Population Genetic Diversity and Differentiation of Mitten Crab, Genus Eriocheir, Based on Microsatellite Markers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction
2.3. Microsatellite Markers and PCR Amplification
2.4. Statistical Analysis
3. Results
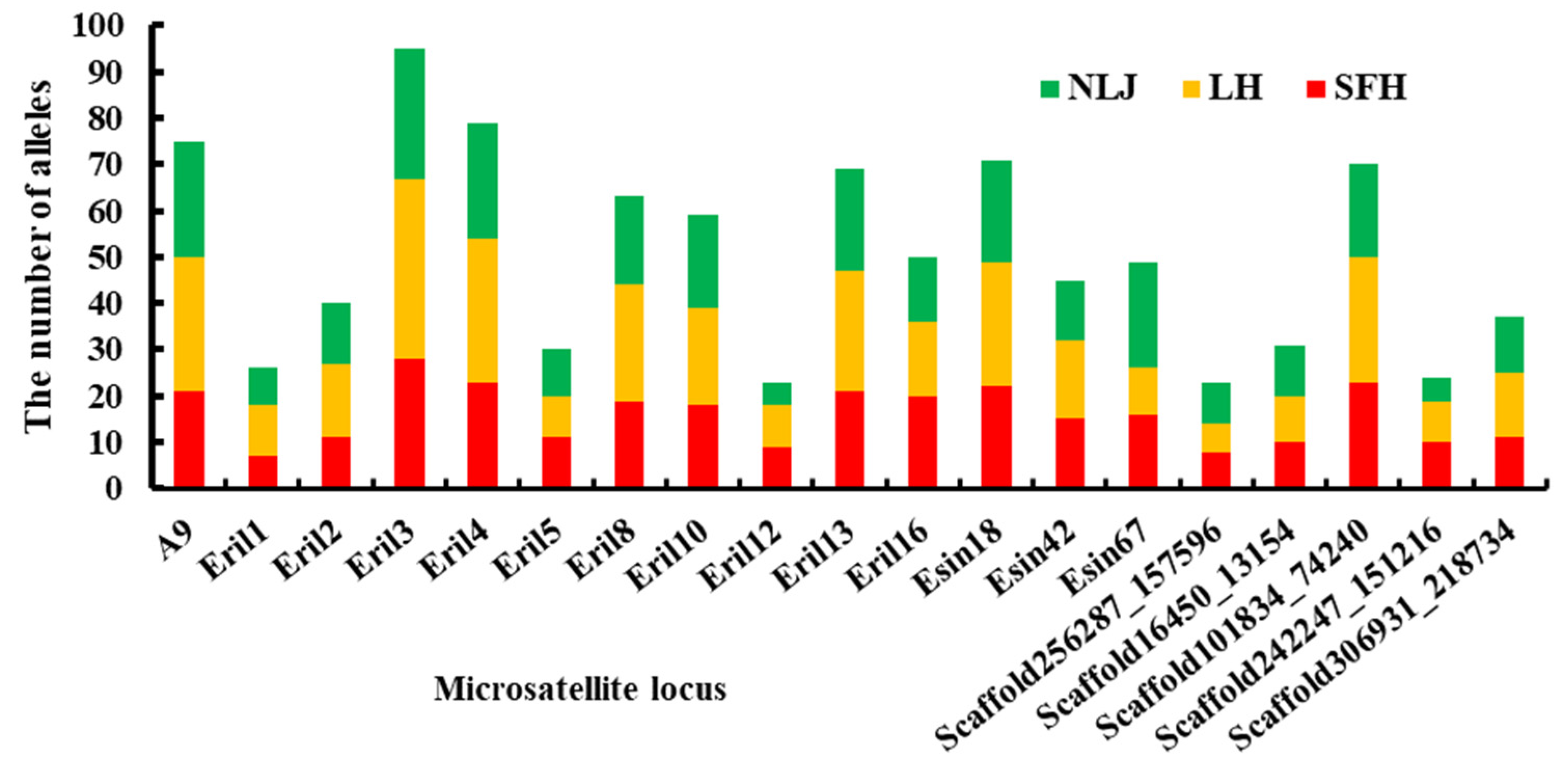
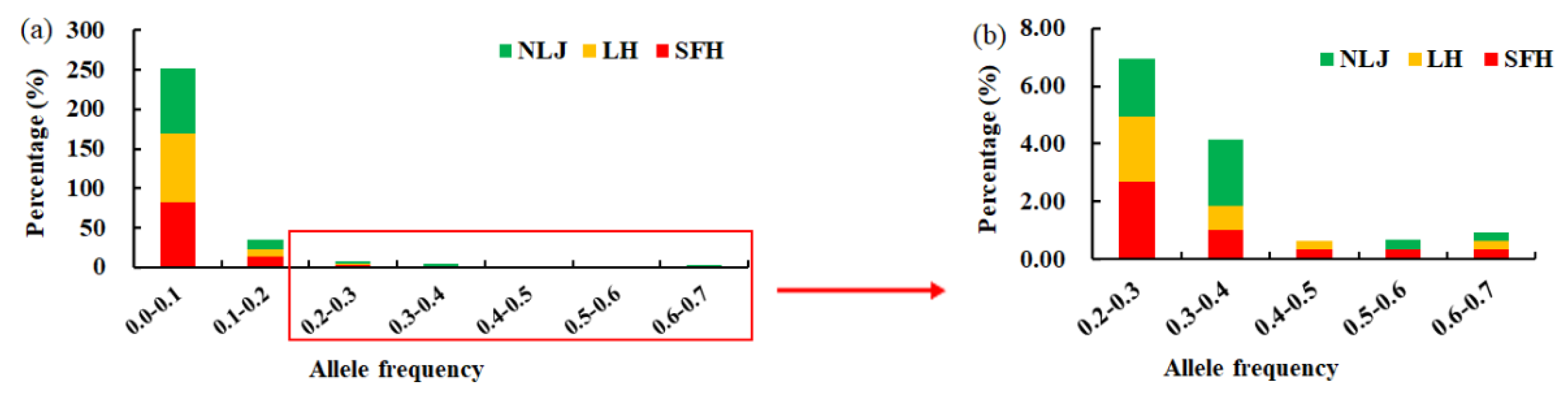
3.1. Genetic Diversity of Microsatellite Loci
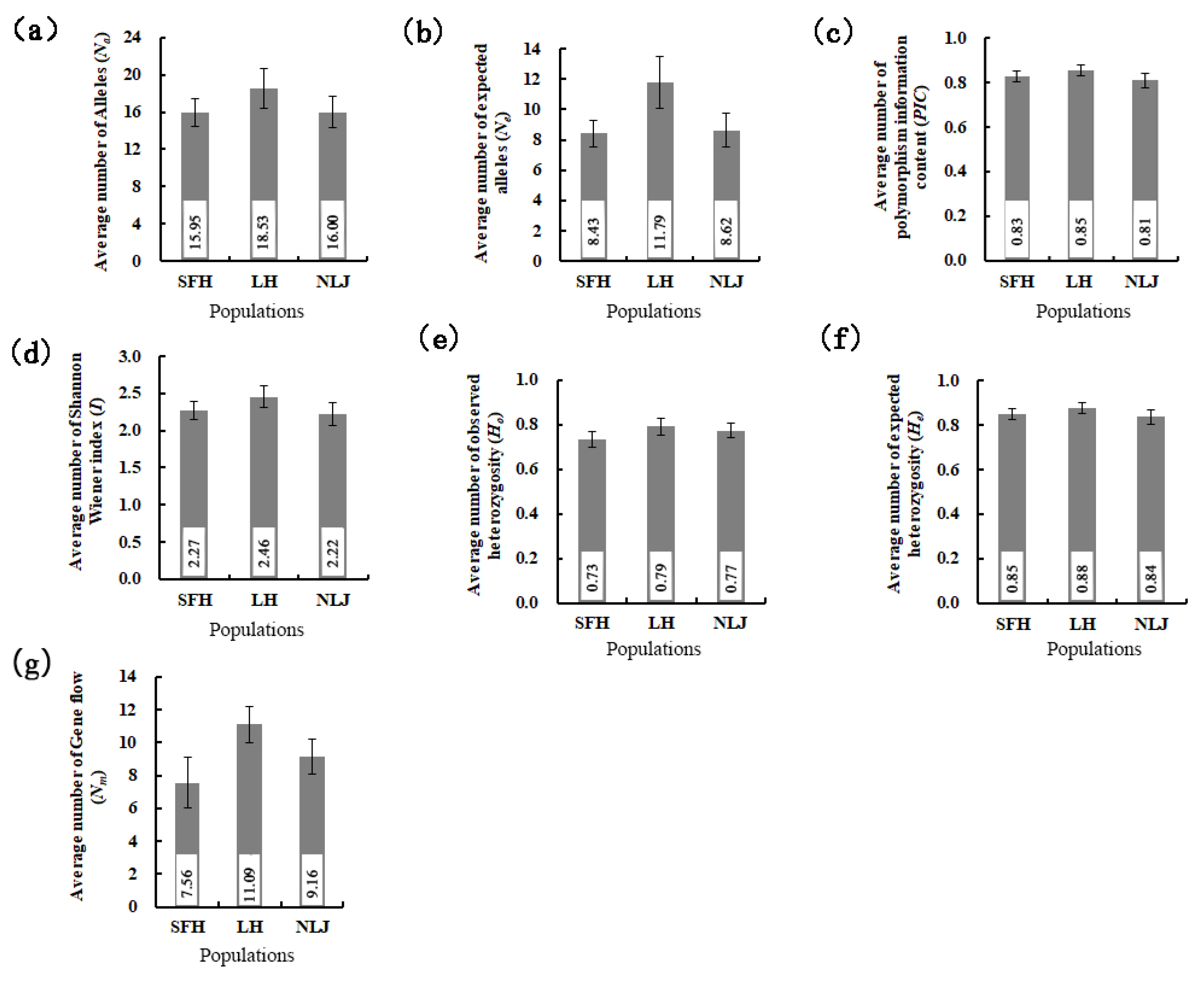
3.2. Genetic Diversity of Eriocheir s. s. Populations
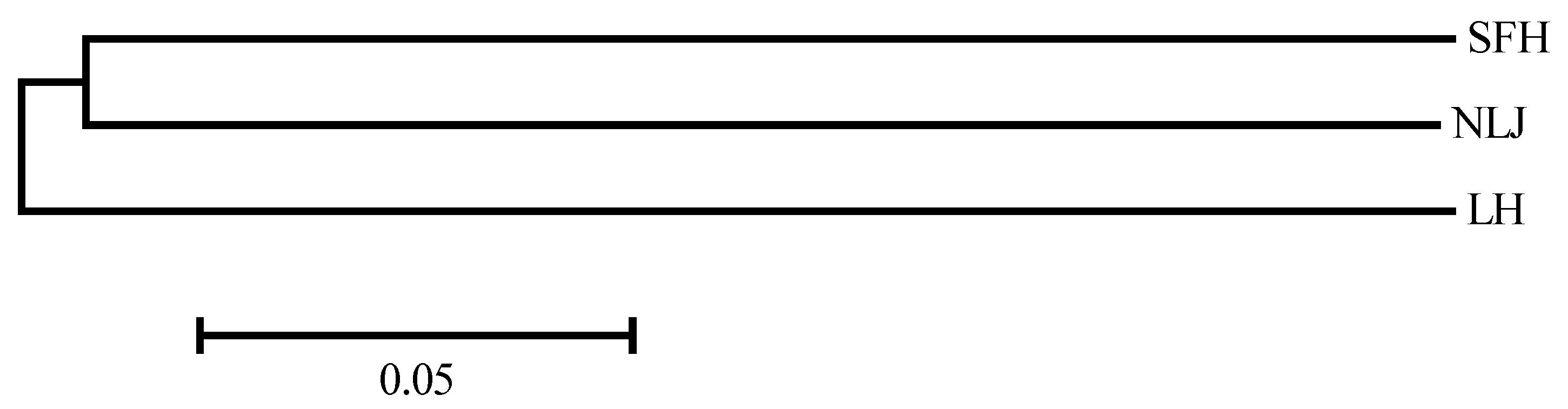
3.3. Genetic Differentiation of Eriocheir s. s. Populations
4. Discussion
4.1. Genetic Diversity
4.2. Genetic Differentiation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, J.W.; Chu, K.H. Genome scan of the mitten crab Eriocheir sensu stricto in East Asia: Population differentiation, hybridization and adaptive speciation. Mol. Phylogenet. Evol. 2012, 64, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Luo, L.; Guo, K.; Zhang, R.; Zhang, X.B.; Kong, L.J.; Zhao, Z.G. Body weight profile and proximate composition of adult Suifenhe Eriocheir sensu stricto. Prog. Fish. Sci. 2022, 43, 61–69. (In Chinese) [Google Scholar] [CrossRef]
- Wu, X.G.; Cheng, Y.X.; Sui, L.Y.; Zeng, C.S.; Southgate, P.C.; Yang, X.Z. Effect of dietary supplementation of phospholipids and highly unsaturated fatty acids on reproductive performance and offspring quality of Chinese mitten crab, Eriocheir sinensis (H. Milne-Edwards), female broodstock. Aquaculture 2007, 273, 602–613. [Google Scholar] [CrossRef]
