Effects of Alkalinity on the Antioxidant Capacity, Nonspecific Immune Response and Tissue Structure of Chinese Mitten Crab Eriocheir sinensis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crabs and Rearing Conditions
2.2. Sample Preparations
2.3. Biochemical Analysis
2.4. Hepatopancreas Histological Analysis
2.5. Statistical Analysis
3. Results
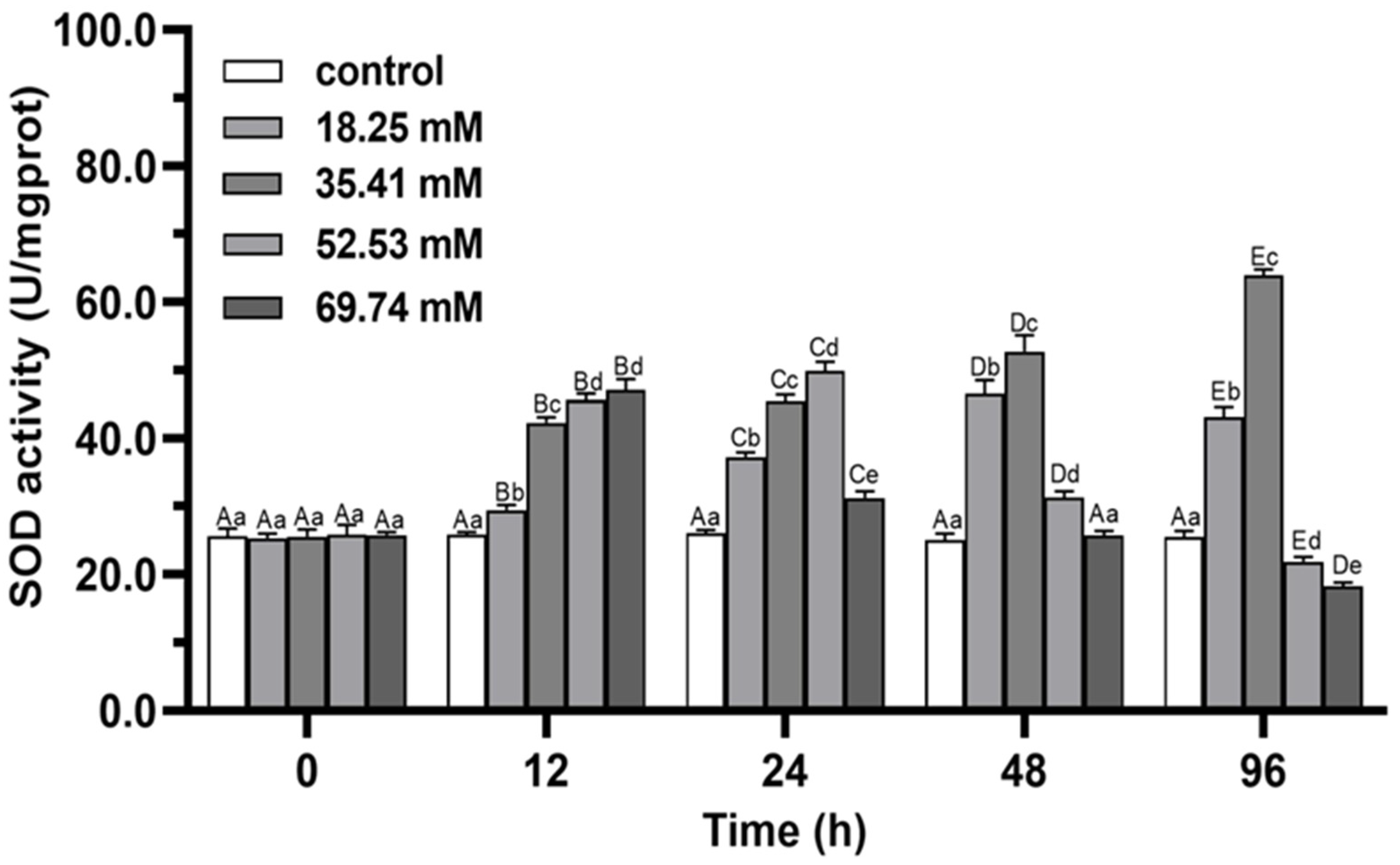
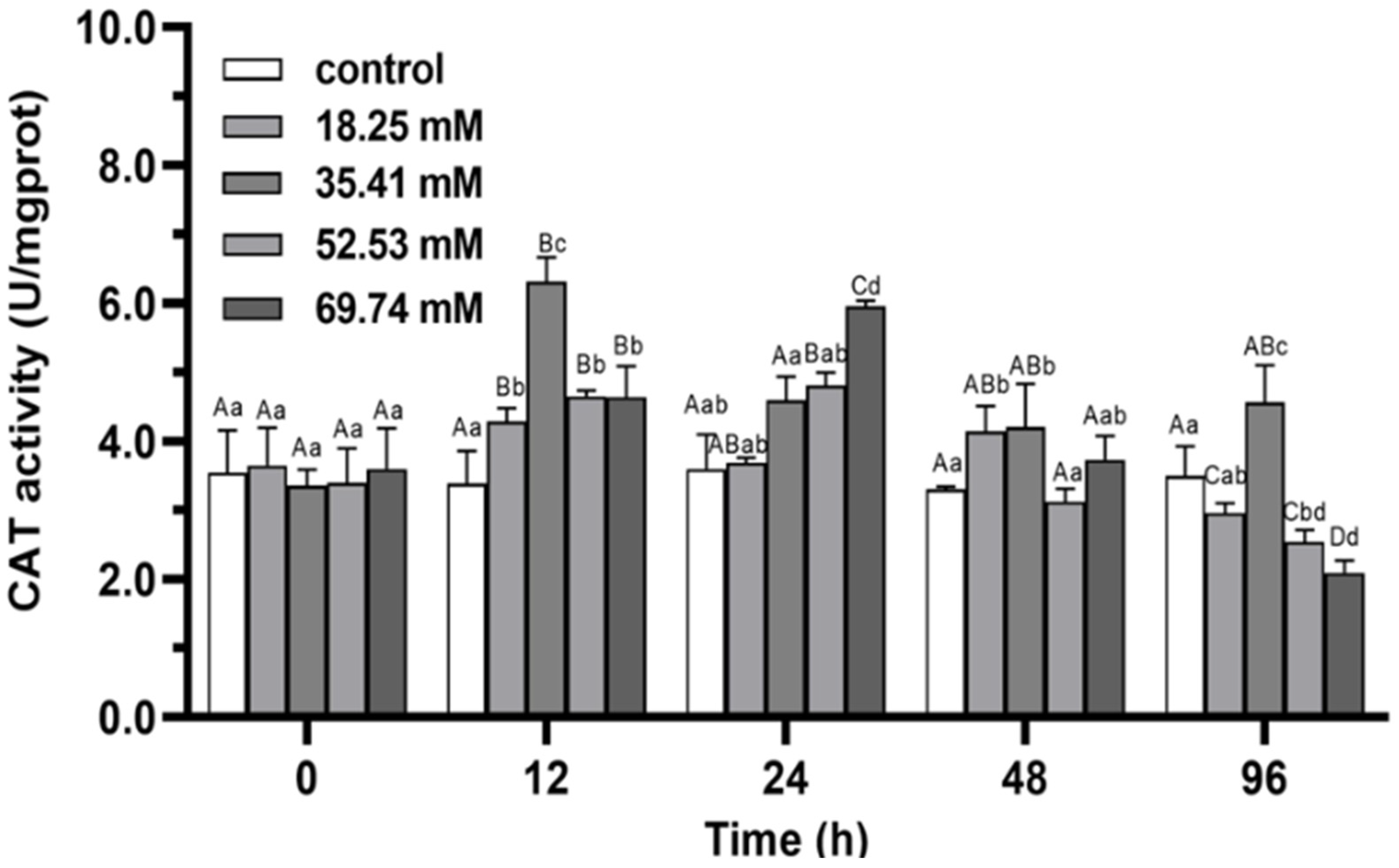
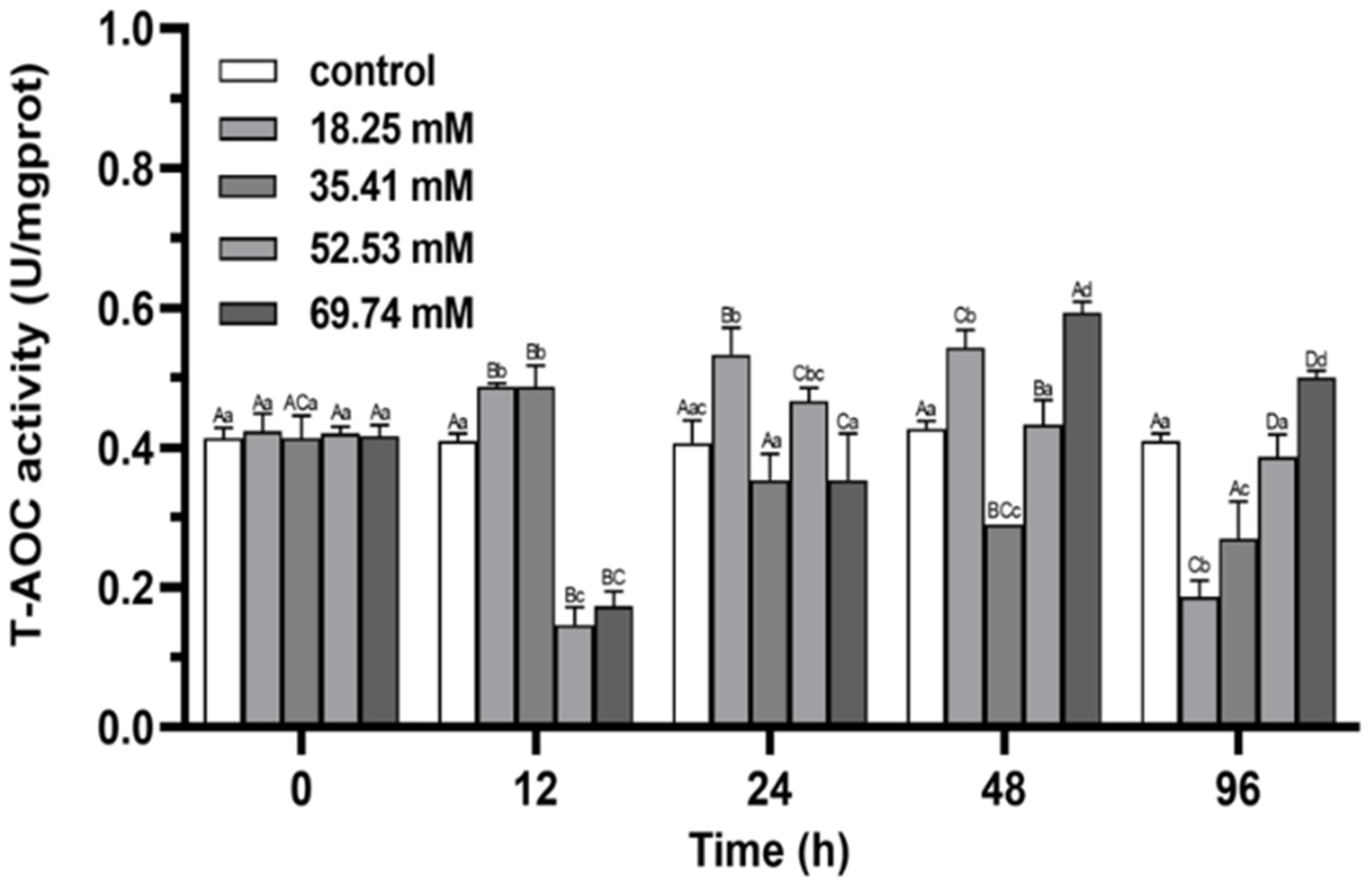
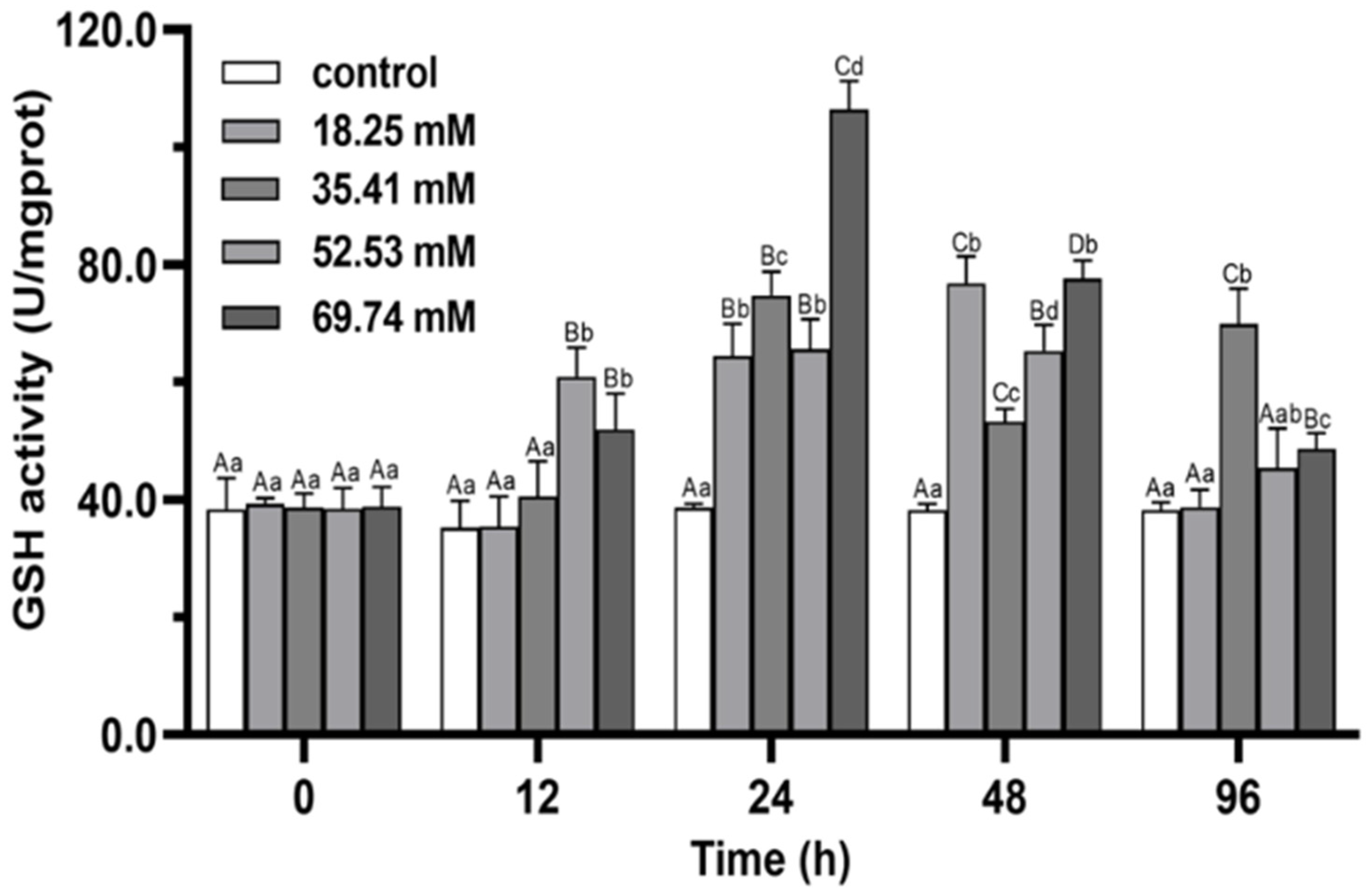
3.1. Antioxidant Enzymes
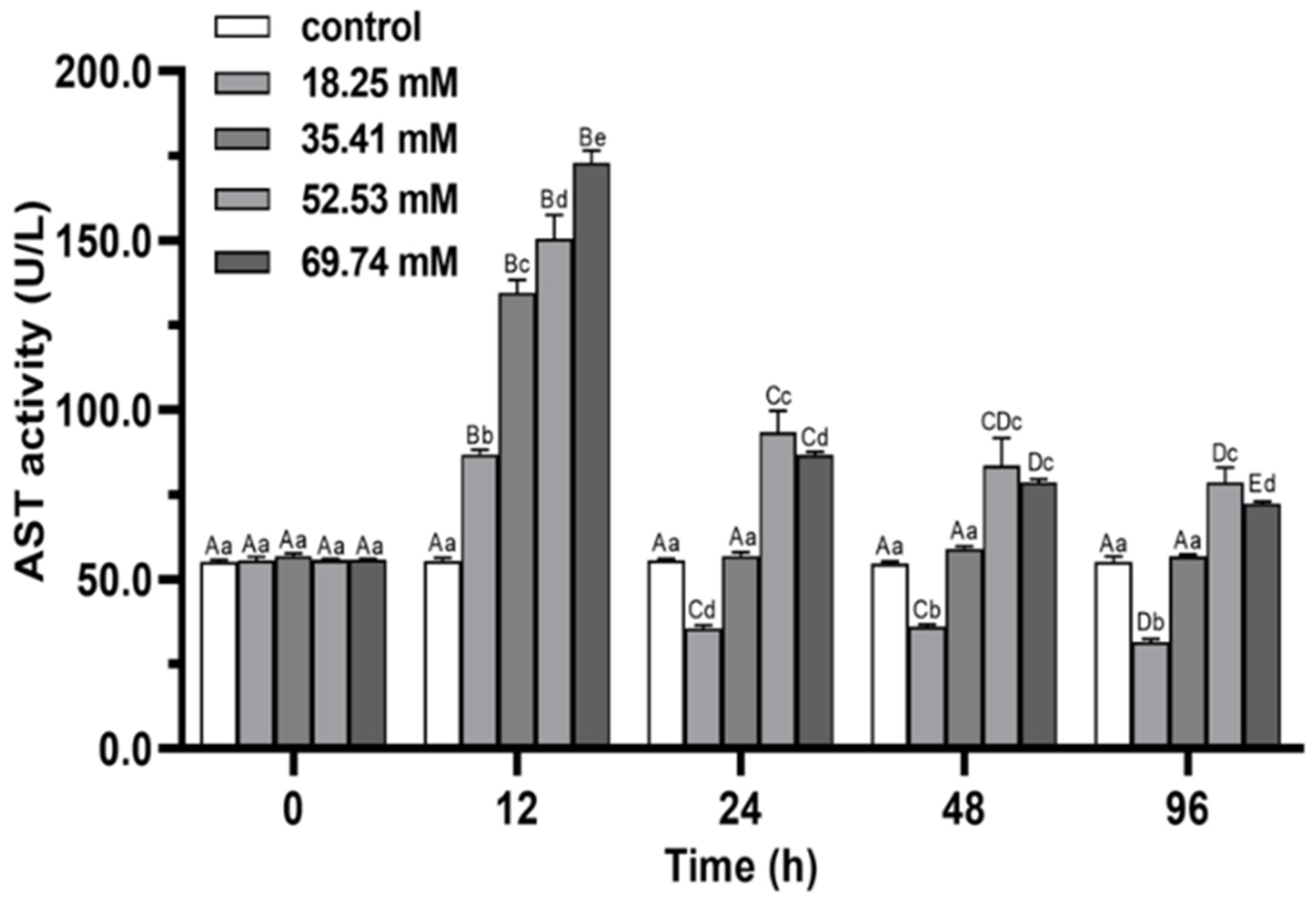
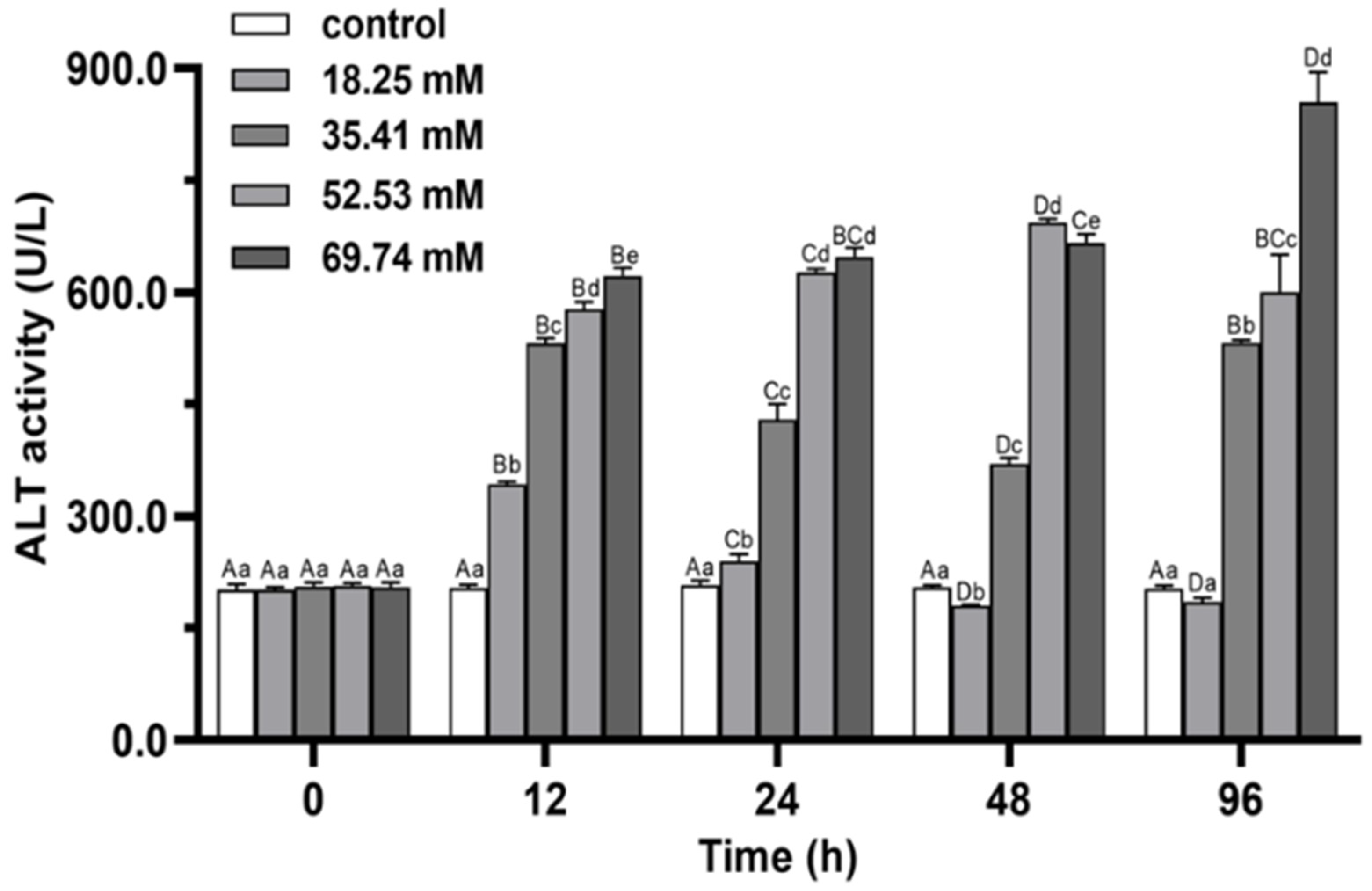
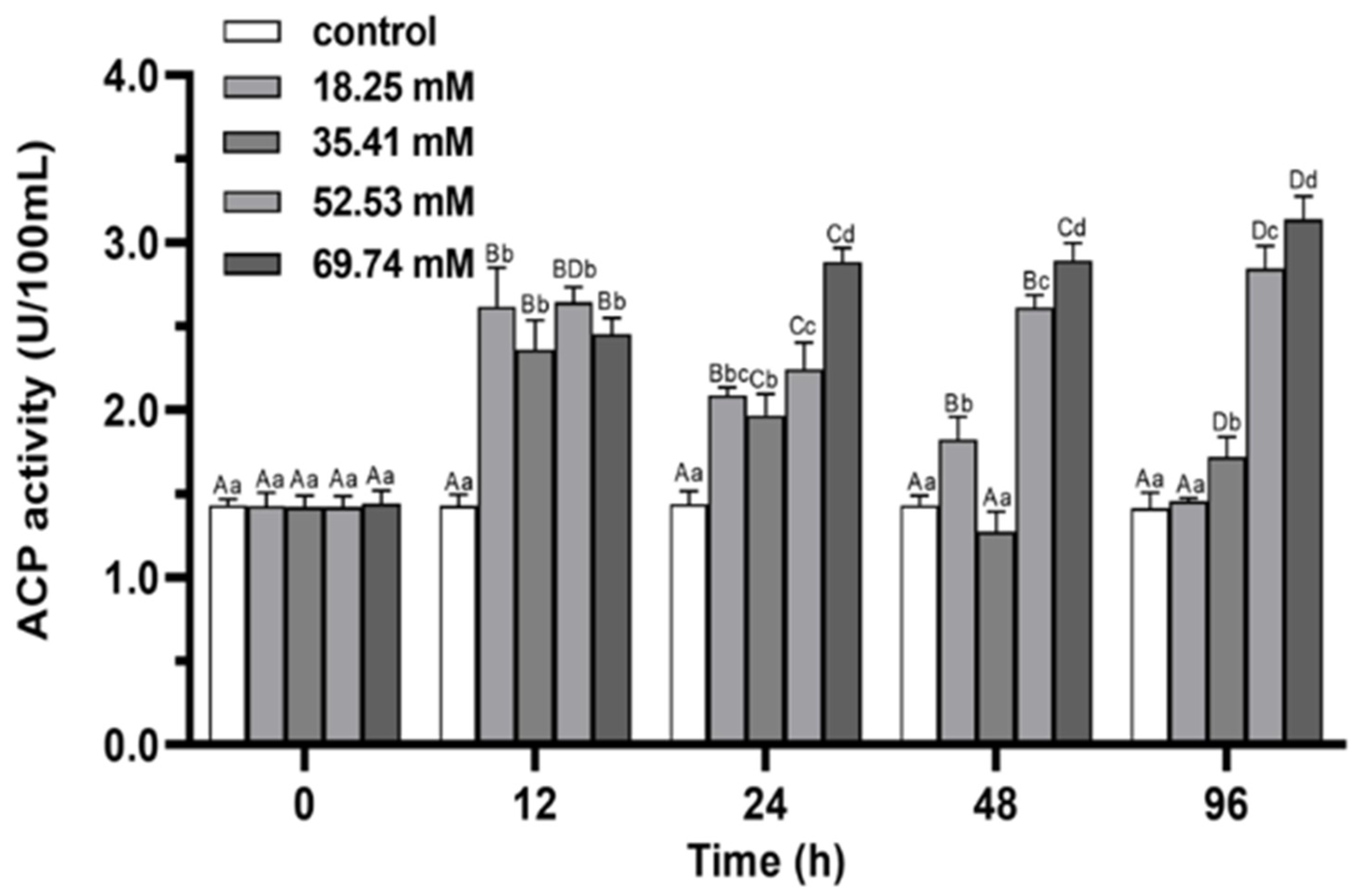
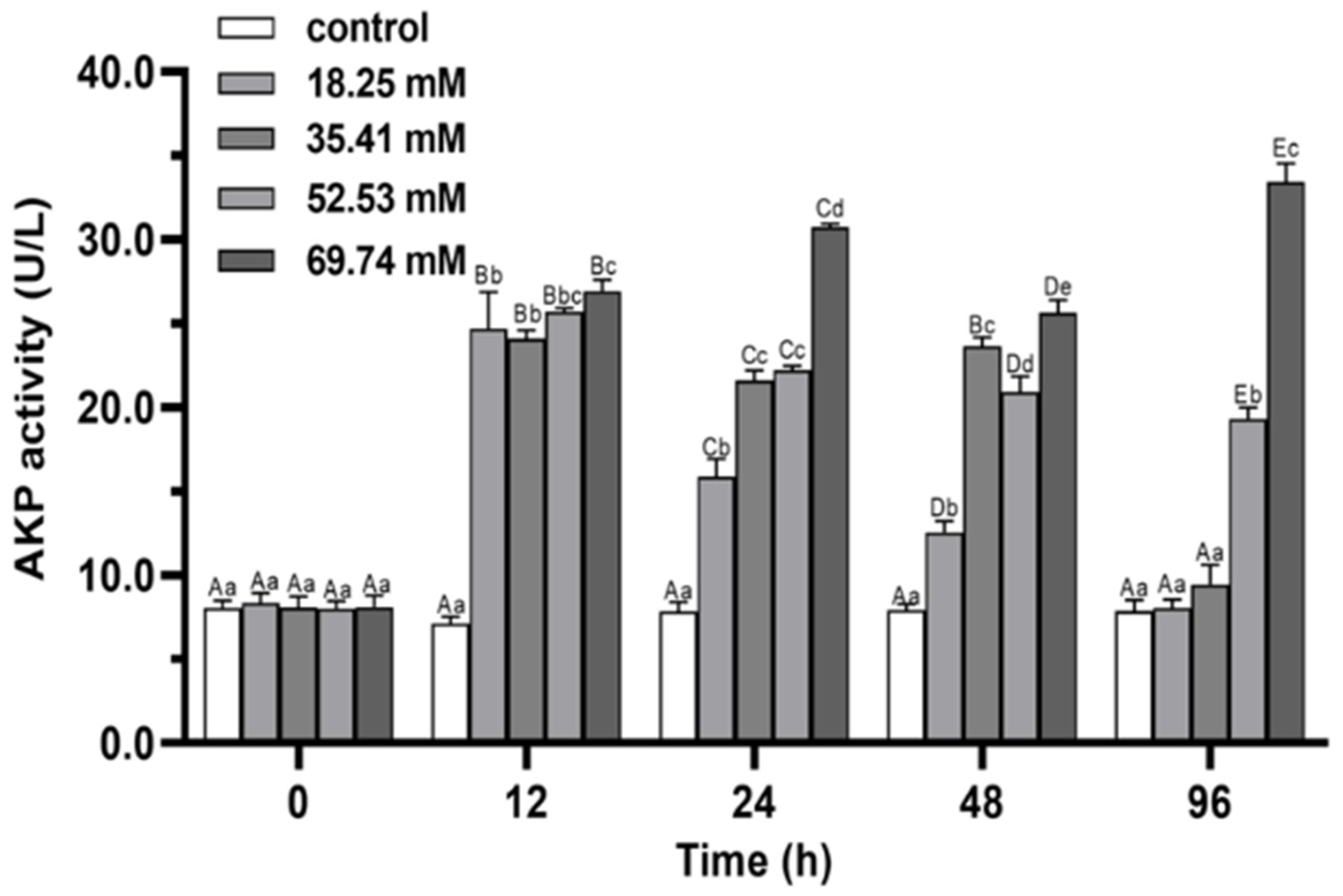
3.2. Immune Enzymes
3.3. Hepatopancreatic Histological Observation
4. Discussion
4.1. Antioxidant Capacity and Non-Specific Immune Functions of E. sinensis under Alkalinity Stress
4.2. Effects of Alkalinity on the Hepatopancreas Structure of E. sinensis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sumner, M.E.; Naidu, R. Sodic Soils: Distribution, Properties, Management, and Environmental Consequences; Oxford University Press: New York, NY, USA, 1998; pp. 1–232. [Google Scholar]
- Shang, X.; Geng, L.; Yang, J.; Zhang, Y.; Xu, W. Transcriptome analysis reveals the mechanism of alkalinity exposure on spleen oxidative stress, inflammation and immune function of Luciobarbus capito. Ecotoxicol. Environ. Saf. 2021, 225, 112748. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.F.; Xu, W.; Geng, L.W.; Bai, Y.Y. A review of effects of saline-alkalinity and pH on growth and development in fish. Chin. J. Fish. 2012, 225, 62–64. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.Y.; Yao, Z.L.; Lai, Q.F.; Yang, P.H.; Wang, H.; Wu, G.X. Advances in saline-alkali adaptation physiology of aquatic animals. J. Yangtze Univ. (Nat. Sci. Ed.) 2015, 12, 44–47. [Google Scholar] [CrossRef]
