Abstract
Skin injury causes fibroblast dysfunction and lowers collagen production. Safe, functional ingredients such as vitamin C (Vit C) and fish hydrolyzed collagen (HC) have been used to alleviate this problem. Defatted HC from salmon (Oncorhynchus nerka) skin could be a potential functional ingredient with skin nourishment activity. This study aimed to investigate the combined effects of HC and Vit C on the proliferation and migration of human dermal fibroblast (HDF). Molecular weight ranging from 102 Da to 10,175 Da and high imino acid content were found in HC. HC (0–800 µg/mL) or vitamin C (Vit C) (0.01–100 µg/mL) was applied for HDF treatment. Higher cell proliferation was found by adding HC at 50 µg/mL or Vit C at 0.01 µg/mL compared to the control and those treated with both compounds at other levels (p < 0.05). Cells treated with HC (50 µg/mL) combined with Vit C (0.01 µg/mL) (HC+Vit C) showed higher proliferation, migration, and lamellipodia formation of HDF cells than those treated with HC or Vit C alone. Moreover, all the samples tested could stimulate the proliferation and migration of HDF cells via FAK/Akt and ERK/p38 MAPK signaling pathways. Thus, HC combined with Vit C could be a promising functional ingredient for skin nourishment and would healing.
1. Introduction
Collagen, especially type I collagen, is found in the skin [1]. In general, fibroblasts, the most abundant cells of the skin dermis, play a vital role in the collagen synthesis of skin [2]. Cell dysfunction, due to internal and external factors, is associated with skin injury and causes a reduction in collagen production. Several scientific reports indicated that peptides from fish collagen could stimulate skin cell proliferation and alleviate skin injury from reactive oxygen species [2,3,4]. Due to the low absorption of native collagen, hydrolyzed collagen (HC) from fish skin has been extensively used as a functional ingredient with various bioactivities such as antidiabetic, wound-healing, antioxidant, and DPP-IV inhibitory activities [5,6,7,8] without religious constraint.
Salmon has become popular in many countries, especially in the form of slices known as ‘sashimi.’ To make the fillet or slices, the skin must be removed. Those skins are discarded or used as animal feed. To increase their value, they can be used for the production of HC with bioactivities. Although HC from salmon skin could provide several bioactivities, size and amino acid sequence played a major role in target bioactivity. Recently, mixed proteases (3% papain and 4% Alcalase) have been employed [9], in which high antioxidant activities were obtained. Since salmon skin contained a high amount of fat, fat removal of hydrolysate using a disk stack centrifugal separator for three cycles, followed by isopropanol extraction, were carried out, in which fat content of HC could be totally removed [9]. This reflected that defatted HC with augmented oxidative stability could be an appropriate functional ingredient for food applications or nutraceuticals.
HC from salmon skins has also been used for skin nourishment [10]. Previously, Woonnoi et al. [10] revealed that HC from salmon skin prepared by two-step hydrolysis could promote keratinocyte proliferation and migration. Apart from HC, vitamin C (Vit C) is well-known as a cofactor for collagen synthesis and a primary antioxidant [11]. Mohammed et al. [12] demonstrated that Vit C could inhibit pro-inflammatory responses and enhance the proliferation of human neonatal dermal fibroblasts in the wound healing process. Thus, the use of HC in combination with Vit C could increase the proliferation and migration of fibroblast cells, thus accelerating wound healing. Nevertheless, no information concerning the effect of defatted HC from salmon skin in combination with Vit C on the proliferation and migration of human fibroblast cells exists. Thus, the purposes of this study were (1) to characterize molecular weight distribution and amino acids composition of HC and (2) to elucidate the combined effects between HC and Vit C on human fibroblast cell proliferation and wound healing.
2. Materials and Methods
2.1. Chemicals and Sockeye (Oncorhynchus nerka) Salmon Skin
Alcalase (EC 3.4.21.62) (2.4 unit/g) from Bacillus licheniformis and papain from papaya (Carica papaya) latex (E.C.3.4.22.2) (3 units/g) were obtained from Siam Victory Chemicals Co, Ltd. (Bangkok, Thailand). Human dermal fibroblast (HDF) cell was supplied by Sigma-Aldrich (St. Louis, MO, USA). Cell culture medium, including Dulbecco’s modified Eagle medium (DMEM), fetal bovine serum (FBS), and antibiotics, were acquired from Gibco (Carlsbad, CA, USA). Other chemicals used for cell culture were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Antibodies against ERK, FAK, p-ERK, and p-FAK were obtained from Abcam (Cambridge, UK). Antibodies against p38, p-p38, p-Akt, Akt, and β-actin were procured from Cell Signaling Technology (Danvers, MA, USA). Frozen sockeye salmon skins were obtained from the Nissui (Thailand) Co., Ltd., Songkhla, Thailand.
2.2. Preparation of Salmon Skin
Frozen stacking layers of skins were cut using an electric sawing machine to gain the size of 3 × 3 cm2. Thereafter, the resulting skins were placed in a stainless tank and thawed using running water, in which the temperature of the skin–water mixture was less than 10 °C. After thawing, cut skins were pretreated with 0.05 mol/L NaOH (1:10, w/v) to remove non-collagenous proteins and subsequently washed using running water, as detailed by Nilsuwan et al. [9]. The resulting skins were mixed with 0.05 mol/L citric acid (1:10, w/v) for 1 h, and the resulting swollen skins were further washed with running water to remove the acid in the skin until the pH of washed skin became neutral.
