Abstract
A monthly survey of the feeding selectivity of Ruditapes philippinarum in the Yalu River Estuary in 2020–2021 was conducted using high-throughput sequencing identification and visual grading technology. The results showed that the most-dominant species in the water of the shellfish culture area and in the stomachs of R. philippinarum was Karlodinium veneficum in those years. The selectivity index (E) indicated that R. philippinarum avoided consuming Bacillariophyta, Chrysophyta and Cryptophyta throughout the year and preferentially consumed Dinophyta and Chlorophyta. In 2020, the annual average biomass of Dinophyta, Bacillariophyta, Chlorophyta, Dictyochophyta, Cryptophyta and Chrysophyta in the stomach contents of R. philippinarum was 54:14:16:1:10:4; it was 41:12:28:0:1:17 in 2021. The annual average biomass ratio of picophytoplankton, nanophytoplankton and microphytoplankton in the stomachs of R. philippinarum was 13:48:39 in 2020; it was 14:66:20 in 2021. R. philippinarum actively fed on nanophytoplankton and avoided picophytoplankton. Among the phytoplankton of different sizes and groups that R. philippinarum prefer to feed, chemical oxygen demand (COD) and organic phosphorus (DOP) have a significant negative effect on the nanophytoplankton community, pH has a positive effect on the Dictyochophyta community and COD and the inorganic nitrogen to phosphorus ratio (DI-N/P) have a significant positive effect on the Chlorophyta community.
1. Introduction
Aquaculture, which is one of the fastest-growing food-producing sectors in the world, will play a critical role in narrowing the gap between rising consumer demand for seafood and declines in the supply of wild-caught seafood products. Many countries have thus made the development of aquaculture a national priority. Filter-feeding shellfish have become increasingly common in China’s coastal waters because of the continual increase in the scale of filter-feeding shellfish culture. Unlike farmed fish that can be fed diverse feeds, the main food source for filter-feeding shellfish culture is natural phytoplankton [1,2]; however, filter-feeding shellfish are selective feeders [3,4]. Large phytoplankton tend to be selectively ingested via the ctenoid gills of filter-feeding shellfish; after entering the intestinal tract, phytoplankton are assimilated selectively through the cilia. Unassimilated phytoplankton are embedded in the mucus and excreted as feces. Both of these selection mechanisms are affected by various properties of phytoplankton, including their size, shape, mobility, viscosity, toxicity and nutrient composition [4,5,6,7,8,9,10,11]. The selective feeding mechanism of shellfish is complex, and phytoplankton retention rates and preferences vary among regions, seasons and environments [2].
Ruditapes philippinarum, Mollusca, Bivalvia, Veneroida, Veneridae, Ruditapes, is dioecious. It is one of the four major shellfish species cultured in China, and it is widely distributed along the coast of China. Its rapid growth and short culture period, coupled with its high adaptability, make it an excellent species for artificial high-density culture. It has great commercial value and has been introduced all over the world. The Yalu River Estuary of Dandong City, Liaoning Province, China, is one of the main regions for R. philippinarum culture, and it accounts for 18% of China’s total output of R. philippinarum. However, R. philippinarum yields have decreased steadily in recent years because of slowing growth rates, reductions in meat quality, and increases in mortality, and this poses a major threat to the sustainable and healthy development of the shellfish aquaculture industry. Previous studies have found that the phytoplankton in the Yalu River Estuary has small particle size components and weak nutrient supply capacity, which may affect the nutritional reserve and healthy growth of R. philippinarum. The aim of this study was to evaluate the feeding preferences of R. philippinarum for phytoplankton of different sizes and from different groups during different periods to evaluate whether nanophytoplankton and Dinophyta have contributed to the decline in R. philippinarum yields.
Phytoplankton are typically identified by observing morphological characters with the aid of a microscope. Given the limitations in the resolution of traditional microscopes, many small phytoplankton, especially picophytoplankton, have not been detected in previous studies, and thus are understudied. Here, high-throughput sequencing identification and visual grading technology were used to characterize the monthly feeding preferences of R. philippinarum for different types and sizes of phytoplankton in the Yalu River Estuary in 2020 and 2021. Our results provide new insights that could be used to identify possible approaches for ensuring the sustainable development of R. philippinarum culture in the Yalu River Estuary.
2. Materials and Methods
2.1. Sample Collection
Samples of R. philippinarum and phytoplankton were collected from the fixed stations in March–December 2020 and March–October and December 2021 (in November 2021, due to epidemic, we could not go out to sea for sampling, so there are no data) in the Yalu River Estuary, which is located in the northern part of the Yellow Sea (Figure 1). In 2020, it was only sampled at Station 1, and in 2021 at Station 1 and Station 2. Because the average water depth of our sampling site was approximately 5 m, environmental DNA samples of phytoplankton were only collected from the surface of the seawater at a depth of 0.5 m. Using a 5 L card cover type water collector to collect seawater about 0.5 m below the water surface, at least 1 L of seawater was vacuum-filtered through 0.22-μm cellulose acetate membranes and subsequently folded and flash-frozen at –80 °C until further processing in the laboratory. At the same time, 1 kg of R. philippinarum was collected from each station, and 30 stomach contents of R. philippinarum, about 0.5 g, were collected from each station every month for high-throughput molecular sequencing.
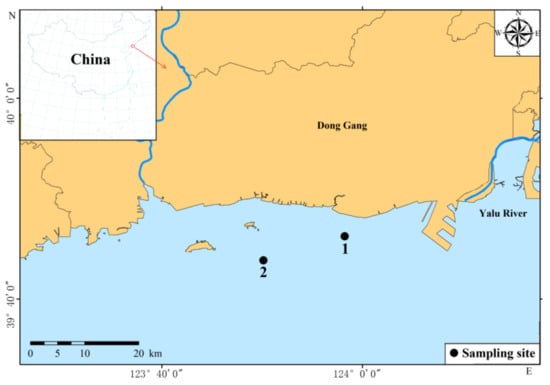
Figure 1.
Sampling sites in Yalu River Estuary, China.
The pretreatment, preservation, detection and quality control methods for the environmental samples were implemented with reference to “Marine Survey Specifications” (GB/T12763-2007) and “Marine Monitoring Specifications” (GB17378-2007). The measured parameters were seawater depth (Dep), water temperature (T), salinity (SST), pH, dissolved oxygen (DO), chemical oxygen demand (COD), total nitrogen (TN), total phosphorus (TP), silicate (SiO3), dissolved inorganic nutrients (nitrite (NO2), nitrate (NO3), ammonia nitrogen (NH4), inorganic phosphorus (DIP), and inorganic nitrogen (DIN)), the inorganic nitrogen-to-phosphorus ratio (DI-N/P), organic nitrogen (DON), organic phosphorus (DOP) and the organic nitrogen-to-phosphorus ratio (DO-N/P).