- Galat, D.L.; Post, G.; Keefe, T.J.; Bouck, G.R. Histological changes in the gill, kidney and liver of Lahontan cutthroat trout, Salmo clarki henshawi, living in lakes of different salinity-alkalinity. J. Fish Biol. 2010, 27, 533–552. [Google Scholar] [CrossRef]
- Wang, H.; Fang, W.H.; Lai, Q.F. Effects of concentrations of Ca2+ and Mg2+ on survival and growth of Penaeus chinensis. J. Fish. Sci. China 2000, 7, 82–86. [Google Scholar] [CrossRef]
- Zhang, L.P.; Xia, J.; Hu, Z.F. Situation and problem analysis of water resources security in China. Resour. Environ. Yangtze Basin 2009, 18, 116–120. [Google Scholar]
- Ondrasek, G.; Rengel, Z. Environmental salinization processes: Detection, implications & solutions. Sci. Total Environ. 2021, 754, 142432. [Google Scholar] [CrossRef]
- Liang, C.F. Tolerance, Growth and Branchal mRNA Expression of Na+/HCO3− Cotransporter and CA of the First Selected Generation of Oreochromis Niloticus in Saline and Alksaline Water; Shanghai Ocean University: Shanghai, China, 2015; pp. 17–23. [Google Scholar]
- Liu, F.; Li, J.; Li, J.T.; Ge, Q.Q.; Ge, H.X.; Shen, M.M. Effects of carbonate alkalinity stress on the survival, growth, reproduction, and immune enzyme activities of Exopalaemon carinicauda. J. Fish. Sci. China 2016, 23, 1137–1147. [Google Scholar] [CrossRef]
