Age, Growth and Population Structure Analyses of the Berryteuthis magister shevtsovi in the Japan Sea by Statolith Microstructure
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Basic Biological Determination and Statolith Extraction
2.3. Statolith Processing and Aging
2.4. Data Analysis
3. Results
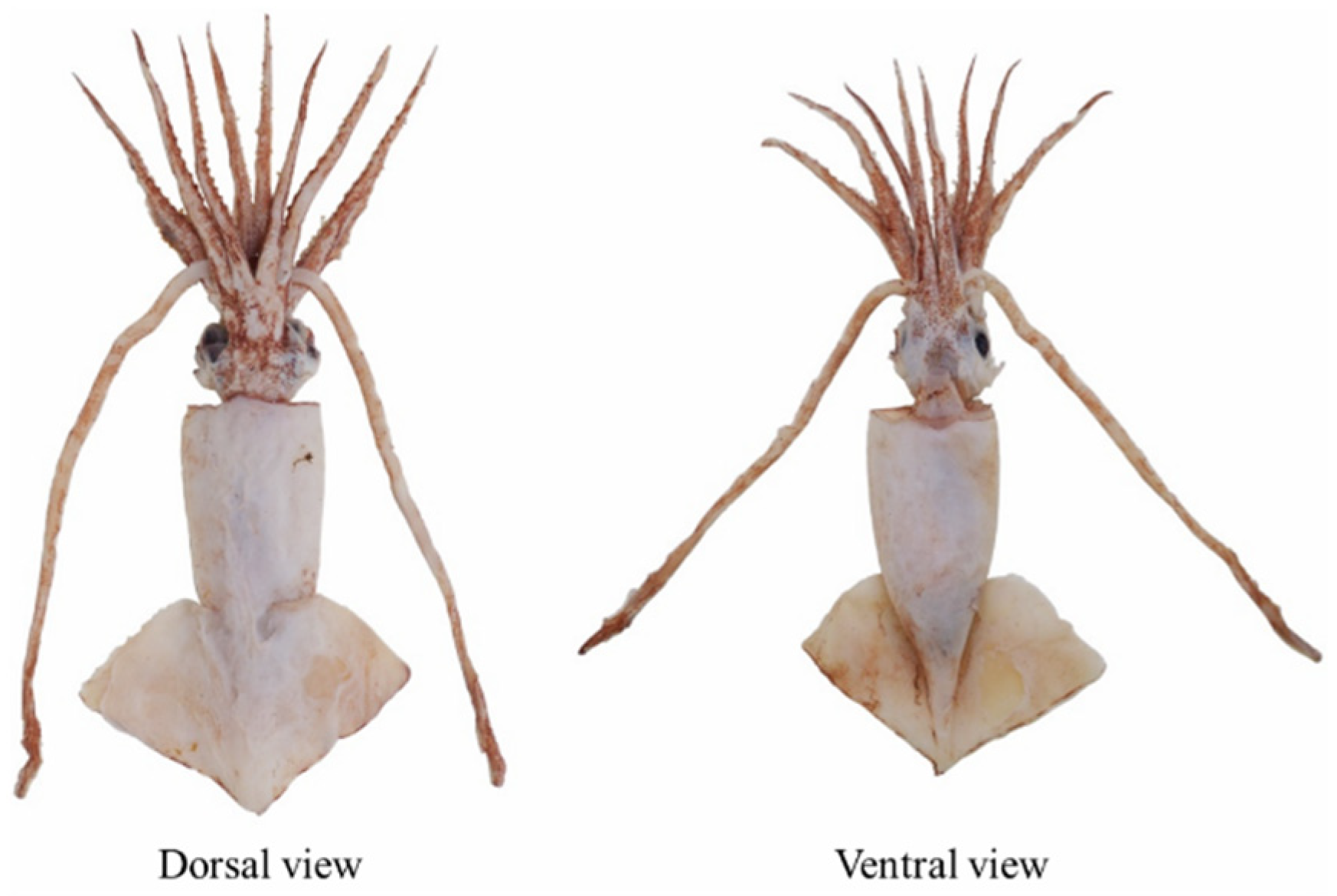
3.1. Individual Composition and Shape Features
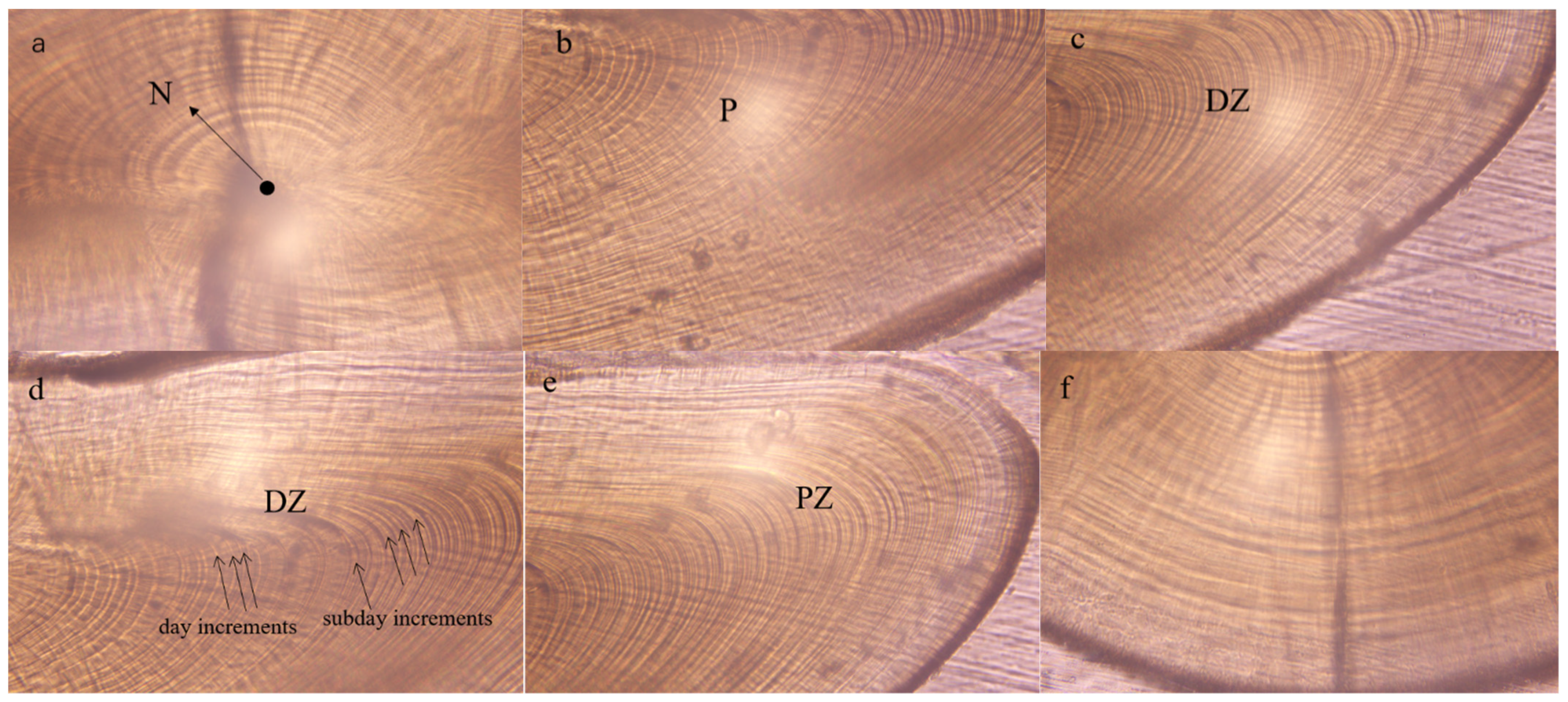
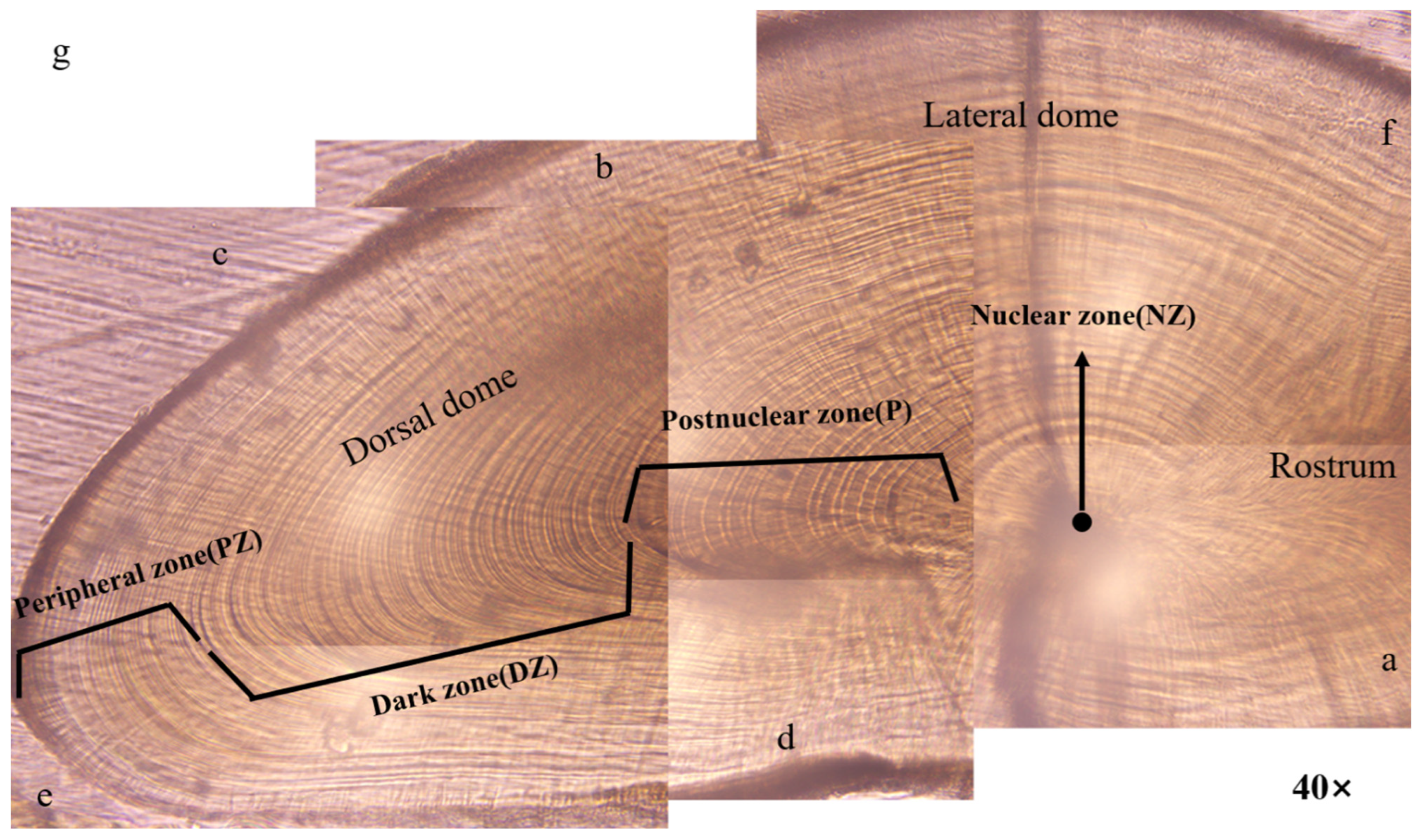
3.2. Microstructures of the Statolith
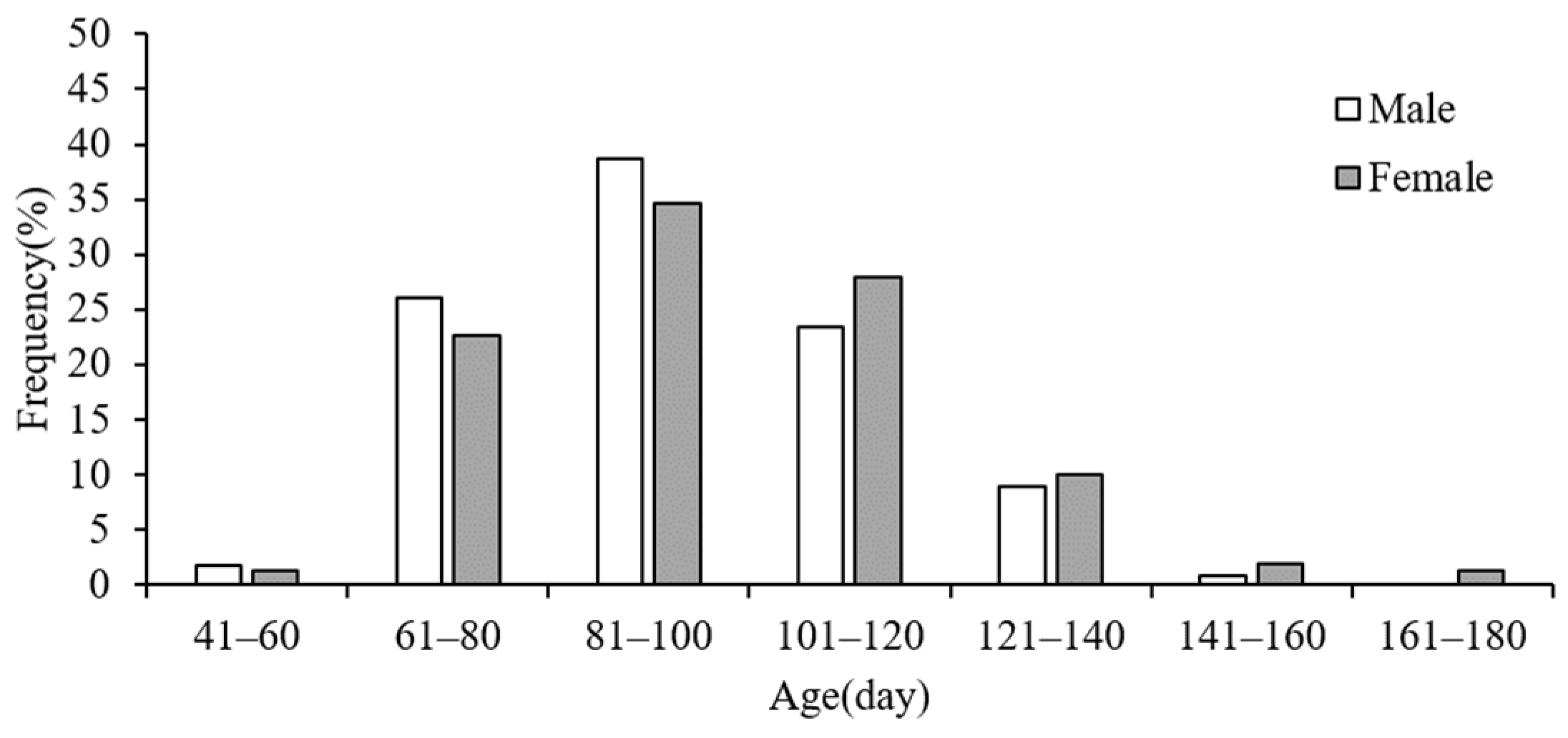
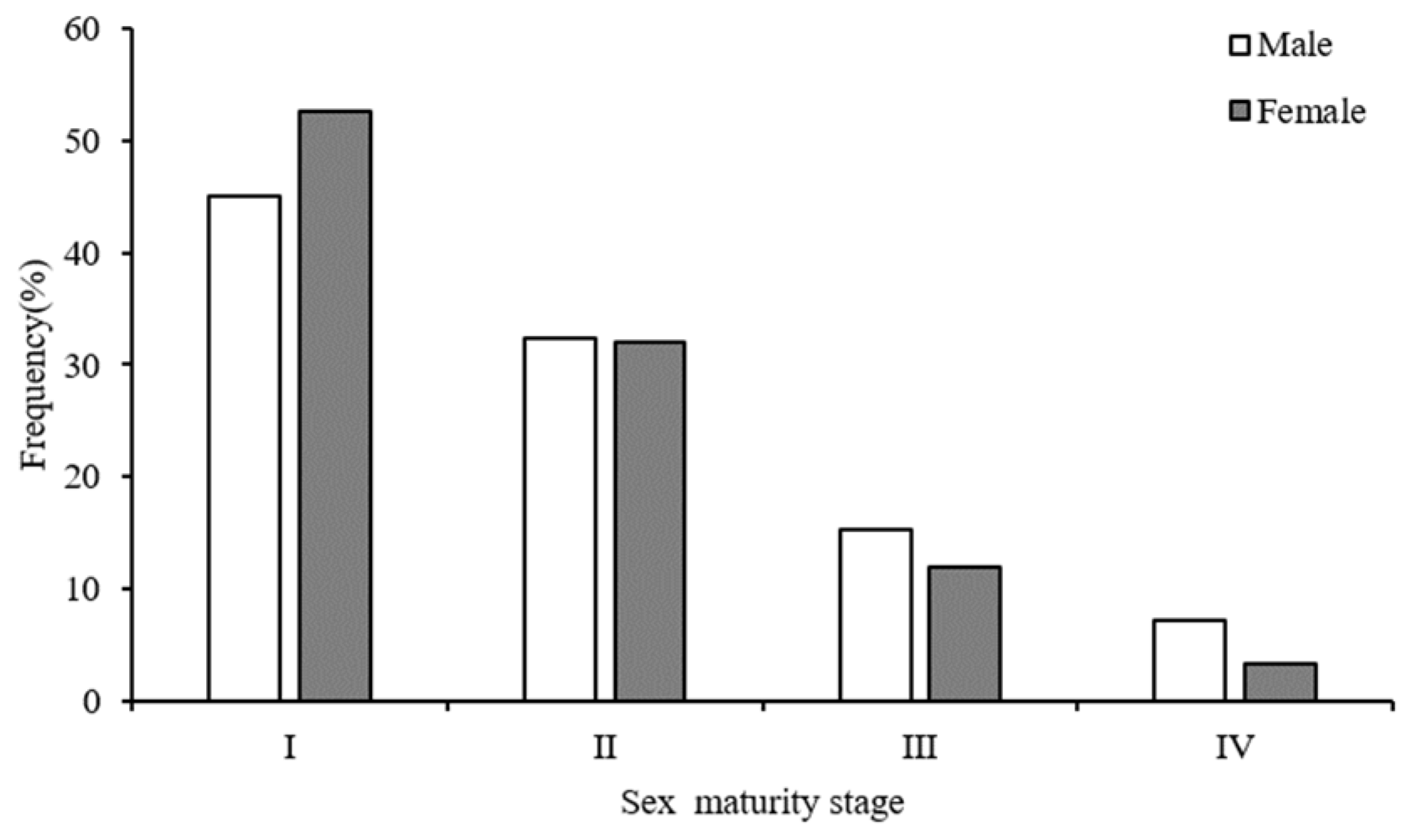
3.3. Age Structure and Maturity
3.4. Hatching Date and Group
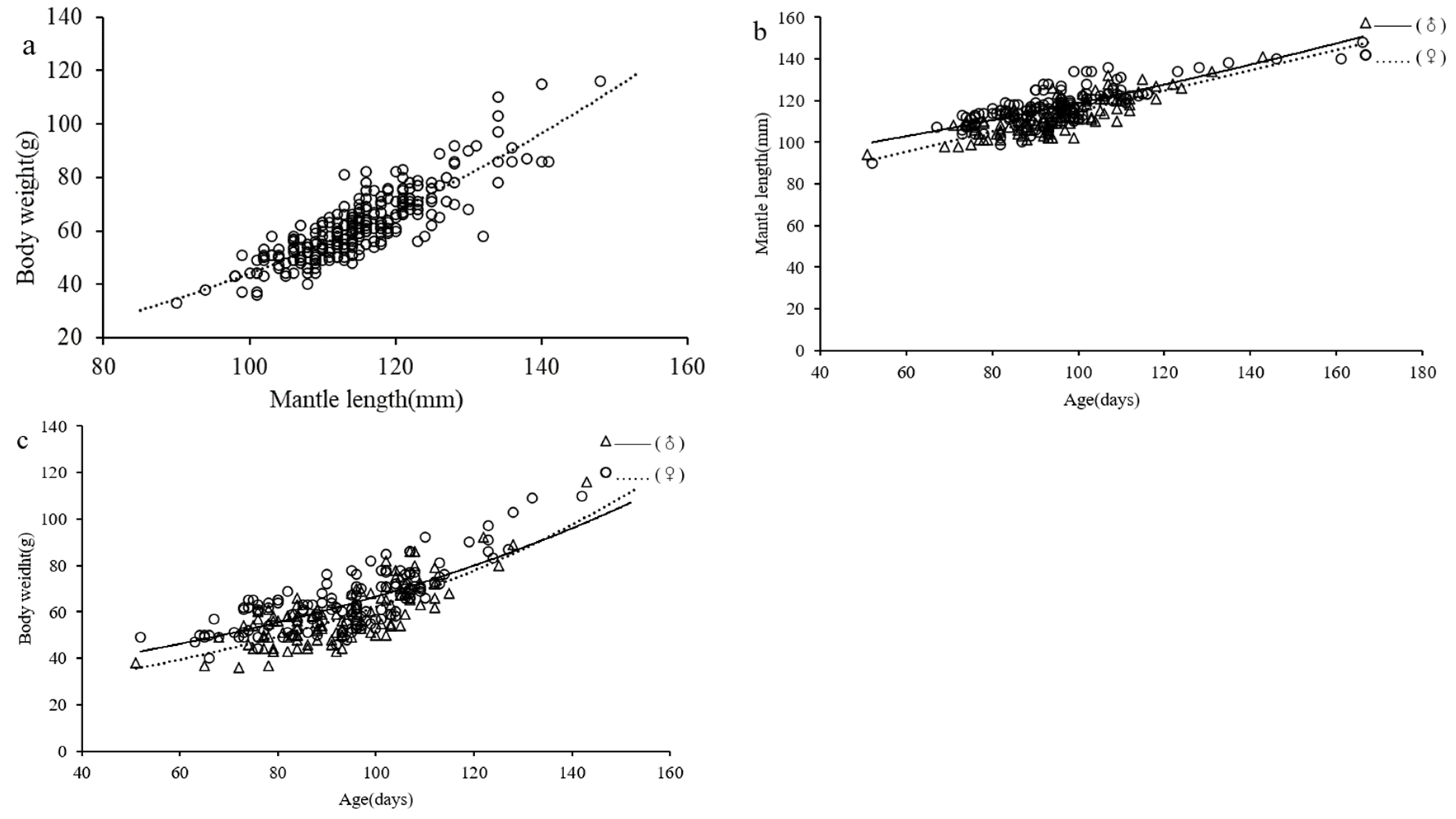
3.5. Growth Pattern
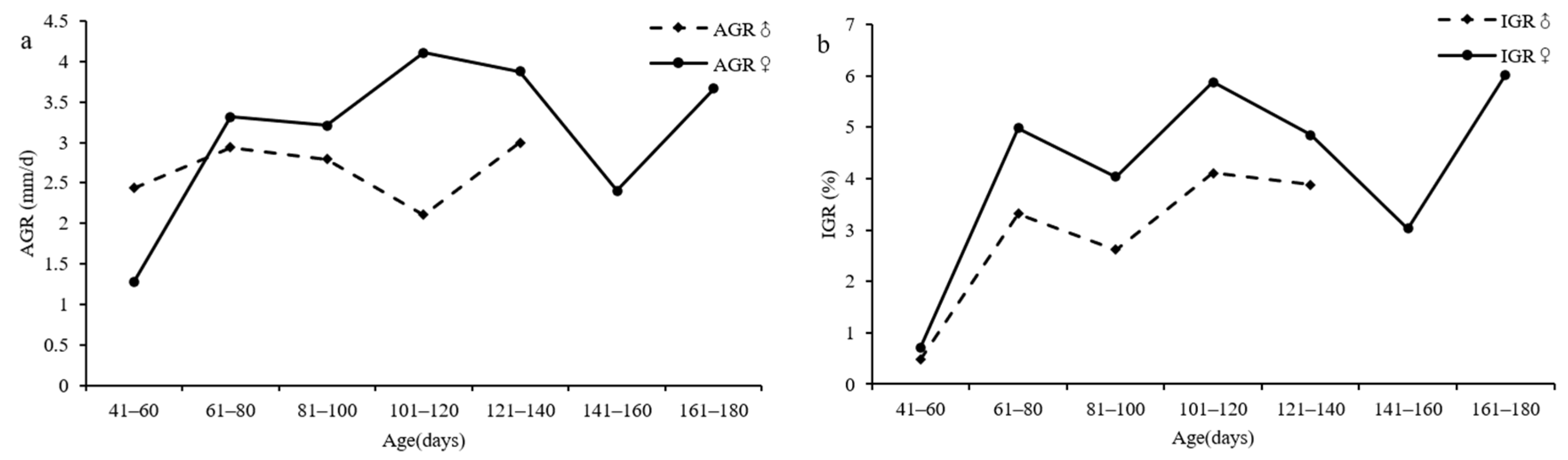
3.6. Growth Rate
4. Discussion
4.1. Morphological Characteristics
4.2. Statolith Microstructure
4.3. Age Structure and Maturity
4.4. Hatching Date and Population Structure
4.5. Growth Models
4.6. Growth Rate
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katugin, O.N. A new subspecies of the schoolmaster gonate squid, Berryteuthis magister (Cephalopoda: Gonatidae), from the Japan Sea. Veliger 2000, 43, 82–97. [Google Scholar]
- Wang, H.H.; He, T.; Lu, H.J.; Chen, Z.Y.; Ning, X.; Chen, X.J. Fisheries biology characteristics of Berryteuthis magister shevtsovi in the Japan Sea. Chin. J. Ecol. 2021, 40, 2467–2477. [Google Scholar]
- Nesis, K.N. Population dynamics of the Berryteuthis magister Commander (Berry) in the Western Bering Sea during the autumn spawning season. Ruthenica 1995, 5, 55–69. [Google Scholar]
- Chen, X.J.; Liu, B.L.; Wang, Y.G. Cephalopods in the World; China Ocean Press: Beijing, China, 2009; pp. 273–306. [Google Scholar]
- Chen, X.Y.; Lu, H.J.; He, J.R.; Wang, H.H.; Liu, K.; Chen, Z.Y.; Chen, X.J. Analysis of Beak Morphological Characteristic of Berryteuthis magister shevtsovi in Japan Sea. Chin. J. Zool. 2021, 56, 918–928. [Google Scholar]
- Zhu, W.B.; Chen, X.Y.; Lu, H.J.; Chen, Z.Y.; Ning, X.; Cui, G.C.; Guo, A.; Chen, X.J. Analysis of pigmentation characteristics on beak of Berryteuthis magister shevtsovi in the Japan Sea based on artificial neural networks. J. Fish. China 2022, 46, 950–958. [Google Scholar]
