Abstract
Serranus scriba is a common member of the coastal fish community in the Adriatic Sea, but knowledge about its feeding ecology is scarce. The aim of this paper is to present new evidence about its food preferences and feeding habits. An innovative non-destructive method of fecal pellet analysis was used for this study. This method does not require sacrificing specimens and the fish can be released back into the sea alive after the laboratory work. The results demonstrated that S. scriba mainly preys on decapods, followed by polychaetes, isopods, fish, mollusks and swarming shrimps. The calculated index of trophic diversity (ITD) value of 0.89 indicates that it is an opportunistic feeder that feeds on a wide range of different prey. According to the calculated trophic level of 3.43, which is higher than that of other members of the community, S. scriba is also an important piscivorous predator. With age, S. scriba undergoes an ontogenetic shift. The proportion of crustaceans, gastropods and polychaetes decreases with age and body size, while the proportion of fish increases.
1. Introduction
The northern Adriatic Sea is a shallow coastal sea, where infralittoral habitats are home to rich fish assemblages, with many members closely associated with the seabed [1]. Anthropogenic activities can lead to habitat disturbances, changes in fish community structure and changes in ecosystem functioning [2,3,4,5]. Therefore, some fish groups can be used as a tool to assess changes in habitat conditions. Currently, robust methodologies are being developed in an effort to assess the most suitable indicator species for the evaluation of the status of coastal fish assemblages [6].
The most important coastal fish families in the Mediterranean Sea in terms of abundance and frequency of occurrence include Gobiidae, Labridae, Bleniidae, Sparidae, Tripterygiidae, Atherinidae, Mugilidae, Mullidae, Pomacentridae, Sygnathidae and Serranidae [1]. The family Serranidae includes 538 species from temperate and tropical seas [7]. This study focuses on one of the smaller members of the group, the most common member of the family in the coastal area of the Mediterranean, the painted comber (Serranus scriba (Linnaeus, 1758)). S. scriba is a subtropical species that inhabits rocky habitats with algal covers and seagrass meadows, distributed in the Mediterranean Sea, the Black Sea and the eastern Atlantic Ocean from the Bay of Biscay to Mauritania [8,9]. S. scriba is very common throughout the Mediterranean Sea, and is usually found at a depth of 0 to 30 m [10]. Normally it does not grow larger than 200 mm, but the largest specimen captured to date measured 323 mm and weighed 456.7 g [11]. S. scriba is not commercially exploited in the Mediterranean area, but it is often caught as by-catch by recreational fishermen [12]. Despite its wide distribution, the ecology and feeding habits of the species are poorly known. According to some studies, S. scriba is often predating on crustaceans, especially decapods [10] and smaller necto-benthic and crypto-benthic fish species [13,14]. It has also been documented that the removal of a mesopredator such as S. scriba, may result in the proliferation of small fishes and could, thus, affect the populations of small invertebrates [15]. Studies on the feeding ecology of S. scriba in the Adriatic Sea have not been conducted yet, although species is widespread and common in the area. S. scriba is supposed to play an important role in the trophic web [16,17,18]; therefore, there is a need for in-depth knowledge of its food and feeding habits.
Feeding ecology describes the diverse feeding modes and morphological, physiological and senso-neural adaptations to the prey type and the prey abundance in the habitat [19,20,21] and contributes to the understanding of resource partitioning [22,23], habitat selection [21], predation [24,25,26,27], evolution [28], competition, trophic ecology [29,30] and energy transfer within and between ecosystems [31,32,33].
The feeding habits of fish species are usually based on the examination of stomach contents [34,35,36], which requires a large number of sacrificed fish. Therefore, it is ethically questionable and particularly unsuitable for the study of endangered or rare fish, and species with low population densities or inhabiting marine protected areas [37,38,39]. Alternatives to traditionally used method of stomach content analysis are non-lethal methods, among which the most effective method is stomach flushing [40]. Although stomach flushing is one of the most efficient non-lethal methods used to date [40,41,42], this procedure can cause mortality of up to 60% in some fish species [42] and can have a negative effect on fish condition [41]. Most non-lethal methods cannot be regarded as non-destructive, because the process of obtaining samples is still quite invasive and can cause injuries or even death [42]. In our study, we used a non-destructive method that is less invasive and does not harm the fish.
The aim of this study is to present the first data on the feeding ecology of S. scriba in the northern Adriatic Sea and to propose the application of a recently developed non-destructive and non-lethal method for isolating undigested prey from feces in order to study the fish diet. The main goals of the paper are (i) to identify and categorize the prey items of S. scriba in the northern Adriatic Sea, (ii) to estimate the trophic level (TROPH) of S. scriba and compare it with other species in the community and that respective to other studies, and (iii) to calculate the index of trophic diversity (ITD).
2. Materials and Methods
2.1. Study Area
The Gulf of Trieste is the northernmost part of the Adriatic Sea, stretching across the coasts of Italy, Slovenia, and Croatia. It extends from Cape Savudrija to Grado and is partially enclosed by the Istrian peninsula to the south. It covers an area of 551 km2 and includes a water volume of 9.5 km3 [43]. The Gulf is very shallow, with an average depth of only 18.7 m and a maximum depth of 37 m. The area is known for the highest amplitudes between high and low tides (average = 88 cm; [44]) and the lowest temperatures in winter [43]. Water temperature and salinity are strongly influenced by river outflows. In winter, the temperature can drop down to 6 °C and in summer it warms up to 26 °C, while the average salinity is around 37–38 [45,46]. Between April and September, temperature stratification occurs in the water column, with a seasonal thermocline in spring [43].
The Slovenian part of the Gulf of Trieste accounts for one third of the entire surface of the Gulf [47]. The coastal relief varies from steep rocky cliffs to gradual sloping beaches. The lower part of the coast, in particular, has already been heavily modified due to anthropogenic development and urbanization. Today, only one fifth of the coastline remains in its natural state [44,47]. The bottom along the coast is mainly rocky and consists of alternating layers of flysch, sandstone and soft marl.
2.2. Fieldwork
Between August 2020 and June 2021, 150 specimens of S. scriba were collected in shallow Slovenian waters, at a depth range of 0.5–5 m. The sampling sites were selected according to the benthic habitat type, since S. scriba predominantly inhabits a rocky bottom with different algal cover, sometimes also bordering with seagrass meadows (mostly Cymodocea nodosa). Fish were caught at eight locations in the Slovenian part of the Adriatic Sea (Figure 1, Table 1). The majority of specimens were caught in front of the National Institute of Biology-Marine Biology Station in Piran, for a total of 44 fish, 22 of which were caught in autumn between the end of September and October 2020 and 22 in spring, between the end of April and June 2021. The rest were caught at other localities (Table 1). Additionally, at the Cape Piran, Cape Ronek and Pacug sites, the parallel transect method [1,48] was used to monitor the density of painted comber populations. A measuring tape (50 m long) was laid on the rocky bottom at depths ranging from 1.5 to 4 m. After a few minutes, when the fish had become accustomed to the diver’s presence [49], all the fish were counted 1 m to the left and then 1 m to the right of the transect line. The data were transformed into densities expressed per 100 m2.
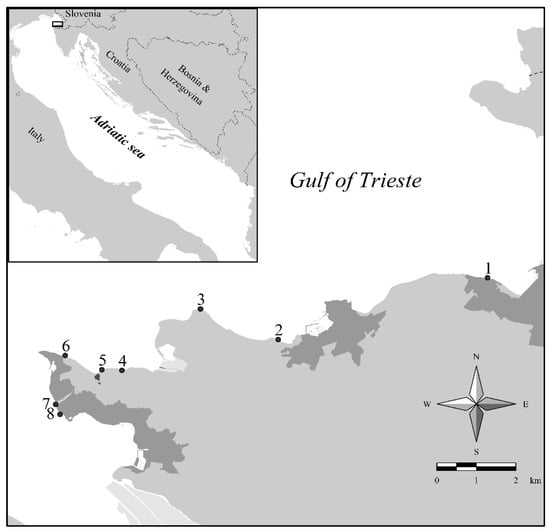
Figure 1.
Map of the study area with 8 sampling sites for Serranus scriba (for the names of the sites see Table 1).

Table 1.
Coordinates of the sampling sites for S. scriba in Slovenian coastal waters, sampling dates and number of fish collected.