2.2. Genomic DNA Extraction
The metagenome of the eukaryotic phytoplankton was extracted using the cetyltrimethylammonium bromide (CTAB) method. The filter membrane and visceral mass of R. philippinarum were cut into small pieces and placed in a 1.5-mL centrifuge tube; 500 μL of CTAB lysate (2% CTAB; 100 mmol/L Tris-Cl, pH 8.0; 1.4 mmol/LNaCl; and 10 mmol/L EDTA), 1 μL of β-mercaptoethanol and 5–10 μL of protease were added and left at 55 °C for 1–1.5 h. Following centrifugation, the liquid was removed and placed in a new centrifuge tube; the supernatant was then extracted twice using the phenol-chloroform method. The supernatant was collected, and twice the volume of pre-cooled absolute ethanol was added and left to precipitate for 2–3 h. After collecting the precipitate, it was washed with 75% ethanol to obtain phytoplankton genomic DNA. The concentration and purity of DNA were determined using 1% agarose gel electrophoresis and an ultraviolet spectrophotometer (Unico Instrument Co., Ltd, Shanghai, China); the samples were stored in a refrigerator at –20 °C for subsequent use.
2.3. PCR Amplification of the 18S rDNA V4 Variable Region
Total genomic DNA from the seawater samples was extracted using the CTAB/SDS method. The primers used in this study were V4(F/R), which are self-developed primers for amplification of the 18S rDNA V4 region of eukaryotic phytoplankton. The upstream primer was V4-F (5ʹ-GCGGTAATTCCAGCTCCAATA-3ʹ), and the downstream primer was V4-R (5ʹ-GATCCCHWACTTTCGTTCTTGA-3ʹ) [12]. The primers were designed with different labels and sent to Shanghai Shenggong Biological Company for synthesis. The PCR reaction system was 50 μL, including 5 μL of PCR buffer, 8 μL of dNTP mixture and 2 μL of downstream primers (10 μmol/L) each, 2 μL of template DNA, 2.5 U Taq DNA polymerase and sterile water. The amplification reactions were conducted on a PE 9700 PCR instrument (Perkin Elmer, Waltham, MA, USA). The thermal cycling conditions were as follows: pre-denaturation at 94 °C for 3 min; 33 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 45 s, elongation at 72 °C for 45 s and a final extension at 72 °C for 5 min. PCR products were detected by 1% agarose gel electrophoresis. Sequencing libraries were generated using the NEB Next® Ultra™ DNA Library Prep Kit for Illumina (NEB, San Diego, CA, USA) per the manufacturer’s instructions; index codes were subsequently added. Library quality was assessed on a Qubit@ 2.0 Fluorometer (Thermo Scientific, Waltham, MA, USA) and an Agilent Bioanalyzer 2100 system. The library was sequenced on an Illumina HiSeq 2500 platform, and 250-bp and 300-bp paired-end reads were generated. The experimental reagents were all from Tianjin Kemiou Chemical Reagent Co., Ltd, Tianjin, China.
2.4. Data Analysis
2.4.1. Quality Control and Analysis of Sequencing Data
The raw data obtained were spliced using FLASH software. Quality control of the spliced sequences was carried out in QIIME software to obtain high-quality data. To ensure the accuracy of our sequencing analysis, more than 90% of our dataset comprised high-quality data. Sequence analyses were performed using Uparse software (Uparse v7.0.1001, http://drive5.com/uparse/, accessed on 22 February 2021, Independent Investigator, Edgar, R.C., Tiburon, CA, USA). Sequences with ≥97% similarity with a given operational taxonomic unit (OTU) were assigned to that OTU [13]. The representative sequence for each OTU was screened for further annotation. The Silva Database (http://www.arb-silva.de/, accessed on 2 November 2021) was used to annotate taxonomic information with the RDP classifier algorithm (v2.2, http://sourceforge.net/projects/rdp-classifier/, accessed on 11 April 2022, Microbial Genomics and Bioinformatics Research Group and Ribocon GmbH, Bremen, Germany). Non-algal OTUs were removed given that we were exclusively interested in the abundance and richness of eukaryotic phytoplankton.
2.4.2. Dominance
Previous studies have shown that the relative proportion of eukaryotic phytoplankton sequences is closely related to the biomass ratio of phytoplankton populations [14,15,16,17]; thus, the proportion of each eukaryotic phytoplankton sequence can be used as an indicator of the biomass ratio. Dominant species were identified according to the equivalent spherical diameter [14,15,16,18,19,20,21], and species with a phytoplankton dominance over 0.1% in each sample were considered dominant. The sum of the eukaryotic phytoplankton sequences with an equivalent spherical diameter greater than 20 μm was used as the microphytoplankton centralized statistic; the sum of the eukaryotic phytoplankton sequences with an equivalent spherical diameter of 3–20 μm was used as the nanophytoplankton centralized statistics; and the sum of the eukaryotic phytoplankton sequence with an equivalent spherical diameter of less than 3 μm was used as a centralized statistic for picophytoplankton. The proportions of the different size-fractionated phytoplankton were determined according to the numbers of sequences derived from each phytoplankton population.
The dominance index Y represents the distribution of biological individuals within a community and is also used as an indicator of biodiversity (Equation (1)):
where nx is the sum of the sequences of x algae in all samples; N is the sum of all algae sequences; and fx is the occurrence frequency of x algae in all samples. Species with a dominance greater than 0.1% at each station were considered overall dominant species according to the cell-size biomass ratio.
Ivlev’s selection index E was used to analyze the feeding selectivity of R. philippinarum in the Yalu River Estuary.
where ri represents the percentage of phytoplankton i in the stomachs of R. philippinarum, and pi represents the biomass percentage of phytoplankton i in the water. The range of E is [−1,1]. When E is −1, R. philippinarum avoids consuming phytoplankton i; when E is 1, R. philippinarum preferentially consumes phytoplankton i; and when E is close to 0, R. philippinarum neither avoids nor preferentially consumes phytoplankton i.
2.5. Statistical Analysis
The sampling method of environmental parameters refers to the Chinese National Standard: Ocean Survey Specification (GB/T 12763.4-2007), and the measurement method refers to the Chinese National Standard: Ocean Monitoring Specification (GB 17378-2007), as shown in Table 1. The dominance of the dominant phytoplankton species was represented by boxplots; the significant difference between environmental factors and particle size groups was analyzed by the Pearson statistical test; and the statistical results were represented by heatmaps. The formula calculation, data analysis and distribution map were all completed by WPS Office and Origin 2021 software.

Table 1.
Sampling methods to measure the environmental parameters.
3. Results
3.1. Phytoplankton Community Structure in the Stomachs of Shellfish and Seawater
3.1.1. Dominant Species
From 2020 to 2021, the top five dominant species and those with dominance in the stomachs of R. philippinarum and the water of the Yalu River Estuary are shown in Figure 2. Among them, Karlodinium veneficum had the highest average dominance in the two years, and it was the first dominant species in the water and stomachs in 2020 and stomachs in 2021, with an annual dominance of 0.12, 0.21 and 0.36, respectively. In 2021, the first dominant species in the water was Akashiwo sanguinea, with an annual dominance of 0.09, of which the highest dominance in autumn was 0.33, and the annual dominance of K. veneficum was 0.03. Chattonella subsalsa was the second dominant species in the stomachs in 2021, with an annual dominance of 0.18, of which the highest dominance, 0.47, was in summer. Teleaulax amphioxeia was the third dominant species in the water in 2021, with the highest dominance in spring at 0.12, followed by Micromonas pusilla as the fourth dominant species, with the highest dominance in summer, which was 0.10. According to the two-year investigation results, among the top five dominant species, the average dominance of K. veneficum and C. subsalsa in the water was lower than that in the stomachs, while the other three dominant species were higher than that in the stomachs.