- Trotsenko, B.G.; Pinchukov, M.A. Mesoscale distribution features of the purpleblack squid Sthenoteuthis oualaniensis with reference to the structure of the upper quasi-homogeneous layer in the West India Ocean. Oceanology 1994, 34, 380–385. [Google Scholar]
- Perales, R.C.; Almansa, E.; Aurora, B.; Beatriz, C.F.; José, I.; Francisco, J.S.; José, F.C.; Carmen, R. Age Validation in Octopus vulgaris Beaks Across the Full Ontogenetic Range: Beaks as Recorders of Life Events in Octopuses. J Shellfish. Res. 2014, 33, 481–493. [Google Scholar] [CrossRef]
- Radtke, R.L. Chemical and structural characteristics of statoliths from the short-finned squid Illex illecebrosus. Mar. Biol. 1983, 76, 47–54. [Google Scholar] [CrossRef]
- Bettencourt, V.; Guerra, A. Growth increments and biomineralization process in cephalopod statoliths. J. Exp. Mar. Biol. Ecol. 2000, 248, 191–205. [Google Scholar] [CrossRef]
- Semmens, J.; Moltschaniwskyj, N. An examination of variable growth in the loliginid squid Sepioteuthis lessoniana: A whole animal and reductionist approach. Mar. Ecol. Prog. Ser. 2000, 193, 135–141. [Google Scholar] [CrossRef]
- Lu, H.J.; Chen, X.J.; Fang, Z. Comparison of the beak morphologic growth characteristics between two spawning populations of Illex argentinus in Southwest Atlantic Ocean. J. Ocean Univ. China 2012, 42, 33–40. [Google Scholar]
- Agus, B.; Carugati, L.; Bellodi, A.; Cannas, R.; Cau, A.; Cera, J.; Coluccia, E.; Melis, R.; Ruiu, S.; Cuccu, D. Molecular and Biological Analysis on Ommastrephes caroli Findings in the Central Western Mediterranean Sea (Sardinian Waters) Including First Age Investigation Using Eye Lenses and Beaks. Front. Mar. Sci. 2021, 8, 683856. [Google Scholar] [CrossRef]
- Arkhipkin, A.I.; Shcherbich, Z.N. Thirty years’ progress in age determination of squid using statoliths. J. Mar. Biol. Assoc. UK 2012, 92, 1389–1398. [Google Scholar] [CrossRef]
