is subjected to seasonal monsoons; during the southwest monsoon (summer) the surface circulation is directed northward, while the direction of circulation reverses during the northeast monsoon (winter) [47,62]. The sampling interval of BD1-07 and BD1-013 (glass eels) coincided with the southwest monsoon, indicating that these individuals could have been transported via a northward current from the South Pacific. In addition, A. marmorata from the Ambon region, an area where individuals of the North and South Pacific populations co-exist [4], could be transported by the Indonesia Throughflow via the Makassar Strait [63] towards Vietnam. To date, glass eels have not been found in Quang Tri [26]. Indeed, the QT-219 sample was an elver collected during the northeast monsoon. Therefore, it is possible that the QT-219 individual may have made its way from Binh Dinh to Quang Tri via the northward circulation in the South China Sea. This assumption was reinforced by the differences in size among sampled A. marmorata between Binh Dinh (glass eels) and Quang Tri (yellow eels). Interestingly, QT-219 was part of a clade with Taiwan-24 (AB279089.1), Taiwan-43 (AB279103.1) and other individuals from South Pacific populations. Therefore, we cannot rule out the possibility that BD1-07, BD1-013 and QT-219 were transported directly to coastal Vietnam from the spawning ground in the South Pacific Ocean by unknown northward flows. Regardless of the mechanism, we conclude that our evidence for some A. marmorata in central Vietnam to have originated from the South Pacific population is compelling.
It is clear that A. marmorata from the North and South Pacific populations migrate to Vietnam via seasonally dependent routes. In order to combat increased population pressures resulting from overfishing of this species in the South East Asia region, we recommend that the Vietnamese government revise their regulations with regard to a restricted fishing season, imposing catch size limits or requiring fishing licenses. Additionally, introducing policies which facilitate the passage of glass eels over dams, reduce anthropogenic influences on glass eel recruiment and restrict the collection of glass eels will significantly aid in the management and conservation of A. marmorata in Vietnam.
5. Conclusions
The giant mottled eel population in central coastal Vietnam is panmictic with high genetic diversity, and consists of individuals from both the North and South Pacific populations. Future investigations into the migration patterns of A. marmorata individuals which are genetically similar to the South Pacific populations must be conducted to guide eel conservation and management of wild eel stocks for aquaculture in Vietnam.
Supplementary Materials
The supplementary materials are available online at https://www.mdpi.com/article/10.3390/fishes7050286/s1, Figure S1: The neighbor-joining tree for Anguilla marmorata based on the mitochondrial control region, Figure S2: Haplotype networks between BD1 and BD2, Figure S3: Haplotype networks between BD1 and QN.
Author Contributions
A.T.N.: conceptualization, methodology, investigation, writing—original draft, revision, project administration. H.T.D.: methodology, investigation. H.T.Q.: methodology. S.H.: methodology. P.M.L.: conceptualization, writing—review, revision and editing. E.L.D.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant (B2020-DHH-09) from the Vietnamese Ministry of Education and Training to Anh Tuan Nguyen.