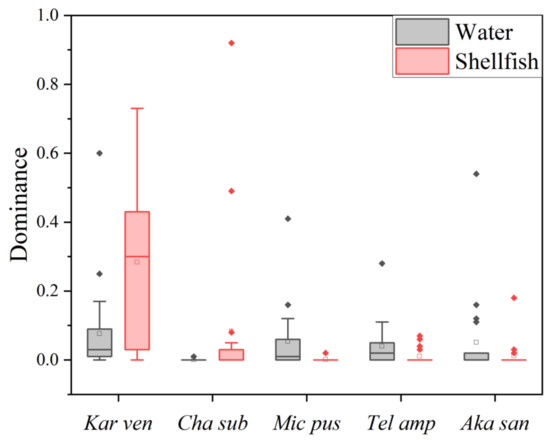
Figure 2.
The top five dominant species and dominance in the stomachs of R. philippinarum in the water of the Yalu River Estuary. Vertical bars are the mean ± SE. Kar van: Karlodinium veneficum; Cha sub: Chattonella subsalsa; Mic pus: Micromonas pusilla; Tel amp: Teleaulax amphioxeia; Aka san: Akashiwo sanguinea.
3.1.2. Phytoplankton Groups
The dominant species (Y > 0.02) of phytoplankton in various groups in the stomachs of R. philippinarum and the water in the Yalu River Estuary in 2020 and 2021 are shown in Table 2. In 2020, the most-dominant species of Bacillariophyta in the stomachs of R. philippinarum was Guinardia striata (its dominance peaked in October at 0.72); the dominance in the water was 0.001, where it had not reached dominance. The most-dominant species of Bacillariophyta in the water was Thalassiosira nordenskioeldii (its dominance peaked in May at 0.28); the dominance in the stomachs was 0.002, where it had not reached dominance. In 2021, the most-dominant species of Bacillariophyta in the stomachs of R. philippinarum was Bacillariophyta sp. GSL077 (its dominance peaked in December at 0.29), and it was not a dominant species in water. The most-dominant species of Bacillariophyta in water was Chaetoceros sp. NIES-3971 (its dominance peaked in March at 0.66); the dominance in the stomachs was 0.01.

Table 2.
Dominant species of various groups of phytoplankton in the stomach contents of R. philippinarum and water of the Yalu River Estuary in 2020–2021.
In 2020, the most-dominant species of Dinophyta in the stomachs of R. philippinarum was K. veneficum (its dominance peaked in April at 0.73), and the most-dominant species in water was also K. veneficum (its dominance peaked in August at 0.60). In 2021, the most-dominant species of Dinophyta in the stomachs of R. philippinarum was K. veneficum (its dominance peaked in May at 0.69), while the dominance in water was 0.03. The most-dominant species of Dinophyta in water was A. sanguinea (its dominance peaked in September at 0.54), and it was not a dominant species in the stomachs.
In 2020, the most-dominant species of Chlorophyta in the stomachs of R. philippinarum was Picochlorum sp. (its dominance peaked in June at 0.46); the dominance in the water was 0.002, where it had not reached dominance. The most-dominant species of Chlorophyta in water was Ostreococcus tauri (its dominance peaked in July at 0.84); the dominance in the stomachs was 0.01, where it had not reached dominance. In 2021, the most-dominant species of Chlorophyta in the stomachs of R. philippinarum was Pterosperma cristatum (its dominance peaked in March at 0.39); the dominance in the water was 0.003, where it had not reached dominance. The most-dominant species of Chlorophyta in water was M. pusilla (its dominance peaked in August at 0.16), and it was not a dominant species in the stomachs.
In 2020, the most-dominant species of Cryptophyta in the stomachs of R. philippinarum was T. amphioxeia (its dominance peaked in September at 0.07), and its dominance in water was 0.02. The most-dominant species of Cryptophyta in water was Cryptomonadales sp. (its dominance peaked in November at 0.23), and its dominance in the stomachs was only 0.02. In 2021, the most-dominant species of Cryptophyta in the stomachs was T. amphioxeia (its dominance peaked in May at 0.28); however, no Chlorophyta in the stomach contents reached dominance.
Overall, the biomass ratio of Dinophyta, Bacillariophyta, Chlorophyta, Dictyochophyta, Cryptophyta, Chrysophyta and unknown algae in the stomachs was 54:14:16:1:10:4:1 and 41:12:28:0:1:17:1 in 2020 and 2021, respectively. Chlorophyta accounted for the highest proportion in June; Bacillariophyta accounted for the highest proportion in November; and Dinophyta accounted for the highest proportion in other months. In 2021, only March, May, June and October had the highest proportion of Dinophyta. The proportion of biomass in water was 41:26:17:1:11:2:2 and 25:44:12:1:15:2:1, respectively, in 2020 and 2021. In 2020, the proportion of Dinophyta was the highest in March, August, September and December; in 2021, only June and September had the highest proportion of Dinophyta (Figure 3).
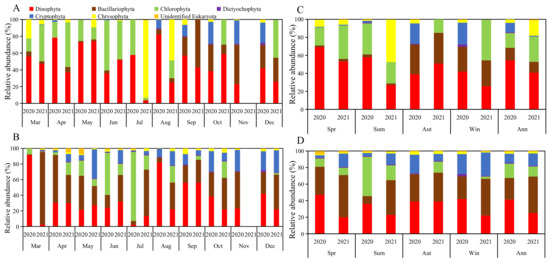
Figure 3.
The biomass proportion of various groups of phytoplankton in 2020 and 2021: (A) monthly in the stomachs; (B) monthly in the water; (C) seasonal in the stomachs; (D) seasonal in the water.
3.1.3. Phytoplankton Sizes
The dominant species (Y > 0.02) of phytoplankton of different sizes in the stomachs of R. philippinarum and the water in the Yalu River Estuary in 2020 and 2021 are shown in Table 3. In 2020, the most-dominant picophytoplankton species in the stomachs of R. philippinarum was Picochlorum sp.; the most-dominant picophytoplankton species in water was O. tauri. In 2021, the most-dominant picophytoplankton species in the stomachs of R. philippinarum was Bacillariophyta sp. GSL077; the most-dominant picophytoplankton species in water was M. pusilla.

Table 3.
Dominant species of phytoplankton of different sizes in the stomach contents of R. philippinarum and water of the Yalu River Estuary in 2020–2021.
