Effect of Water Area and Waterweed Coverage on the Growth of Pond-Reared Eriocheir sinensis
Abstract
1. Introduction
2. Materials and Methods
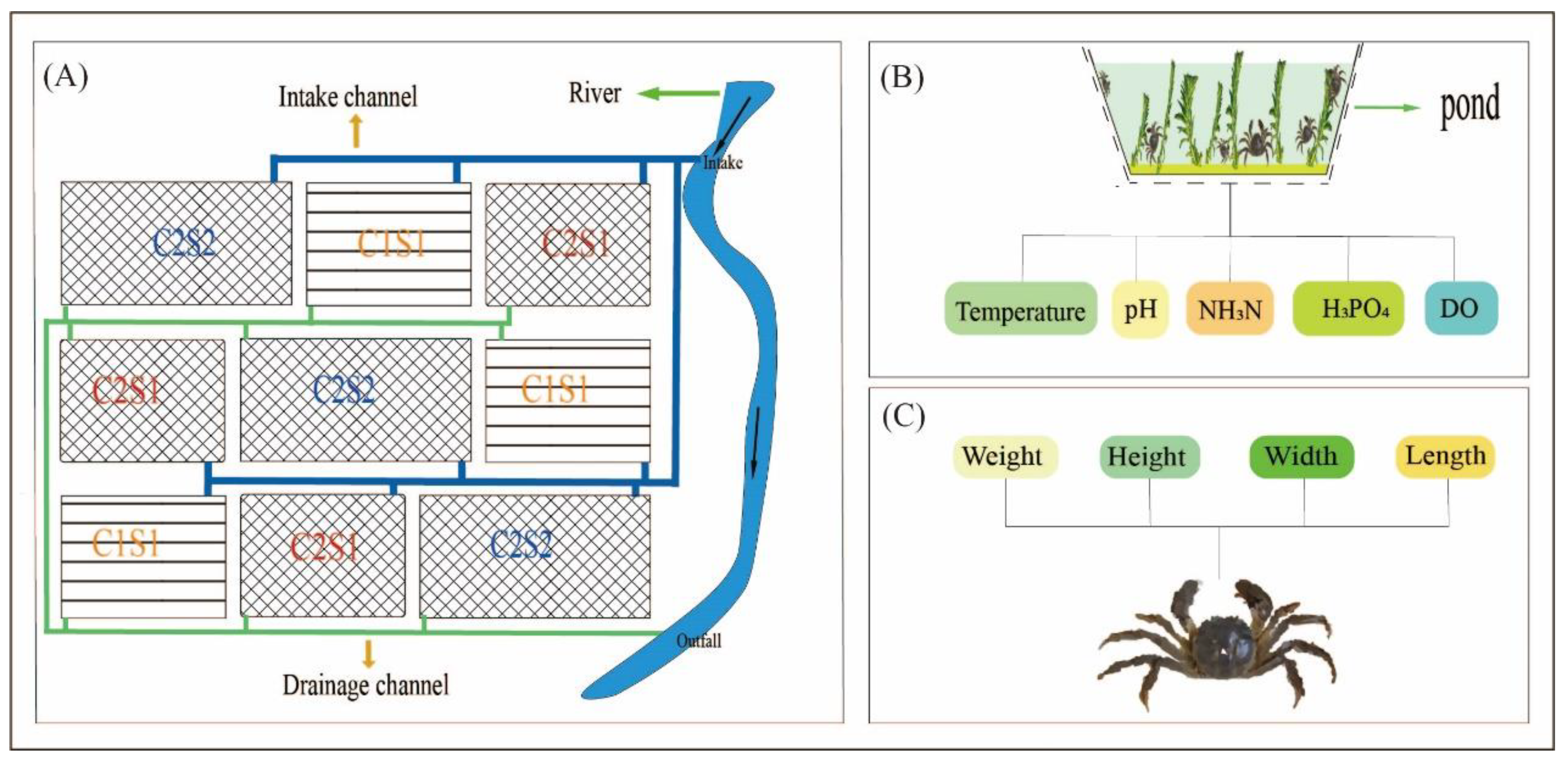
2.1. Experimental Design
2.2. Monitoring of the Water Characteristics
2.3. Data Collection for the E. sinensis
2.4. Statistical Analysis
3. Results
3.1. Fluctuation Characteristic of Water Environment Quality
3.1.1. pH Change of Pond Water
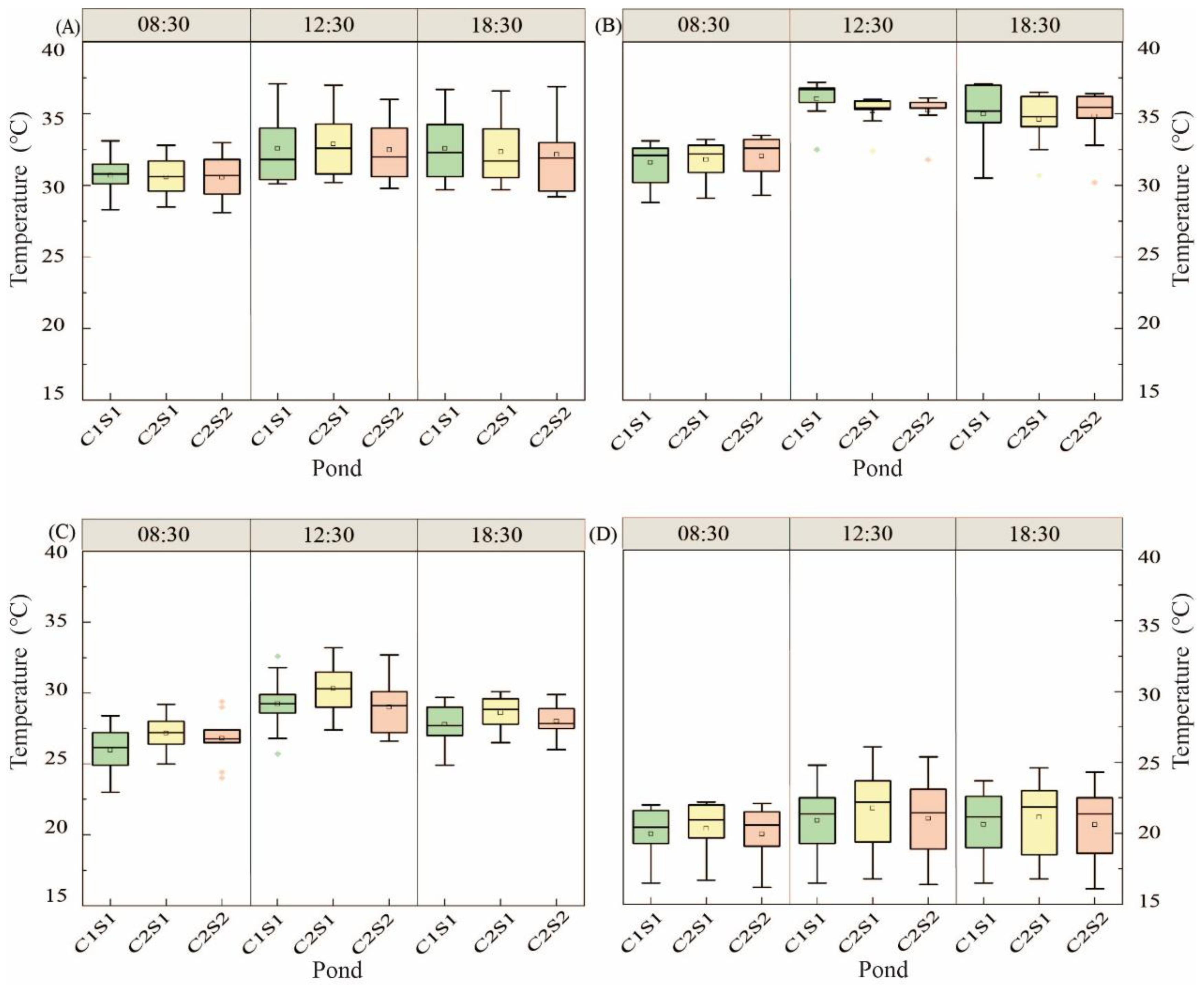
3.1.2. Water Temperature Change
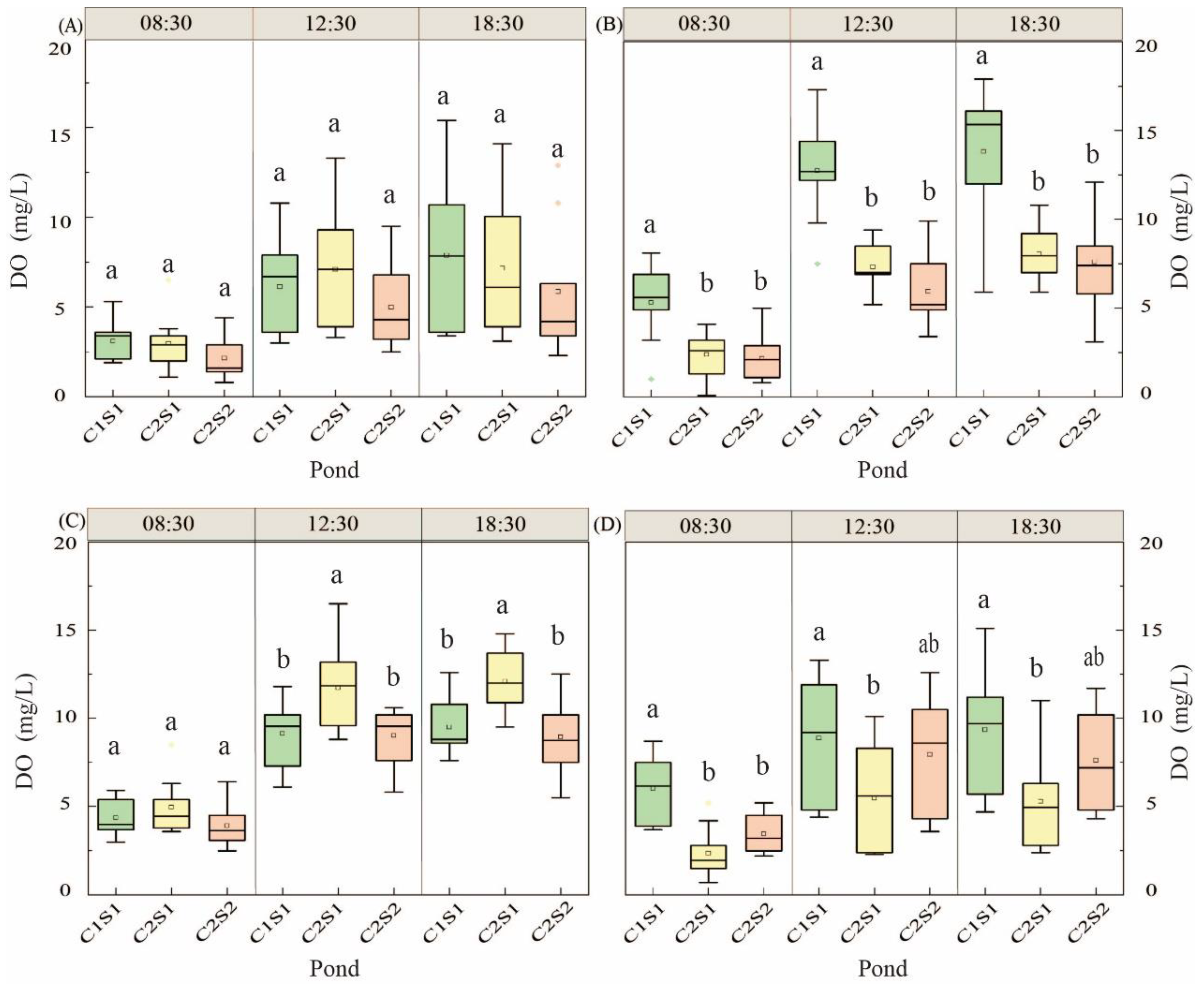
3.1.3. Change of Dissolved Oxygen in Water
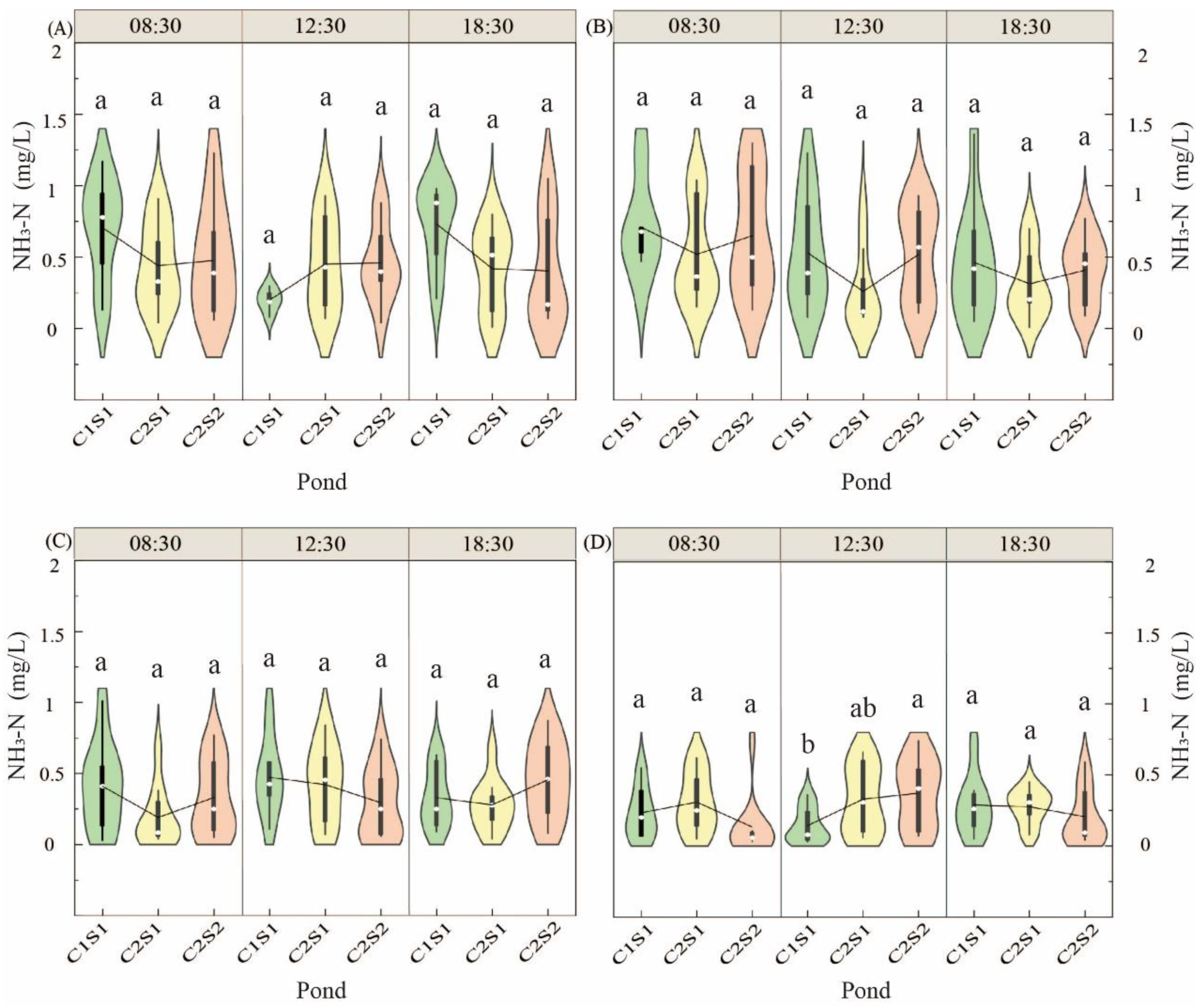
3.1.4. Ammonium-Nitrogen Change
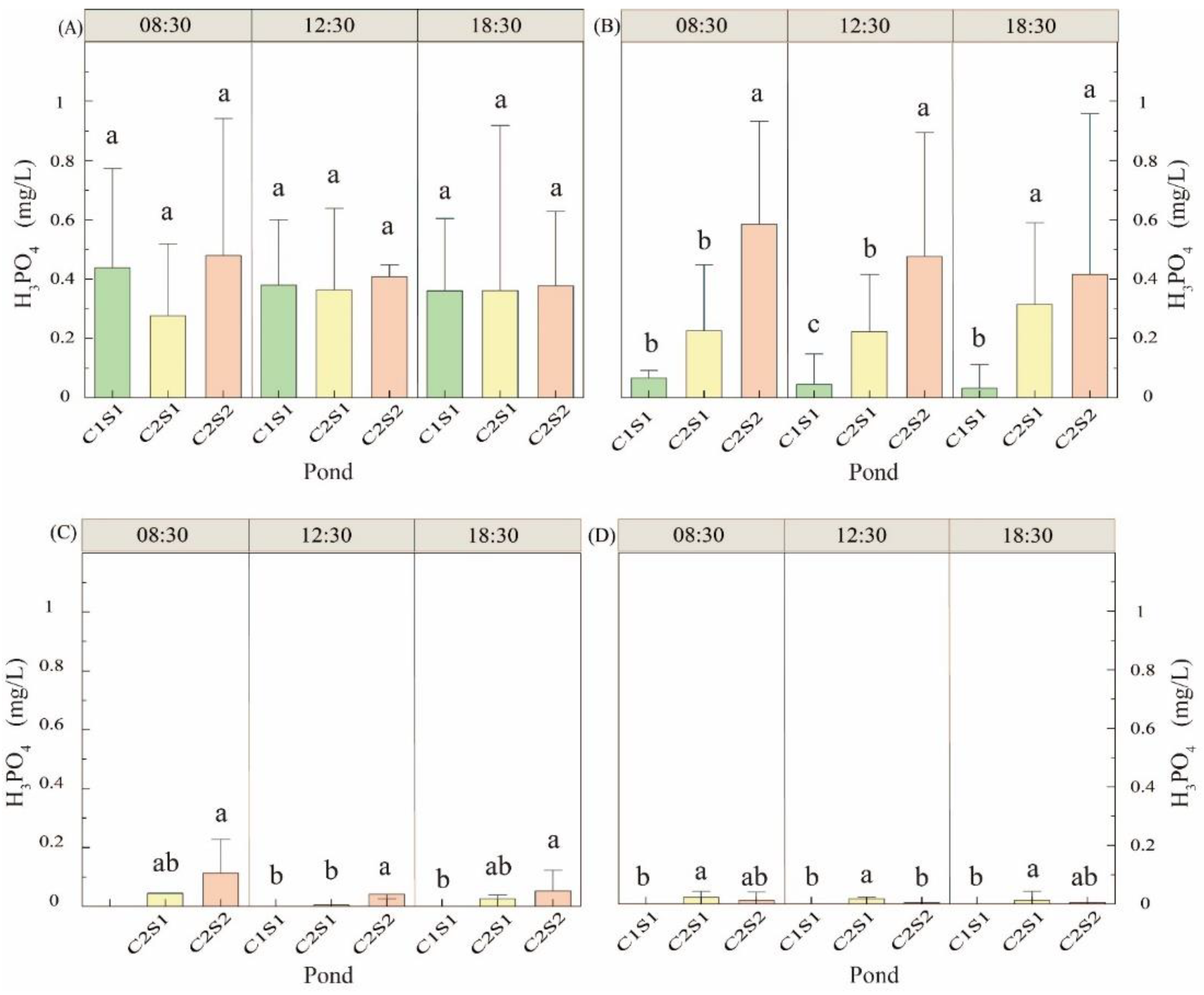
3.1.5. Orthophosphate Change
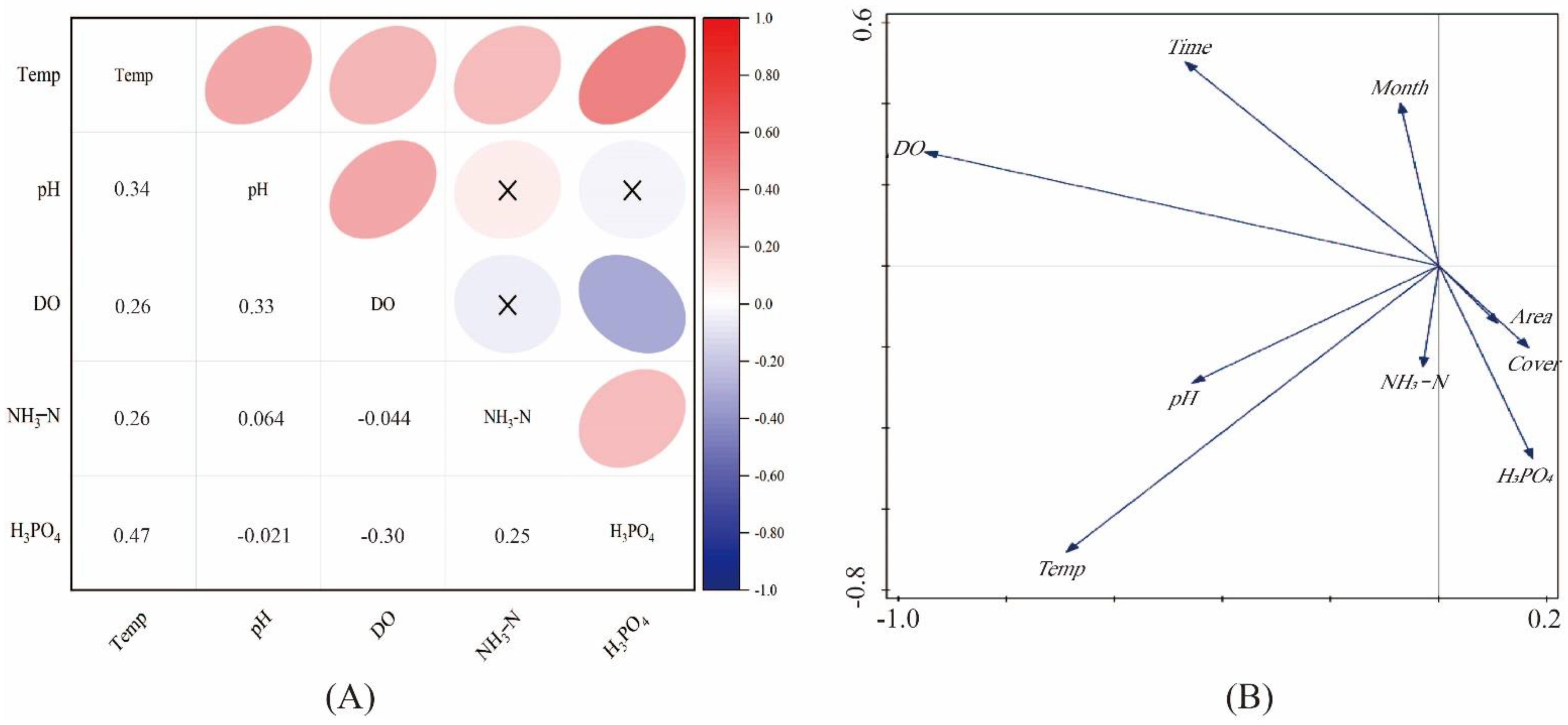
3.2. Relationship between Environmental Factors
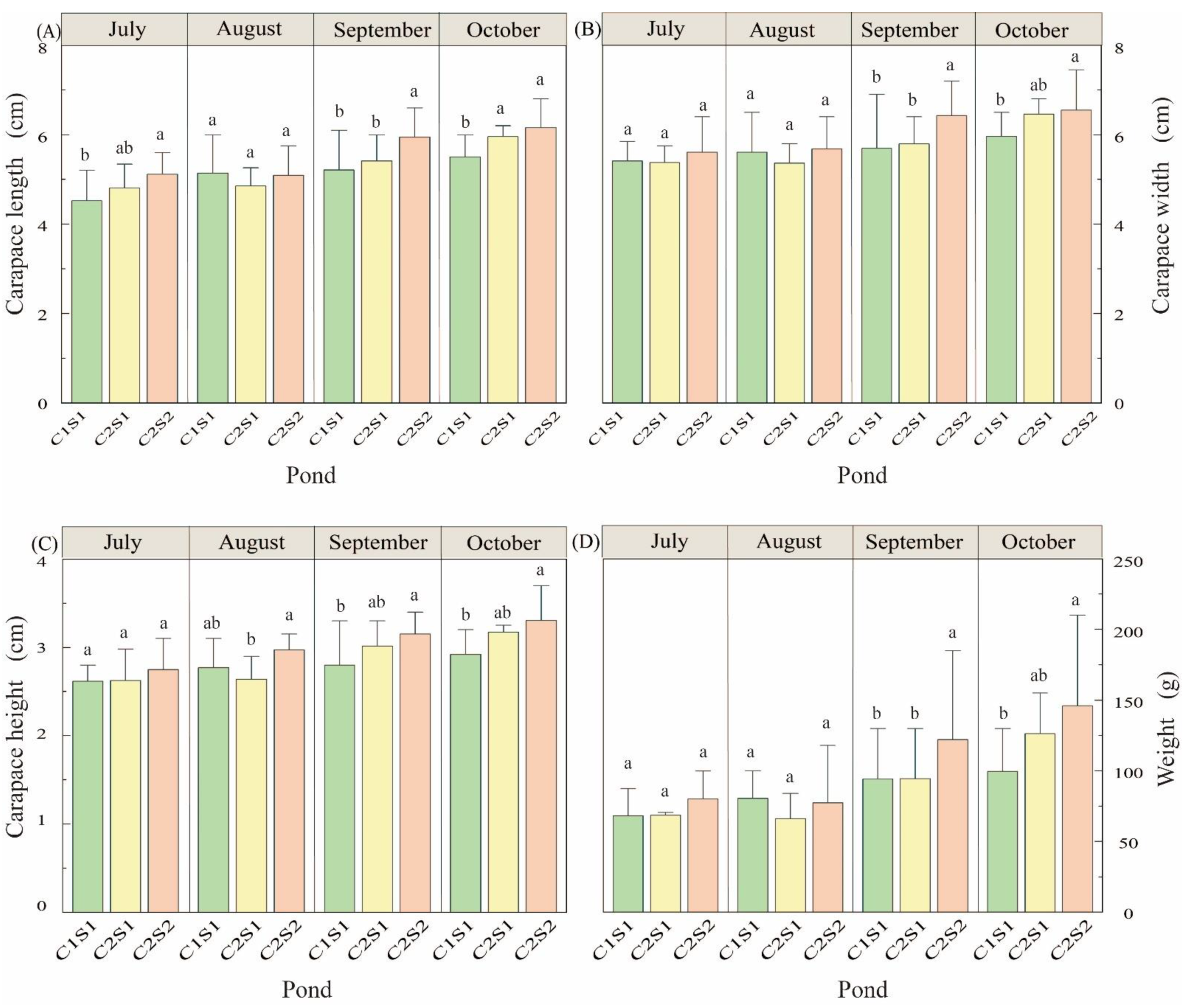
3.3. The Morphological Characteristics and Weight of E. sinensis
3.4. The Edible-Part Index of E. sinensis
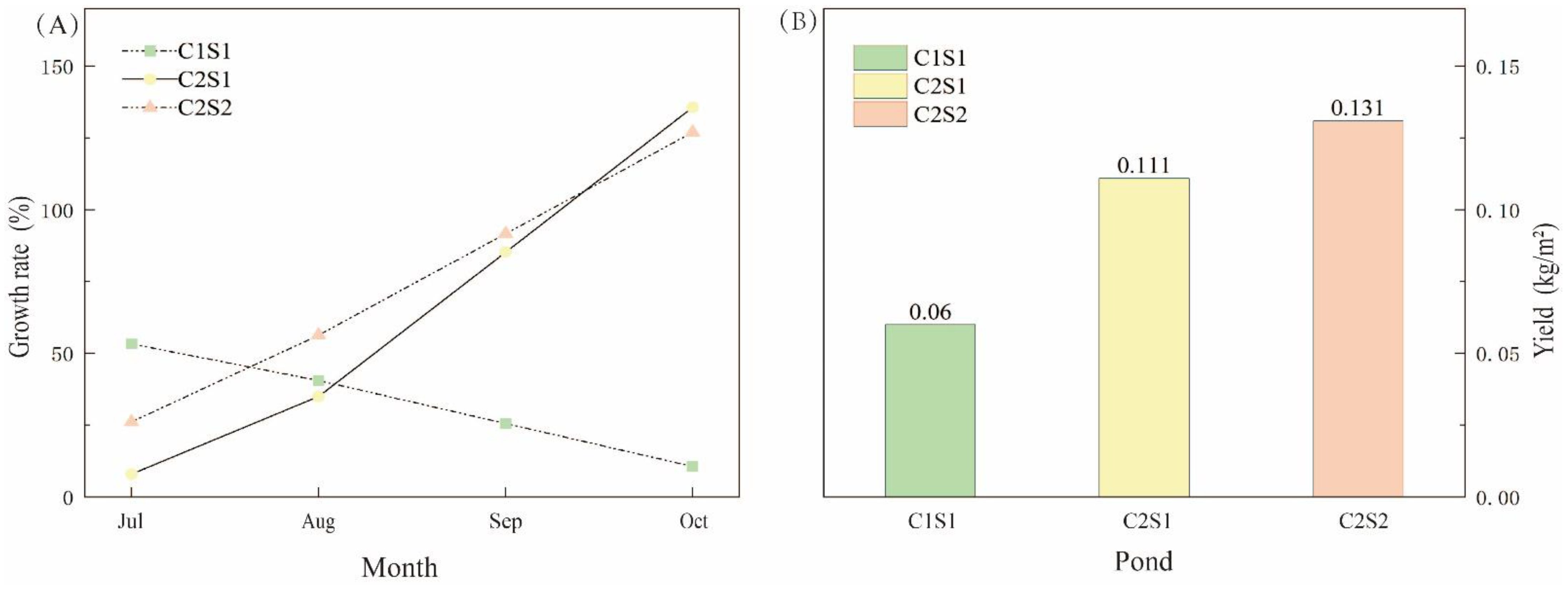
3.5. The Yields of E. sinensis
4. Discussion
4.1. The Effect of Waterweed Coverage on the Growth of Crabs
4.2. The Effect of the Surface Area of the Ponds on the Growth of Crabs
4.3. The Effect of Other Factors on the Growth of Crabs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, P. Analysis on the Technical Efficiency of Freshwater Aquaculture in China-Based on SFA Empirical Analysis of Freshwater Aquaculture Areas. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2014. [Google Scholar]