- Lu, H.J.; Wang, C.J.; Chen, X.J. Preliminary study on the biological characteristics of Sthenoteuthis oualaniensis in the high seas nearby the equator of eastern Pacific during April to June. J. Shanghai Ocean Univ. 2014, 23, 441–447. [Google Scholar]
- Dong, Z.Z. Cephalopod Biology of the World’s Oceanic Economy; Shandong Science and Technology Press: Jinan, China, 1991; pp. 27–105. [Google Scholar]
- Fang, Z.; Xu, L.; Chen, X.; Liu, B.; Li, J.; Chen, Y. Beak growth pattern of purpleback flying squid Sthenoteuthis oualaniensis in the eastern tropical Pacific equatorial waters. Fish. Sci. 2015, 81, 443–452. [Google Scholar] [CrossRef]
- Arkhipkin, A.I. Reproductive System Structure, Development and Function in Cephalopods with a New General Scale for Maturity Stages. J. Northwest Atl. Fish. Sci. 1985, 12, 63–74. [Google Scholar] [CrossRef]
- Chen, X.; Lu, H.; Liu, B.; Chen, Y. Age, growth and population structure of jumbo flying squid, Dosidicus gigas, based on statolith microstructure off the Exclusive Economic Zone of Chilean waters. J. Mar. Biol. Assoc. UK 2010, 91, 229–235. [Google Scholar] [CrossRef]
- Lu, H.-J.; Chen, X.-J. Age, growth and population structure of Illex argentinus based on statolith microstructure in Southwest Atlantic Ocean. J. Fish. China 2012, 36, 1049–1056. [Google Scholar] [CrossRef]
- Lu, H.J.; Zhang, X.; Tong, Y.H.; Tang, Y.; Liu, K.; Liu, W.; Chen, X.J. Statolith microstructure and growth characteristics of Sthenoeuthis oualaniensis in the Xisha Islands waters of the South China Sea. J. Fish. China 2020, 44, 767–776. [Google Scholar]
- Yatsu, A.; Midorikawa, S.; Shimada, T.; Uozumi, Y. Age and growth of the neon flying squid, Ommastrephes bartrami, in the North Pacific ocean. Fish. Res. 1997, 29, 257–270. [Google Scholar] [CrossRef]
- Liu, B.L.; Chen, X.J.; Chen, Y.; Hu, G.Y. Determination of squid age using upper beak rostrum sections: Technique improvement and comparison with the statolith. Mar. Biol. 2015, 162, 1685–1693. [Google Scholar] [CrossRef]
- Rodhouse, P.G.; Hatfield, E.M.C. Dynamics of Growth and Maturation in the Cephalopod Illex argentinus de Castellanos, 1960 (Teuthoidea: Ommastrephidae). Philos. Trans. R. Soc. Lond. B Biol. Sci. 1990, 329, 229–241. [Google Scholar]
