Using Merged Pre-Fishery Abundance as a Parameter Evaluating the Status of Atlantic Salmon and Anadromous Brown Trout Populations: A Norwegian Case Study
Abstract
1. Introduction
2. Materials and Methods
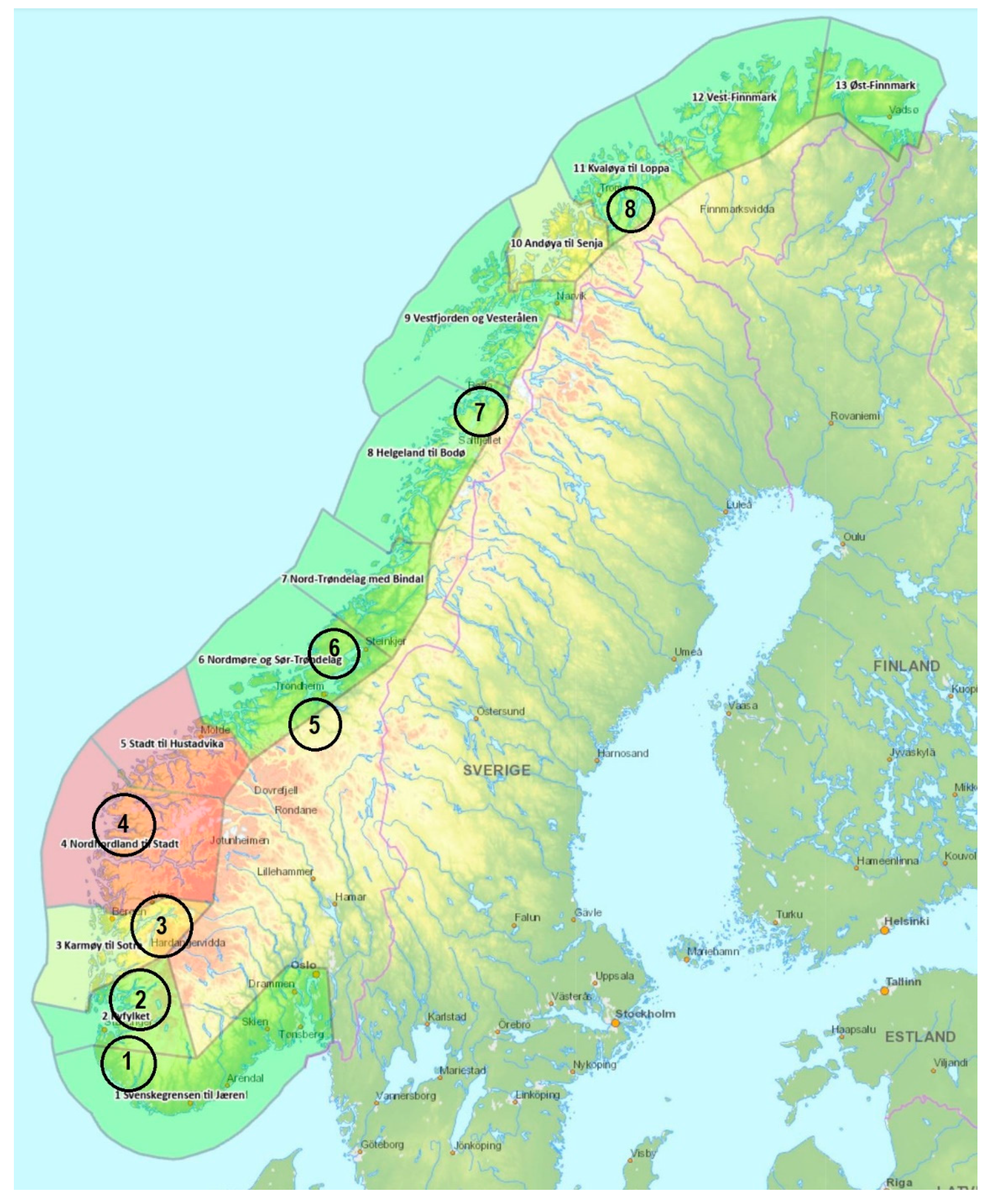
2.1. Study Area
2.2. Physical River Data
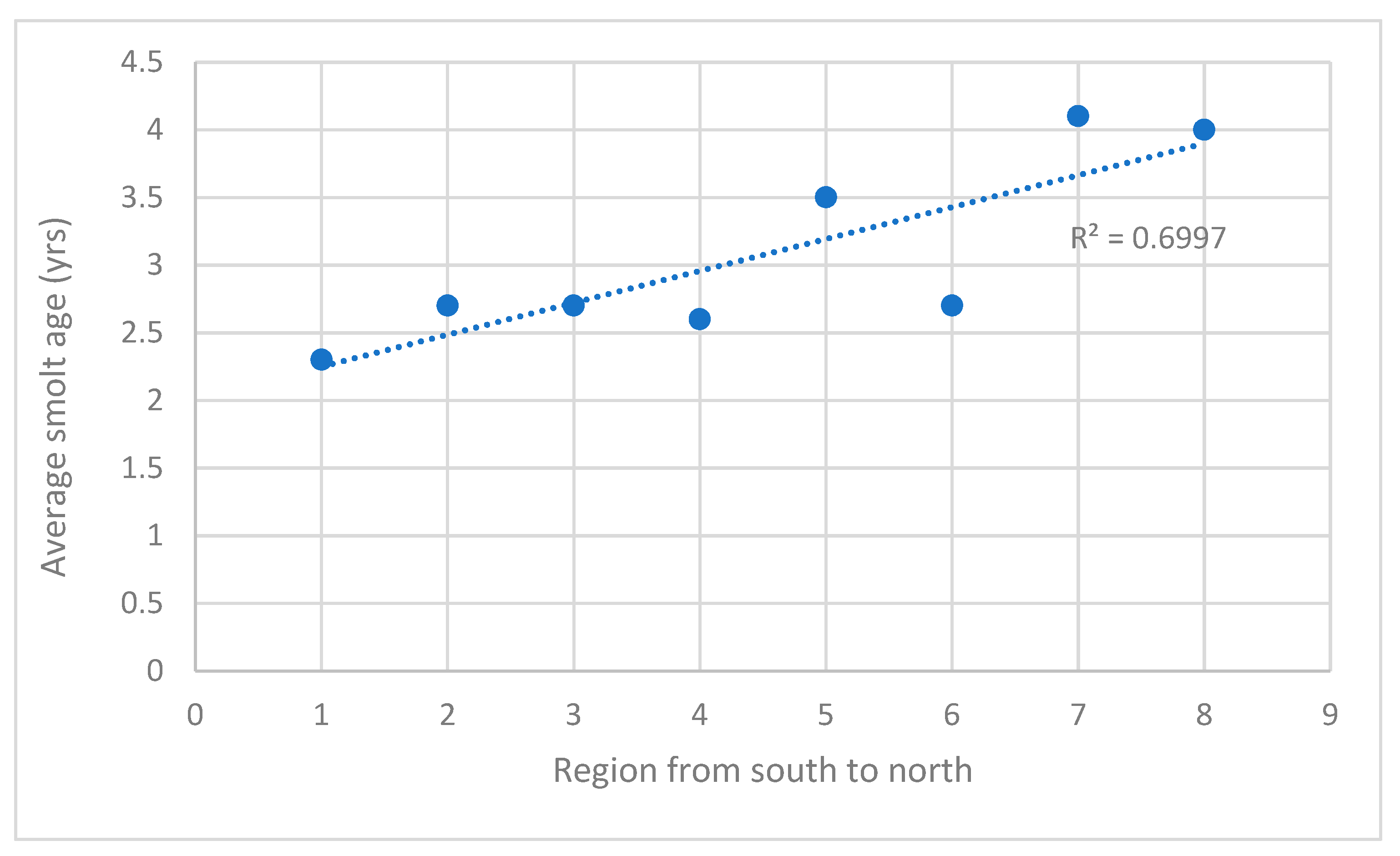
2.3. Average Smolt Age
2.4. Salmon Populations’ Spawning Target
2.5. Estimating PFA
2.6. Snorkelling
2.7. River Catch Data
2.8. Underwater Video Surveillance
2.9. Statistical Methods
3. Results
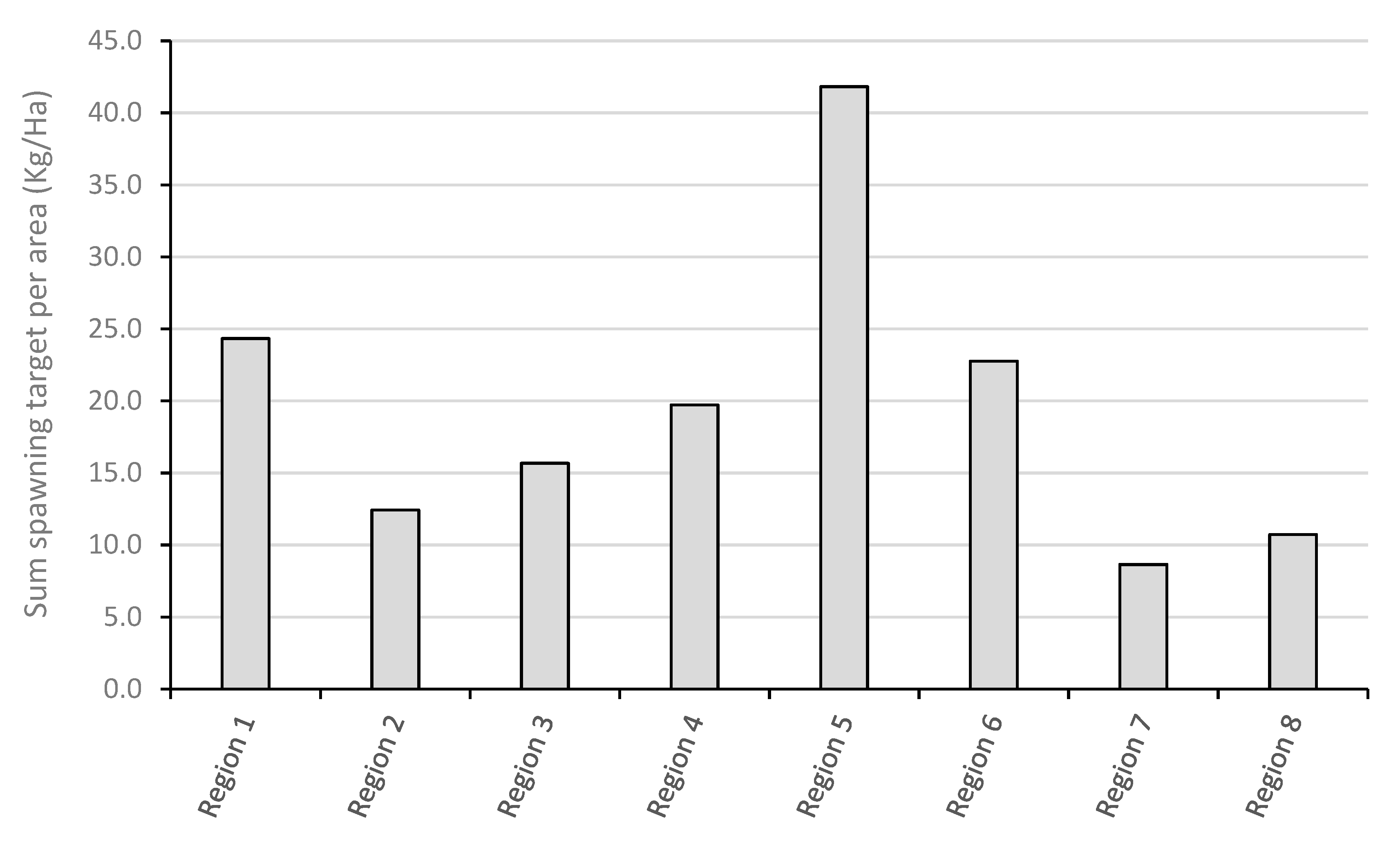
3.1. Comparing the Salmon Spawning Target in the Eight River Regions in 2019
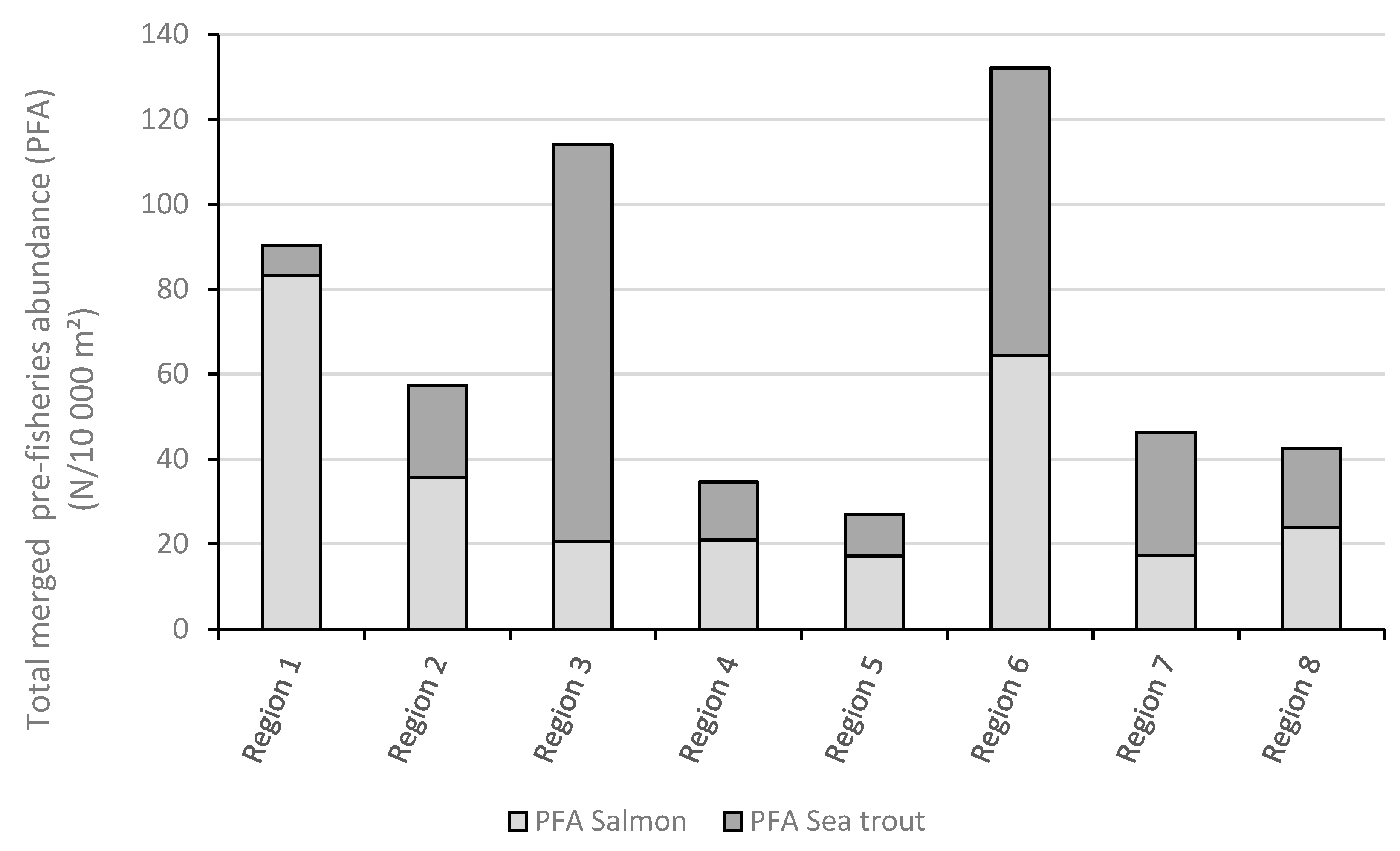
3.2. Comparing the Total PFA in the Eight Geographical Regions in 2019
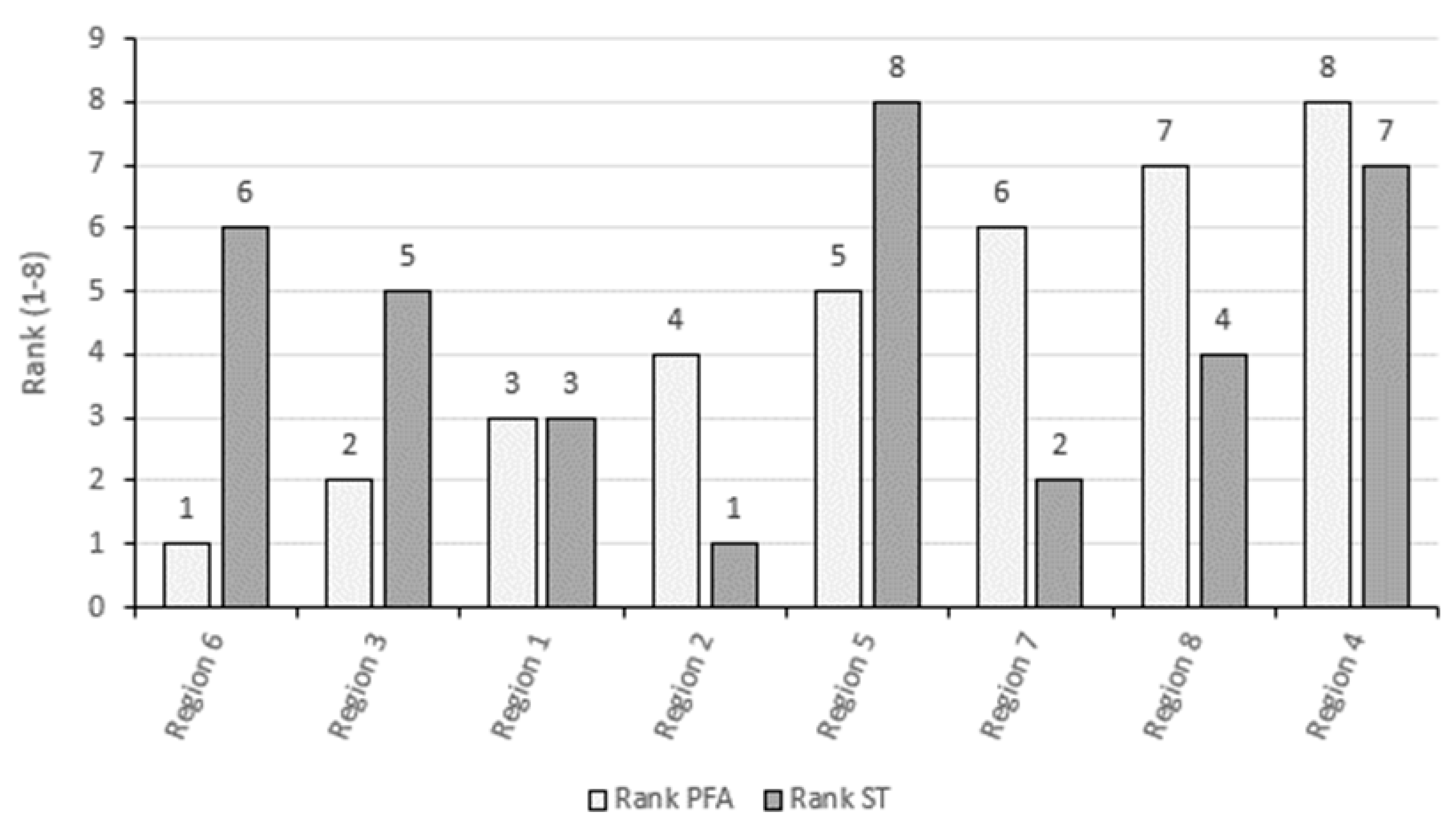
3.3. Comparing the ST and the PFA Approach in 2019
3.4. Average Smolt Age
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verspoor, E.; de Leániz, C.G.; McGinnity, P. Genetics and habitat management. In The Atlantic Salmon. Genetics, Conservation and Management; Verspoor, E., Stradmeyer, L., Nielsen, J., Eds.; Blackwell Publishing: Oxford, UK, 2007; pp. 399–424. [Google Scholar]