In 2020, the most-dominant nanophytoplankton species in the stomach of R. philippinarum and the water was K. veneficum. In 2021, the most-dominant nanophytoplankton species in water was also K. veneficum, and the most-dominant nanophytoplankton species in water was Chaetoceros sp. NIES-3971.
In 2020, the most-dominant microphytoplankton species in the stomach of R. philippinarum was G. striata; the most-dominant microphytoplankton species in the water was Dissodinium pseudolunula (its abundance peaked in March at 0.57), and the dominance in the stomachs was 0.003, where it had not reached dominance. In 2021, the most-dominant microphytoplankton species in the stomachs of R. philippinarum was P. cristatum; the most-dominant microphytoplankton species in water was A. sanguinea.
Overall, the biomass ratio of picophytoplankton, nanophytoplankton and microphytoplankton in the stomachs of R. philippinarum was 13:48:39 and 14:66:20 in 2020 and 2021, respectively. In 2020, picophytoplankton accounted for the highest proportion in June, microphytoplankton accounted for the highest proportion in March, September and October, and nanophytoplankton accounted for the highest proportion in other months. In 2021, only March had the highest proportion of microphytoplankton, and nanophytoplankton accounted for the highest proportion in other months. The proportion of biomass in water was 19:44:37 and 22:39:39 in 2020 and 2021, respectively. In 2020, the proportion of nanophytoplankton was the highest in April, May, August, November and December. In 2021, only March, May and July had the highest proportion of nanophytoplankton (Figure 4).
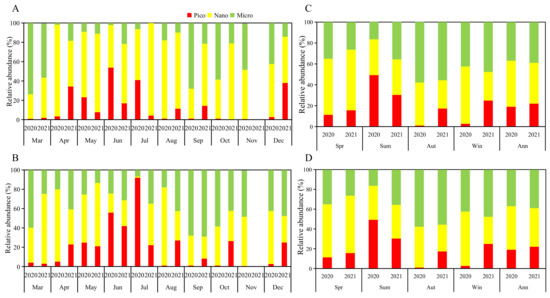
Figure 4.
The biomass proportion of phytoplankton of different sizes in 2020 and 2021: (A) monthly in the stomachs; (B) monthly in the water; (C) seasonal in the stomachs; (D) seasonal in the water.
3.2. Selectivity Index (E) Value
3.2.1. Selectivity for Phytoplankton Groups
The selectivity index of R. philippinarum to various groups of phytoplankton in the water in the Yalu River Estuary was shown in Figure 5, and the situation of E values greater than 0 is shown in Figure 6. Judging from the proportion of E values greater than 0 in the whole year, R. philippinarum preferred to feed on Dinophyta and Chlorophyta; Bacillariophyta, Chrysophyta, Cryptophyta and Dictyochophyta were negative. Among Bacillariophyta, the proportion of E values greater than 0 in winter in 2020 and 2021 was higher than 0.5, so R. philippinarum may prefer to eat on Bacillariophyta in winter. Among Dictyochophyta, the proportion of E values greater than 0 in spring and autumn of 2020 was higher than 0.5, so R. philippinarum may like eating Dictyochophyta better than others in spring and autumn. Among Cryptophyta, the proportion of E values greater than 0 in winter in 2020 was higher than 0.5, so R. philippinarum may prefer to feed on Cryptophyta in winter. Among Chrysophyta, the proportion of E values greater than 0 in spring of 2020 and spring and summer of 2021 was higher than 0.5, so R. philippinarum may prefer to feed on Chrysophyta in spring and summer.
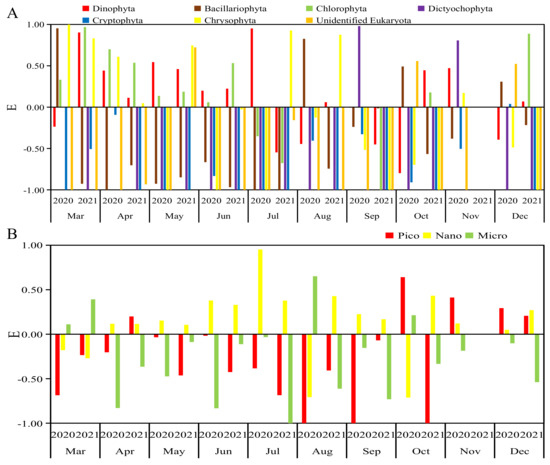
Figure 5.
Selectivity index of R. philippinarum for phytoplankton in the Yalu River Estuary in 2020–2021: (A) various groups; (B) various sizes.
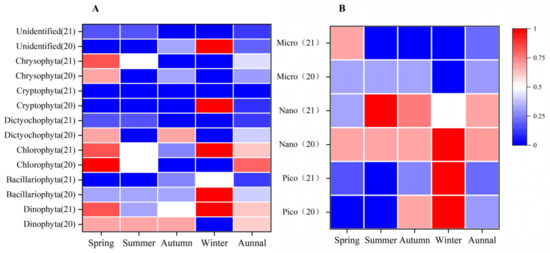
Figure 6.
Frequency distribution of the situation of E values greater than 0: (A) various groups; (B) various sizes.
3.2.2. Selectivity for Phytoplankton of Different Sizes
Judging from the proportion of E values greater than 0 in the whole year, R. philippinarum preferred to feed on nanophytoplankton; picophytoplankton and microphytoplankton were negative. In the picophytoplankton community, the proportion of E values greater than 0 in autumn and winter of 2020 and winter of 2021 was higher than 0.5, so R. philippinarum may like consuming picophytoplankton better than others in autumn and winter. In the spring of 2021, the proportion of E values of microphytoplankton larger than 0 was higher than 0.5, so R. philippinarum may prefer to eat on microphytoplankton in spring (Figure 5 and Figure 6).
3.3. Relationship between the Environmental Factors and Feeding Selectivity
The average values of environmental parameters for each season in 2020 and 2021 are shown in Table 4. NO3, NH4, DIN, TN, and TP gradually increase with the change in seasons, while DON and DOP had little change throughout the year. DI-N/P, COD and T were the highest in summer, followed by spring and autumn, and lowest in winter.

Table 4.
The average values of environmental parameters for each season in 2020 and 2021.
The relationship between the phytoplankton dominance and environmental factors in the water at Station 1 in 2020 and Station 1 and Station 2 in 2021 is shown in Figure 7. Among them, DO, SST and DO-N/P had positive effects on the nanophytoplankton community, but none of them reached significance, while COD and DOP had significant negative effects on the nanophytoplankton community. pH, SST and DON had positive effects on Dinophyta community, and pH had a significant effect on Dinophyta. NO3, DIN and DI-N/P had negative effects on the Dinophyta community, but none of them reached significance. COD and DI-N/P had significant positive effects on the Chlorophyta community, while NH4, DO and DIP had negative effects on the Chlorophyta community, but none of them reached significance.