- Froese, R.; Thorson, J.T.; Reyes, R.B. A Bayesian approach for estimating length-weight relationships in fishes. J. Appl. Ichthyol. 2013, 30, 78–85. [Google Scholar] [CrossRef]
- Jackson, G.D. Application and Future Potential of Statolith Increment Analysis in Squids and Sepioids. Can. J. Fish. Aquat. Sci. 1994, 51, 2612–2625. [Google Scholar] [CrossRef]
- Arkhipkin, A.I. Age and growth of the mesopelagic squid Ancistrocheirus lesueurii (Oegopsida: Ancistrocheiridae) from the central-east Atlantic based on statolith microstructure. Mar. Biol. 1997, 129, 103–111. [Google Scholar] [CrossRef]
- Malcolm, H. Modeling and Quantitative Methods in Fisheries; Chapman and Hall/CRC: New York, NY, USA, 2001; pp. 227–232. [Google Scholar]
- Hiramatsu, K. Application of Maximum Likelihood Method and AIC to Fish Population Dynamics. In Fish Population Dynamics and Statistical Models; Matsumiya, Y., Ed.; Koseisha Koseikaku: Tokyo, Japan, 1993; pp. 9–21. [Google Scholar]
- Imai, C.; Sakai, H.; Katsura, K.; Honto, W.; Hida, Y. Growth model for the endangered cyprinid fish Tribolodon nakamurai based on otolith analyses. Fish. Sci. 2002, 68, 843–848. [Google Scholar] [CrossRef]
- Arkhipkin, A.I.; Roa-Ureta, R. Identification of ontogenetic growth models for squid. Mar. Freshw. Res. 2005, 56, 371–386. [Google Scholar] [CrossRef]
- Okutani, T.; Tagawa, M.; Horikawa, H. Cephalopods from Continental Shelf and Slope around Japan; Japan Fisheries Resource Conservation Society: Tokyo, Japan, 1988. [Google Scholar]
- Tang, F.H.; Shi, Y.R.; Zhu, J.X.; Wu, Z.L.; Wu, Y.M.; Cui, X. Influence of marine environment factors on temporal and spatial distribution of Japanese common squid fishing grounds in the Japan Sea. J. Fish. China 2015, 22, 1036–1043. [Google Scholar]
- Zhou, Y.Q. Study on the maximum swimming speed of fishes. J. Fish. China 1985, 9, 105–120. [Google Scholar]
- Alexander, I.A.; Vyacheslav, A.B.; Andrey, V.V. Distribution and growth in juveniles of the squid Berryteuthis magister (Cephalopoda, Gonatidae) in the western Bering Sea. Sarsia 1998, 83, 45–54. [Google Scholar]