Before specimen collection, we took 15 min each time to observe feeding behavior. We were interested in how S. scriba approaches and grasps its prey, where it hunts and what interactions they have with each other and with other species. During the observation, the snorkeler calmly floated on the surface of the water, carefully observing the action while moving the bait along the seafloor.
Fish were collected by snorkeling, using a barbless hook (size 6, Crivit Lasercut Selection) with a bait. During the first few days of fieldwork, different baits were tested, from pieces of squid, snails of the genus Gibbula, chunks of anchovy, to hermit crabs, which proved to be the best bait to attract S. scriba. The hook was attached to a 3 m long nylon line (0.20 mm, 3.1 kg, Crivit Specimen Line) hanging from a modified wooden pole. After the fish took the bait (hook and bait were completely in its mouth), we achieved that the hook was anchored in the oral cavity of the fish with a short pull on the nylon. Therefore, the fish did not swallow the hook, which could lead to internal injuries of the esophagus or stomach and thus death. As soon as the fish was hooked, we pulled it over the surface of the water as quickly as possible and took it off the hook. On one snorkeling session, 1–15 S. scriba individuals were caught and placed in buckets of filtered seawater. Each fish was placed in its own bucket with filtered seawater (through a 125-µm sieve) to remove any impurities that might affect the results. The specimens were transported to the laboratory of the National Institute of Biology-Marine Biology Station in Piran as soon as possible (in less than one hour after the sampling was completed).
2.3. Laboratory Work
At the laboratory of the National Institute of Biology-Marine Biology Station in Piran, each bucket was labeled with the serial number of the fish and equipped with an aerator to supply air. The aerator was placed above the bottom of the bucket to prevent the feces from fragmenting due to air bubbles.
The fish were then accurately weighed using the Sartorius CP 225D balance and measured with caliper to the nearest millimeter. Total body length (TL, length from the tip of the snout to the end of the caudal fin), standard body length (SL, length from the tip of the snout to the base of the tail) and fork length (FL, length from the tip of the snout to the center of the fork in the tail), were measured. The measured fish were classified into age groups of 1, 2, 3 and 4 years according to the study of Tuset et al. [50].
The buckets were then left covered for at least 24 h. During this period, all fish digested the prey and defecated. After 24 h, no feces containing prey items were excreted; only white, “empty” feces were excreted by a few fish after this period. Thin, white, stringy feces are an indicator of an empty gut. The fecal pellets were then carefully removed using a modified pipette and stored in 70% ethanol. Fecal pellets from each fish were stored separately in a labeled vial with the serial number of the fish. The contents of the bucket were filtered through a 125 µm sieve to capture any remaining pieces of prey and fish that were released back into coastal waters. Almost all the fish survived the procedure, but two out of 150 fish died in the buckets due to internal injuries after swallowing the hook. When released, the fish were in good condition and swam away immediately.
The contents of the fecal pellets were examined under an Olympus SZx16 stereo microscope with an Olympus DP74 camera. Fecal pellets consist of undigested prey items and peritrophic membranes. The undigested prey items were determined to the lowest possible taxonomic level and counted. The number of prey specimens was identified by the presence of its typical body parts, such as carapace or claws, in the sample. For example, when we found a pair of claws or a carapace of an anomurid crab of the genus Pisidia, we assumed that the fish had caught and digested just one Pisidia sp. specimen. In addition to taxa with hard body parts, taxa with soft bodies were also recognized by their undigested parts such as outer body layers (e.g., polychaetes or fish eggs). The prey items were identified using identification keys for the marine fauna of the Mediterranean and northwestern Europe [51,52,53]. Each prey was measured and photographed under an Olympus SZx16 stereo microscope with an Olympus DP74 camera. Fish species were determined through the identification of otoliths in pellets, using the Atlas of Otoliths for the Western Mediterranean [54] and the AFORO online database [55]. According to the formulas in the AFORO online data base [55], we calculated the total length of prey fish using the lengths of the otoliths.
2.4. Data Analyses
The prey frequency of occurrence (prey occurrence in the fecal pellets) (%PF; [56]) was calculated as follows: %PF = ns/NS, where ns represents the number of fecal pellets with prey s and NS the number of total fecal pellets. Additionally, the numerical index (%PN; [56]) of prey in the fecal pellets was calculated: %PN = ni/NI ∗ 100, where ni represents the total number of prey belonging to taxon i, and NI represents the total number of all prey in all taxonomic units.
The prey were divided into four categories: main prey, secondary prey, complementary prey and accidental prey (Table 2), as listed by Hureau [57].

Table 2.
Prey categories according to Hureau [57] (%PF = frequency of occurrence, %PN = numerical index).
Diet diversity was expressed by the index of trophic diversity (ITD), which is a modified Shannon-Wiener diversity index (H′; [58]):
ITD = 1 − H′.
The ITD value ranges from 0 to 1, where 0 means no diversity and 1 means maximum diversity. ITD was calculated at the consistent taxonomic level that is at the order level.
TrophLab, a stand-alone Microsoft access for estimating trophic levels, was downloaded from www.fishbase.org (accessed on 20 July 2021) [59] and used to calculate the TROPH index of the species studied. The trophic levels of S. scriba were calculated [59,60] as:
where DCij represents the proportion of prey j in the diet of species i and TROPHj represents the partial trophic level of prey j. To demonstrate the importance of each taxon in the diet, the SIMPER function in the R programming environment [61] was used:
where x represents the abundance of prey taxon i in samples j and k. The index is the sum of the individual contributions of all prey taxa of species S:
𝑇𝑅𝑂𝑃𝐻𝑖 = 1 + Σ𝐷𝐶𝑖𝑗 ∗ 𝑇𝑅𝑂𝑃𝐻𝑗,
[𝑖𝑗𝑘] = 𝑎𝑏𝑠(𝑥[𝑖𝑗] − 𝑥[𝑖𝑘])/𝑠𝑢𝑚(𝑥[𝑖𝑗] + 𝑥[𝑖𝑘]),
d[jk] = sum (i = 1…S) d[ijk].
The SIMPER function performs a pairwise comparison of prey groups and returns the average contributions of each taxon to the overall Bray-Curtis diversity index (Available at: https://www.rdocumentation.org/packages/vegan/versions/2.4-2/topics/SIMPER; accessed on 26 July 2021). Spearman’s correlation and multivariate analysis (Bray-Curtis similarity for differences between fishes, locations and between ages for S. scriba) were performed in R (R 4.0.2 software package; R Development Core Team 2008, Vienna, Austria) using the PRIMER v7+ (PERMANOVA software, Albany, New Zeland) [61,62] package. A p-value of <0.05 was chosen to determine the statistical significance of the trend.
3. Results
3.1. S. scriba Density and Biometry
On performed visual parallel transects, S. scriba density varied between 6 and 12 ind./100 m2 at different localities and depths. The highest density was calculated at Cape Ronek, where 11–12 ind./100 m2 were observed at 1.5 m depth (Table 3). Average density at Cape Ronek was 11 ind./100 m2 and 6.5 ind./100 m2 in Pacug and 7 ind./100 m2 at Cape Piran (Table 3). Based on the results for the Slovenian part of the Gulf of Trieste, the calculated average density of S. scriba is 8.34 ind./100 m2. The total length of the 150 specimens caught, ranged from 108 mm to 217 mm, while the weight ranged from 17 g to 163 g (Table 4). According to the length structure of the fish, we estimated that most fish were 1 or 2 years old (Table 5), indicating that the majority of caught fish were juveniles.

Table 3.
Painted comber densities at sampling locations in Slovenian waters.

Table 4.
Average minimal and maximal sizes (TL = total length, FL = fork length, SL = standard length) and weight of 150 specimens of S. scriba.

Table 5.
Total length (TL) and groups of 150 specimens of S. scriba.