- Chen, K.; Li, E.C.; Yu, N.; Du, Z.Y.; Chen, L.Q. Biochemical composition and nutritional value analysis of Chinese mitten crab, Eriocheir sinensis, grown in pond. Glo. Adv. Res. J. Agric. Sci. 2013, 2, 164–173. [Google Scholar]
- Bureau of Fisheries; Ministry of Agriculture and Rural Affairs. PRC.; National Fisheries Technology Extension Center; China Society of Fisheries. 2021 China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2021; p. 36. (In Chinese) [Google Scholar]
- Sui, L.Y.; Zhang, F.M.; Wang, X.M.; Bossier, P.; Sorgeloos, P.; Hänfling, B. Genetic diversity and population structure of the Chinese mitten crab Eriocheir sinensis, in its native range. Mar. Biol. 2009, 156, 1573–1583. [Google Scholar] [CrossRef]
- Zhang, L.S.; Zhai, J.J.; Wang, D.D. Ecological and morphological characteristics of populations of Chinese mitten crab (Eriocheir sinensis) from Changjiang, Oujiang and Liaohe Rivers and the quality identification of their young crabs. Fish. Sci. Tech. Info. 2000, 27, 200–205. (In Chinese) [Google Scholar] [CrossRef]
- Wang, S.H.; Zhao, Z.G.; Luo, L.; Zhang, R.; Guo, K.; Xu, W. A Comprehensive study of growth performance of Chinese mitten handed crab (Eriocheir sinensis) and water quality in rice-juveniles co-culture in Heilongjiang Province. Chin. J. Fish. 2022, 35, 86–92. (In Chinese) [Google Scholar]
- Li, Y.S.; Li, S.F.; Xu, G.Y.; Ling, W.H. Comparison of growth performance of Chinese mitten crab (Eriocheir sinensis) in pen culture from the Yangtze and Liaohe river systems. J. Shanghai Fish. Univ. 2000, 9, 189–193. (In Chinese) [Google Scholar] [CrossRef]