- Ferguson, A.; Reed, T.E.; Cross, T.F.; McGinnity, P.; Prodöhl, P.A. Anadromy, potamodromy and residency in brown trout Salmo trutta: The role of genes and the environment. J. Fish Biol. 2019, 95, 692–718. [Google Scholar] [CrossRef]
- Thorstad, E.B.; Økland, F.; Aarestrup, K.; Heggberget, T.G. Factors affecting the within-river spawning migration of Atlantic salmon, with emphasis on human impacts. Rev. Fish Biol. Fish. 2007, 18, 345–371. [Google Scholar] [CrossRef]
- Ford, J.S.; Myers, R.A. A Global Assessment of Salmon Aquaculture Impacts on Wild Salmonids. PLoS Biol. 2008, 6, e33. [Google Scholar] [CrossRef]
- Gargan, P.; Forde, G.; Hazon, N.; Russell, D.; Todd, C. Evidence for sea lice-induced marine mortality of Atlantic salmon (Salmo salar) in western Ireland from experimental releases of ranched smolts treated with emamectin benzoate. Can. J. Fish. Aquat. Sci. 2012, 69, 343–353. [Google Scholar] [CrossRef]
- Skaala, Ø.; Sjøtun, K.; Dahl, E.; Husa, V.; Bjørge, A.; Uiblein, F. Interactions between salmon farming and the ecosystem: Lessons from the Hardangerfjord, western Norway. Mar. Biol. Res. 2014, 10, 199–202. [Google Scholar] [CrossRef]