3.2. Feeding Habits of S.scriba
A total of 32 taxa were identified as prey items in the fecal pellets of S. scriba (Table 6). The most abundant prey of S. scriba were crustaceans (%PN = 69.21%, %PF = 98.67%), followed by polychaetes (%PN = 12.63%, %PF = 40.67%), mollusks (gastropoda: %PN = 4.66%, %PF = 17.33%; bivalvia: %PN = 0.67%, %PF = 2.67%) and fish (%PN = 6.82%, %PF = 20.67%; Table 6). Among crustaceans, the most abundant and also most frequent prey items were decapods (PN% = 46.75%, %PF = 96.67%), followed by isopods (PN% = 13.64%, %PF = 37.33%). The most common prey items found was Pisidia sp. which alone accounted for 18.80% of all prey. Polychaetes, mainly vagile species (suborder Errantia), accounted for 12.63% of prey items, which is the second most frequent prey (Table 2 and Table 6). Teleost fishes (6.82% of total prey items) constituted the complementary prey of 1st order (Table 2). The following fish species were identified (see Table 6): Atherina hepsetus Linnaeus, 1758, Gobius fallax Sarato, 1889, Symphodus ocellatus (Linnaeus, 1758), S. cinereus (Bonnaterre, 1788), Pomatoschistus bathi Miller, 1982, Mullus surmuletus Linnaeus, 1758 and Gobius cruentatus Gmelin, 1789. In some cases, only fish vertebrae were found in the fecal pellets and species identification was not possible. In addition to prey with hard parts, prey with soft body structure were also identified in the feces such as fish eggs (N% = 5.99, %PF = 11.33%) and cuticles of polychaete worms. The diet of S. scriba specimens was compared between age groups. The majority of the captured S. scriba in our study were less than 3 years old and less than 173 mm long (TL). While the adult specimens of S. scriba prey mainly on decapods, isopods and fish, the diet of juveniles consists of polychaetes and small crustaceans such as mysids, amphipods and shrimps. Two-year old and older individuals tend to supplement their diet with epibenthic and nectobenthic fish, which coincides with the increase of the trophic level with age (up to 3+) (Table 7). The proportion of fish in the diet increased from an initial 3.97% at age 1 to around 10% by age 4. The proportion of crustaceans decreased from 71.52% to 40.66% between the 1st and the 4th year (Figure 2). The proportion of eggs in the diet increased from 1.99% in 1-year-old individuals to 35.16% in 4-year-old individuals. The average calculated trophic diversity index (ITD) was 0.89 (on a scale of 0 to 1, where 0 means no diversity and 1 means highest diversity). No statistically significant difference was found between the diet and age/length composition of S. scriba at the different sampling sites (Bray-Curtis, p < 0.05).

Table 6.
Numerical index (%PN) and frequency of occurrence (%PF) of particular prey taxa of S. scriba in the northern Adriatic Sea.

Table 7.
Trophic level (TROPH) of different age groups of S. scriba.
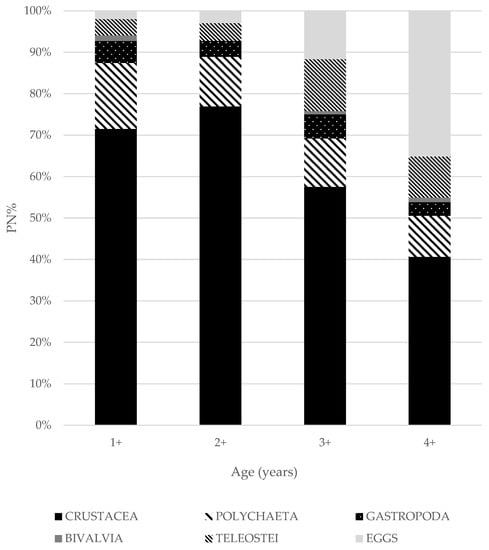
Figure 2.
Comparison of prey proportions (%PN) in S. scriba diet.
During the fieldwork, it was noted that S. scriba specimens are more active in the morning and evening, when they were observed to be feeding actively. Our personal observations also demonstrated that S. scriba usually monitors visually open areas and upon detection of a passing prey (or a bait) performs a short but very fast burst chase and then upon completion of prey pursuit occupies another waiting spot. The waiting spot was usually within heterogenous rocky reefs, but we also observed them waiting in Posidonia oceanica meadows or hiding in algae. In many cases, S. scriba was observed lurking behind a rock or under a boulder, waiting for a prey to come close enough to grab it and suck it into their mouths. The prey was consumed with a quick suction. If the prey was too large, it was usually spat out several times before consumption. This activity often attracted several other S. scriba, who then competed for the prey. Larger, dominant S. scriba often exhibited aggressive behavior and chased off smaller specimens. On the other hand, on a few occasions, younger S. scriba (juvenile and subadult individuals) were also observed cooperating and hunting between rocks in groups of up to 6 individuals. Regarding interspecific interactions, in a few cases S. ocellatus was observed to clean parasites from S. scriba. The latter stood vertically with their heads down and allowed S. ocellatus to remove parasites.
4. Discussion
4.1. Advantages and Disadvantages of Non-Destructive Methods of Sampling and Analyses
A recently developed non-destructive method [63] was tested in this study on S. scriba to detect prey in fecal pellets. The advantage of the fecal examination method is that it can be used on smaller-sized fish, even smaller than 15 mm [63], and that the survival of all specimens makes these procedures suitable for use in protected marine areas and for the study of protected species or species with low population densities [37,40], and also renders the research more ethical without compromising quality. Another modern method that is also non-lethal and frequently used for diet estimation is stable isotope analysis; however, this method cannot provide taxonomic resolution and is more useful for describing a long-term assimilated diet [64]. This method can be complementary to stomach contents analysis or analysis of feces. Advantage of this non-destructive method is also shorter handling time, since a shorter handling time means a higher chance of survival and a less stressful experience for the animal [42,63]. The method is easy to use on less mobile, small and medium-sized coastal fishes, as the fish can be placed in buckets overnight and complex aquarium equipment is not needed. The method is far more complicated to use on open-water fish or larger fish, which cannot be housed in buckets or similar small containers. Nevertheless, most members of coastal fish assemblages are small to medium in size, and therefore the feeding habits of many species can be studied using this method.
While the fieldwork for the capture of fish proved to be particularly time consuming, since it required 4 to 6 h of snorkeling to catch 10 specimens of S. scriba, it should be noted that long capturing times may be connected with the use of a barbless hook, which can reduce catch efficiency, but it also significantly reduces unhooking time, stress and hooking injuries [65,66,67]. Another important factor is the size of the hook, as larger hooks decrease the incidence of deep hooking and consequently prevent serious injuries and bleeding [68,69,70,71]. In our study, we used large and thin hooks (size 6). Only 2 fish out of 150 died due to hooking. Thus, our fishing technique proved to be suitable for the non-destructive diet analysis method. The diameter of the nylon line is another important factor. We used nylon line, 0.20 mm in diameter, because S. scriba has sharp teeth that can bite through thinner thread.
In other studies, S. scriba were caught using traditional fishing gears, such as floating nets and longlines, but these methods are more likely to injure or kill the fish [10,14,17,72,73,74]. Working with live fish requires appropriate living conditions for the captured specimens, which means regular water changes, adequate oxygen levels and appropriate water temperature.
During our sampling surveys, mostly juvenile and sub-adult fish were caught, since young fish are less wary and more curious than older, experienced fish. Due to the predominance of juveniles in the sample, the data on trophic levels and prey proportions for all S. scriba individuals are biased to some extent, so it is better to consider trophic levels and prey proportions separately for each length (age) class.
Prey items in the fecal pellets of S. scriba were sometimes so decomposed that identification down to the lowest taxonomic units was impossible, but because their prey consisted mainly of crustaceans, polychaetes, fish and other taxa with hard, distinguishable body parts that are not so easily decomposed, identification of family, class or order was still possible (see Figure 3 and Figure 4). Even some soft parts of prey, such as the cuticle of polychaetes and fish eggs, were found, demonstrating that species with softer bodies can also be recognized in feces. The quality of the fecal examination method was tested on five dead adult specimens of S. scriba obtained as bycatch from fishermen. These individuals were dissected and the stomach contents were isolated to compare the stage of decomposition in the stomach and feces of S. scriba. We were able to confirm that the stage of decomposition was similar. Fish exanimated by the non-destructive method probably defecated faster due to stress and consequently the prey did not decompose well, making it easier for us to determine the prey [75]. This was confirmed by the finding of whole juvenile Gobius sp. (Figure 4B), whole tanaids of species Tanais dulongii (Figure 3D), whole sphaeromatids (Figure 3C), anthurids and some whole Pisidia sp. in the feces. We did not find any soft or hard prey in the stomachs examined that was not also found in the feces. Soft prey was also not found in the diet of S. scriba by the authors of other studies, regardless of the method used [10,14,17]. Thus, the non-destructive method does not lead to worse results. Even with the traditional method of stomach content analysis, there is always a possibility of overestimating the proportions of prey that decompose more slowly [35,76,77].