- Yang, F.Y.; Li, X.J.; Zhao, C.S.; Chen, Y.; Yang, X.Q.; Sun, L.M. Factors influencing the growth of Litopenaeus vannamei in carbonate alkaline waters of northeast China. J. Hydroecol. 2007, 27, 42–46. [Google Scholar]
- Lin, T.T.; Lai, Q.F.; Yao, Z.L.; Lu, J.X.; Zhou, K.; Wang, H. Combined effects of carbonate alkalinity and pH on survival, growth and haemocyte parameters of the Venus clam Cyclina sinensis. Fish Shellfish Immunol. 2013, 35, 525–531. [Google Scholar] [CrossRef]
- Zeng, F.Y.; Luo, K.; Luan, S.; Cao, B.X.; Lu, X.; Tan, J. Analysis of growth and survival among different families of Litopenaeu vannamei in the chloride typed alkaline water. J. Fish. Sci. China 2018, 25, 308–315. [Google Scholar] [CrossRef]
- Zheng, W.G.; Zhang, Z.Q.; Zhang, M.Z. Study on tolerance of Carassius auratus Pengze fingerlings to salinity and alkalinity. J. Jimei Univ. (Nat. Sci.) 2004, 2, 127–130. [Google Scholar] [CrossRef]
- Qi, T.T.; Liu, J.; Zhao, P.S.; Ge, B.M.; Liu, Q.N.; Jiang, S.H.; Wang, Z.; Zhang, H.; Tang, B.; Ding, G.; et al. A novel modulation of physiological regulation in cultured Chinese mitten crab (Eriocheir japonica sinensis) in response to consistent salinity changes. Gene 2020, 756, 144914. [Google Scholar] [CrossRef]
- Wang, X.D.; Huang, Z.P.; Wang, C.L.; Qi, C.L.; Gu, Z.M.; Li, E.C.; Qin, J.G.; Chen, L.Q. A comparative study on growth and metabolism of Eriocheir sinensis juveniles under chronically low and high pH stress. Front. Physiol. 2020, 11, 885. [Google Scholar] [CrossRef]
- Qiang, J.; Duan, X.J.; Zhu, C.K.; He, J.; Bao, J.W.; Tao, Y.F.; Zhu, H.J.; Xu, P. Selenium-cultured potamogeton maackianus in the diet can alleviate oxidative stress and immune suppression in Chinese mitten crab (Eriocheir sinensis) under copper exposure. Front. Physiol. 2020, 11, 713. [Google Scholar] [CrossRef]