2.3. Preparation of Defatted Hydrolyzed Collagen from Salmon Skin
Swollen skins were used for HC production as tailored by Nilsuwan et al. [9]. Hydrolysis was conducted using mixed proteases (3% papain and 4% Alcalase) at pH 8 and 60 °C for 240 min. After the hydrolysis process, the reaction was terminated [3]. The obtained HC solution was subjected to defatting using a disk stack centrifugal separator (SPX FLOW Technology Italia S.p.A., Milan, Italy) with a feed rate of 2.0 L/min for 9 cycles. Thereafter, the supernatant (HC solution) was lyophilized using a freeze-dryer (Model Duratop™ lP/Dura Dry™ lP, FTS® System, Inc., Stone Ridge, NY, USA). Lyophilized HC powder was mixed with isopropanol (1:10 (w/v)) and ultrasonicated using the ultrasonic equipment (Sonics, Model VC750, Sonics & Materials, Inc., Newtown, CT, USA) at an amplitude of 70% with pulsed mode (on/off at 10 s) for 10 min and subsequently stirred at 4–8 °C for 20 min. The mixture was then centrifuged using a refrigerated centrifuge (Himac CR22N, Hitachi, Tokyo, Japan) at 10,000× g for 20 min at 4 °C and the HC pellet was collected. The defatting process was conducted in the same manner for three cycles. Isopropanol present in the HC pellet was removed with the aid of blowing air at 25 °C for 30 min. The defatting HC powder was used for further study.
2.4. Molecular Weight (MW) Distribution
Sephadex G-25 gel filtration column (GE Healthcare Bio-Science AB, Uppsala, Sweden) was used for separating peptides in HC. The fractions (3 mL) were collected, and A220 and A280 were monitored. The MW of the selected fraction was calculated in comparison with those of standard markers, including blue dextran (2,000,000 Da), insulin chain B (3495.89 Da), vitamin B12 (1355.4 Da), glycine–tyrosine (238.25 Da) and tyrosine (181.2 Da).
2.5. Amino Acid Analysis
The amino acid composition of the HC was determined using an amino acid analyzer (MLC-703; Atto Co., Tokyo, Japan) [13]. The content of amino acids was reported as g/100 g HC.
2.6. Effect of HC and Vitamin C on Cell Proliferation and Migration of Human Fibroblast (HDF) Cell
2.6.1. Cell Culture
Human dermal fibroblast (HDF) cells were cultured in the T-75 flasks with complete Dulbecco’s modified Eagle medium (DMEM) medium with 10% fetal bovine serum (FBS), 100 IU/mL penicillin, and 100 µg/mL streptomycin and 2 mM L-glutamine [14]. Cells were incubated in a 5% CO2 humidified incubator (Binder Model C 170, Binder Inc., Bohemia, NY, USA) at 37 °C and subsequently subcultured using 0.25% trypsin-EDTA solution at 80–90% confluence.
2.6.2. Cell Proliferation Assays
HC (0, 50, 100, 200, 400, and 800 µg/mL) and vitamin C (Vit C) (0.01, 0.1, 1, 10, and 100 µg/mL) solutions were tested for cytotoxicity toward cells. Those concentrations of both samples had no cytotoxicity when tested using MTT and double-stranded DNA (dsDNA) assays [10]. Those concentrations were used for the proliferation study. To elucidate the combined impact between HC and Vit C, HC and Vit C at the selected concentration were used. The result was reported as % cell proliferation by comparing to control (without any treatment).
2.6.3. Cell Migration Assays
HDF cells (5 × 105 cells/well) were added to 6-well plates, and the wound was made by scratching using a sterilized tip, as detailed by Chotphruethipong et al. [5]. Cells were treated with the selected samples, including HC and Vit C and HC+Vit C at the selected level. Thereafter, cells were incubated for 24 and 48 h. Wound gaps were imaged using an inverted microscope (EVOS™ XL Core, Thermo Fisher Scientific, Waltham, MA, USA) and calculated by ImageJ software (version 14.1, NIH, Bethesda, MD, USA). The result was expressed as % wound area by comparing to that found at 0 h [15].
2.6.4. Lamellipodia Formation
Lamellipodia formation was determined as described by Chotphruethipong et al. [14]. HDF cells (5 × 103 cells/well) were seeded in 6-well plates and treated with HC and Vit C and HC+Vit C at the selected level for 24 h. Thereafter, phalloidin-rhodamine and Hoechst 33342 dyes were used for staining lamellipodia formation and DNA of cells, respectively [14]. After staining, cells were visualized by a fluorescent microscope (Olympus IX70 with DP50, Shinjuku-ku, Tokyo, Japan). Lamellipodia formation was reported as relative fluorescence intensity to that of control. Moreover, the percentage of the number of positive cells (cells showing lamellipodia) was calculated as follows:
Percentage = (number of positive cells/total cells) × 100
2.6.5. Western Blot Analysis
After treatment with the selected samples, cells (1 × 106 cells/35-mm dish) were lysed and collected as tailored by Chotphruethipong et al. [16]. The proteins (30 µg) were subjected to SDS-PAGE and transferred onto PVDF membranes. 5% skimmed milk or 3% BSA was used to block non-specific proteins. Subsequently, primary antibodies against p38 (Cell Signaling, 9212S), p-p38 (Cell Signaling, 4511S), ERK1/2 (Abcam, ab184699), p-ERK1/2 (Abcam, ab50011), p-FAK (Abcam, ab4792), FAK (Abcam, ab40794), Akt (Cell Signaling, 9272S), and p-Akt (Cell Signaling, 9271S) were added into membranes, followed by adding with HRP-conjugated secondary antibody for 1.5 h. The interest proteins appeared as dark bands [16]. The amount of expression of proteins was calculated using ImageJ software (version 14.1, NIH, Bethesda, MD, USA) after being normalized by β-actin (Cell Signaling, 4967S) (control). Thereafter, phosphorylated proteins, including p-ERK1/2, p-FAK, p-Akt, and p-p38, were normalized with total protein.