- Freeman, S.; Vigoda-Gadot, E.; Sterr, H.; Schultz, M.; Korchenkov, I.; Krost, P.; Angel, D. Public attitudes towards marine aquaculture: A comparative analysis of Germany and Israel. Environ. Sci. Policy 2012, 22, 60–72. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, B. Study on Influence Factors of Aquaculture and Safety of Aquatic Products. Heilongjiang Agric. Sci. 2014, 114–118. [Google Scholar] [CrossRef]
- MARD. China Fishery Statistics Yearbook; China Agricultural Press: Beijing, China, 2020; p. 24. [Google Scholar]
- Wang, H.; Feng, G.; Wu, B.; Hu, H.; Zhuang, P. Research on Management and Countermeasures of Sustainable Utilization of Eriocheir sinensis of Yangtze Population. Chin. J. Agric. Resour. Reg. Plan. 2019, 40, 93–100. [Google Scholar]
- Peng, J. Effects of Cultural Water Environment on Survival and Growthof Juvenile and Adult Eriocheir sinesis. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2017. [Google Scholar]
- Peng, J.; Xu, Z.; Liu, B.; Duan, C.; Tang, Y.; Zhao, Y. Effect of Temperature Stress on the Survival of Juvenile Chinese Mitten Crab (Eriocheir sinensis). Iran. J. Fish. Sci. 2019, 18, 763–774. [Google Scholar] [CrossRef]
- Allen, K.O. Effects of Stocking Density and Water Exchange Rate on Growth and Survival of Channel Catfish Ictalurus Punctatus (Rafinesque) in Circular Tanks. Aquaculture 1974, 4, 29–39. [Google Scholar] [CrossRef]