- Zhao, X.J.; Yang, Z.G.; Cheng, Y.X. Effects of cadmium alone and in combination with pH on bioaccumulation, tissue structure, and enzyme activity of the Chinese mitten crab, Eriocheir sinensis. Comp. Biochem. Physiol. C 2021, 245, 109025. [Google Scholar] [CrossRef]
- Wang, T.Y.; Yang, C.; Zhang, T.T.; Liang, H.L.; Ma, Y.C.; Wu, Z.X.; Sun, W.T. Immune defense, detoxification, and metabolic changes in juvenile Eriocheir sinensis exposed to acute ammonia. Aquat. Toxicol. 2021, 240, 105989. [Google Scholar] [CrossRef]
- Yang, Y.H.; Li, M.S.; Luo, L.; Wang, S.H.; Zhang, R.; Guo, K.; Liu, J.Y.; Li, H.T.; Zhao, Z.G. Study on toxicity of salinity and alkalinity on Eriocheir sinensis. J. Northeast Agric. Univ. 2022, 53, 36–41. [Google Scholar] [CrossRef]
- Wang, H.; Lai, Q.; Fang, W.; Wang, J. Study on Aquaculture in Different Type of Saline Alkali Water. In Proceedings of the Forum on Fishery Science and Technology, Guangzhou, China, 22–25 September 2003; p. 303. [Google Scholar]
- Athanasios, V.; Thomais, V.; Manos, D.; Michael, S. Molecular biomarkersof oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol. Environ. Saf. 2006, 64, 178–189. [Google Scholar] [CrossRef]
- Wu, Y.S.; Lee, M.C.; Huang, C.T.; Kung, T.C.; Huang, C.Y.; Nan, F.H. Effects of traditional medical herbs “minor bupleurum decoction” on the non-specific immune responses of white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 2017, 64, 218–225. [Google Scholar] [CrossRef]