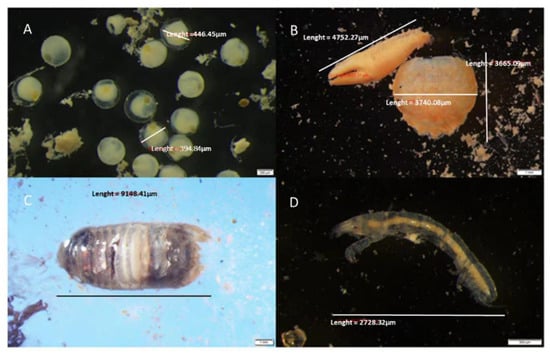
Figure 3.
Prey items isolated from feces of S. scriba: (A) unidentified eggs, (B) carapace and claws of Pisidia sp., (C) isopod from family Sphaeromatidae and (D) Tanais dulongii.
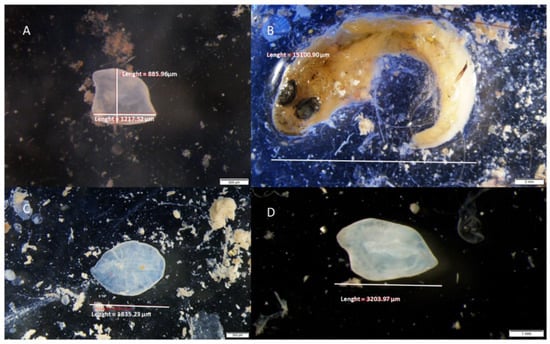
Figure 4.
Otoliths and undigested Gobius sp. found in fecal pellets of S. scriba: (A) otolith of Gobius cruentatus, (B) Gobius sp., (C) otolith of Atherina hepsetus and (D) Gobius fallax.
Some differences in the results of previous studies are due to the fact that they did not always use standardized methodology to study feeding ecology in ichthyology. To determine the importance of the prey, it is best to use a numerical index as well as frequency of occurrence and categorize the prey as regards to these two criteria (see Table 2). These parameters applied for food spectra analysis are set accordingly and are used to quantitatively describe and graphically represent diet [35,78,79,80].
4.2. Feeding Habits and Trophic Levels of S. scriba in the Northern Adriatic and in Other Mediterranean Areas
Our results indicate that S. scriba in the northern Adriatic Sea feeds on both slow-moving and fast-moving prey [13]. The calculated average trophic diversity index value (ITD) indicates a highly diverse diet. The calculated trophic levels demonstrated that in general, adults feed on higher trophic level than juveniles. This change in diet during development is referred to as an ontogenetic shift and has been confirmed for another Serranus species [81]. According to literature data [50], around 50% of individuals are sexually mature at 173 mm, which indicates that the majority of individuals used in the study were juveniles. Given the observed trend towards an increasing proportion of fish in the diet with body size, it is reasonable to assume that this proportion and therefore trophic level is even higher for older and larger S. scriba. The value of trophic level for 4-year-old S. scriba and older is, however, most likely biased due to the very small sample of these individuals and is not representative.
According to the analyses of otoliths found in the fecal pellets of S. scriba (see Figure 4), G. fallax was highlighted as the most common fish prey (Table 6), a finding that is attributed to its high abundance in the area [31] and its suitable shape and size for consumption by S. scriba. The suitable size of the prey is determined by limits of visual detection and size of the jaw apparatus [82,83,84,85]. Consuming one large prey means better energy efficiency than feeding on numerous small prey, but it is more time consuming, as prey handling time is longer [81]. Even though fish prey is not as important as regards to %PN and %PF, their mass and volume are larger than other prey and they are therefore a very important part of the diet. Piscivores generally reorient their prey head-first and lying on their side before swallowing it [84,86]. Therefore, the greatest width of the prey fish when swallowed is body depth (i.e., maximum distance between the dorsal and ventral portions of the fish). Prey with lower body depth is preferred, as it is easier to catch and consume [86]. Larger individuals of S. scriba are able to prey on fish species with greater body depth (S. ocellatus) (Figure 4), while smaller individuals prefer fish with shallow body-depth (Gobius sp.), as they are easier to capture and consume. Price et al. [87] have shown that handling time increases with prey body depth. Longer handling time increases the risk of losing the prey and being exposed to predators [88]; therefore, greater body depth is an anti-predatory adaptation [86].
Predators most often prey on fish that are well below the maximum ingestible size [82]. In wrasses, we noticed another anti-predatory behavior; in the presence of S. scriba, lateral positioning and display of the dorsal and ventral fin have been observed both in the wild and in captivity (pers. observation). Such a display makes the prey fish appear larger and is often sufficient to deter the predator from swallowing it [87,89]. Wrasses have a greater body depth than gobies at the same body length; therefore, wrasses are consumed by larger individuals of S. scriba, as observed in our study. Interestingly, smaller wrasses such as Symphodus ocellatus were observed to be removing parasites from S. scriba, although the latter is its potential predator (our study). Such behavior, i.e., approaching potentially dangerous clients, has been studied on cleaning gobies [90,91,92].
Our observations regarding specimens of S. scriba often predating from behind rocks, or lurking under boulders, are in accordance with previous studies [93,94]. The observed hunting behavior is known as the “sit and wait” predation mode and “burst chase” prey pursuit mode [19,94]. Vandewalle et al. [94] described that the majority of individuals occupy waiting spot above or within the algal cover or an overhang within heterogeneous rocky reefs, but they have also been observed hiding in algal thalli or Posidonia oceanica meadows, bordering sandy substrate.
S. scriba were observed searching for smaller crustaceans in cavities, holes and crevices under stones. Therefore, it was not surprising that crabs of the genus Pisidia were the most common prey items among the preyed crustaceans (see Figure 3), occurring in more than 50% of the examined samples (%PF = 56.67%, %PN = 18.80%). A crab, Pilumnus hirtellus, occurred in more than one tenth of the samples (% PF = 12.67%, %PN = 3.16%), while other crustaceans in the fecal pellets were mostly too decomposed to be identified to a species level. Isopods were a secondary common prey, mostly species of the family Sphaeromatidae, which are common inhabitants of rocky bottom communities [95]. Among complementary prey, well-preserved, almost whole specimens of Tanais dulongii (Tanaidacae), a widely distributed amphipod species along the entire Slovenian coast [96], were found.
Analyses of the diet of S.scriba have been conducted in different parts of the Mediterranean Sea and Canary Islands (Table 8 and Table 9). In general, all the results confirm that crustaceans, especially decapods, are the main food source for S. scriba, while fish mainly represent secondary food. The highest proportion of fish in the diet was observed in a study from the Canary Islands (%PN = 22.64%), while this proportion was the lowest in our study (%PN = 6.82%). In terms of frequency of occurrence, fish were present in 38.32% of the stomachs of S. scriba from the Canary Islands, 30.1% of those from the Tyrrhenian Sea and 20.67% of the fecal pellets of S. scriba from the northern Adriatic (see Table 8). The proportion of polychaetes in the diet of S. scriba was significantly higher in the northern Adriatic compared to other areas (%PN = 12.65%, %PF = 40.67%). In the Tyrrhenian Sea and in the Atlantic, authors observed a significantly higher proportion of caridean shrimps than observed in our study.

Table 8.
Diet of Serranus scriba in various parts of the Mediterranean and Canary Islands (%PN = numerical index, %PF = frequency of occurrence).

Table 9.
Trophic levels of Serranus scriba in the Mediterranean Sea.