- Dagmara, W.; Monika, N. Gonad Maturity in Female Chinese Mitten Crab Eriocheir sinensis from the Southern Baltic Sea: The First Description of Ovigerous Females and the Embryo Developmental Stage. Oceanologia 2014, 56, 779–787. [Google Scholar] [CrossRef]
- Kong, L.; Cai, C.; Ye, Y.; Chen, D.; Wu, P.; Li, E.; Chen, L.; Song, L. Comparison of non-volatile compounds and sensory characteristics of Chinese mitten crabs (Eriocheir sinensis) reared in lakes and ponds: Potential environmental factors. Aquaculture 2012, 364–365, 96–102. [Google Scholar] [CrossRef]
- Jin, G.; Xie, P.; Li, Z. Effects of the Stocking Density and Body Size of the Mitten Crab (Eriocheir sinensis) on Aquatic Plant Biomass. J. Freshw. Ecol. 2001, 16, 341–345. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Z.; Yang, X.; Zhu, S. Effects of the Coverage of Waterweed in Pond on the Growth of Eriocheir sinensis. Anim. Husb. Feed Sci. 2014, 35, 66–67. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, S.; Yue, W.; Cheng, X.; Peng, Z.; Wang, J.; Wang, C. Effects of Elodea nuttallii on Growth and Nutritional Quality of Chinese Mitten Crab, Eriocheir sinensis. J. Fish. Sci. China 2017, 24, 91–99. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Liang, X.; Cui, Y. Stocking Models of Chinese Mitten Crab (Eriocheir japonica sinensis) in Yangtze Lakes. Aquaculture 2006, 255, 456–465. [Google Scholar] [CrossRef]