- Skaala, Ø.; Johnsen, G.H.; Lo, H.; Borgstrøm, R.; Wennevik, V.; Hansen, M.M.; Merz, J.E.; Glover, K.A.; Barlaup, B.T. A conservation plan for Atlantic salmon (Salmo salar) and anadromous brown trout (Salmo trutta) in a region with intensive industrial use of aquatic habitats, the Hardangerfjord, western Norway. Mar. Biol. Res. 2014, 10, 308–322. [Google Scholar] [CrossRef]
- Forseth, T.; Barlaup, B.T.; Finstad, B.; Fiske, P.; Gjøsæter, H.; Falkegård, M.; Hindar, A.; Mo, T.A.; Rikardsen, A.H.; Thorstad, E.B.; et al. The major threats to Atlantic salmon in Norway. ICES J. Mar. Sci. 2017, 74, 1496–1513. [Google Scholar] [CrossRef]
- Hindar, K.; Hutchings, J.A.; Diserud, O.; Fiske, P. Stock, recruitment and exploitation. In Atlantic Salmon Ecology; Aas, Ø., Einum, S., Klementsen, A., Skurdal, J., Eds.; Blackwell Publishing: Oxford, UK, 2011; pp. 299–332. [Google Scholar]
- Forseth, T.; Fiske, P.; Barlaup, B.; Gjøsæter, H.; Hindar, K.; Diserud, O.H. Reference point based management of Norwegian Atlantic salmon populations. Environ. Conserv. 2013, 40, 356–366. [Google Scholar] [CrossRef]