The trophic levels for S. scriba in different parts of the Mediterranean Sea were calculated (see Table 9) and ranged from 3.43 ± 0.52 in the northern Adriatic (our study) to 3.94 +/± −0.63 in the Aegean Sea [29]. The TROPH values for the same species may vary between sampling sites, seasons and years [97], and such changes in feeding habits may be influenced by changes in habitats [97] and prey availability [98]. The TROPH value also varies between different sizes and phases of the predator’s biological cycle [99] as well as sampling time [48,99]. These parameters should be taken into account when interpreting and comparing results for the same geographic area. Indeed, if we compare two results from the Aegean Sea [14,100], we may assume that the differences in TROPH values are probably due to differences in fish body lengths (see Table 9), which may explain the difference in the values for the same region. Moreover, the trophic level for S. scriba calculated in our study (TROPH = 3.43 ± 0.53) is most similar to that of Labrus merula (TROPH = 3.47 ± 0.55), Symphodus ocellatus (TROPH = 3.4 ± 0.51), Mullus surmuletus (TROPH = 3.44 ± 0.53), Diplodus annularis (TROPH = 3.4 ± 0.46), Diplodus sargus (TROPH = 3.38 ± 0.51) and Diplodus vulgaris (3.5 ± 0.46), as calculated by Stergiou and Karpouzi [29]. All these species feed on decapod crustaceans, polychaetes, bivalves and echinoderms [29], which means that S. scriba may compete with them for available food resources. Furthermore, according to Stergiou and Karpouzi [29], the trophic level of S. scriba adults (3.7 ± 0.58) is higher than the trophic levels of other members of the coastal fish community (i.e., Sparidae, Labridae, Bleniidae and Gobiidae), that are abundant in the northern Adriatic Sea [1]. Therefore, S. scriba can be considered as a top predator of the community. As one of the most abundant piscivorous species on the rocky bottom in the coastal zone of Slovenian waters, it could play an important role as a predator of the goby family, especially of Gobius cruentatus and Gobius fallax, which are among the most numerous fish species in the area [101]. S. scriba feeds also on juvenile Chromis chromis,, a key fish species with an important role in transferring carbon, nitrogen and phosphorus from pelagic systems to the littoral zone [102,103].
4.3. Implications for Conservation
Fish are a crucial bioindicator of the ecological integrity of aquatic systems at different levels, from microhabitat to catchment; thus, they represent an important monitoring tool [104]. Species that are suitable bioindicators have high specificity and fidelity, i.e., they are found only in a particular type of environment and are widespread and abundant in that environment [105]. Because S. scriba is site-faithful [74], widespread throughout the Mediterranean [73], easy to collect and identify [104], and feeds opportunistically, it is suitable as a bioindicator species.
Data on feeding habits are essential for species and habitat conservation [103,106]. S. scriba is known as one of the nine most aggressive predators in the Adriatic fish communities, as defined by approaching, attacking and lure ingestion [93]. Aggressive predators play a very important role in the environment, as their behavior is the primary organizing force shaping the assembly of fish communities and driving preference and occupancy of heterogeneous and homogenous benthic habitats [104]. The most aggressive predators in Adriatic fish communities were found to be nine taxa of families Serranidae (3), Gobidae (3), Sparidae (2) and Labridae (1) [93].
The Adriatic Sea is a heavily exploited part of the Mediterranean basin, where the number of large apex predators, such as sharks and rays, have declined dramatically over the past two centuries [107] and mesopredators (mid-range predators in the middle of a trophic level [3] that typically prey on smaller animals), have taken over their role [108]. The loss of an aggressive mesopredator, such as S. scriba, may result in a drastic change in the fish community, including an increase in prey populations, and may have a major impact on the ecosystem as a whole [109]. S. scriba is an abundant opportunistic predator in the coastal fish community and is helping to maintain stability of the ecosystem [110,111] due to its generalist foraging strategy. Although nowadays the species does not require special protection, efforts should be made to maintain the overall variety and diversity of marine habitat types in the northern Adriatic Sea. Anthropogenic impacts on marine ecosystems should be monitored regularly and appropriate conservation actions taken before populations declines are recorded. For monitoring fish assemblages, it is recommended to use non-destructive methods such as a visual census whenever possible.
5. Conclusions
S. scriba is an important opportunistic mesopredator of the northern Adriatic rocky bottom fish communities. It preys on a wide range of different prey such as small epibenthic invertebrates and small coastal fishes (e.g., gobies, wrasses and Atherina spp.). While younger S. scriba tend to feed on small invertebrates such as polychaetes, mysids, and shrimp, they later undergo an ontogenetic shift and feed on a higher trophic level (with fish and decapod crustaceans). This paper supports previous research on the feeding habits of S. scriba and confirms the usefulness of the new non-lethal method [63] for studying the diet of small- to medium-sized coastal fish.
Author Contributions
Conceptualization, A.L., L.L. and D.T.; methodology, A.L., L.L. and D.T.; investigation, A.L., L.L., D.T. and M.O.-B.; writing—original draft preparation, A.L., D.T. and M.O.-B.; writing—review and editing, A.L., L.L., D.T. and M.O.-B.; project administration and funding acquisition, A.L., L.L., D.T. and M.O.-B. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support from the Slovenian Research Agency (ARRS), research core funding No. P1-0237.
Institutional Review Board Statement
Our research was conducted at the Marine Biology Station Piran of the National Institute of Biology of Slovenia, where researchers have the government authorization for fish culture and experimentation. Moreover, Serranus scriba is not on the list of protected species in Slovenia and therefore no special permits are required for catching and studying the species. We confirm that ethical cost of the research is balanced by the scientific value of the research. Knowledge about food and feeding habits of S. scriba is important for understanding local ecosystem dynamics, it helps us identify changes in the environment at an early change and contributes to the general advancement of knowledge. The authors assure that the present research complies with the commonly accepted ‘3Rs’: replacement of animals by alternatives wherever possible, reduction in number of animals used, and refinement of experimental conditions and procedures to minimize the harm to animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available, since they originate from the research program funded by the Slovenian Research Agency (ARRS).
Acknowledgments
The authors would like to thank Milijan Šiško, Tihomir Makovec, Leon Lojze Zamuda, Luka Preložnik, Jure Zaman, Urška Bizjak, Gaja Jenko, Mojca Pungerčar, Doroteja Erhatič and Romina Bonaca for their assistance during the fieldwork and laboratory work. Special thanks to Valentina Pitacco and Milijan Šiško for their help in the statistical analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Orlando-Bonaca, M.; Lipej, L. Factors affecting habitat occupancy of fish assemblage in the Gulf of Trieste (Northern Adriatic Sea). Mar. Ecol. 2005, 26, 42–53. [Google Scholar] [CrossRef]