- Olusoji, O.A.B.; Anifowose, O.J.; Sodamola, M.Y. Length-Weight Relationships, Condition Factor and Fecundity of the West Africa Freshwater Crab, Sudanonautes africanus (Milne-Edwards 1883), in Western Nigeria. West. Afr. J. Appl. Ecol. 2009, 16, 65–74. [Google Scholar] [CrossRef][Green Version]
- Wu, X.; Cheng, Y.; Sui, L.; Yang, X.; Nan, T.; Wang, J. Biochemical Composition of Pond-reared and Lake-stocked Chinese Mitten Crab Eriocheir sinensis (H. Milne-Edwards) Broodstock. Aquac. Res. 2007, 38, 1459–1467. [Google Scholar] [CrossRef]
- Wang, Q. Analysis on Climatic Conditions of Yellow River Crabs Growth. J. Agric. Catastrophology 2013, 13, 32–35. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, X.; Dai, D. Algal Boom Prevention and Control in Intensive Culture Ponds of Eriocheir sinensis by Management of Elodea nuttallii Planting. J. Water Ecol. 2019, 40, 73–80. [Google Scholar]
- Feng, T.; Liu, J.; Zhang, T.; Ye, S.; Li, Z. Study on Pond-Culture Technology of Chinese Mitten Crab (Eriocheir sinensis) in Lake-Reclaimed Paddy of Honghu Lake. Freshw. Fish. 2010, 40, 66–71. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, J.; Zhang, F.; Shen, X.; Lin, H.; Wang, I.; Zhu, L. Distinguishing Cyanobacteria Bloom and Aquatic Plants in Lake Taihu Based on Hyperspectral Imager for the Coastal Ocean Images. Remote Sens. Tech. Appl. 2016, 31, 879–885. [Google Scholar] [CrossRef]