Institutional Review Board Statement
All animal manipulations were approved by the Animal Ethics Committee of Hue University, Vietnam (permit No. HUVN0015/QĐ-DHH).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
This study would not have been possible without great help from my colleagues. We would like to thank Duc Nghia Vo, Van Huy Nguyen, Duc Thanh Nguyen, Tan Xinh Huynh, Van Khanh Vo, Thoi Tuy Ngo and Nam Thang Ha (Hue University) for their assistance during the period of sampling in Vietnam. The authors wish to thank Graham McCulloch (University of Otago) and Phan Thi Thao Nguyen (Institute of Biotechnology) for advice on data interpretation and reporting.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ege, V. A revision of the genus Anguilla Shaw: A systematic, phylogenetic and geographical study. Dana Rep. 1939, 16, 1–256. [Google Scholar]
- Kuroki, M.; Aoyama, J.; Miller, M.; Yoshinaga, T.; Shinoda, A.; Hagihara, S.; Tsukamoto, K. Sympatric spawning of Anguilla marmorata and Anguilla japonica in the western North Pacific Ocean. J. Fish Biol. 2009, 74, 1853–1865. [Google Scholar] [CrossRef]
- Ishikawa, S.; Tsukamoto, K.; Nishida, M. Genetic evidence for multiple geographic populations of the giant mottled eel Anguilla marmorata in the Pacific and Indian oceans. Ichthyol. Res. 2004, 51, 343–353. [Google Scholar] [CrossRef]
- Minegishi, Y.; Aoyama, J.; Tsukamoto, K. Multiple population structure of the giant mottled eel, Anguilla marmorata. Mol. Ecol. 2008, 17, 3109–3122. [Google Scholar] [CrossRef] [PubMed]
- Tabeta, O.; Tanimoto, T.; Takai, T.; Matsui, I.; Imamura, T. Seasonal occurrence of anguillid elvers in Cagayan River, Luzon Island, the Philippines. Bull. Jpn. Soc. Sci. Fish 1976, 42, 421–426. [Google Scholar] [CrossRef]
- Budimawan. The early life history of the tropical eel Anguilla marmorata (Quog & Gaimard, 1824) from four Pacific estuaries, as revealed from otolith microstructural analysis. J. Appl. Ichthyol. 1997, 13, 57–62. [Google Scholar]
- Arai, T.; Aoyama, J.; Limbong, D.; Tsukamoto, K. Species composition and inshore migration of the tropical eels Anguilla spp. recruiting to the estuary of the Poigar River, Sulawesi Island. Mar. Ecol. Prog. Ser. 1999, 188, 299–303. [Google Scholar] [CrossRef]
- Arai, T.; Limbong, D.; Otake, T.; Tsukamoto, K. Metamorphosis and inshore migration of tropical eels Anguilla spp. in the Indo-Pacific. Mar. Ecol. Prog. Ser. 1999, 182, 283–293. [Google Scholar] [CrossRef]
- Arai, T.; Limbong, D.; Otake, T.; Tsukamoto, K. Recruitment mechanisms of tropical eels Anguilla spp. and implications for the evolution of oceanic migration in the genus Anguilla. Mar. Ecol. Prog. Ser. 2001, 216, 253–264. [Google Scholar] [CrossRef]
- Marui, M.; Arai, T.; Miller, M.J.; Jellyman, D.J.; Tsukamoto, K. Comparison of early life history between New Zealand temperate eels and Pacific tropical eels revealed by otolith microstructure and microchemistry. Mar. Ecol. Prog. Ser. 2001, 213, 273–284. [Google Scholar] [CrossRef]
- Arai, T. Do we protect freshwater eels or do we drive them to extinction? SpringerPlus 2014, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Arai, T. How have spawning ground investigations of the Japanese eel Anguilla japonica contributed to the stock enhancement? Rev. Fish Biol. Fish 2014, 24, 75–88. [Google Scholar] [CrossRef]