- IRP; Fletcher, S.; Lu, Y.; Alvarez, P.; McOwen, C.; Baninla, Y.; Fet, A.M.; He, G.; Hellevik, C.; Klimmek, H.; et al. Governing Coastal Resources. In Implications for a Sustainable Blue Economy; Report of the International Resource Panel; United Nations Environment Programme: Nairobi, Kenya, 2021. [Google Scholar]
- Airoldi, L.; Balata, D.; Beck, M.W. The Gray Zone: Relationships between habitat loss and marine diversity and their applications in conservation. J. Exp. Mar. Biol. Ecol. 2008, 366, 8–15. [Google Scholar] [CrossRef]
- Elliott, M.; Borja, A.; Cormier, R. Activity-footprints, pressures-footprints and effects-footprints—Walking the pathway to determining and managing human impacts in the sea. Mar. Pol. Bul. 2020, 155, 111201. [Google Scholar] [CrossRef] [PubMed]
- Borja, A.; Menchaca, I.; Garmendia, J.M.; Franco, J.; Larreta, J.; Sagarminaga, Y.; Schembri, Y.; González, R.; Antón, R.; Micallef, T.; et al. Big Insights From a Small Country: The Added Value of Integrated Assessment in the Marine Environmental Status Evaluation of Malta. Front. Mar. Sci. 2021, 8, 638232. [Google Scholar] [CrossRef]
- Sarrazin, V.; Kuhs, V.; Kullmann, B.; Kreutle, A.; Pusch, C.; Thiel, R. A sensitivity-based procedure to select representative fish species for the Marine Strategy Framework Directive indicator development, applied to the Greater North Sea. Ecol. Indic. 2021, 131, 108161. [Google Scholar] [CrossRef]
- Nelson, J.S. Fishes of the World; John Wiley and Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Bauchot, M.L. Poissons osseux. Fiches FAO d’identification pour les besoins de la pêche. (rev. 1). Méditerranée et mer Noire. Zone Pêche. 1987, 37, 891–1421. (In French) [Google Scholar]
- Maigret, J.; Ly, B.; Maigret, S. Marine fishes of Mauritania. France Sci. Nat. 1986, 213, 77. [Google Scholar]
- Moreno-Lopes, A.; Tuset, J.V.; González Garcia-Diaz, M.M. Feeding habits of Serranus scriba (Osteichthyes, Serranidae). Bol. Mus. Munic. Funchal. 2002, 53, 5–17. [Google Scholar]
- Zorica, B.; Pallaoro, A.; Sinovčić, G.; Keč, V.Č. Recent data of maximum age and length of painted comber Serranus scriba (Linnaeus, 1758) in Mediterranean Sea. Acta Adriat. 2010, 51, 223–226. [Google Scholar]
- Heemstra, P.C.; Anderson, W.D., Jr.; Lobel, P.S. Groupers (seabasses, creolefish, coney, hinds, hamlets, anthiines, and soapfishes). In FAO Species Identification Guide for Fishery Purpouses. The Living Marine Resources of the Western Central Atlantic; Bony Fishes Part 1 (Acipenseridae to Grammatidae); Food and agriculture organization of the United Nations: Rome, Italy, 2013; Volume 2, pp. 1308–1369. [Google Scholar]
- Arculeo, M.; Froglia, C.; Riggio, S. Food partitioning between Serranus scriba and Scorpaena porcus (Perciformes) on the infralittoral ground of the South Tyrrhenian Sea. Cybium 1993, 17, 251–258. [Google Scholar]
- Vasiliki, M. An Estimation of the Diet of the Species Serranus scriba (Linnaeus, 1758) in the Area of Nisiopi, in South-West Lesvos. J. Environ. Eng. Sci. 2016, 5, 593–600. [Google Scholar] [CrossRef]
- Deudero, S.; Pinnegar, J.K.; Polunin, N.V.C. Spatial variation and ontogenic shifts in the isotopic composition of Mediterranean littoral fishes. Mar. Biol. 2004, 145, 971–981. [Google Scholar] [CrossRef]
- Heemstra, P.C.; Randall, J.E. Groupers of the world. FAO Fish. Synop. 1993, 16, 1. [Google Scholar]
- Pinnegar, J.K.; Polunin, N.V.C. Contributions of stable-isotope data to elucidating food webs of Mediterranean rocky littoral fishes. Oecologia 2000, 122, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Labropoulou, M.; Eleftheriou, A. The foraging ecology of two pairs of congeneric demersal fish species: Importance of morphological characteristics in prey selection. J. Fish. Biol. 1997, 50, 324–340. [Google Scholar] [CrossRef]
- Saikia, S.K. On the methodology of feeding ecology in fish. Eur. J. Ecol. 2016, 2, 35–46. [Google Scholar] [CrossRef][Green Version]
- Ferry, L.; Cailliet, G. Sample size and data analysis: Are we characterizing and comparing diet properly. In Proceedings of the Feeding, Ecology and Nutrition in Fish. International Congress on the Biology of Fishes, San Francisco, CA, USA, 14–18 July 1996; pp. 71–80. [Google Scholar]
- Wootton, J.T. Effects of disturbance on species diversity: A multitrophic perspective. Am. Nat. 1998, 152, 803–825. [Google Scholar] [CrossRef]
- Ross, S.T. Resource partitioning in fish assemblages: A review of field studies. Copeia 1986, 2, 352–388. [Google Scholar] [CrossRef]
- Guedes, A.P.P.; Araújo, F.G. Trophic resource partitioning among five flatfish species (Actinopterygii, Pleuronectiformes) in a tropical bay in south-eastern Brazil. J. Fish Biol. 2008, 72, 1035–1054. [Google Scholar] [CrossRef]
- Wetherbee, B.M.; Cortés, E.; Bizzarro, J.J. Food consumption and feeding habits. In Biology of Sharks and Their Relatives, 1st ed.; Jeffrey, C., Carrier, J.A., Musick, M.R., Heithaus, Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 225–246. [Google Scholar]
- Motta, P.J.; Wilga, C.D. Advances in the study of feeding behaviors, mechanisms, and mechanics of sharks. In The Behavior and Sensory Biology of Elasmobranch Fishes: An Anthology in Memory of Donald Richard Nelson, 1st ed.; Timothy, C.T., Samuel, H.G., Eds.; Springer Science-Business Media, B.V.: Dordrecht, The Netherlands, 2001; pp. 131–156. [Google Scholar]
- Martin, R.A.; Hammerschlag, N.; Collier, R.S.; Fallows, C. Predatory behaviour of white sharks (Carcharodon carcharias) at Seal Island, South Africa. J. Mar. Biolog. Assoc. 2005, 85, 1121–1136. [Google Scholar] [CrossRef]
- Frid, A.; Marliave, J. Predatory fishes affect trophic cascades and apparent competition in temperate reefs. Biol. Lett. 2010, 6, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Collar, D.C.; O’Meara, B.C.; Wainwright, P.C.; Near, T.J. Piscivory limits diversification of feeding morphology in centrarchid fishes. Int. J. Org. Evol. 2009, 63, 1557–1573. [Google Scholar] [CrossRef]
- Stergiou, K.I.; Karpouzi, V.S. Feeding habits and trophic levels of Mediterranean fish. Rev. Fish Biol. Fish. 2002, 11, 217–254. [Google Scholar] [CrossRef]
- Svanback, R.; Bolnick, D.I. Intraspecific competition drives increased resource use diversity within a natural population. Proc. R. Soc. 2007, 274, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Murakami, M. Reciprocal subsidies: Dynamic interdependence between terrestrial and aquatic food webs. Proc. Natl. Acad. Sci. USA 2001, 98, 166–170. [Google Scholar] [CrossRef]
- Baxter, C.V.; Fausch, K.D.; Murakami, M.; Chapman, P.L. Fish invasion restructures stream and forest food webs by interrupting reciprocal prey subsidies. Ecology 2004, 85, 2656–2663. [Google Scholar] [CrossRef]
- Baxter, C.V.; Fausch, K.D.; Saunders, C.W. Tangled webs: Reciprocal flows of invertebrate prey link streams and riparian zones. Freshw. Biol. 2005, 50, 201–220. [Google Scholar] [CrossRef]
- Hynes, H.B.N. The food of fresh-water sticklebacks (Gasterosteus aculeatus and Pygosteus pungitius), with a Review of Methods Used in Studies of the Food of Fishes. J. Anim. Ecol. 1950, 19, 36–58. [Google Scholar] [CrossRef]