- Yu, Y.; Wan, J.; Liang, X.; Wang, Y.; Liu, X.; Mei, J.; Sun, N.; Li, X. Effects of Protein Level on the Production and Growth Performance of Juvenile Chinese Mitten Crab (Eriocheir sinensis) and Environmental Parameters in Paddy Fields. Water 2022, 14, 1941. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, K.; Li, H.; Zhang, W.; Zhu, P.; Wang, X.; Cui, X.; Rongbing, F. The Purification Effect of Nitrogen and Phosphorus and Allelopathic Effect on Polluted Water by Elodea nuttallii. J. Agron. Environ. Sci. 2011, 30, 554–558. [Google Scholar]
- Liu, Y. Study on the Influence of Elodea nuttallii Harvesting on Water Environment in Intensive Culture Ponds of Eriocheir sinensis. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2019. [Google Scholar]
- Cai, J.; Shen, Q.; Zheng, X. Advancement in Researches of Ammonia Pollution Hazards on Aquaculture and Its Treatment Technology. J. Zhejiang Ocean Univ. (Nat. Sci.) 2010, 29, 167–172. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, B.; Gao, C.; Liu, T.; Li, X.; Zu, X. Effects of Waterweed Density on Water Quality and Growth of Palaemonetes Sinensis in Ponds. Chin. Agric. Sci. Bul. 2022, 38, 100–105. [Google Scholar]
- Pang, Y.; Niu, C.; Wu, L.; Song, Y.; Song, X.; Shi, A.Y.; Shi, X.; Wu, Z.W.; Tang, B.; Yang, X.; et al. Comprehensive Utilization of Land Resources for Photovoltaic Power Generation to Culture Chinese Mitten Crab (Eriocheir sinensis): Growth Performance, Nutritional Composition and Tissue Color. Fishes 2022, 7, 207. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Zhang, S.; Lian, Y.; Ding, H.; Du, X.; Li, Z.; De Silva, S.S. Sustainable Farming Practices of the Chinese Mitten Crab (Eriocheir sinensis) around Hongze Lake, Lower Yangtze River Basin, China. Ambio 2016, 45, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xiao, Z.; Jiang, H.; Zhao, X.; Sun, Z.; Yu, Y. Effect of Initial Size and Stocking Density on Growth and Yield of Chinese Mitten-handed Crab Eriochier sinensis in Rice Fields. Fish. Sci. 2009, 28, 36–39. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Q.; Lei, X.Y.; Zhang, D.M.; Wang, S.; Wan, J.W.; Liu, H.J.; Chen, Y.K.; Zhao, Y.I.; Wang, G.Q.; et al. Effects of different stocking densities on the growth performance and antioxidant capacity of Chinese mitten crab (Eriocheir sinensis) in rice crab culture system. Aquac. Int. 2022, 30, 883–898. [Google Scholar] [CrossRef]
- Chen, J.; Ma, X.; Wang, W.; Yong, Y.; Tao, C. The Study of Relationships between Growth, Molt and Accumulated Temperature of the Chinese Mitten Crab (Eriocheir sinensis). J. Shanghai Ocean Univ. 2016, 25, 675–683. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Ma, X. Ecological Breeding of River Crab; China Agricultural Press: Beijing, China, 2010. [Google Scholar]
- Huang, S.; Wang, Z.; Mao, H.; Wang, C. Observation on Molting and Growth of Adult Chinese Mitten Crab Reared in the Laboratory Condition. J. Shanghai Ocean Univ. 2014, 23, 359–365. [Google Scholar]