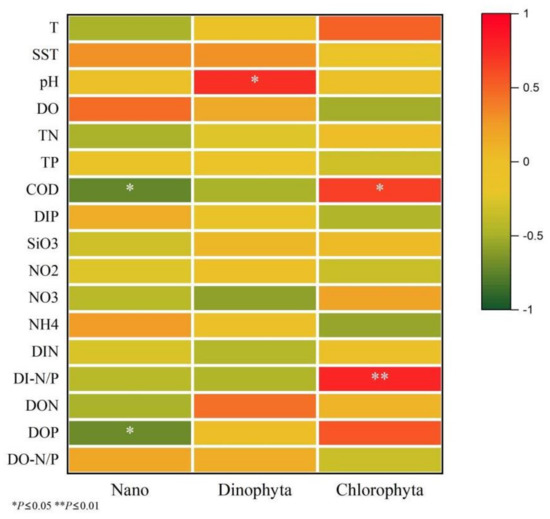
Figure 7.
The relationship between the phytoplankton dominance and environmental factors in water.
4. Discussion
The proportion of Dinophyta biomass in the water was higher than that of Bacillariophyta in 2020, and the proportion of Bacillariophyta was higher than that of Dinophyta in 2021, while the proportion of Dinophyta biomass in the stomachs of R. philippinarum was far higher than that of Bacillariophyta in 2020 and 2021. At the same time, from the ratio of E values greater than 0, it can be found that the R. philippinarum avoided consuming most Bacillariophyta, Chrysophyta, Dictyochophyta and Cryptophyta and preferred consuming Dinophyta, and Chlorophyta throughout the year in the shellfish culture area of the Yalu River Estuary. This is consistent with the feeding habits of wild shellfish in that area as observed by Pan [22], in which feeding selectivity was higher for Dinophyta and Chrysophyta than for Bacillariophyta and Chlorophyta. In this study, R. philippinarum avoided feeding on Bacillariophyta in spring. Lavaud et al. stated that Pecten maximus cease consuming Bacillariophyta when their density in water is high [23]. Bougrier et al. indicated that Ostrea chilensis avoids consuming small Bacillariophyta [24]. The second most-dominant species observed in spring was T. nordenskioeldii, and the first dominant species observed was Chaetoceros sp. NIES-3971 in the water, which had high cell density in the water and they belong to Bacillariophyta with a relatively small particle size, so R. philippinarum chose to avoid eating. In addition, there were great differences between the different Bacillariophyta entering the digestive glands of shellfish [22], which may lead to R. philippinarum avoiding Bacillariophyta in Yalu River Estuary.
Langdon and Waldock [25] believed that filter-feeding shellfish ingested less or no toxin-producing phytoplankton, while Jiang et al. stated that shellfish are insensitive to most toxic phytoplankton and can ingest and accumulate toxins without affecting their feeding selectivity [26]. Different species of bivalve mollusks have relatively different feeding choices to toxic phytoplankton. Mytilus edulis rarely have negative responses when ingesting paralytic shellfish poisoning (PSP) toxin-producing phytoplankton (Alexandrium sp.), but scallops, oysters and clams decrease in different degrees, which may be related to the difference in sensitivity of shellfish neuron cells to PSP [27,28,29]. In this study, it was found that the first predominant species of K. veneficum in the water in the shellfish culture area of the Yalu River Estuary had hemolysin toxin, and was the first dominant species in the stomachs of R. philippinarum. A. sanguinea with hemolytic toxin was also the dominant species in the water and stomachs. Therefore, the negative response of R. philippinarum to toxic algae may not be great.
This study found that DI-N/P had a positive effect on Chlorophyta, while NH4 had a negative effect on Chlorophyta. In spring and summer, DI-N/P was significantly higher than that in autumn and winter, and the NH4 content was significantly lower than that in autumn and winter, so the content of Chlorophyta in water was higher. Langdon and Waldock indicated that filter-feeding juvenile oysters eat phytoplankton containing specific fatty acids [25]. Navarro et al. also found that Dinophyta had a higher fatty acid content than Bacillariophyta, and high fatty acid can promote Argopecten purpuratus to feed [30]. Ding et al. also indicated that unsaturated fatty acid was an important factor for the growth of many bivalves [31]. Sun et al. found that Picochlorum sp. contains higher unsaturated fatty acids, and this study found that Picochlorum sp. was the dominant species in the stomachs of R. philippinarum in 2020 and 2021 [32]. All these reasons may make R. philippinarum prefer to eat Dinophyta and Chlorophyta.
Molina et al. showed that Limnoperna fortunei does not feed on Cryptophyta [33], and Loret et al. found that Pecten maximus selectively feeds on Cryptophyta [34]. In our study, R. philippinarum avoided consuming Cryptophyta in spring, summer and autumn, but preferentially consumed Cryptophyta in winter. This finding might be explained by differences in the properties of shellfish species. Alternatively, this could be caused by the failure to detect the cells of Cryptophyta in the stomachs of mussels given that their fragile cell walls can be easily broken during digestion [35]. Shumway et al. also indicated that Cryptophyta cells may be completely digested and absorbed by bivalve mollusks during their passage through the intestinal tract [3]. In this study, it was found that the proportion of Cryptophyta in winter was relatively higher compared with other seasons, and the water temperature in winter was low. It is possible that Cryptophyta can stay in the stomach of R. philippinarum for a long time, so the detected proportion was higher in winter than other seasons. Therefore, although some fragile Cryptophyta may not be capable of being detected in the stomachs of bivalve mollusks through microscopy, they appear to be an important food source for natural populations of bivalves [3,34,35,36].
The biomass ratio of picophytoplankton, nanophytoplankton and microphytoplankton in the stomachs of R. philippinarum in the shellfish culture area of the Yalu River Estuary was 13:48:39 and 14:66:20 in 2020 and 2021, respectively, which indicated that R. philippinarum preferred consuming nanophytoplankton. Cranford et al. found that bivalve mollusks could easily feed on nanophytoplankton and that the retention rate of 2–8 μm phytoplankton was high [1]. Strohmeier et al. found that the retention rate of phytoplankton by Mytilus edulis gradually increased with the size of phytoplankton, and the maximum retention rate was observed for 7–35 μm phytoplankton [37]. Rosa et al. found that M. edulis preferred algae that were greater than 4 μm in size [38]. Dunphy et al. showed that O. chilensis retains 6 μm phytoplankton less efficiently than the 15 μm phytoplankton, which is consistent with the findings of our study [39]. Møhlenberg et al. showed that the retention rate of phytoplankton by M. edulis decreased rapidly for phytoplankton less than 4 μm in size [40]. Tammes et al. indicated that M. edulis could not effectively retain phytoplankton smaller than 2.5 μm [41]. Zhang measured the rejection rate of 2 μm phytoplankton in M. edulis, Crassostrea gigas and Azumapecten farreri by simulating on-site running water, which was 19%, 17% and 8%, respectively, and the rejection rate of particles below 2 μm was even lower [2]. These findings are consistent with the observation that R. philippinarum avoided consuming picophytoplankton in the shellfish culture area of the Yalu River Estuary.