2.7. Statistical Analysis
A completely randomized design (CRD) was used for the entire study. Experimentation and analysis were conducted in triplicate (n = 3). Analysis of variance (ANOVA) was conducted, and the differences among the samples were determined using Duncan’s multiple range test at p < 0.05 level. The analysis was performed with an SPSS package (SPSS for windows, SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Molecular Weight (MW) Distribution of HC
MW distribution of HC is shown in Figure 1. Three dominant peaks of A280 representing proteins or peptides having aromatic amino acids with MW of 8728 Da, 5509 Da, and 1883 Da were detected in defatted HC from salmon skin. In addition, three major peaks of A220, including peptides having MW of 102 Da, 2195 Da, and 4726 Da, were observed. Peptides with large MW (>5 kDa) were also found in HC.

Figure 1.
Elution profiles of defatted hydrolyzed collagen (HC) from salmon skin using SephadexTM G-25 gel filtration chromatography.
3.2. Amino Acid Composition of HC
Amino acid compositions of HC are presented in Table 1. HC had Gly as the dominant amino acid (21.8 g/100 g HC), which was equivalent to 360 residues/1000 residues. Pro (8.81 g/100 g HC) and Glu (7.63 g/100 g HC) were also detected at high content, followed by Arg (7.18 g/100 g HC) and Asp (5.79 g/100 g HC), respectively.

Table 1.
Amino acid composition of defatted hydrolyzed collagen (HC) from salmon skin.
3.3. HDF Fibroblast Cell Proliferation as Affected by Hc or Vit C at Various Concentrations
The effect of hydrolyzed collagen (HC) at various levels on the proliferation of HDF cells is shown in Figure 2a. All levels of HC tested had no cytotoxicity, except at the level of 800 µg/mL (78.73 ± 6.97), which showed slightly decreased proliferation of the cell as compared to the control (without HC) (p < 0.05). Cell proliferation was also augmented as the levels of HC increased up to 100 µg/mL (p < 0.05). Nevertheless, there was no difference in cell proliferation of HC at levels of 50 (124.48 ± 6.03) and 100 µg/mL (123.25 ± 6.48) (p > 0.05).
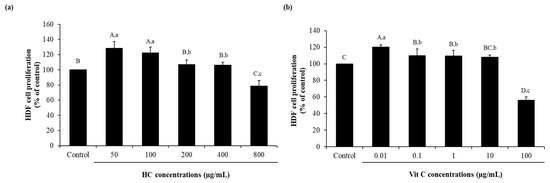
Figure 2.
Effect of HC from salmon skin (a) and vitamin C (Vit C) (b) at different levels on the proliferation of human dermal fibroblast (HDF) cells. Bars represent standard deviation (n = 3). Different uppercase letters on bars indicate significant differences between different samples tested and control (p < 0.05). Different lowercase letters on bars indicate significant differences among samples tested with HC or Vit C at different concentrations (p < 0.05).
The effectiveness of vitamin C (Vit C) at various levels on the proliferation of HDF cells is depicted in Figure 2b. Vit C, especially at low levels (0.01 µg/mL to 1 µg/mL), could promote the proliferation of cells more effectively than the control (p < 0.05), as evidenced by higher cell proliferation, while the use of Vit C at 10 µg/mL could promote cell proliferation and had a similar result to the control (p > 0.05). Among the levels of Vit C tested, the highest cell proliferation was found for Vit C-treated cells at 0.01 µg/mL (120.13 ± 2.72) (p < 0.05), while no difference in proliferation of cells was observed when Vit C ranging from 0.1 µg/mL to 10 µg/mL (107.90 ± 2.76 to 109.88 ± 8.02) (p > 0.05) was used. Furthermore, high level of Vit C (100 µg/mL) (56.20 ± 3.83) led to the decreased proliferation of cell (p < 0.05). Since HC at 50 µg/mL and Vit C at 0.01 µg/mL had the highest efficacy for cell proliferation, they were selected for further study.
3.4. Impact of HC in Combination with Vit C at the Selected Level on Proliferation of HDF Cells
As shown in Figure 3a, all samples tested had no cytotoxicity on HDF cells, as indicated by the cell integrity of all samples tested (Figure 3b). Additionally, all samples showed higher cell proliferation than the control (p < 0.05), except HC, which showed similar results to the control (p > 0.05). When comparing cell proliferation between Vit C-treated cells and HC-treated cells using both compounds at an optimal level, no difference in cell proliferation was found between both samples (p > 0.05), suggesting that the efficiency in stimulating HDF proliferation of HC at 50 µg/mL was similar to Vit C at 0.01 µg/mL. When HC (50 µg/mL) combined with Vit C (0.01 µg/mL) (HC+Vit C) was used, higher cell proliferation (127.00 ± 2.79) was observed as compared to Vit C (121.70 ± 0.84) or HC (121.97 ± 0.99) alone (p < 0.05). The result was confirmed with the dsDNA assay (Figure 3c), in which HC+Vit C could induce a marked proliferation of cells as ascertained by higher cell proliferation as compared to HC or Vit C (p < 0.05). Furthermore, staining with Hoechst 33342 fluorescent dye also indicated the number of DNA (Figure 3d). Cells treated with HC or Vit C, especially HC+Vit C, showed a higher number of DNA as compared to that of control.