- Hyslop, E.J. Stomach contents analysis—A review of methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Cortes, E. A critical review of methods of studying fish feeding based on analysis of stomach contents: Application to elasmobranch fishes. Can. J. Fish. Aquat. Sci. 1997, 54, 726–738. [Google Scholar] [CrossRef]
- Baker, A.M.; Fraser, D.F. A method for securing the gut contents of small, live fish. Trans. Am. Fish. Soc. 1976, 105, 520–522. [Google Scholar] [CrossRef]
- Crossman, E.J.; Hamilton, J.G. An apparatus for sampling gut contents of large, living fishes. Environ. Biol. Fishes 1978, 3, 297–300. [Google Scholar] [CrossRef]
- Light, R.W.; Adler, P.H.; Arnold, D.E. Evaluation of gastric lavage for stomach analyses. N. Am. J. Fish. Manag. 1983, 3, 81–85. [Google Scholar] [CrossRef]
- Kamler, J.F.; Pope, K.L. Nonlethal methods of examining fish stomach contents. Rev. Fish. Sci. Aquac. 2001, 9, 1–11. [Google Scholar] [CrossRef]
- Hartleb, C.F.; Moring, J.R. An improved gastric lavage device for removing stomach contents from live fish. Fish. Res. 1995, 24, 261–265. [Google Scholar] [CrossRef]
- Meehan, W.R.; Miller, R.A. Stomach flushing: Effectiveness and influence on survival and condition of juvenile salmonids. J. Fish. Res. Board Can. 1978, 35, 1359–1363. [Google Scholar] [CrossRef]
- Boicourt, W.C.; Kuzmić, M. The inland sea: Circulation of Chesapeake Bay and the Northern Adriatic. Mar. Ecol. Prog. Ser. 1999, 303, 81–129. [Google Scholar] [CrossRef]
- Ogrin, D. Podnebje in izredni vremenski dogodki ob Tržaškem zalivu pred letom 1841. Geogr. Obz. 2012, 3, 23–30. (In Slovene) [Google Scholar]
- Bailey, R.G. Ecoregions—The Ecosystem Geography of the Oceans and Continents, 2nd ed.; Springer: New York, NY, USA, 1998; pp. 196–200. [Google Scholar]
- Bićanić, Z. Undersurface Salinity Minimum Participation in the Process of Making Deep Adriatic Sea-water. Hrvat. Geogr. Glas. 1998, 60, 123–134. [Google Scholar]
- Turk, R. An assessment of the vulnerability of the Slovene coastal belt and its categorisation in view of (in)admissible human pressure, various activities and land-use. Ann. Ser. Hist. Nat. 1999, 9, 37–50. [Google Scholar]
- Lipej, L.; Orlando-Bonaca, M. Assessing Blennioid fish populations in the shallow Gulf of Trieste: A comparison of four in situ methods. Period. Biol. 2006, 108, 23–29. [Google Scholar]
- Labrosse, P.; Kulbicki, M.; Ferraris, J. Underwater visual fish census surveys: Proper use and implementation. In Reef Resources Assessment Tools; Secretariat of the Pacific Community: Noumea, New Caledonia, 2002. [Google Scholar]
- Tuset, V.M.; Garcia-Diaz, M.M.; Gonzalez, J.A.; Lorente, M.J.; Lozano, I.J. Reproduction and growth of the painted comber Serranus scriba (Serranidae) of the Marine Reserve of Lanzarote Island (Central-Eastern Atlantic). Estuar. Coast. Shelf Sci. 2005, 64, 335–346. [Google Scholar] [CrossRef]
- Riedl, R. Fauna e flora del Mediterraneo. Dalle Alghe ai Mammiferi; una Guida Sistematica alle Specie che Vivono nel mar Mediterraneo, 1st ed.; Franco Muzzio Editore: Padova, Italy, 1991. (In Italian) [Google Scholar]
- Falciai, L.; Minervini, R. Guida dei Crostacei Decapodi d’Europa, 1st ed.; Franco Muzzio Editore: Padova, Italy, 1992. [Google Scholar]
- Hayward, J.P.; Ryland, S.J. Handbook of the Marine Fauna of North-West Europe, 2nd ed.; Oxford University Press: Oxford, UK, 2017; (In Italian). [Google Scholar] [CrossRef]
- Tuset, V.M.; Lombarte, A.; Assis, C.A. Otolith atlas for the western Mediterranean, north and central eastern Atlantic. Sci. Mar. 2008, 72, 7–198. [Google Scholar] [CrossRef]
- Lombarte, A.; Chic, Ò.; Parisi-Baradad, V.; Olivella, R.; Piera, J.; Garcia-Ladona, E. A web-based environment for shape analysis of fish otoliths. The AFORO database. Sci. Mar. 2006, 70, 147–152. [Google Scholar] [CrossRef]
- Macdonald, J.S.; Green, R.H. Redundancy of variables used to describe importance of prey species in fish diets. Can. J. Fish. Aquat. 1983, 40, 635–637. [Google Scholar] [CrossRef]
- Hureau, J.C. Biologie Comparée de Quelques Poissons Antarctiques (Nototheniidae). Musée Océanographique: Monaco-Ville, Monaco, 1970; p. 68. (In French) [Google Scholar]
- Shannon, C.E.; Wiener, W. The Mathematical Theory of Communication, 1st ed.; University of Illinois Press: Urbana, IL, USA, 1963. [Google Scholar]
- Pauly, D.; Froese, R. Trophic levels of fishes. In FishBase 2000: Concepts, Design and Data Sources, 1st ed.; The International Center for Living Aquatic Resources Management (ICLARM): Manila, Philippines, 2000; p. 127. [Google Scholar]
- Pauly, D.; Froese, R.; Palomares, M.L. Fishing down aquatic food webs: Industrial fishing over the past half-century has noticeably depleted the topmost links in aquatic food chains. Am. Sci. 2000, 88, 46–51. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Austral. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. Primer v6: User Manual/Tutorial. Plymouth; PRIMER-E: Auckland, New Zealand, 2006. [Google Scholar]
- Trkov, D.; Lipej, L. A non-destructive method for assessing the feeding habits of coastal fish. Mediterr. Mar. Sci. 2019, 20, 453–459. [Google Scholar] [CrossRef]
- Zorica, B.; Ezgeta-Balić, D.; Vidjak, O.; Vuletin, V.; Šestanovič, M.; Isajović, I.; Čikeš Keč, V.; Vrgoč, N.; Harod, C. Diet Composition and Isotopic Analysis of Nine Important Fisheries Resources in the Eastern Adriatic Sea (Mediterranean). Front. Mar. Sci. 2021, 8, 609432. [Google Scholar] [CrossRef]
- Muoneke, M.I.; Childress, W.M. Hooking mortality: A review for recreational fisheries. Rev. Fish. Sci. Aquac. 1994, 2, 123–156. [Google Scholar] [CrossRef]
- Wilde, G.R. Tournament-associated mortality in black bass. Fisheries 1998, 23, 12–22. [Google Scholar] [CrossRef]
- Alos, J.; Palmer, M.; Grau, A.M.; Deudero, S. Effects of hook size and barbless hooks on hooking injury, catch per unit effort, and fish size in a mixed-species recreational fishery in the western Mediterranean Sea. ICES J. Mar. Sci. 2008, 65, 899–905. [Google Scholar] [CrossRef][Green Version]
- Broadhurst, M.K.; Gray, C.A.; Reid, D.D.; Wooden, M.E.L.; Young, D.J.; Haddy, J.A.; Damiano, C. Mortality of key fish species released by recreational anglers in an Australian estuary. J. Exp. Mar. Biol. Ecol. 2005, 321, 171–179. [Google Scholar] [CrossRef]
- Carbines, G.D. Large hooks reduce catch-and-release mortality of blue cod, Parapercis colias in the Marlborough Sounds of New Zealand. N. Am. J. Fish. Manag. 1999, 19, 81–85. [Google Scholar] [CrossRef]
- Bacheler, N.M.; Buckel, J.A. Does hook type influence the catch rate, size, and injury of grouper in a North Carolina commercial fishery? Fish. Res. 2004, 69, 303–311. [Google Scholar] [CrossRef]
- Cooke, S.J.; Barthel, B.L.; Suski, C.D.; Siepker, M.J.; Philipp, D.P. Influence of circle hook size on hooking efficiency, injury, and size selectivity of bluegill with comments on circle hook conservation benefits in recreational fisheries. N. Am. J. Fish. Manag. 2005, 25, 211–219. [Google Scholar] [CrossRef]
- Chopin, F.S.; Arimoto, T.; Inoue, Y. A comparison of the stress response and mortality of sea bream Pagrus major captured by hook and line and trammel net. Fish. Res. 1996, 28, 277–289. [Google Scholar] [CrossRef]
- Alos, J.; Palmer, M.; Balle, S.; Grau, A.M.; Morales-Nin, B. Individual growth pattern and variability in Serranus scriba: Bayesian analysis. ICES J. Mar. Sci. 2010, 67, 502–512. [Google Scholar] [CrossRef]
- March, D.; Palmer, M.; Alós, J.; Grau, A.; Cardona, F. Short-term residence, home range size and diel patterns of the painted comber Serranus scriba in a temperate marine reserve. Mar. Ecol. Prog. Ser. 2010, 400, 195–206. [Google Scholar] [CrossRef]