- Dekker, W. Worldwide decline of eel resources necessitates immediate action: Quebec declaration of concern. Fisheries 2003, 28, 28–30. [Google Scholar]
- Jacoby, D.M.; Casselman, J.M.; Crook, V.; DeLucia, M.B.; Ahn, H.; Kaifu, K.; Kurwie, T.; Sasal, P.; Silfvergrip, A.M.C.; Smith, K.G.; et al. Synergistic patterns of threat and the challenges facing global anguillid eel conservation. Glob. Ecol. Conserv. 2015, 4, 321–333. [Google Scholar] [CrossRef]
- Marini, M.; Pedrosa-Gerasmio, I.R.; Santos, M.D.; Shibuno, T.; Daryani, A.; Romana-Eguia, M.R.R.; Wibowo, A. Genetic diversity, population structure and demographic history of the tropical eel Anguilla bicolor pacifica in Southeast Asia using mitochondrial DNA control region sequences. Glob. Ecol. ConserV. 2021, 26, e01493. [Google Scholar] [CrossRef]
- Robinet, T.; Guyet, S.; Marquet, G.; Mounaix, B.; Olivier, J.M.; Tsukamoto, K.; Pierre, V.; Feunteun, E. Elver invasion, population structure and growth of marbled eels Anguilla marmorata in a tropical river on Réunion Island in the Indian Ocean. Environ. Biol. Fishes 2003, 68, 339–348. [Google Scholar] [CrossRef]
- Maes, G.E.; Pujolar, J.; Hellemans, B.; Volckaert, F.A. Evidence for isolation by time in the European eel (Anguilla anguilla L.). Mol. Ecol. 2006, 15, 2095–2107. [Google Scholar] [CrossRef] [PubMed]
- Gagnaire, P.A.; Minegishi, Y.; Zenboudji, S.; Valade, P.; Aoyama, J.; Berrebi, P. Within-population structure highlighted by differential introgression across semipermeable barriers to gene flow in Anguilla marmorata. Evol. Int. J. Org. Evol. 2011, 65, 3413–3427. [Google Scholar] [CrossRef] [PubMed]
- Minegishi, Y.; Gagnaire, P.A.; Aoyama, J.; Bosc, P.; Feunteun, E.; Tsukamoto, K.; Berrebi, P. Present and past genetic connectivity of the Indo-Pacific tropical eel Anguilla bicolor. J. Biogeogr. 2012, 39, 408–420. [Google Scholar] [CrossRef]
- Fahmi, M.R.; Solihin, D.D.; Shao, Z.; Pouyaud, L.; Berrebi, P. Population genetic structure of the tropical eel Anguilla bicolor in Indonesian waters based on microsatellite markers. Folia Zool. 2015, 64, 87–96. [Google Scholar] [CrossRef]
- Watanabe, S.; Aoyama, J.; Nishida, M.; Tsukamoto, K. Evaluation of the population structure of Anguilla bicolor bicolor using total number of vertebrae and the mtDNA control region. Coast. Mar. Sci. 2005, 29, 165–169. [Google Scholar]
- Gubili, C.; Schabetsberger, R.; Poellabauer, C.; Bates, B.; Wagstaff, R.M.; Woodward, L.M.; Sichrowsky, U.; Scheck, A.; Boseto, D.T.; Feunteun, E.; et al. High genetic diversity and lack of pronounced population structure in five species of sympatric Pacific eels. Fish. Manag. Ecol. 2019, 26, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, J. Life history and evolution of migration in catadromous eels (Genus Anguilla). Aqua-BioSci. Monogr. 2009, 2, 1–42. [Google Scholar] [CrossRef]
- Lutaenko, K.A. Coastal marine biodiversity of Vietnam (South China Sea), its current problems and major threats. In Proceedings of the Russia-China Bilateral Workshop, Valadivostok, Russia, 10–11 October 2019. [Google Scholar]
- Nguyen, A.T.; Tsukamoto, K.; Lokman, P.M. Composition and distribution of freshwater eels Anguilla spp. in Vietnam. Fish Sci. 2018, 84, 987–994. [Google Scholar] [CrossRef]
- Tuan, N.T.; Duat, H.V. An overview of the Anguillid eel culture in Vietnam. J. Aquac. Mar. Biol. 2021, 10, 96–101. [Google Scholar] [CrossRef]