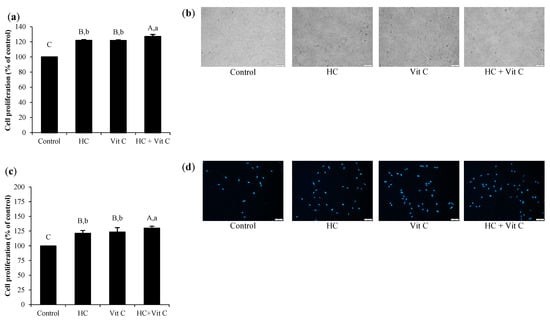
Figure 3.
Effect of HC, Vit C, and HC combined with Vit C (HC+Vit C) at the selected levels on the proliferation of HDF cells determined using MTT assay (a) and cell morphology images (b). Double-stranded DNA (dsDNA) assay (c) and DNA staining images (d) after treatment with the different samples. The scale bar is 100 μm, and the magnification used is ×10. Bars represent standard deviation (n = 3). Different uppercase letters on bars indicate significant differences between different samples tested and control (p < 0.05). Different lowercase letters on bars indicate significant differences among different samples tested (p < 0.05).
3.5. Impact of HC in Combination with Vit C on Migration and Lamellipodia Formation of HDF Cells
The impact of HC, Vit C, and HC+Vit C at the selected level on cell migration is illustrated in Figure 4. Within the first 24 h of treatment, the efficacy of HC-treated cells and Vit C-treated cells was similar to the control (p > 0.05) (Figure 4a,b). Nevertheless, the increased efficacy of cell migration was found for cells treated with HC+Vit C, as evidenced by the decreased area of the wound compared to other treatments (p < 0.05). It indicated that HC+Vit C had an excellent ability to heal the wound, in which the wound gap was reduced by 55.68%, compared with that of 0 h (Figure 4b). With the extended incubation time (48 h), a decrease in wound gap was observed for all samples. Moreover, three samples (HC, Vit C, and HC+Vit C) had higher efficacy of wound healing than the control (p < 0.05). Nonetheless, the efficacy of wound healing among the three samples was not different (p > 0.05) (Figure 4a,b), and the wound gap was reduced to 34.08%–35.90% as compared to that of 0 h.
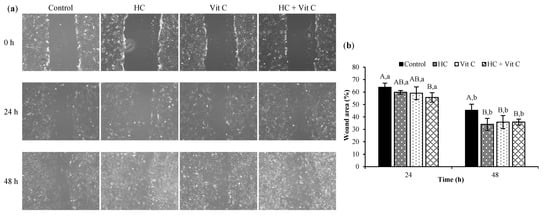
Figure 4.
Effect of HC, Vit C, and HC combined with Vit C (HC+Vit C) at the selected levels on HDF migration at 0, 24, and 48 h. Cell morphology with a magnification of ×10 (a) and % wound area (b). Bars represent standard deviation (n = 3). Different uppercase letters on bars indicate significant differences among different samples tested within the same time (p < 0.05). Different lowercase letters on bars indicate significant differences between time tested within the same samples (p < 0.05).
Lamellipodia formation of HDF cells after treatment with HC or Vit C or HC+Vit C at the selected level is shown in Figure 5. The finding demonstrated that all samples could enhance lamellipodia formation of HDF cells compared to the control (p < 0.05), as witnessed by higher relative fluorescence intensity (Figure 5b) and higher positive cells (Figure 5c) compared to the control (p < 0.05). Nevertheless, the efficacy of lamellipodia formation in HC-treated cells has similar to that of Vit C-treated cells (p > 0.05). Via treatment with the HC+Vit C sample, the drastically increased formation of lamellipodia was found (p < 0.05). Increasing lamellipodia formation after HC+Vit C treatment suggested that the combination of HC and Vit C was more effective than HC or Vit C alone.
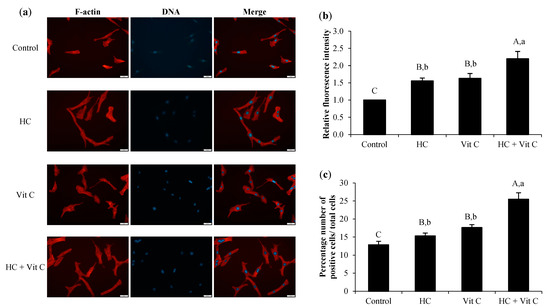
Figure 5.
Effect of HC, Vit C, and HC combined with Vit C (HC+Vit C) at the selected levels on lamellipodia formation of HDF cells using a scale bar of 50 μm and magnification of ×20 (a), the relative fluorescence intensity of lamellipodia after treatment with different samples (b), and percentage of a number of positive cells/total cells (c). Bars represent standard deviation (n = 3). Different uppercase letters on bars indicate significant differences between different samples tested and control (p < 0.05). Different lowercase letters on bars indicate significant differences among different samples tested (p < 0.05).
3.6. Effect of HC, Vit C, and HC+Vit C on Migration of HDF Cells via FAK/Akt Signaling Pathway
The expression levels of phosphorylated FAK (p-FAK) and Akt (p-Akt) of different samples are shown in Figure 6. All samples tested had higher levels of p-FAK than the control (p < 0.05). When comparing all the samples tested, a higher level of p-FAK was found in HC+Vit C and Vit C samples (p < 0.05) (Figure 6a,b). When the expression level of p-Akt among the samples tested was compared (Figure 6a,c), a marked increase in p-Akt level was found for the HC+Vit C sample (p < 0.05) (Figure 6c), while HC and Vit C samples showed similar p-Akt level (p > 0.05) (Figure 6c).