- Li, Y.J.; Chai, X.; Wu, H.; Jing, W.X.; Wang, L. The response of metallothionein and malondialdehyde after exclusive and combined Cd/Zn exposure in the crab Sinopotamon henanense. PLoS ONE 2013, 8, e80475. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.L.; Zhou, G.; Pan, J.L.; Li, Y.H.; Lu, Q.P.; Zhou, J.; Li, X.G. Effects of Astragalus polysaccharides on antioxidant abilities and non-specific immune responses of Chinese mitten crab, Eriocheir sinensis. Aquac. Int. 2017, 25, 1333–1343. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, K.; Li, Z.Y.; Chai, X.X.; Fu, X.Y.; Kholodkevich, S.; Kuznetsova, T.; Chen, C.; Ren, N.Q. Effescts of acute diclofenac exposure on intestinal histology, antioxidant defense, and microbiota in freshwater crayfish (Procambarus clarkii). Chemosphere 2021, 263, 128130. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.L.; Chen, L.Q.; Gu, S.Z.; Liu, C.; Long, Z.Q.; Zhang, W. Effects of ammonia exposure on immunity indicators of haemolymph a histological structure of hepatopancreas in Chinese mitten crab (Eriocheir sinensis). J. Fish. Sci. China 2007, 3, 412–418. [Google Scholar] [CrossRef]
- Castex, M.; Lemaire, P.; Wabete, N.; Chim, L. Effect of dietary probiotic Pediococcus acidilacticion antioxidant defences and oxidative stress status of shrimp Litopenaeus stylirostris. Aquaculture 2009, 294, 306–313. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, J.J.; Li, Q.; Jiang, R.L.; Yu, N.; Qin, J.G.; Chen, L.Q. Effects of perfluorooctane sulfonate on the immune responses and expression of immune-related genes in Chinese mitten-handed crab Eriocheir sinensis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 172–173, 13–18. [Google Scholar] [CrossRef]