The consumption of bivalve shellfish is determined by the filtration rate and particle-retention efficiency of the gills, which is the main organ for the transportation of seawater and the retention of particles. The feeding of shellfish is closely related to the structure of the gills and the movement of cilia on the gill filaments [42,43]. Jørgensen and Ward et al. found that the retention rate of phytoplankton was severely reduced when the anterior cilia of M. edulis were inactivated, which indicates that the anterior cilia are critically important for shellfish feeding [44,45]. Riisgård measured the retention rate of phytoplankton by six filter-feeding shellfish and found that the retention rate of phytoplankton larger than 4 μm in shellfish with larger anterior cilia, such as Geukensia demissa, Spisula solidissima, Brachidontes exustus and Mercenaria mercenaria, was 100%; by contrast, the retention rate of phytoplankton larger than 4 μm in shellfish with small anterior cilia or no anterior cilia, such as Crassostrea virginica and Argopecten irradians, was 75%–85% [43].
R. philippinarum possesses large anterior cilia; consequently, it preferred consuming large phytoplankton (nanophytoplankton and microphytoplankton). Among the six filter-feeding shellfish species studied by Riisgård [43], only G. demissa could significantly retain 0.2–2 μm phytoplankton, which may stem from the greater compactness of the cilia of G. demissa compared with the other five species examined. Wang found that the distance between the lateral cilia of R. philippinarum, Argopecten irradians, A. farreri and C. gigas was less than 1 μm, which is consistent with the proportion of picophytoplankton detected in the stomachs of R. philippinarum in our study (18%) [42]. Some researchers have suggested that filter-feeding shellfish prefer phytoplankton with greater concentrations of organic nutrients, and phytoplankton with low concentrations of organic nutrients are excreted as feces; picophytoplankton are typically not consumed by bivalves because of their small size and low nutritional quality [46,47].
This study found that the retention rate of picophytoplankton by R. philippinarum in the Yalu River Estuary was greater in autumn and winter than in summer and R. philippinarum preferred feeding on Chaetoceros calcitrans and Syndiniales sp. among picophytoplankton. Wei argued that C. calcitrans was an ideal opening food source for bivalves [48], and Lora-Vilchis also found that C. calcitrans was rich in some specific fatty acids; it also has a better effect as an initial feed for the larvae of Pinna rudis Linnaeus [49], which indicates that the picophytoplankton C. calcitrans can be ingested by juveniles. Ward et al. showed that slender or tri-radial phytoplankton may be more easily trapped by bivalves than spherical phytoplankton of the same volume [4,50]. C. calcitrans is mostly chain-like in water and is more likely to be trapped and fed; these considerations might explain why R. philippinarum preferentially consumed picophytoplankton in the Yalu River Estuary in autumn and winter [51,52,53,54,55].
The research area in the study was the main breeding area for R. philippinarum, in recent years, slow growth, decreased meat quality, and increased mortality have generally occurred, but no diseases, pollution, or red tides have been found in this sea area.The results showed that R. philippinarum avoided feeding on picophytoplankton, and the nanophytoplankton that actively fed was K. veneficum with low nutrition and toxicity in the study. Due to the quantitative selectivity strategy of R. philippinarum on feed microalgae, it filtered and fed on the dominant K. veneficum as much as possible in the case of low effective feed concentration. In addition, the increasing density of R. philippinarum culture resulted in the slow growth of R. philippinarum and increased mortality in the region. Therefore, it is recommended that the cultivation density should be reasonably controlled within the range of cultivation capacity in this region to maintain the healthy and sustainable cultivation of R. philippinarum.
5. Conclusions
In the Yalu River Estuary, R. philippinarum avoided consuming most Bacillariophyta, Chrysophyta, Dictyochophyta and Cryptophyta and preferentially fed on Dinophyta and Chlorophyta. R. philippinarum had a particularly strong preference for consuming K. veneficum, which was the most-dominant species in the water.
The annual average biomass ratio of picophytoplankton, nanophytoplankton and microphytoplankton in the stomachs of R. philippinarum was 13:48:39 in 2020 and 14:66:20 in 2021. R. philippinarum actively fed on nanophytoplankton and avoided picophytoplankton.
Among the phytoplankton of different grain sizes and groups that R. philippinarum prefer to feed, COD and DOP have a significant negative effect on the nanophytoplankton community, pH has a positive effect on the Dictyochophyta community, and COD and DI-N/P have a significant positive effect on the Chlorophyta community.
Author Contributions
Conceptualization, Y.L.; data curation, G.S.; formal analysis, K.W. and S.L.; investigation, G.S.; project administration, J.W.; software, Y.L.; supervision, Z.W.; validation, L.S.; writing—original draft, Y.L.; writing—review and editing, L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Liaoning revitalization talents program (XLYC1907109); outstanding young scientific and technological personnel of Dalian (2019RJ09); Modern Agro-industry Technology Research System (CARS-49); and key research and development project of Liaoning Province, China (2018228004).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank our colleagues for their assistance in collecting samples and Liwen Bianji for editing the language of a draft of this manuscript.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cranford, P.J.; Li, W.; Strand, Ø.; Strohmeier, T. Phytoplankton Depletion by Mussel Aquaculture: High Resolution Mapping, Ecosystem Modeling and Potential Indicators of Ecological Carrying Capacity. ICES CM Doc 2008/ H: 12. International Council for the Exploration of the Sea, Copenhagen. 2008. Available online: http://ices.dk/sites/pub/CM%20Doccuments/CM-2008/H/H1208.pdf (accessed on 26 May 2022).
- Zhang, J.H. Effect of Filter Feeding Shellfish Mariculture on the Ecosystem and the Evaluation of Ecology Carrying Capacity (Graduate School of Chinese Academy of Sciences (Institute of Oceanography), Qing Dao, Shan Dong, China). Personal Communication. 2008. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=2008117213.nh&DbName=CDFD2008 (accessed on 10 June 2022).