- Xu, J.W.; Chan, T.Y.; Tsang, L.M.; Chu, K.H. Phylogeography of the mitten crab Eriocheir sensu stricto in East Asia: Pleistocene isolation, population expansion and secondary contact. Mol. Phylogenet. Evol. 2009, 52, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Wu, L.; Liu, J.S.; Zhang, C.Y.; Lin, M.X.; Wang, J.; Wang, C.H. Morphological differences and genetic admixture in mitten crabs in the Tumenjiang River. J. Fish. Sci. China. 2016, 23, 555–564. (In Chinese) [Google Scholar] [CrossRef]
- Wang, C.H.; Li, C.H.; Li, S.F. Mitochondrial DNA-inferred population structure and demographic history of the mitten crab (Eriocheir sensu stricto) found along the coast of mainland China. Mol. Ecol. 2008, 17, 3515–3527. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, H.; Wu, X.G.; He, J.; Dong, P.S.; Wang, Y.P.; Cheng, Y.X. Genetic variation of wild and cultured populations of chinese mitten crab Eriocheir sinensis from the Yangtze, Huanghe, and Liaohe river basins using microsatellite marker. Oceanol. Limnol. Sin. 2015, 46, 958–968. (In Chinese) [Google Scholar] [CrossRef]
- Li, C.H.; Li, S.F. Phylogenesis of populations of mitten crabs (Eriocheir sinesis, Eriocheir japonicus) in six river systems of mainland China: Morphology discriminant analysis. J. Fish. Sci. China 1999, 23, 337–342. (In Chinese) [Google Scholar]
- Li, S.F.; Zou, S.M. Phylogenesis of populations of mitten crabs (Eriocheir sinesis, Eriocheir japonicus) in six river systems of mainland China: RAPD fingerprinting marker. J. Fish. Sci. China 1999, 23, 325–330. (In Chinese) [Google Scholar]