- Anon. Status of Wild Atlantic Salmon in Norway 2020. Vitenskapelig råd for Lakseforvalting. In Norwegian with Summary in English. 147p. 2020. Available online: https://www.vitenskapsradet.no/ (accessed on 15 June 2022).
- Potter, E.C.E.; Crozier, W.W.; Schön, P.-J.; Nicholson, M.D.; Maxwell, D.L.; Prévost, E.; Erkinaro, J.; Gudbergsson, G.; Karlsson, L.; Hansen, L.P.; et al. Estimating and forecasting pre-fishery abundance of Atlantic salmon (Salmo salar L.) in the Northeast Atlantic for the management of mixed-stock fisheries. ICES J. Mar. Sci. 2004, 61, 1359–1369. [Google Scholar] [CrossRef]
- Krkošek, M.; Lewis, M.A.; Volpe, J.P.; Morton, A. Fish farms and sea lice infestations of wild juvenile salmon in the Broughton Archipelago—A rebuttal to Brooks (2005). Rev. Fish. Sci. 2006, 14, 1–11. [Google Scholar] [CrossRef]
- Kristoffersen, A.B.; Qviller, L.; Helgesen, K.O.; Vollset, K.W.; Viljugrein, H.; Jansen, P.A. Quantitative risk assessment of salmon louse-induced mortality of seaward-migrating post-smolt Atlantic salmon. Epidemics 2018, 23, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Klemetsen, A.; Amundsen, P.A.; Dempson, J.B.; Jonsson, B.; Jonsson, N.; O’Connell, M.F.; Mortensen, E. Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): A review of aspects of their life histories. Ecol. Freshw. Fish 2003, 12, 1–59. [Google Scholar] [CrossRef]