- Hong, Y.H.; Huang, Y.; Yan, G.G.; Pan, C.; Zhang, J.L. Antioxidative status, immunological responses, and heat shock protein expression in hepatopancreas of Chinese mitten crab, Eriocheir sinensis under the exposure of glyphosate. Fish Shellfish Immunol. 2019, 86, 840–845. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Q.; Pang, Y.Y.; Song, X.Z.; Zhou, N.; Wang, J.; He, L.; Lv, J.H.; Song, Y.M.; Cheng, Y.X.; et al. The protective effects of melatonin on oxidative damage and the immune system of the Chinese mitten crab (Eriocheir sinensis) exposed to deltamethrin. Sci. Total Environ. 2019, 653, 1426–1434. [Google Scholar] [CrossRef]
- Hong, Y.H.; Huang, Y.; Yan, G.W.; Huang, Z.Q. Effects of deltamethrin on the antioxidant defense and heat shock protein expression in Chinese mitten crab, Eriocheir sinensis. Environ. Toxicol. Pharmacol. 2019, 66, 1–6. [Google Scholar] [CrossRef]
- Noguchi, N.; Watanabe, A.; Shi, H. Diverse functions of antioxidants. Free Radic. Res. 2000, 33, 809–817. [Google Scholar] [CrossRef]
- Bagnyukova, T.V.; Storey, K.B.; Lushchak, V.I. Adaptive response of antioxidant enzymes to catalase inhibition by aminotriazole in goldfifish liver and kidney. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2005, 142, 335–341. [Google Scholar] [CrossRef]
- Pan, L.Q.; Wu, Z.W.; Zhang, H.X. Effects of heavy metal ions on transaminases activities of Litopenaeu vannamei. J. Ocean Univ. China 2005, 35, 195–198. [Google Scholar] [CrossRef]
- Dey, S.; Samanta, P.; Pal, S.; Mukherjee, A.K.; Kole, D.; Ghosh, A.R. Integrative assessment of biomarker responses in teleostean fishes exposed to glyphosate-based herbicide (Excel Mera 71). Emerg. Contam. 2016, 2, 191–203. [Google Scholar] [CrossRef]