- Beulig, A.; Fowler, J. Fish on prozac: Effect of serotonin reuptake inhibitors on cognition in goldfish. Behav. Neurosci. 2008, 122, 426. [Google Scholar] [CrossRef]
- Amundsen, P.A.; Hernandez, J. Feeding studies take guts–critical review and recommendations of methods for stomach contents analysis in fish. J. Fish Biol. 2019, 95, 1364–1373. [Google Scholar] [CrossRef]
- Baker, R.; Buckland, A.; Sheaves, M. Fish gut content analysis: Robust measures of diet composition. Fish Fish. 2014, 15, 170–177. [Google Scholar] [CrossRef]
- Buckland, A.; Baker, R.; Loneragan, N.; Sheaves, M. Standardising fish stomach content analysis: The importance of prey condition. Fish. Res. 2017, 196, 126–140. [Google Scholar] [CrossRef]
- Amundsen, P.A.; Klemetsen, A. Diet, gastric evacuation rates and food consumption in a stunted population of Arctic charr, Salvelinus alpinus L., in Takvatn, northern Norway. J. Fish Biol. 1988, 33, 697–709. [Google Scholar] [CrossRef]
- Greenwell, C.N.; Coulson, P.G.; Tweedley, J.R.; Loneragan, N.R. Regional differences in the feeding of the ambush predator Neosebastes pandus and comparisons of diets in the Scorpaenidae, Triglidae and Platycephalidae. J. Fish Biol. 2018, 93, 95–109. [Google Scholar] [CrossRef]
- Morato, T.; Santos, S.R.; Andrade, P.J. Feeding habits, seasonal and ontogenetic diet shift of blacktail comber, Serranus atricauda (Pisces: Serranidae), from the Azores, Northeastern Atlantic. Fish. Res. 2000, 49, 51–60. [Google Scholar] [CrossRef]
- Gill, A.B. The dynamics of prey choice in fish: The importance of prey size and satiation. J. Fish Biol. 2003, 63, 105–116. [Google Scholar] [CrossRef]
- Mihalitsis, M.; Bellwood, D.R. A morphological and functional basis for maximum prey size in piscivorous fishes. PLoS ONE 2017, 12, e0184679. [Google Scholar] [CrossRef]
- Christensen, B. Predator foraging capabilities and prey antipredator behaviours: Pre-versus postcapture constraints on size-dependent predator-prey interactions. Oikos 1996, 76, 368–380. [Google Scholar] [CrossRef]
- Hoyle, J.A.; Keast, A. The effect of prey morphology and size on handling time in a piscivore, the largemouth bass (Micropterus salmoides). Can. J. Zool. 1987, 65, 1972–1977. [Google Scholar] [CrossRef]
- Reimchen, T.E. Evolutionary attributes of headfirst prey manipulation and swallowing in piscivores. Can. J. Zool. 1991, 69, 2912–2916. [Google Scholar] [CrossRef]
- Price, S.A.; Claverie, T.; Near, T.J.; Wainwright, P.C. Phylogenetic insights into the history and diversification of fishes on reefs. Coral Reefs. 2015, 34, 997–1009. [Google Scholar] [CrossRef]
- Nilsson, P.A.; Brönmark, C. Prey vulnerability to a gape-size limited predator: Behavioural and morphological impacts on northern pike piscivory. Oikos 2000, 88, 539–546. [Google Scholar] [CrossRef]
- Wootton, R.J. Biology of the sticklebacks. Hydrobiology 1978, 63, 434. [Google Scholar] [CrossRef]
- Darcy, G.H.; Maisel, E.; Ogden, J.C. Cleaning preferences of the gobies Gobiosoma evelynae and G. prochilos and the juvenile wrasse Thalassoma bifasciatum. Copeia 1974, 147, 375–379. [Google Scholar] [CrossRef]
- Wicksten, M.K. Behaviour of cleaners and their client fishes at Bonaire, Netherlands Antilles. J. Nat. Hist. 1998, 32, 13–30. [Google Scholar] [CrossRef]
- Soares, M.C.; Bshary, R.; Cardoso, S.C.; Côté, I.M.; Oliveira, R.F. Face your fears: Cleaning gobies inspect predators despite being stressed by them. PLoS ONE 2012, 7, e39781. [Google Scholar] [CrossRef] [PubMed]
- Kruschel, C.; Schultz, S.T. Aggressive predation drives assembly of Adriatic fish communities. Diversity 2020, 12, 130. [Google Scholar] [CrossRef]
- Vandewalle, P.; Casinos, A.; Viladiu, C.; Osse, J. Suction Feeding Strategies of Two Species of Mediterranean Serranidae (Serranus cabrilla and Serranus scriba). Neth. J. Zool. 1999, 49, 81–95. [Google Scholar] [CrossRef]
- Vieira, P.E.; Queiroga, H.; Costa, F.O.; Holdich, D.M. Distribution and species identification in the crustacean isopod genus Dynamene Leach, 1814 along the North East Atlantic-Black Sea axis. ZooKeys 2016, 635, 1–29. [Google Scholar] [CrossRef]
- Fišer, C. Prispevek k poznavanju škarjevk (Tanaidacea: Peracarida: Crustacea) v slovenskem morju. Nat. Slov. 2004, 6, 11–17. (In Slovene) [Google Scholar]
- Karachle, P.K.; Stergiou, K.I. The effect of season and sex on trophic levels of marine fishes. J. Fish Biol. 2008, 72, 1463–1487. [Google Scholar] [CrossRef]
- Politou, C.Y.; Papaconstantinou, C. Feeding ecology of Mediterranean poor cod, Trisopterus minutus capelanus (Lacepede), from the eastern coast of Greece. Fish. Res. 1994, 19, 269–292. [Google Scholar] [CrossRef]
- Tudela, S. Ecosystem effects of fishing in the Mediterranean. FAO Fisheries Department (EP/INT/759/GEF). General fisheries Comission for the Mediterranean Studies and Reviews. 2000, 74, 44. [Google Scholar]
- Karachle, P.K.; Stergiou, K.I. An update on the feeding habits of fish in the Mediterranean Sea (2002–2015). Mediterr. Mar. Sci. 2017, 18, 43–52. [Google Scholar] [CrossRef]
- Lipej, L.; Mavrič, B.; Orlando-Bonaca, M.; Uhan, J.; Makovec, T.; Trkov, D. Raziskave Ribjih Združb v Akvatoriju Krajinskega Parka Strunjan: Zaključno Poročilo 2015; Nacionalni Inštitut za Biologijo: Morska Biološka Postaja: Piran, Slovenija, 2015; p. 32. (In Slovene) [Google Scholar]
- Jennings, S.; Renones, O.; Morales-Nin, B.; Polunin, N.V.C.; Moranta, J.; Coll, J. Spatial variation in the 15N and 13C stable isotope composition of plants, invertebrates and fishes on Mediterranean reefs: Implications for the study of trophic pathways. Mar. Ecol. Progr. Ser. 1997, 146, 109–116. [Google Scholar] [CrossRef]
- Pinnegar, J.K. Why the damselfish Chromis chromis is a key species in the M editerranean rocky littoral—A quantitative perspective. J. Fish Biol. 2018, 92, 851–872. [Google Scholar] [CrossRef]
- Chovanec, A.; Hofer, R.; Schiemer, F. Fish as bioindicators. Trace Met. Contam. Environ. 2003, 6, 639–676. [Google Scholar] [CrossRef]
- Gerhart, A. Bioindicator species and their use in biomonitoring. Env. Mon. 2002, 1, 77–123. [Google Scholar]
- Simpfendorfer, C.A.; Heupel, M.R.; White, W.T.; Dulvy, N.K. The importance of research and public opinion to conservation management of sharks and rays: A synthesis. Mar. Freshw. Res. 2011, 62, 518–527. [Google Scholar] [CrossRef]
- Ferretti, F.; Osio, G.C.; Jenkins, C.J.; Rosenberg, A.A.; Lotze, H.K. Long-term change in a meso-predator community in response to prolonged and heterogeneous human impact. Sci. Rep. 2013, 3, 1057. [Google Scholar] [CrossRef] [PubMed]
- Gül, G.; Demirel, N. Evaluation of the comprehensive feeding strategy and trophic role of overexploited mesopredator species in the Sea of Marmara (northeastern Mediterranean). Estuar. Coast. Shelf Sci. 2021, 259, 107448. [Google Scholar] [CrossRef]
- Gianni, F.; Bartolini, F.; Airoldi, L.; Mangialajo, L. Reduction of herbivorous fish pressure can facilitate focal algal species forestation on artificial structures. Mar. Environ. Res. 2018, 138, 102–109. [Google Scholar] [CrossRef]
- Sergio, F.; Newton, I.; Marchesi, L.; Pedrini, P. Ecologically justified charisma: Preservation of top predators delivers biodiversity conservation. J. Appl. Ecol. 2006, 43, 1049–1055. [Google Scholar] [CrossRef]
- Estes, J.A.; Terborgh, J.; Brashares, S.J.; Power, E.M.; Berger, J.; Bond, J.W.; Carpenter, R.S.; Essington, T.E.; Holt, D.R.; Jackson, B.C.; et al. Trophic downgrading of planet Earth. Science 2011, 333, 301–306. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).