- Lamberg, A.; Strand, R.; Kanstad-Hanssen, Ø. Videoovervåking av Laks og Sjøørret i Granvinsvassdraget i 2017. SNA-Rapport 01/2020; 33p. 2020. (In Norwegian). Available online: http://skandnat.no/wp-content/uploads/2021/01/SNA-rapport-01-2020.pdf (accessed on 1 June 2022).
- Lamberg, A.; Bjørnbet, S.; Berdal, M.; Gjertsen, V.; Strand, R.; Hanssen, K.Ø. Videoovervåking av Laks og Sjøørrret i Orkla i årene 2013 til 2017. SNA-Rapport 11/2018. 69p. 2018. (In Norwegian). Available online: https://skandnat.no/wp-content/uploads/2018/10/Orkla-SNA-rapport-11-2018.pdf (accessed on 1 June 2022).
- Skaala, Ø.; Johnsen, G.H.; Barlaup, B. Prioriterte Strakstiltak for Sikring av Ville Bestander av Laksefisk i Hardangerfjordbassenget i Påvente av Langsiktige Forvaltningstiltak. Report fra Havforskningen. Institute of Marine Research, Bergen, 10/2010, 39p. 2010. (In Norwegian with Abstract in English). Available online: https://www.hi.no/hi/nettrapporter/rapport-fra-havforskningen/2010/hi-rapp_10-2010 (accessed on 25 May 2022).