- Arkhipkin, A.I. Statoliths as ‘black boxes’ (life recorders) in squid. Mar. Freshw. Res. 2005, 56, 573–583. [Google Scholar] [CrossRef]
- Wang, H.H.; Lu, H.J.; He, J.R.; Liu, K.; Chen, X.Y.; Chen, X.J. Microstructures and growth characteristics of statoliths from Sthenoeuthis oualaniensis in the northwest Indian Ocean. Chin. J. Appl. Ecol. 2022, 1–9. [Google Scholar] [CrossRef]
- Chen, X.J.; Liu, B.L. Fishery Resources Biology; Science Press: Beijing, China, 2017; pp. 58–62. [Google Scholar]
- Ma, J.; Chen, X.J.; Liu, B.L.; Lu, H.J.; Li, S.L.; Cao, J. Review of the influence of environment factors on microstructure of statoliths of cephalopod. J. Shanghai Ocean Univ. 2009, 5, 616–622. [Google Scholar]
- Hu, G.Y.; Chen, X.J.; Liu, B.L.; Fang, Z. Microstructure of statolith and beak for Dosidicus gigas and its determination of growth increments. J. Fish. China 2015, 39, 361–370. [Google Scholar]
- Wang, Y.P.; Chen, X.J.; Fang, Z.; Li, J.H.; Li, Z.Q.; Chen, L.F. Statolith Morphology of Jumbo Flying Squid (Dosidicus gigas) in Waters Near the Equator of Eastern Pacific Ocean. J. Oceanol. Limnol. 2019, 147–156. [Google Scholar] [CrossRef]
- Hu, G.; Fang, Z.; Liu, B.; Yang, D.; Chen, X.; Chen, Y. Age, growth and population structure of jumbo flying squid Dosidicus gigas off the Peruvian Exclusive Economic Zone based on beak microstructure. Fish. Sci. 2016, 82, 597–604. [Google Scholar] [CrossRef]
- Liu, B.L.; Chen, X.J.; Li, J.H.; Chen, Y. Age, growth and maturation of Sthenoteuthis oualaniensis in the eastern tropical Pacific Ocean by statolith analysis. Mar. Freshw. Res. 2016, 67, 1973–1981. [Google Scholar] [CrossRef]
- Sukramongkol, N.; Promjinda, S.; Prommas, R. Age and Reproduction of Sthenoteuthis Oualaniensis in the Bay of Bengal. In Proceedings of the Ecosystem-Based Fishery Management in the Bay of Bengal, Phuket, Thailand, 14 December 2007; Department of Fisheries (DOF), Ministry of Agriculture and Cooperatives: Bangkok, Thailand, 2007; pp. 195–205. [Google Scholar]