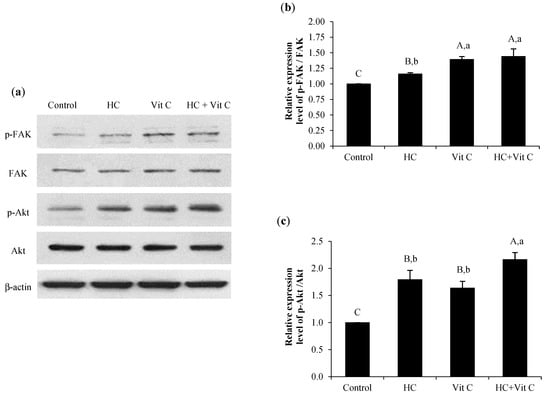
Figure 6.
Effect of HC, Vit C, and HC combined with Vit C (HC+Vit C) at the selected levels on the expression of p-FAK and p-Akt proteins (a) and relative level of p-FAK (b) and p-Akt (c) in HDF cells after treatment with the samples for 24 h. Bars represent standard deviation (n = 3). Different uppercase letters on bars indicate significant differences between different samples tested and control (p < 0.05). Different lowercase letters on bars indicate significant differences among different samples tested (p < 0.05).
3.7. Impact of HC, Vit C, and HC+Vit C on ERK/p38 MAPK Signaling Pathway in HDF Cells
The expression levels of ERK1/2 and p38 MAPK as influenced by the different samples are presented in Figure 7. All samples had an increased expression level of p-ERK1/2. However, their efficacy in activation of p-ERK1/2 was not different from the control (p > 0.05), except for Vit C, which showed a slightly lower level of p-ERK1/2 (Figure 7a,b). When considering the expression level of p-p38, all samples tested showed a higher level of p-p38 than the control (p < 0.05) (Figure 7a,c). Nevertheless, no difference in p-p38 level was found among the samples tested (p > 0.05).
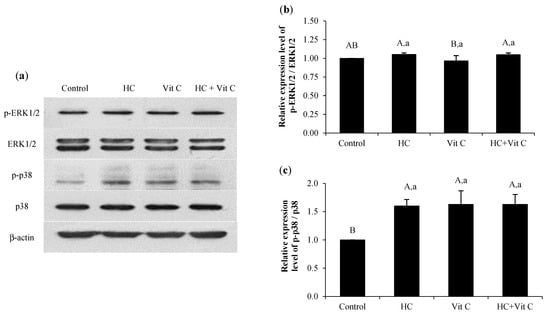
Figure 7.
Effect of HC, Vit C, and HC combined with Vit C (HC+Vit C) at the selected levels on the expression of p-ERK1/2 and p-p38 proteins (a) and relative level of p-ERK1/2 (b) and p-p38 (c) in HDF cells after treatment with the samples for 24 h. Bars represent standard deviation (n = 3). Different uppercase letters on bars indicate significant differences between different samples tested and control (p < 0.05). Different lowercase letters on bars indicate significant differences among different samples tested (p < 0.05).
4. Discussion
The MWs of peptides in HC from the present study (Figure 1) were larger than those of peptides from salmon skin prepared by two-step enzymatic hydrolysis (3% papain prior to 2% Alcalase) [10]. The different processes used probably affected the chain length, MW, and bioactivities of peptides [17]. Short peptides generally possessed greater bioactivities than large counterparts, particularly for activation of fibroblast migration [18]. Moreover, they could be rapidly absorbed into the human body [16]. Apart from the size of peptides, the amino acid sequence plays a vital role in bioactivities. Sato et al. [19] revealed that peptides having Pro-Hyp and Pro-Hyp-Gly could increase wound healing by accelerating the fibroblast migration to the injured tissue. Thus, the size of peptides might be another factor affecting fibroblast cell proliferation.
When the amino acid composition of HC was determined (Table 1), HC obtained from salmon skin in this study had lower imino acids (13.46 g/100 g HC) than that derived from seabass skin prepared using two-step hydrolysis (papain at 0.3 units/g dry matter for 90 min, followed by Alcalase at 0.3 units/g dry matter for 90 min) (20.43 g/100 g HC) [5]. The difference in raw material and enzymatic hydrolysis processes used is the important factor affecting the amino acid composition of hydrolysate [20,21]. Apart from imino acids, hydrophobic amino acids were found at a high content for defatted HC obtained from salmon skin (47.55 g/100 g HC), which might be related to several biological activities of peptides, including fibroblast and osteoblast proliferation [14,16]. Hydrophobic amino acids of HC were roughly 53.61% of total amino acids (Table 1). Pro, Gly, and Ala were dominant hydrophobic amino acids, which likely promoted wound-healing activity [5,22].
When HC was added to HDF cells (Figure 2), it could accelerate cell proliferation, especially at low levels (50–100 µg/mL). The addition of HC at high levels (>100 µg/mL) did not promote the proliferation of HDF cells. In general, HC from fish skin had the ability to scavenge free radicals generated after oxidative stress to facilitate cell survival and proliferation [23,24]. Nevertheless, the use of a high level of HC more likely weakened the structural integrity of fibroblast cells and enhanced intracellular oxidant levels [23], resulting in cell death owing to oxidative stress-induced apoptosis. Moreover, HC at a high level could act as an inhibitor and reduce cell growth [25]. Thus, the use of HC at a low level (Figure 2a), particularly at 50 µg/mL, was an appropriate level for stimulating HDF proliferation. Defatted HC from salmon skin had a high content of hydrophobic amino acids (47.55 g/100 g) (Table 1). Those amino acids might help promote fibroblast proliferation. Apart from hydrophobic amino acids, imino acids were also documented to enhance the proliferation of fibroblast cells [5,10]. Furthermore, arginine was also reported to stimulate fibroblast proliferation [26]. Those amino acids were found in defatted HC from salmon skin, in which the contents of imino acids and arginine were 13.46 g/100 g and 7.18 g/100 g, respectively (Table 1).