- Wang, S.H.; Zhang, C.; Shang, M.; Wu, X.G.; Cheng, Y.X. Genetic diversity and population structure of native mitten crab (Eriocheir sensu stricto) by microsatellite markers and mitochondrial COI gene sequence. Gene 2019, 693, 101–113. [Google Scholar] [CrossRef]
- Guo, J.Y.; Ng, N.K.; Dai, A.; Ng, P.K.L. The taxonomy of three commercially important species of mitten crabs of the genus Eriocheir de Hann, 1835 (Crustacea: Decapod: Brachyura: Grapsidae). Raffles Bull. Zool. 1997, 45, 445–476. [Google Scholar]
- Yang, X.; Kong, L.J.; Zhang, Z.H.; Wang, X.Y.; Zhang, X.B. Experiment report on artificial propagation of Suifen River crab. Fish. Eco. Res. 2006, 3, 42–45. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, X.D.; Cheng, Y.X.; Yang, Y.H.; Wu, X.G. Landmark-based morphometric identification of mitten crab Eriocheir from Suifen River. Fish. Sci. 2021, 40, 501–507. (In Chinese) [Google Scholar]
- Xu, J.W. Population Genetics of Mitten Crabs in Eriocheir sensu stricto. Ph.D. Thesis, Chinese University of Hong Kong, Hong Kong, China, 2005. [Google Scholar]
- Ma, H.T.; Chang, Y.M.; Yu, D.M.; Sun, X.W. Microsatellite variations among four populations of Eriocheir sinensis. Zool. Res. 2007, 28, 126–133. [Google Scholar] [CrossRef]