- Xue, J.; Liu, H.; Jiang, T.; Chen, X.; Yang, J. Shape variation in the carapace of Chinese mitten crabs (Eriocheir sinensis H. Milne Edwards, 1853) in Yangcheng Lake during the year-long culture period. Eur. Zool. J. 2022, 89, 217–228. [Google Scholar] [CrossRef]
| Month | Time | S1C1 | S1C2 | S2C2 | p Value |
|---|---|---|---|---|---|
| July | 08:30 | 7.456 ± 0.289 | 7.470 ± 0.363 | 7.421 ± 0.240 | 0.940 |
| 12:30 | 7.326 ± 0.319 | 7.489 ± 0.277 | 7.423 ± 0.174 | 0.430 | |
| 18:30 | 7.540 ± 0.347 | 7.626 ± 0.273 | 7.438 ± 0.266 | 0.422 | |
| August | 08:30 | 7.488 ± 0.299 | 7.560 ± 0.225 | 7.493 ± 0.325 | 0.822 |
| 12:30 | 7.546 ± 0.331 | 7.577 ± 0.287 | 7.523 ± 0.273 | 0.929 | |
| 18:30 | 7.606 ± 0.331 | 7.632 ± 0.236 | 7.556 ± 0.212 | 0.810 | |
| September | 08:30 | 7.519 ± 0.397 | 7.461 ± 0.371 | 7.748 ± 0.467 | 0.349 |
| 12:30 | 7.743 ± 0.607 | 8.090 ± 0.545 | 7.949 ± 0.520 | 0.470 | |
| 18:30 | 8.154 ± 0.338 | 8.174 ± 0.546 | 8.201 ± 0.373 | 0.973 | |
| October | 08:30 | 6.940 ± 0.263 | 7.140 ± 0.196 | 7.112 ± 0.247 | 0.144 |
| 12:30 | 7.140 ± 0.151 | 7.130 ± 0.183 | 7.240 ± 0.337 | 0.528 | |
| 18:30 | 7.433 ± 0.403 | 7.344 ± 0.505 | 7.350 ± 0.417 | 0.892 |
| Sex | Pond | Gonadosomatic Index (%) | Hepatosomatic Index (%) | Muscle Index (%) | Edible-Part Index (%) | G/L (%) |
|---|---|---|---|---|---|---|
| Famale | C1S1 | 7.594 ± 0.671 ab | 9.650 ± 0.407 a | 17.910 ± 2.135 ab | 35.154 ± 2.784 a | 0.787 ± 0.059 ab |
| C2S1 | 9.411 ± 1.753 a | 9.695 ± 0.523 a | 15.362 ± 1.451 b | 34.468 ± 3.354 a | 0.971 ± 0.181 a | |