When considering the levels of Vit C on cell proliferation (Figure 2b), cells treated with Vit C at a low level (0.01 µg/mL) exhibited greater cell proliferation than those treated with high levels of Vit C (>0.01 µg/mL). Chaitrakoonthong et al. [27] also reported that Vit C at levels higher than 20 µg/mL decreased the cell viability of fibroblast cells. Moreover, the use of Vit C at levels ranging from 0.1 to 4 mM also inhibited cell proliferation. Simultaneously, oxidative species could be formed with increasing levels of Vit C [28]. This might lead to cell death, mainly due to mitochondrial dysfunction, depleting ATP levels, and disrupting cellular redox balance [29,30]. Although Vit C plays an essential role in cellular functions, particularly the stimulation of fibroblast proliferation and collagen synthesis [12], the level of Vit C used is a significant factor affecting cell proliferation. Based on the results from Figure 2, HC at 50 µg/mL and Vit C at 0.01 µg/mL showed the highest efficacy for cell proliferation. Thus, they were chosen to elucidate the combined effect of HC and Vit C (HC+Vit C), HC, or Vit C on cell proliferation using MTT and dsDNA assays (Figure 3).
Based on the findings in Figure 3, the combination between HC and Vit C could enhance cell proliferation effectively as compared to other treatments (p < 0.05) (Figure 3a,c), indicating that HC+Vit C exhibited a synergistic effect on the proliferation of HDF cells. Similar to the result of Benjakul et al. [31], HC from seabass skin incorporated with Vit C could augment L929 mouse fibroblast. Thus, HC, Vit C, or HC combined with Vit C could be used as potential agents for inducing the proliferation of HDF cells.
When HDF cells were used to determine the impact of HC in combination with Vit C on migration and lamellipodia formation of HDF cells, lowered wound gap of cells was found after treatment with all the samples (Figure 4). Nevertheless, the highest efficacy of wound healing was observed in cells treated with HC+Vit C (Figure 4b). This result suggested that HC+Vit C was able to be an effective compound for wound healing. Recently, Chotphruethipong et al. [14] reported that fibroblast is an important cell associated with the proliferation stage in the wound healing process. The augmented migration of HDF cells after treatment with HC+Vit C showed a greater ability to promote migration of HDF cells than HC or Vit C alone. As a result, the gap in the wound was decreased to a greater extent. The result was related to lamellipodia formation (Figure 5), in which higher cells with lamellipodia were found with the addition of HC+Vit C (Figure 5b,c). Defatted HC from salmon skin rich in glycine (21.8 g/100 g) might upsurge tissue regeneration [9,32], resulting in a decreased wound gap and increased lamellipodia formation. Moreover, arginine present in HC had a crucial role in the wound healing process by stimulating fibroblast proliferation and migration [33]. Simultaneously, Vit C incorporation might accelerate collagen synthesis and decrease inflammatory responses at the wound site [33]. Commonly, migration of skin cells was associated with the actin cytoskeleton, such as lamellipodia or filopodia [34]. This indicated that HC, vit C, and HC+Vit C could be the potential supplements used for skin nourishment.
When HDF cells were used to examine the effect of HC, Vit C, or HC+Vit C on the migration of HDF cells via the FAK/Akt signaling pathway, all samples could enhance the efficacy in promoting the migration of HDF cells via the activation of FAK signaling pathway as indicated by higher levels of p-FAK than the control (p < 0.05) (Figure 6a,b). In general, activation of both signaling molecules promotes cell proliferation and motility [15]. During the wound healing process, FAK activation is an essential step in promoting fibroblast migration initiated by the interaction between integrins and extracellular matrix proteins [35]. Increasing expression of p-FAK revealed that the samples tested were able to stimulate fibroblast migration in the wound healing process. Furthermore, all samples could also induce migration of cells via the activation of the Akt signaling pathway (Figure 6a,c). Nevertheless, the highest effectiveness in promoting cell migration was found when HC+Vit C was treated. The result was confirmed by the enhanced cell migration and lamellipodia formation (Figure 4 and Figure 5) when HC+Vit C was used, demonstrating that the combination between HC and Vit C might aid the promotion of cell migration, thus leading to the lowered wound gap.
Additionally, all samples tested had no toxicity on cells, as indicated by similar expression levels of p-ERK1/2 to the control (p > 0.05) (Figure 7a,b). In general, mitogen-activated protein kinases (MAPK) are involved in the various functions of cells, including proliferation, inflammation, migration, and apoptosis [36]. The MAPK can be classified into three groups, including p38, extracellular-signal-regulated protein kinase (ERK/MAPK), and Jun N-terminus kinase (JNK). Augmented expression of p-p38 of all the samples tested suggested that they could promote HDF proliferation and migration through the activation of the p-p38/MAPK signaling pathway, as witnessed by a higher level of p-p38 than the control (p < 0.05) (Figure 7a,c). The finding was associated with the proliferation and migration of cells, as shown in Figure 3 and Figure 4. Thus, the samples tested, including HC, Vit C, and HC+Vit C, had the ability to increase HDF proliferation and migration via the p38 MAPK signaling pathway.
5. Conclusions
Defatted hydrolyzed collagen (HC) from salmon skin contained peptides with large MW (>5 kDa) and small MW (<2 kDa). HC had high contents of imino acids and glycine. The use of HC at the level of 50 µg/mL and vitamin C (Vit C) at the level of 0.01 µg/mL could stimulate human dermal fibroblast proliferation effectively. Nevertheless, the effectiveness was increased when HC and Vit C at the selected level were used in combination (HC+Vit C). HC+Vit C could enhance the proliferation and migration of HDF cells effectively. HC, Vit C, and HC+Vit C augmented the expression of p-Akt, p-FAK, and p-p38 proteins associated with cell proliferation and migration. Therefore, defatted HC from salmon skin can be a functional ingredient or nutraceutical for skin nourishment and wound healing, especially when used in conjunction with Vit C. The optimization of the HC/Vit C ratio should be carried out to obtain the highest combined efficacy of both compounds.