- Chen, Z.Y.; Lu, H.J.; Liu, W.; Liu, K.; Chen, X.J. Beak Microstructure Estimates of the Age, Growth, and Population Structure of Purpleback Flying Squid (Sthenoteuthis oualaniensis) in the Xisha Islands Waters of the South China Sea. Fishes 2022, 7, 187. [Google Scholar] [CrossRef]
- Markaida, U.; Quiñonez-Velázquez, C.; Sosa-Nishizaki, O. Age, growth and maturation of jumbo squid Dosidicus gigas (Cephalopoda: Ommastrephidae) from the Gulf of California, Mexico. Fish. Res. 2004, 66, 31–47. [Google Scholar] [CrossRef]
- Watanabe, H.; Kubodera, T.; Ichii, T.; Sakai, M.; Moku, M.; Seitou, M. Diet and sexual maturation of the neon flying squid Ommastrephes bartramii during autumn and spring in the Kuroshio–Oyashio transition region. J. Mar. Biol. Assoc. UK 2008, 88, 381–389. [Google Scholar] [CrossRef]
- Arkhipkin, A.I.; Steven, E.C.; Jennifer, F.G.; Simon, R.T. Spatial and temporal variation in elemental signatures of statoliths from the Patagonian longfin squid (Loligo gahi). Can. J. Fish. Aquat. 2004, 61, 1212–1224. [Google Scholar] [CrossRef]
- Lu, H.-J.; Ou, Y.-Z.; He, J.-R.; Zhao, M.-L.; Chen, Z.-Y.; Chen, X.-J. Age, Growth and Population Structure Analyses of the Purpleback Flying Squid Sthenoteuthis oualaniensis in the Northwest Indian Ocean by Beak Microstructure. J. Mar. Sci. Eng. 2022, 10, 1094. [Google Scholar] [CrossRef]
- Fedorets, Y.A. Seasonal Distribution of the Squid Berryteuthis magister in thе Western Bering Sea. In Systematics and Ecology of Cephalopods; Zoological Institute of Academy of Sciences of the USSR: Leningrad, Russia, 1983; pp. 129–130. [Google Scholar]
- Fei, H.N.; Zhang, S.Q. Aquatic Resources; China Science and Technology Press: Beijing, China, 1990; pp. 245–254. [Google Scholar]
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2010, 22, 241–253. [Google Scholar] [CrossRef]
- Seiyaboh, E.I.; Harry, G.A.; Izah, S.C. Length-Weight Relationship and Condition Factor of Five Fish Species from River Brass, Niger Delta. Int. J. Agric. Biol. 2016, 4, 37–44. [Google Scholar]
- Hu, X.M.; Xiong, X.J.; Qiao, F.L.; Guo, X.P. Surface current field and seasonal variability in the Kuroshio andadjacent regions derived from satellite-tracked drifter data. Acta Oceanol. Sin. 2008, 30, 11–29. [Google Scholar]