- Qin, Q.; Qin, S.J.; Wang, L.; Lei, W.W. Immune responses and ultrastructural changes of hemocytes in freshwater crab Sinopotamon henanense exposed to elevated cadmium. Aquat. Toxicol. 2012, 106, 140–146. [Google Scholar] [CrossRef]
- Ajima, M.N.O.; Kumar, K.; Poojary, N.; Pandey, P.K. Oxidative stress biomarkers, biochemical responses and Na+-K+-ATPase activities in Nile tilapia, Oreochromis niloticus exposed to diclofenac. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 240, 108934. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cheng, Z.Z. Effects of the anesthetic MS-222 on the AKP, CAT and ACP activities in goldfish. J. Shanghai Ocean Univ. 2010, 19, 327–332. [Google Scholar]
- Zhao, L.L.; Yang, X.Z.; Cheng, Y.X.; Liang, P.; Zhang, J.B.; Hong, Y.H.; Wang, C.; Yang, Z. Effects of Histamine on Survival and Immune Parameters of the Chinese mitten crab, Eriocheir sinensis. J. Shellfish Res. 2012, 31, 827–834. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Yang, Y.B.; Zhang, Y.L.; Hu, K.; Zeng, L.G.; Liu, L.S.; Yan, Z.J.; Yang, X.L.; Chang, O.Q. Effects of avermectin exposure on oxidative stress and histological structure of hepatopancreas in Eriocheir sinensis. Asian J. Ecotoxicol. 2017, 12, 337–347. [Google Scholar] [CrossRef]
- Zhang, X.J.; Lu, H.D.; Tian, Q.Q.; Jia, X.X.; Ren, F.F. Effects of deltamethrin on histopathology of Chinese mitten crab Eriocheir sinensis. Asian J. Ecotoxicol. 2018, 13, 342–351. [Google Scholar] [CrossRef]
- Tao, Y.F.; Qiang, J.; Wang, H. Acute toxicity of low-pH stress and its effect on enzyme activity and histological structure of gill and hepatopancreas in Procambarus clarkia. J. Fish. Sci. China 2016, 23, 1279–1289. [Google Scholar] [CrossRef]