Author Contributions
L.C.: conceptualization, investigation of the experiments, and writing of the original draft. K.N.: investigation of the experiments. P.H. and W.S.: validation and supervision. R.E.A. and N.R.A.: reviewed the manuscript. S.B.: conceptualization, supervision, editing of the manuscript, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Chair Professor Grants (P-20-52297) from the National Science and Technology Development Agency, Thailand.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research was financially supported by the Grants (P-20-52297) from the National Science and Technology Development Agency, Thailand. Prachayacharn program (AGR6502111N) from Prince of Songkla University was also acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yeung, D.A.; Kelly, N.H. The role of collagen-based biomaterials in chronic wound healing and sports medicine applications. Bioengineering 2021, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Fu, Y.; Dai, H.; Wang, Q.; Gao, R.; Zhang, Y. Recent progress in preventive effect of collagen peptides on photoaging skin and action mechanism. Food Sci. Hum. Wellness 2022, 11, 218–229. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Binlateh, T.; Hutamekalin, P.; Sukketsiri, W.; Aluko, R.E.; Benjakul, S. Hydrolyzed collagen from defatted sea bass skin and its conjugate with epigallocatechin gallate: In vitro antioxidant, anti-inflammatory, wound-healing and anti-obesity activities. Food Biosci. 2021, 43, 101303. [Google Scholar] [CrossRef]
- Pozzolini, M.; Millo, E.; Oliveri, C.; Mirata, S.; Salis, A.; Damonte, G.; Arkel, M.; Scarfì, S. Elicited ROS scavenging activity, photoprotective, and wound-healing properties of collagen-derived peptides from the marine sponge Chondrosia reniformis. Mar. Drugs 2018, 16, 465. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Binlateh, T.; Hutamekalin, P.; Sukketsiri, W.; Aluko, R.E.; Benjakul, S. In vitro antioxidant and wound-healing activities of hydrolyzed collagen from defatted Asian sea bass skin as influenced by different enzyme types and hydrolysis processes. RSC Adv. 2021, 11, 18144–18151. [Google Scholar] [CrossRef]
- Jin, R.; Teng, X.; Shang, J.; Wang, D.; Liu, N. Identification of novel DPP–IV inhibitory peptides from Atlantic salmon (Salmo salar) skin. Food Res. Int. 2020, 133, 109161. [Google Scholar] [CrossRef]
- Wu, R.; Wu, C.; Liu, D.; Yang, X.; Huang, J.; Zhang, J.; Liao, B.; He, H. Antioxidant and anti-freezing peptides from salmon collagen hydrolysate prepared by bacterial extracellular protease. Food Chem. 2018, 248, 346–352. [Google Scholar] [CrossRef]
- De Almagro, M.C. The use of collagen hydrolysates and native collagen in osteoarthritis. Am. J. Biomed. Sci. Res. 2020, 6, 530–532. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Chantakun, K.; Chotphruethipong, L.; Benjakul, S. Development of hydrolysis and defatting processes for production of lowered fishy odor hydrolyzed collagen from fatty skin of sockeye Salmon (Oncorhynchus nerka). Foods 2021, 10, 2257. [Google Scholar] [CrossRef]
- Woonnoi, W.; Chotphruethipong, L.; Tanasawet, S.; Benjakul, S.; Sutthiwong, N.; Sukketsiri, W. Hydrolyzed collagen from salmon skin increases the migration and filopodia formation of skin keratinocytes by activation of FAK/Src pathway. Polish J. Food Nutr. Sci. 2021, 71, 323–332. [Google Scholar] [CrossRef]
- D’Aniello, C.; Cermola, F.; Patriarca, E.J.; Minchiotti, G. Vitamin C in stem cell biology: Impact on extracellular matrix homeostasis and epigenetics. Stem. Cells Int. 2017, 2017, 8936156. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Ward, S.; Wayne, J.S.; Brophy, D.F.; Fowler, A.A., III; Yager, D.R.; Natarajan, R. Vitamin C promotes wound healing through novel pleiotropic mechanisms. Int. Wound J. 2016, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Sae-leaw, T.; Benjakul, S.; O’Brien, N.M.; Kishimura, H. Characteristics and functional properties of gelatin from seabass skin as influenced by defatting. Int. J. Food Sci. Technol. 2016, 51, 1204–1211. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Sukketsiri, W.; Aluko, R.E.; Sae-Leaw, T.; Benjakul, S. Effect of hydrolyzed collagen from defatted Asian sea bass (Lates calcarifer) skin on fibroblast proliferation, migration and antioxidant activities. J. Food Sci. Technol. 2021, 58, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Singkhorn, S.; Tantisira, M.H.; Tanasawet, S.; Hutamekalin, P.; Wongtawatchai, T.; Sukketsiri, W. Induction of keratinocyte migration by ECa 233 is mediated through FAK/Akt, ERK, and p38 MAPK signaling. Phytother. Res. 2018, 32, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Chotphruethipong, L.; Binlateh, T.; Hutamekalin, P.; Aluko, R.E.; Tepaamorndech, S.; Zhang, B.; Benjakul, S. Impact of hydrolyzed collagen from defatted sea bass skin on proliferation and differentiation of preosteoblast MC3T3-E1 cells. Foods 2021, 10, 1476. [Google Scholar] [CrossRef] [PubMed]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed collagen—Sources and applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef]