- Zhao, C.X.; Chen, Z.P.; He, X.B.; Deng, Y.S.; Feng, B.; Yan, Y.R. Age, growth and population structure of purple beak flying squid, Sthenoteuthis oualaniensis the south China Sea in spring based on statolith microstructure. Acta Hydrobiol. Sin. 2017, 41, 884–890. [Google Scholar]
- Jereb, P.; Roper, C.F.E. Cephalopods of the World. In An Annotated and Illustrated Catalog of Cephalopod Species Known to Date; Volume 2: Myopsid and Oegopsid Squids; FAO: Rome, Italy, 2010; pp. 315–318. [Google Scholar]
- Arkhipkin, A.; Jereb, P.; Ragonese, S. Growth and maturation in two successive seasonal groups of the short-finned squid, Illex coindetii from the Strait of Sicily (central Mediterranean). ICES J. Mar. Sci. 2000, 57, 31–41. [Google Scholar] [CrossRef]
- Petrić, M.; Škeljo, F.; Šifner, S.K. Age, growth and maturation of Illex coindetii (Cephalopoda: Ommastrephidae) in the eastern Adriatic Sea. Reg. Stud. Mar. Sci. 2021, 47, 101935. [Google Scholar] [CrossRef]
- Mejía-Rebollo, A.; Quiñónez-Velázquez, C.; Salinas-Zavala, C.A.; Salinas, Z.; Markaida, U. Age, growth and maturity of jumbo squid (Dosidicus gigas d’orbigny, 1835) off the Western Coast of the baja California peninsula. Cal. Coop. Ocean. Fish. 2008, 49, 256–262. [Google Scholar]
- Arkhipkin, A.I. Towards identification of the ecological lifestyle in nektonic squid using statolith morphometry. J. Molluscan Stud. 2003, 69, 171–178. [Google Scholar] [CrossRef][Green Version]



| Year | Growth Model | Linear | Power | Exponential | Logarithmic | ||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | AIC | R2 | AIC | R2 | AIC | R2 | AIC | ||
| ML–BW | 0.7362 | 1077.0901 | 0.7420 | 861.2017 | 0.7388 | 861.2090 | 0.7259 | 861.2140 | |
| Females | ML–age | 0.5939 | 1268.3508 | 0.5914 | 1306.3009 | 0.5918 | 1270.1061 | 0.5848 | 1452.1809 |
| BW–age | 0.5686 | 1202.2370 | 0.558 | 1251.1959 | 0.6088 | 1200.7663 | 0.5128 | 1203.5403 | |
| Males | ML–age | 0.6125 | 1273.8008 | 0.5925 | 1278.9351 | 0.6214 | 1269.5682 | 0.5761 | 1270.8557 |