- Xiong, L.W.; Wang, Q.; Qiu, G.F. Large-scale isolation of microsatellites from Chinese mitten crab Eriocheir sinensis via a Solexa genomic survey. Int. J. Mol. Sci. 2012, 13, 16333–16345. (In Chinese) [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Q.; Huang, S.; Mao, H.C.; Zhou, L.; Wang, C.H. Genetic differentiation analysis of the even and odd year populations of Chinese mitten crab. J. Shanghai Ocean Univ. 2013, 22, 657–664. [Google Scholar]
- Xiong, L.W.; Liu, Z.Q.; Yan, Y.L.; Qiu, G.F. The segregation patterns of 37 polymorphism tri-nucleotide microsatellite markers in an F1 family of the Chinese mitten crab (Eriocheir sinensis). J. Fish. China 2015, 39, 174–181. (In Chinese) [Google Scholar] [CrossRef]
- Qiu, G.F.; Xiong, L.W.; Liu, Z.Q.; Yan, Y.L.; Shen, H. A first generation microsatellite-based linkage map of the Chinese mitten crab Eriocheir sinensis and its application in quantitative trait loci (QTL) detection. Aquaculture 2016, 451, 223–231. [Google Scholar] [CrossRef]
- Ma, X.X.; Wang, K.; Guo, W.Z.; Zhang, T.Z. Multiple SSR-PCR techniques and their application in cotton. Mol. Plant Breed. 2007, 5, 648–654. (In Chinese) [Google Scholar] [CrossRef]
- Teng, S.S.; Hu, G.Y.; Fan, J.X.; Chai, X.L.; Xiao, G.Q. Genetic diversity and genetic differentiation analysis of Sinonovacula constricta populations revealed by SNP markers. Acta Hydrobiol. Sin. 2021, 45, 861–870. (In Chinese) [Google Scholar] [CrossRef]
- Wang, S.H.; Luo, L.; Zhang, R.; Guo, K.; Xu, W.; Zhang, X.B.; Zhao, Z.G. Optimization of genetic diversity analysis parameters of mitten handed crab in Suifen River. Chin. J. Fish. 2022. accepted (In Chinese) [Google Scholar]