- Shumway, S.E.; Cucci, T.L.; Newell, R.C.; Yentsch, C.M. Particle selection, ingestion, and absorption in fifilterfeeding bivalves. J. Exp. Mar. Biol. Ecol. 1985, 91, 77–92. [Google Scholar] [CrossRef]
- Ward, J.E.; Shumway, S.E. Separating the grain from the chaffff: Particle selection in suspension- and depositfeeding bivalves. J. Exp. Mar. Biol. Ecol. 2004, 300, 83–130. [Google Scholar] [CrossRef]
- Ten Winkel, E.H.; Davids, C. Food selection by Dreissena polymorpha Pallas (Mollusca: Bivalvia). Freshw. Biol. 1982, 12, 553–558. [Google Scholar] [CrossRef]
- Baker, S.M.; Levinton, J.S.; Ward, J.E. Particle transport in the zebra mussel, Dreissena polymorpha (Pallas). Biol. Bull. 2000, 199, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.S.; Ross, A.H.; Hayden, B.J. Comparison of assimilation efficiency on diets of nine phytoplankton species of the greenshell mussel Perna canaliculus. J. Shellfish. Res. 2006, 25, 887–892. [Google Scholar]
- Safi, K.A.; Hewitt, J.E.; Talman, S.G. The effect of high inorganic seston loads on prey selection by the suspension feeding bivalve, Atrina zelandica. J. Exp. Mar. Biol. Ecol. 2007, 344, 136–148. [Google Scholar] [CrossRef]
- Safi, K.A.; Hayden, B. Differential grazing on natural planktonic populations by the mussel Perna canaliculus. Aquat. Biol. 2010, 11, 113–125. [Google Scholar] [CrossRef]
- Espinosa, E.P.; Cerrato, R.M.; Wikfors, G.H.; Allam, B. Modeling food choice in the two suspension-feeding bivalves, Crassostrea virginica and Mytilus edulis. Mar. Biol. 2016, 163, 40. [Google Scholar] [CrossRef]
- Rosa, M.; Ward, J.E.; Holohan, B.A.; Shumway, S.E.; Wikfors, G.H. Physicochemical surface properties of microalgae and their combined effects on particle selection by suspension-feeding bivalve molluscs. J. Exp. Mar. Biol. Ecol. 2017, 486, 59–68. [Google Scholar] [CrossRef]
- Song, L.; Wu, J.; Liu, W.D.; Song, Y.; Wang, N. Diversity of marine nanophytoplankton and picophytoplankton in Changxing Island offshore waters of Bohai Sea. Res. Environ. Sci. 2016, 29, 1635–1642. [Google Scholar] [CrossRef]
- Song, L.; Wu, J.; Du, J.; Li, N.; Song, G.J.; Wang, K.; Sun, M.; Wang, P. The characteristics and distribution of eukaryotic phytoplankton community in Liaodong Bay, China. Ocean. Sci. J. 2019, 54, 183–203. [Google Scholar] [CrossRef]
- Prokopowich, C.D.; Gregory, T.R.; Crease, T.J. The correlation between rDNA copy number and genome size in eukaryotes. Genome 2003, 46, 48–50. [Google Scholar] [CrossRef]
- Zhu, F.; Massana, R.; Nota, F.; Marie, D.; Vaulot, D. Mapping of picoeucaryotes in marine ecosystems with quantitative PCR of the 18S rRNA gene. FEMS Microbiol. Ecol. 2005, 52, 79–92. [Google Scholar] [CrossRef]
- Godhe, A.; Asplund, M.E.; Harnstrom, K.; Saravanan, V.; Tyagi, A.; Karunasagar, I. Quantification of diatom and dinoflagellate biomasses in coastal marine seawater samples by Real-Time PCR. Appl. Env. Microb. 2008, 74, 7174–7182. [Google Scholar] [CrossRef]
- Song, L.; Bi, X.D.; Song, G.J.; Du, J.; Wu, J.H.; Wang, Z.S.; Hu, C.K. Size-fractionated eukaryotic microalgae and its influencing factors Dachangshan Island and its adjacent waters. China Environ. Sci. 2020, 40, 2627–2634. [Google Scholar] [CrossRef]
- Liu, R.Y. Checklist of Marine Biota of China Seas, 1st ed.; Science Press: Beijing, China, 2008; pp. 301–870. [Google Scholar]
- Xu, X.; Yu, Z.M.; Cheng, F.J.; He, L.Y.; Cao, X.H.; Song, X.X. Molecular diversity and ecological characteristics of the eukaryotic phytoplankton community in the coastal waters of the Bohai sea, China. Harmful Algae 2017, 61, 13–22. [Google Scholar] [CrossRef]
- Yu, J. Establishment of Efficient Detection Technology for Plankton Diversity and Its Application in Brown Tide Research in Bohai Sea. (Ocean University of China, Qing Dao, Shan Dong, China). Personal Communication. 2014. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1014328607.nh&DbName=CMFD2015 (accessed on 20 April 2022).
- Jiang, X.J. Analysis of Picoplankton Abundance and Study of Genetic Diversity of Microeukaryotes in the in the North Yellow Sea. (Ocean University of China, Qing Dao, Shan Dong, China). Personal Communication. 2009. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=2009171363.nh&DbName=CMFD2009 (accessed on 6 May 2022).
- Pan, H.Z. Study on Feeding Selectivity of Different Phytoplankton Assemblages by Several Typical Bivalve Species in the Yellow Sea and Bohai Sea. (Jinan University, Guang Zhou, Guang Dong, China). Personal Communication. 2020. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1021514063.nh&DbName=CMFD2021 (accessed on 20 April 2022).
- Lavaud, R.; Artigaud, S.; Grand, F.L.; Donval, A.; Soudant, P.; Flye-Sainte-Marie, J.; Strohmeier, T.; Strand, O.; Leynaert, A.; Beker, B.; et al. New insights into the seasonal feeding ecology of Pecten maximus using pigments, fatty acids and sterols analyses. Mare Ecol. Prog. Ser. 2018, 590, 109–129. [Google Scholar] [CrossRef]
- Bougrier, S.; Hawkins, A.J.S.; Héral, M. Preingestive selection of different microalgal mixtures in Crassostrea gigas and Mytilus edulis, analysed by flow cytometry. Aquaculture 1997, 150, 123–134. [Google Scholar] [CrossRef]
- Langdon, C.J.; Waldock, M.J. The effect of algal and artificial diets on the growth and fatty acid composition of Crassostrea gigas Spat. J. Mar. Biol. Assoc. UK 1981, 61, 431–448. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, L.; Li, Y.; Zhang, J.; Tan, Z.; Wu, H.; Jiang, T.; Lu, S. Occurrence of marine algal toxins in oyster and phytoplankton samples in Daya Bay, South China Sea. Chemosphere 2017, 183, 80–88. [Google Scholar] [CrossRef]
- Bricelj, V.M.; Lee, J.H.; Cembella, A.D. Influence of dinoflagellate cell toxicity on uptake and loss of paralytic shellfish toxins in the northern quahog Mercenaria mercenaria. Mar. Ecol. Prog. 1991, 74, 33–46. [Google Scholar] [CrossRef]
- Navarro, J.M.; Labraña, W.; Chaparro, O.R.; Cisternas, B.; Ortíz, A. Physiological Constraints in Juvenile Ostrea chilensis Fed the Toxic Dinoflagellate Alexandrium catenella. Estuaries Coasts 2016, 39, 1133–1141. [Google Scholar] [CrossRef]