| BW–age | 0.6025 | 1299.3064 | 0.6030 | 1349.9067 | 0.6397 | 1299.0834 | 0.5540 | 1300.8069 | |
| Age-Class (d) | Sample Number | Mantle Length | Body Weight | |||||
|---|---|---|---|---|---|---|---|---|
| Average of ML (mm) | IGR | AGR (mm−1) | Average of BW (g) | IGR | AGR (g−1) | |||
| females | 41–60 | 2 | 120.00 | 0.18 | 0.21 | 67.50 | 0.70 | 1.27 |
| 61–80 | 34 | 115.15 | 1.44 | 1.68 | 62.06 | 4.98 | 3.32 | |
| 81–100 | 52 | 116.77 | 1.86 | 2.22 | 63.34 | 4.04 | 3.21 | |
| 101–120 | 42 | 117.38 | 2.39 | 2.84 | 64.97 | 5.87 | 4.11 | |
| 121–140 | 15 | 120.93 | 1.74 | 2.13 | 62.27 | 4.84 | 3.88 | |
| 141–160 | 3 | 111.67 | 1.92 | 2.10 | 63.67 | 3.02 | 2.40 | |
| 161–180 | 2 | 113.00 | 1.18 | 1.33 | 65.00 | 6.01 | 3.67 | |
| males | 41–60 | 2 | 110.00 | 1.09 | 1.20 | 59.00 | 0.47 | 2.44 |
| 61–80 | 29 | 109.24 | 2.15 | 2.31 | 54.17 | 3.32 | 2.94 | |
| 81–100 | 43 | 110.84 | 1.56 | 1.79 | 56.14 | 2.61 | 2.79 | |
| 101–120 | 26 | 114.00 | 1.70 | 2.05 | 61.62 | 4.10 | 2.11 | |
| 121–140 | 10 | 119.10 | 1.23 | 1.47 | 71.10 | 3.88 | 3.00 | |
| 141–160 | 1 | 121.00 | / | / | 75.00 | / | / | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Ou, Y.; Teng, Y.; Chen, Z.; Chen, X. Age, Growth and Population Structure Analyses of the Berryteuthis magister shevtsovi in the Japan Sea by Statolith Microstructure. Fishes 2022, 7, 215. https://doi.org/10.3390/fishes7050215
Lu H, Ou Y, Teng Y, Chen Z, Chen X. Age, Growth and Population Structure Analyses of the Berryteuthis magister shevtsovi in the Japan Sea by Statolith Microstructure. Fishes. 2022; 7(5):215. https://doi.org/10.3390/fishes7050215
Chicago/Turabian StyleLu, Huajie, Yuzhe Ou, Yurong Teng, Ziyue Chen, and Xinjun Chen. 2022. "Age, Growth and Population Structure Analyses of the Berryteuthis magister shevtsovi in the Japan Sea by Statolith Microstructure" Fishes 7, no. 5: 215. https://doi.org/10.3390/fishes7050215
APA StyleLu, H., Ou, Y., Teng, Y., Chen, Z., & Chen, X. (2022). Age, Growth and Population Structure Analyses of the Berryteuthis magister shevtsovi in the Japan Sea by Statolith Microstructure. Fishes, 7(5), 215. https://doi.org/10.3390/fishes7050215