| C2S2 | 7.135 ± 0.301 b | 10.097 ± 1.330 a | 20.111 ± 1.263 a | 37.344 ± 2.286 a | 0.712 ± 0.067 b | |
| Male | C1S1 | 2.091 ± 0.080 a | 9.428 ± 0.110 b | 19.329 ± 1.351 ab | 30.847 ± 1.321 b | 0.222 ± 0.011 a |
| C2S1 | 1.345 ± 0.039 b | 10.070 ± 0.217 a | 17.887 ± 1.127 b | 29.302 ± 0.940 b | 0.134 ± 0.006 b | |
| C2S2 | 2.195 ± 0.491 a | 9.758 ± 0.276 ab | 21.815 ± 1.864 a | 33.768 ± 1.594 a | 0.226 ± 0.056 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Peng, J.; Chen, J.; Zhao, Y.; Ding, Y.; Dai, J.; Hu, Z.; Huang, T.; Dong, M.; Xu, Z. Effect of Water Area and Waterweed Coverage on the Growth of Pond-Reared Eriocheir sinensis. Fishes 2022, 7, 282. https://doi.org/10.3390/fishes7050282
Tang Y, Peng J, Chen J, Zhao Y, Ding Y, Dai J, Hu Z, Huang T, Dong M, Xu Z. Effect of Water Area and Waterweed Coverage on the Growth of Pond-Reared Eriocheir sinensis. Fishes. 2022; 7(5):282. https://doi.org/10.3390/fishes7050282
Chicago/Turabian StyleTang, Yongcheng, Jiao Peng, Jiahao Chen, Yunlin Zhao, Yi Ding, Jingyi Dai, Zhiyuan Hu, Tian Huang, Meng Dong, and Zhenggang Xu. 2022. "Effect of Water Area and Waterweed Coverage on the Growth of Pond-Reared Eriocheir sinensis" Fishes 7, no. 5: 282. https://doi.org/10.3390/fishes7050282
APA StyleTang, Y., Peng, J., Chen, J., Zhao, Y., Ding, Y., Dai, J., Hu, Z., Huang, T., Dong, M., & Xu, Z. (2022). Effect of Water Area and Waterweed Coverage on the Growth of Pond-Reared Eriocheir sinensis. Fishes, 7(5), 282. https://doi.org/10.3390/fishes7050282