- Jonsson, B.; Jonsson, N.; Hansen, L.P. Atlantic salmon straying from the River Imsa. J. Fish Biol. 2003, 62, 641–657. [Google Scholar] [CrossRef]
- Källo, K.; Baktoft, H.; Kristensen, M.L.; Birnie-Gauvin, K.; Aarestrup, K. High prevalence of straying in a wild brown trout (Salmo trutta) population in a fjord system. ICES J. Mar. Sci. 2022, 79, 1539–1547. [Google Scholar] [CrossRef]
- Birnie-Gauvin, K.; Thorstad, E.B.; Aarestrup, K. Overlooked aspects of the Salmo salar and Salmo trutta lifecycles. Rev. Fish Biol. Fish. 2019, 29, 749–766. [Google Scholar] [CrossRef]
- Van Zwol, J.A.; Neff, B.D.; Wilson, C.C. The effect of competition among three salmonids on dominance and growth during the juvenile life stage. Ecol. Freshw. Fish 2012, 21, 533–540. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Ecology of Atlantic Salmon and Brown Trout. Habitat as a Template for Life Histories; Springer: New York, NY, USA, 2011. [Google Scholar]
- Jensen, A.J.; Diserud, O.H.; Finstad, B.; Fiske, P.; Thorstad, E.B. Early-season brown trout (Salmo trutta) migrants grow and survive better at sea. J. Fish Biol. 2022, 100, 1419–1431. [Google Scholar] [CrossRef]
- Thorstad, E.B.; Todd, C.D.; Uglem, I.; Bjørn, P.A.; Gargan, P.G.; Vollset, K.W.; Halttunen, E.; Kålås, S.; Berg, M.; Finstad, B. Marine life of the sea trout. Mar. Biol. 2016, 163, 47. [Google Scholar] [CrossRef]
- Jensen, A.J.; Finstad, B.; Fiske, P. The cost of anadromy: Marine and freshwater mortality rates in anadromous Arctic char and brown trout in the Arctic region of Norway. Can. J. Fish. Aquat. Sci. 2019, 76, 2408–2417. [Google Scholar] [CrossRef]
- Rideout, R.M.; Tomkiewicz, J. Skipped Spawning in Fishes: More Common than You Might Think. Mar. Coast. Fish. Dyn. Manag. Ecosyst. Sci. 2011, 3, 176–189. [Google Scholar] [CrossRef]
- Flemming, I.A. Reproductive strategies of Atlantic salmon: Ecology and evolution. Rev. Fish Biol. Fish. 1996, 6, 379–416. [Google Scholar] [CrossRef]
- Arnekleiv, J.V.; Kjærstad, G.; Rønning, L.; Davidsen, J.G.; Sjursen, A.D. Studies on Freshwater Biology in the River Stjørdalsvassdraget in 2009–2013. NTNU Vitenskapsmuseet Naturhistorisk Rapport 2014-3, 82p. 2014. (In Norwegian with Abstract in English). Available online: https://www.ntnu.no/documents/10476/401393002/2014-3+Rapport+Stj%C3%B8rdalsvassdraget.pdf/8ea46943-9794-4382-b1e7-fa9c7f68fec6 (accessed on 24 April 2022).
- Hindar, K.; Diserud, O.; Fiske, P.; Forseth, T.; Jensen, A.J.; Ugedal, O.; Jonsson, N.; Sloreid, S.-E.; Arnekleiv, J.V.; Saltveit, S.J.; et al. Spawning Targets for Atlantic Salmon Populations in Norway. NINA Report 226. 78p. 2007. (In Norwegian with English summary). Available online: http://www.nina.no/archive/nina/pppbasepdf/rapport/2007/226.pdf (accessed on 20 May 2022).
- Vollset, K.W.; Skoglund, H.; Barlaup, B.T.; Pulg, U.; Gabrielsen, S.-E.; Wiers, T.; Skår, B.; Lehmann, G.B. Can the river location within a fjord explain the density of Atlantic salmon and sea trout? Mar. Biol. Res. 2014, 10, 268–278. [Google Scholar] [CrossRef]
- Skoglund, H.; Wiers, T.; Normann, E.S.; Stranzl, S.; Landro, Y.; Pulg, U.; Postler, C.; Velle, G.; Gabrielsen, S.-E.; Lehmann, G.B.; et al. Gytefisktelling av Laks og Sjøaure og Uttak av Rømt Oppdrettslaks i Elver på Vestlandet Høsten 2018. LFI-Report nr 359. 19p. 2018. (In Norwegian). Available online: https://norceresearch.brage.unit.no/norceresearch-xmlui/bitstream/handle/11250/2647900/LFI_359.pdf?sequence=4 (accessed on 20 May 2022).
- Hellen, B.A.; Kambestad, M.; Johnsen, G.H. Habitatkartlegging og Forslag til Tiltak for Sjøaure i Utvalgte Vassdrag ved Hardangerfjorden. Rådgivende Biologer AS Rapport nr. 1781. 251p. 2013. (In Norwegian). Available online: https://www.radgivende-biologer.no/rapporter/ar-2013/habitatkartlegging-og-forslag-til-tiltak-for-sjoaure-i-utvalgte-vassdrag-ved-hardangerfjorden/ (accessed on 10 May 2022).
- Orell, P.; Erkinaro, J.; Karppinen, P. Accuracy of snorkelling counts in assessing spawning stock of Atlantic salmon, Salmo salar, verified by radio-tagging and underwater video monitoring. Fish. Manag. Ecol. 2011, 18, 392–399. [Google Scholar] [CrossRef]
- Svenning, M.-A.; Lamberg, A.; Dempson, B.; Strand, R.; Hanssen, Ø.K.; Fauchald, P. Incidence and timing of wild and escaped farmed Atlantic salmon (Salmo salar) in Norwegian rivers inferred from video surveillance monitoring. Ecol. Freshw. Fish 2016, 26, 360–370. [Google Scholar] [CrossRef]
- Kanstad-Hanssen, Ø.; Gjertsen, V.; Bentsen, V.; Lamberg, A. Oppvandring av Sjøvandrende Laksefisk i Fisketrappa i Målselvfossen i 2016. Ferskvannsbiologen Rapport 2017-06; 15p. 2017. (In Norwegian). Available online: http://skandnat.no/wp-content/uploads/2021/05/Rapport-2017-06-Malselv-2016-Endelig.pdf (accessed on 25 April 2022).