- Song, Y.; Wu, C.; Zhang, X.; Bian, W.; Liu, N.; Yin, S.; Yang, M.; Luo, M.; Tang, J.; Yang, X. A short peptide potentially promotes the healing of skin wound. Biosci. Rep. 2019, 39, BSR20181734. [Google Scholar] [CrossRef]
- Sato, K.; Asai, T.T.; Jimi, S. Collagen-derived di-peptide, prolylhydroxyproline (Pro-Hyp): A new low molecular weight growth-initiating factor for specific fibroblasts associated with wound healing. Front. Cell Dev. Biol. 2020, 8, 1243. [Google Scholar] [CrossRef]
- Ali, A.M.M.; Benjakul, S.; Kishimura, H. Molecular characteristics of acid and pepsin soluble collagens from the scales of golden carp (Probarbus jullieni). Emir. J. Food Agric. 2017, 29, 450–457. [Google Scholar] [CrossRef]
- Kakko, T.; Damerau, A.; Nisov, A.; Puganen, A.; Tuomasjukka, S.; Honkapää, K.; Tarvainen, M.; Yang, B. Quality of protein isolates and hydrolysates from Baltic Herring (Clupea harengus membras) and Roach (Rutilus rutilus) produced by pH-shift processes and enzymatic hydrolysis. Foods 2022, 11, 230. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Soliman, M.; Kotb, S.; Ali, M.M. Evaluation of fish skin as a biological dressing for metacarpal wounds in donkeys. BMC Vet. Res. 2020, 16, 472. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Clin. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Chotphruethipong, L.; Sukketsiri, W.; Battino, M.; Benjakul, S. Conjugate between hydrolyzed collagen from defatted seabass skin and epigallocatechin gallate (EGCG): Characteristics, antioxidant activity and in vitro cellular bioactivity. RSC Adv. 2021, 11, 2175–2184. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, M.; Kazimierczak, P.; Vivcharenko, V.; Koziol, M.; Przekora, A. Effect of vitamin C/hydrocortisone immobilization within curdlan-based wound dressings on In vitro cellular response in context of the management of chronic and burn wounds. Int. J. Mol. Sci. 2021, 22, 11474. [Google Scholar] [CrossRef]
- Fujiwara, T.; Kanazawa, S.; Ichibori, R.; Tanigawa, T.; Magome, T.; Shingaki, K.; Miyata, S.; Tohyama, M.; Hosokawa, K. L-arginine stimulates fibroblast proliferation through the GPRC6A-ERK1/2 and PI3K/Akt pathway. PLoS ONE 2014, 9, e92168. [Google Scholar] [CrossRef]
- Chaitrakoonthong, T.; Ampornaramveth, R.; Kamolratanakul, P. Rinsing with L-ascorbic acid exhibits concentration-dependent effects on human gingival fibroblast in vitro wound healing behavior. Int. J. Dent. 2020, 2020, 4706418. [Google Scholar] [CrossRef]
- Mata, A.M.O.F.D.; Carvalho, R.M.D.; Alencar, M.V.O.B.D.; Cavalcante, A.A.D.C.M.; Silva, B.B.D. Ascorbic acid in the prevention and treatment of cancer. Rev. Assoc. Med. Bras. 2016, 62, 680–686. [Google Scholar] [CrossRef]
- Chakraborty, A.; Jana, N.R. Vitamin C-conjugated nanoparticle protects cells from oxidative stress at low doses but induces oxidative stress and cell death at high doses. ACS Appl. Mater. Interfaces 2017, 9, 41807–41817. [Google Scholar] [CrossRef]
- Wu, Y.K.; Tu, Y.K.; Yu, J.; Cheng, N.C. The influence of cell culture density on the cytotoxicity of adipose-derived stem cells induced by L-ascorbic acid-2-phosphate. Sci. Rep. 2020, 10, 104. [Google Scholar] [CrossRef]
- Benjakul, S.; Karnjanapratum, S.; Visessanguan, W. Hydrolysed collagen from Lates calcarifer skin: Its acute toxicity and impact on cell proliferation and collagen production of fibroblasts. Int. J. Food Sci. Technol. 2018, 53, 1871–1879. [Google Scholar] [CrossRef]
- Sá, O.M.D.S.; Lopes, N.N.F.; Alves, M.T.S.; Caran, E.M.M. Effects of glycine on collagen, PDGF, and EGF expression in model of oral mucositis. Nutrients 2018, 10, 1485. [Google Scholar] [CrossRef] [PubMed]
- Barchitta, M.; Maugeri, A.; Favara, G.; Magnano San Lio, R.; Evola, G.; Agodi, A.; Basile, G. Nutrition and wound healing: An overview focusing on the beneficial effects of curcumin. Int. J. Mol. Sci. 2019, 20, 1119. [Google Scholar] [CrossRef]
- Ruggiero, C.; Lalli, E. Targeting the cytoskeleton against metastatic dissemination. Cancer Metastasis Rev. 2021, 40, 89–140. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.K.; Cheng, Y.; Liang Cheng, M.; Yu, L.; Mu, M.; Li, H.; Liu, Y.; Zhang, B.; Yao, Y.; Guo, H.; et al. Focal adhesion kinase regulates fibroblast migration via integrin beta-1 and plays a central role in fibrosis. Sci. Rep. 2016, 6, 19276. [Google Scholar] [CrossRef]
- Huang, C.; Jacobson, K.; Schaller, M.D. MAP kinases and cell migration. J. Cell Sci. 2004, 117, 4619–4628. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).