- Chen, C.A.; Ablan, M.C.A.; McManus, J.W.; Diepernk Bell, J.; Tuan, V.S.; Cabanban, A.S.; Shao, K.T. Population structure and genetic variability of six bar wrasse (Thallasoma hardwicki) in northern South China Sea revealed by mitochondrial control region sequences. Mar. Biotechnol. 2004, 6, 312–326. [Google Scholar] [CrossRef]
- Adamkewicz, S.; Harasewych, M. Systematics and biogeography of the genus Donax (Bivalvia: Donacidae) in eastern North America. Am. Malacol. Bull. 1996, 13, 97–103. [Google Scholar]
- Hall, T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Fu, Y.X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 1997, 147, 915–925. [Google Scholar] [CrossRef]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Soriano, A.; Ramos-Onsins, S.E.; Rozas, J.; Calafell, F.; Navarro, A. Statistical power analysis of neutrality tests under demographic expansions, contractions and bottlenecks with recombination. Genetics 2008, 179, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 1–19. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Honda, S.; Muthmainnah, D.; Suryati, N.K. Exchanging Information on Catadromous Eels in South East Asia. SEAFDEC-Newsletter 2016, 39, 8–9. [Google Scholar]
- Moritz, C.; Dowling, T.; Brown, W. Evolution of animal mitochondrial DNA: Relevance for population biology and systematics. Annu. Rev. Ecol. Evol. Syst. 1987, 18, 269–292. [Google Scholar] [CrossRef]
- Stoneking, M.; Hedgecock, D.; Higuchi, R.G.; Vigilant, L.; Erlich, H.A. Population variation of human mtDNA control region sequences detected by enzymatic amplification and sequence-specific oligonucleotide probes. Am. J. Hum. Genet. 1991, 48, 370. [Google Scholar]
- Avise, J. Molecular Markers, Natural History and Evolution, 2nd ed.; Sinauer & Associates: Sunderland, MA, USA, 2004. [Google Scholar]
- Frankham, R.; Ballou, S.E.J.D.; Briscoe, D.A.; Ballou, J.D. Introduction to Conservation Genetics; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Zhang, D.X.; Hewitt, G.M. Nuclear DNA analyses in genetic studies of populations: Practice, problems and prospects. Mol. Ecol. 2003, 12, 563–584. [Google Scholar] [CrossRef]
- Miller, M.J.; Mochioka, N.; Otake, T.; Tsukamoto, K. Evidence of a spawning area of Anguilla marmorata in the western North Pacific. Mar. Biol. 2002, 140, 809–814. [Google Scholar]
- Tsukamoto, K.; Chow, S.; Otake, T.; Kurogi, H.; Mochioka, N.; Miller, M.J.; Aoyama, J.; Kimura, S.; Watanabe, S.; Yoshinaga, T.; et al. Oceanic spawning ecology of freshwater eels in the western North Pacific. Nat. Commun. 2011, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Yambot, A.V.; Zhang, H.; Hung, C.L. Sympatric spawning but allopatric distribution of Anguilla japonica and Anguilla marmorata: Temperature-and oceanic current-dependent sieving. PLoS ONE 2012, 7, e37484. [Google Scholar] [CrossRef] [PubMed]
- Wakiya, R.; Itakura, H.; Kaifu, K. Age, growth and sex ratios of the giant mottled eel, Anguilla marmorata, in freshwater habitats near its northern geographic limit: A comparison to tropical regions. Zool. Stud. 2019, 58, 34. [Google Scholar]
- Hu, J.; Kawamura, H.; Hong, H.; Qi, Y. A review on the currents in the South China Sea: Seasonal circulation, South China Sea warm current and Kuroshio intrusion. J. Oceanogr. 2000, 56, 607–624. [Google Scholar] [CrossRef]
- Xue, H.; Chai, F.; Pettigrew, N.; Xu, D.; Shi, M.; Xu, J. Kuroshio intrusion and the circulation in the South China Sea. J. Geophys. Res. Oceans 2004, 109, 1978–2012. [Google Scholar] [CrossRef]