- Skoglund, H.; Vollset, K.W.; Barlaup, B.; Lennox, R. Gytefisktelling av Laks og Sjøaure på Vestlandet—Status og Utvikling i Perioden 2004–2018. (Estimation of Spawning Population of Atlantic Salmon and Sea Trout in Western Norway—Status and Development in the Period 2004–2018). NORCE Report nr. 357, Bergen, Norway, 44p. 2019. (In Norwegian with Abstract in English). Available online: https://norceresearch.brage.unit.no/norceresearch-xmlui/bitstream/handle/11250/2647898/LFI_357.pdf?sequence=4 (accessed on 27 April 2022).
- Holte, E.; Skoglund, H.; Solem, Ø.; Kanstad-Hanssen, Ø.; Kambestad, M.; Lamberg, A.; Muladal, R.; Sollien, P.V.; Hellen, B.A.; Ulvan, E.M. Overvåking av Gytebestander av Laks og Sjøørret i Norge, 2019. NINA Rapport 1849, 226p. 2020. (In Norwegian with Abstract In Norwegian). Available online: https://brage.nina.no/nina-xmlui/handle/11250/2657851 (accessed on 1 June 2022).
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1984; p. 718. [Google Scholar]
- Stien, L.H.; Nilsson, J.; Bui, S.; Fosseidengen, J.E.; Kristiansen, T.S.; Øverli, Ø.; Folkedal, O. Consistent melanophore spot patterns allow long-term individual recognition of Atlantic salmon Salmo salar. J. Fish Biol. 2017, 91, 1699–1712. [Google Scholar] [CrossRef]
- Pulg, U.; Vollset, K.W.; Lennox, R.J. Linking habitat to density-dependent population regulation: How spawning gravel availability affects abundance of juvenile salmonids (Salmo trutta and Salmo salar) in small streams. Hydrobiologia 2019, 841, 13–29. [Google Scholar] [CrossRef]
- Einum, S. Salmonid population dynamics: Stability under weak density dependence? Oikos 2005, 110, 630–633. [Google Scholar] [CrossRef]
- Hindar, K.; Tufto, J.; Sættem, L.M.; Balstad, T. Conservation of genetic variation in harvested salmon populations. ICES J. Mar. Sci. 2004, 61, 1389–1397. [Google Scholar] [CrossRef]
- Hansen, M.M.; Skaala, Ø.; Jensen, L.F.; Bekkevold, D.; Mensberg, K.-L.D. Gene flow, effective population size and selection at major histocompatibility complex genes: Brown trout in the Hardanger Fjord, Norway. Mol. Ecol. 2007, 16, 1413–1425. [Google Scholar] [CrossRef]
- Lamberg, A.; Kvitvær, T. Videoovervåking av laks og sjøørret i Mundheimselva i 2018. NA Report 14/2018, 68p. 2018. (In Norwegian). Available online: https://skandnat.no/2022/01/20/videoovervaking-av-laks-og-sjoorret-i-mundheimselva-i-2018/ (accessed on 1 June 2022).
- Degerman, E.; Leonardsson, K.; Lundqvist, H. Coastal migrations, temporary use of neighbouring rivers, and growth of sea trout (Salmo trutta) from nine northern Baltic Sea rivers. ICES J. Mar. Sci. 2012, 69, 971–980. [Google Scholar] [CrossRef]
- Limburg, K.E.; Waldman, J.R. Dramatic Declines in North Atlantic Diadromous Fishes. BioScience 2009, 59, 955–965. [Google Scholar] [CrossRef]
- Hersoug, B. Why and how to regulate Norwegian salmon production?–The history of Maximum Allowable Biomass (MAB). Aquaculture 2021, 545, 737144. [Google Scholar] [CrossRef]
- Misund, A.U. From a natural occurring parasitic organism to a management object: Historical perceptions and discourses related to salmon lice in Norway. Mar. Policy 2019, 99, 400–406. [Google Scholar] [CrossRef]
- Vollset, K.W.; Dohoo, I.; Karlsen, Ø.; Halttunen, E.; Kvamme, B.O.; Finstad, B.; Wennevik, V.; Diserud, O.H.; Bateman, A.; Friedland, K.D.; et al. Disentangling the role of sea lice on the marine survival of Atlantic salmon. ICES J. Mar. Sci. 2017, 75, 50–60. [Google Scholar] [CrossRef]
- Myksvoll, M.S.; Sandvik, A.D.; Albretsen, J.; Asplin, L.; Johnsen, I.A.; Karlsen, Ø.; Kristensen, N.M.; Melsom, A.; Skardhamar, J.; Ådlandsvik, B. Evaluation of a national operational salmon lice monitoring system—From physics to fish. PLoS ONE 2018, 13, e0201338. [Google Scholar] [CrossRef]
- Diserud, O.H.; Fiske, P.; Karlsson, S.; Glover, K.A.; Næsje, T.; Aronsen, T.; Bakke, G.; Barlaup, B.T.; Erkinaro, J.; Florø-Larsen, B.; et al. Natural and anthropogenic drivers of escaped farmed salmon occurrence and introgression into wild Norwegian Atlantic salmon populations. ICES J. Mar. Sci. 2022, 79, 1363–1379. [Google Scholar] [CrossRef]