- Köhler, A.; Lauritzen, B.; Jansen, D.; Böttcher, P.; Tegoliwa, L.; Krüner, G.; Broeg, K. Detection of P-Glycoprotein Mediated MDR/MXR in Carcinus Maenas Hepatopancreas by Immuno-Gold-Silver Labeling. In Proceedings of the Agu Fall Meeting, AGU Fall Meeting Abstracts, San Francisco, CA, USA, 6–10 December 1998; Volume 387, pp. 175–180. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Wang, S.; Zhao, Z.; Luo, L.; Zhang, R.; Guo, K.; Zhang, L.; Yang, Y. Effects of Alkalinity on the Antioxidant Capacity, Nonspecific Immune Response and Tissue Structure of Chinese Mitten Crab Eriocheir sinensis. Fishes 2022, 7, 206. https://doi.org/10.3390/fishes7040206
Li M, Wang S, Zhao Z, Luo L, Zhang R, Guo K, Zhang L, Yang Y. Effects of Alkalinity on the Antioxidant Capacity, Nonspecific Immune Response and Tissue Structure of Chinese Mitten Crab Eriocheir sinensis. Fishes. 2022; 7(4):206. https://doi.org/10.3390/fishes7040206
Chicago/Turabian StyleLi, Mingshuai, Shihui Wang, Zhigang Zhao, Liang Luo, Rui Zhang, Kun Guo, Lanlan Zhang, and Yuhong Yang. 2022. "Effects of Alkalinity on the Antioxidant Capacity, Nonspecific Immune Response and Tissue Structure of Chinese Mitten Crab Eriocheir sinensis" Fishes 7, no. 4: 206. https://doi.org/10.3390/fishes7040206
APA StyleLi, M., Wang, S., Zhao, Z., Luo, L., Zhang, R., Guo, K., Zhang, L., & Yang, Y. (2022). Effects of Alkalinity on the Antioxidant Capacity, Nonspecific Immune Response and Tissue Structure of Chinese Mitten Crab Eriocheir sinensis. Fishes, 7(4), 206. https://doi.org/10.3390/fishes7040206