- Arai, T.; Taha, H.; Mohd-Riduan, M.N.; Mokti, S.S.A. Molecular and morphological evidence for the identity of the giant mottled eel, Anguilla marmorata in Southeast Asia. Trop. Ecol. 2020, 61, 429–436. [Google Scholar] [CrossRef]
- Kuroki, M.; Miller, I.J.; Feunteun, E.; Sasal, P.; Pikering, T.; Han, Y.S.; Faliex, E.; Acou, A.; Dessier, A.; Schabetsberger, R.; et al. Distribution of anguillid leptocephali and possible spawning areas in the South Pacific Ocean. Prog. Oceanogr. 2020, 180, 102234. [Google Scholar] [CrossRef]
- Schabetsberger, R.; Økland, F.; Aarestrup, K.; Kalfatak, D.; Sichrowsky, U.; Tambets, M.; Dall’Olmo, G.; Kaiser, R.; Miller, P.I. Oceanic migration behaviour of tropical Pacific eels from Vanuatu. Mar. Ecol. Prog. Ser. 2013, 475, 177–190. [Google Scholar] [CrossRef]
- Lee, T.; Fournier, S.; Gordon, A.L.; Sprintall, J. Maritime Continent water cycle regulates low-latitude chokepoint of global ocean circulation. Nat. Commun. 2019, 10, 2103. [Google Scholar] [CrossRef]
- Pattiaratchi, C.B.; Siji, P. Variability in Ocean Current Around Australia. 2020. Available online: https://www.imosoceanreport.org.au/time-series/environment/current-variability/ (accessed on 29 June 2022).
- Ganachaud, A.; Cravatte, S.; Melet, A.; Schiller, A.; Holbrook, N.J.; Sloyan, B.M.; Widlansky, M.J.; Bowen, M.J.; Verron, J.; Wiles, P.; et al. The Southwest Pacific Ocean circulation and climate experiment (SPICE). J. Geophys. Res. Oceans 2014, 119, 7660–7686. [Google Scholar] [CrossRef]
- Kessler, W.S.; Cravatte, S. Mean circulation of Coral Sea. J. Geophys. Res. Oceans 2013, 118, 6385–6410. [Google Scholar] [CrossRef]
- Gordon, A.L.; Susanto, R.D.; Vranes, K. Cool Indonesian Throughflow as a consequence of restricted surface layer flow. Nature 2003, 425, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Susanto, R.D.; Fang, G.; Soesilo, I.; Zheng, Q.; Qiao, F.; Wei, Z.; Sulistyo, B. New surveys of a branch of the Indonesian throughflow. Eos 2010, 91, 261–263. [Google Scholar] [CrossRef]
- Susanto, R.D.; Wei, Z.; Adi, A.T.; Fan, B.; Li, S.; Fang, G. Karimata throughflow from December 2007 to November 2008. Acta Oceanol. Sin. 2013, 32, 1–6. [Google Scholar] [CrossRef]
- Susanto, R.D.Z.; Wei, T.R.; Adi, Q.; Zheng, G.; Fang, B.; Fan, A.; Supangat, T.; Agustiadi, S.; Li, M.; Trenggono, M.; et al. Oceanography Surrounding Krakatau Volcano. Oceanography 2016, I29, 264–272. [Google Scholar]
- Dao, H.T.; Smith-Keune, C.; Wolanski, E.; Jones, C.M.; Jerry, D.R. Oceanographic currents and local ecological knowledge indicate and genetics does not refute, a contemporary pattern of larval dispersal for the ornate spiny lobster, Panulirus ornatus in the south-east Asian archipelago. PLoS ONE 2015, 10, e0124568. [Google Scholar] [CrossRef]
- Williams, K.C. Spiny lobster ecology and exploitation in the South China Sea region. In Proceedings of the Australian Centre for International Agriculture Research, the Institue of Oceanography, Nha Trang, Vietnam, 16 November 2004. [Google Scholar]
- Wyrtki, K. Physical oceanography of the Indian Ocean. In The Biology of the Indian Ocean; NAGA Report: San Diego, CA, USA, 1961. [Google Scholar]
- Arai, T.; Taha, H. Contrasting patterns of genetic population structure in tropical freshwater eels of genus Anguilla in the Indo-Pacific. Heliyon 2021, 7, e07097. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).