- Stensland, S.; Dugstad, A.; Navrud, S. The Recreational value of Atlantic salmon angling under different fishing regulations. Fish. Manag. Ecol. 2021, 28, 362–372. [Google Scholar] [CrossRef]
- Jensen, A.J.; Finstad, B.; Fiske, P.; Hvidsten, N.A.; Rikardsen, A.H.; Saksgård, L. Timing of smolt migration in sympatric populations of Atlantic salmon (Salmo salar), brown trout (Salmo trutta), and Arctic char (Salvelinus alpinus). Can. J. Fish. Aquat. Sci. 2012, 69, 711–723. [Google Scholar] [CrossRef]
- Råd, O. Breeding distribution and habitat selection of Red-breasted Mergansers in freshwater in western Norway. Wildfowl J. 1980, 31, 53–56. [Google Scholar]
- Svenning, M.-A.; Fagermo, S.E.; Barrett, R.T.; Borgstrøm, R.; Vader, W.; Pedersen, T.; Sandring, S. Goosander predation and its potential impact on Atlantic salmon smolts in the River Tana estuary, northern Norway. J. Fish Biol. 2005, 66, 924–937. [Google Scholar] [CrossRef]
- Landa, A.; Guidos, S. Bycatch in local fishery disrupts natural reestablishment of Eurasian otter in western Norway. Conserv. Sci. Pract. 2020, 2, e208. [Google Scholar] [CrossRef]
- Berg, O.K.; Berg, M. The duration of sea and freshwater residence of the sea trout, Salmo trutta, from the Vardnes River in northern Norway. Environ. Biol. Fishes 1989, 24, 23–32. [Google Scholar] [CrossRef]

| Region | River | Method | Number of Lakes | Winter Habitat Sea Trout |
|---|---|---|---|---|
| 1 | Bjerkreimselva | Video in two fish ladders | 6 | Yes |
| 2 | Frafjordelva | Snorkelling | 1 | Yes |
| 2 | Dirdalselva | Snorkelling | ||
| 2 | Espedalselva | Snorkelling | 2 | Yes |
| 2 | Forsandåna | Snorkelling | ||
| 2 | Årdalselva | Snorkelling | 1 | Yes |
| 2 | Hålandselva | Snorkelling | ||
| 2 | Vikedalselva | Snorkelling | ||
| 2 | Rødneelva (Sandeid) | Snorkelling | ||
| 3 | Eidfjordvassdraget | Snorkelling | 1 | Yes |
| 3 | Etneelva | Snorkelling/trap | 1 | Yes |
| 3 | Granvinsvassdraget | Snorkelling/video | 1 | Yes |
| 3 | Jondalselva | Snorkelling | ||
| 3 | Kinso | Snorkelling | ||
| 3 | Omvikelva | Snorkelling/video | ||
| 3 | Rosendalselvene | Snorkelling | ||
| 3 | Sima | Snorkelling | ||
| 3 | Steinsdalelva | Snorkelling | 1 (estuary) | |
| 3 | Uskedalselva | Snorkelling | ||
| 4 | Nausta | Video in fish ladder/snorkelling | ||
| 4 | Gaula (Sunnfjord) | Video in fish ladder | ||
| 5 | Orkla | Video/snorkelling | Yes | |
| 6 | Stordalselva | Video | 1 | Yes |
| 6 | Norddalselva | Video | Yes | |
| 7 | Saltdalsleva | Snorkelling | 1 | Yes |
| 7 | Beiarelva | Snorkelling | Yes | |
| 8 | Målselva | Video in fish ladder/snorkelling | Yes |
| Region | River Length (km) Accessible to Anadromous Fish | Annual Mean Water Discharge (m³/s) | River Area (m²) Accessible to Anadromous Fish | Precipitation Field (km²) | Smolt Age (Years) |
|---|---|---|---|---|---|
| 1 | 52.0 | 54 | 1.774.413 | 703 | 2.3 |
| 2 | 56.8 | 108 | 2.602.491 | 1253 | 2.7 |
| 3 | 74.0 | 131 | 1.542.336 | 1722 | 2.7 |
| 4 | 26.4 | 76 | 1.833.010 | 908 | 2.6 |
| 5 | 88.0 | 67 | 4.522.770 | 3051 | 3.5 |
| 6 | 36.8 | 17 | 1.724.595 | 374 | 2.7 |
| 7 | 101.2 | 100 | 4.728.820 | 2396 | 4.1 |
| 8 | 112.2 | 171 | 5.000.000 | 3015 | 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamberg, A.; Imsland, A.K.D. Using Merged Pre-Fishery Abundance as a Parameter Evaluating the Status of Atlantic Salmon and Anadromous Brown Trout Populations: A Norwegian Case Study. Fishes 2022, 7, 264. https://doi.org/10.3390/fishes7050264
Lamberg A, Imsland AKD. Using Merged Pre-Fishery Abundance as a Parameter Evaluating the Status of Atlantic Salmon and Anadromous Brown Trout Populations: A Norwegian Case Study. Fishes. 2022; 7(5):264. https://doi.org/10.3390/fishes7050264
Chicago/Turabian StyleLamberg, Anders, and Albert Kjartansson D. Imsland. 2022. "Using Merged Pre-Fishery Abundance as a Parameter Evaluating the Status of Atlantic Salmon and Anadromous Brown Trout Populations: A Norwegian Case Study" Fishes 7, no. 5: 264. https://doi.org/10.3390/fishes7050264
APA StyleLamberg, A., & Imsland, A. K. D. (2022). Using Merged Pre-Fishery Abundance as a Parameter Evaluating the Status of Atlantic Salmon and Anadromous Brown Trout Populations: A Norwegian Case Study. Fishes, 7(5), 264. https://doi.org/10.3390/fishes7050264