- Villanueva, P.A.; Navarro, J.M. Pre-ingestive selection efficiency in two populations of the razor clam Tagelus dombeii with different histories of exposure to paralytic shellfish poisoning (PSP). Mar. Freshw. Behav. Phy. 2016, 49, 291–300. [Google Scholar] [CrossRef]
- Navarro, J.M.; Leiva, G.E.; Martinez, G.; Aguilera, C. Interactive effects of diet and temperature on the scope for growth of the scallop Argopecten purpuratus during reproductive conditioning. J. Exp. Mar. Biol. Ecol. 2000, 247, 67–83. [Google Scholar] [CrossRef]
- Ding, R.Y.; Cha, D.J.; Xie, Y.X.; Xin, Q.D.; Li, X.Y.; Li, Y.; Cui, H.W.; Pan, H.Z.; Tan, Z.J.; Jiang, T. Feeding Selectivity of Phytoplankton by Four Bivalve Species in Aquaculture Area of Qinhuangdao [J/OL]. J. Yantai Univ. (Nat. Sci. Eng. Ed.) 2022, 1–11. [Google Scholar] [CrossRef]
- Sun, X.M.; Yang, F.F.; Wu, J.Y.; Li, T.; Xiang, W.Z. Evaluation of the oil-producing performance of oleaginous Picochlorum species. Fish. Mod. 2014, 41, 43–48. [Google Scholar] [CrossRef]
- Molina, F.R.; Paggi, J.C.; Devercelli, M. Zooplanktophagy in the natural diet and selectivity of the invasive mollusk Limnoperna fortunei. Biol. Invasions 2010, 12, 1647–1659. [Google Scholar] [CrossRef]
- Loret, P.; Pastoureaud, A.; Cedric, B.; Delesalle, B. Phytoplankton composition and selective feeding of the pearl oyster Pinctada margaritifera in the Takapoto lagoon (Tuamotu Archipelago, French Polynesia): In situ study using optical microscopy and HPLC pigment analysis. Mar. Ecol. Prog. Ser. 2000, 199, 55–67. [Google Scholar] [CrossRef]
- Cucci, T.L.; Shumway, S.E.; Newell, R.C.; Selvin, R.; Yentsch, C.M. Flow cytometry: A new method for characterization of differential ingestion, digestion and egestion by suspension feeders. Mar. Ecol. Prog. Ser. 1985, 24, 201–204. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, L.; Zhang, F.; Fang, X.; Lu, L.; Zhang, J.; Wang, W.; Qu, K.; Chai, C. Selective feeding of bay scallop Argopecten irradians on phytoplankton community revealed by HPLC analysis of phytopigments in Bohai Sea, China. J. Oceanol. Limnol. 2019, 37, 1747–1755. [Google Scholar] [CrossRef]
- Strohmeier, T.; Strand, Ø.; Alunno-Bruscia, M.; Duinker, A.; Cranford, P.J. Variability in particle retention efficiency by the mussel Mytilus edulis. J. Exp. Mar. Biol. Ecol. 2012, 412, 96–102. [Google Scholar] [CrossRef]
- Rosa, M.; Ward, J.E.; Ouvrard, M.; Holohan, B.A.; Espinosa, E.P.; Shumway, S.E.; Allam, B. Examining the physiological plasticity of particle capture by the blue mussel, Mytilus edulis (L.): Confounding factors and potential artifacts with studies utilizing natural seston. J. Exp. Mar. Biol. Ecol. 2015, 473, 207–217. [Google Scholar] [CrossRef]
- Dunphy, B.J.; Hall, J.A.; Jeffs, A.G.; Wells, R.M.G. Selective particle feeding by the Chilean oyster, Ostrea chilensis; implications for nursery culture and broodstock conditioning. Aquaculture 2006, 261, 594–602. [Google Scholar] [CrossRef]
- Møhlenberg, F.; Riisgård, H.U. Efficiency of particle retention in 13 species of suspension feeding bivalves. Ophelia 1978, 17, 239–246. [Google Scholar] [CrossRef]
- Tammes, P.M.L.; Dral, A.D.G. Observations on the straining of suspensions by mussels. Arch. Meerl. Zool 1956, 11, 87–112. [Google Scholar] [CrossRef]
- Wang, F.; Dong, S.L.; Fan, R.Q.; Gao, L. The Observation of the Gills of Four Species Filtering-Feeding Bivalve Using Scanning Electron Microscope. J. Ocean. Univ. Qing Dao 1998, 2, 73–74; 76–77. [Google Scholar] [CrossRef]
- Riisgård, H.U. Efficiency of particle retention and filtration rate in 6 species of Northeast American bivalves. Mar. Ecol. Prog. Ser. 1988, 45, 217–223. [Google Scholar] [CrossRef]
- Jørgensen, C.B. Comparative studies on the function of gills in suspension feeding bivalves, with special reference to effects of serotonin. Biol. Bull. 1976, 151, 331–343. [Google Scholar] [CrossRef]
- Ward, J.E.; Sanford, L.P.; Newell, R.I.E.; MacDonald, B.A. A new explanation of particle capture in suspension-feeding bivalve molluscs. Limnol. Oceanogr. 1998, 43, 741–752. [Google Scholar] [CrossRef]
- Naddafi, R.; Pettersson, K..; Eklöv, P. The effe.ect of seasonal variation in selective feeding by zebra mussels (Dreissena polymorpha) on phytoplankton community composition. Freshw. Biol. 2007, 52, 823–842. [Google Scholar] [CrossRef]
- Dong, B.; Xue, Q.Z.; Li, J. The effect of temperature on the filtration rate, clearance rate and absorption efficiency of manila clam. Ruditapes Philippinarum. Mar. Fish. Res. 2000, 1, 37–42. Available online: https://doi.org/10.3969/j.issn.1000-7075.2000.01.007 (accessed on 3 May 2022).
- Wei, X.M.; Xu, Z.C.; He, J.J. Effects of temperature and specific gravity of seawater on the multiplication of calcareous chaetoceros. TaiWan Strait 1986, 1, 97–100. Available online: https://doi.org/CNKI:SUN:TWHX.0.1986-01-015 (accessed on 21 March 2022).
- Lora-Vilchis, M.C.; Cruz, R.V.; Reynoso-Granados, T.; Voltolina, D. Evaluation of five microalgae diets for juvenile pen shells Atrina maura. J. World Aquacult Soc. 2004, 35, 232–236. [Google Scholar] [CrossRef]
- Li, F.X. Size Fraction of Phytoplankton and Ecological Contribution of Microbial Loop in Larre-Scale Bivalve Mariculture Area. (Shanghai Ocean University, Shang Hai, China). Personal Communication. 2019. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1019667251.nh&DbName=CMFD2020 (accessed on 20 May 2022).
- Bayne, B.L.; Widdows, J.; Newell, R.I.E. Physiological measurements on estuarine bivalve molluscs in the field. Biol. Benthic Org. 1977, 57–68. [Google Scholar] [CrossRef]
- Rubenstein, D.E.; Koehl, M.A.R. The mechanisms of filter feeding: Some theoretical considerations. Am. Nat. 1977, 111, 981–994. [Google Scholar] [CrossRef]
- Labarbera, M. Particle capture by a Pacific brittle star: Experimental test of the aerosol suspension feeding model. Science 1978, 201, 1147–1149. [Google Scholar] [CrossRef]
- Jørgensen, C.B. Fluid mechanical aspects of suspension feeding. Mar. Ecol. Prog. Ser. 1983, 11, 89–103. [Google Scholar] [CrossRef]
- Silvester, N.R.; Sleigh, M.A. Hydrodynamic aspects of particle capture by Mytilus. J. Mar. Biol. Assoc. UK 1984, 64, 859–879. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).