- Li, Q.Q.; Wu, X.G.; He, J.; Liu, Q.; Cheng, Y.X. Can genetic diversity be maintained during mass selection of the Chinese mitten crab, Eriocheir sinensis? Aquac. Res. 2018, 49, 1606–1615. [Google Scholar] [CrossRef]
- Hänfling, B.; Weetman, D. Characterization of microsatellite loci for the Chinese mitten crab, Eriocheir sinensis. Mol. Ecol. Notes 2003, 3, 15–17. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Shi, Y.H.; Le, G.W. Isolation and characterization of polymorphic microsatellites from Chinese mitten crab, Ericheir sinensis. Mol. Ecol. Notes 2006, 6, 838–839. [Google Scholar] [CrossRef]
- Yan, C.M.; Zhang, Y.B.; Zheng, W.; Sun, X.W.; Chang, Y.M.; Mao, R.X.; Liu, H.J.; Li, Z.Q.; Xiao, Z.G. Genetic relationship in wild carps using microsatellite markers. J. Northeast Agric. Univ. 2011, 42, 102–106. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, Y.; Xu, P.; Lu, C.Y.; Sun, X.W. Genetic analysis of grass carp populations from three major watersheds based on 26 microsatellite markers. J. Fish. Sci. China 2011, 18, 1011–1020. (In Chinese) [Google Scholar] [CrossRef]
- Hu, Y.T.; Jiang, H.; Duan, G.Q.; Zhou, H.X.; Ling, J.; Wang, H. Genetic diversity analysis of Pelteobagrus fulvidraco from two major drainage systems in Anhui Province based on microsatellite markers. South China Fish. Sci. 2020, 16, 33–41. (In Chinese) [Google Scholar] [CrossRef]
- Han, Z.K.; Liu, P.; Li, J.; Gao, B.Q.; Chen, P. SSR analysis of selected populations of inbred Portunus trituberculatus. J. Fish. China 2012, 36, 25–31. (In Chinese) [Google Scholar] [CrossRef]
- Huang, X.S.; Xu, Y.; Hu, X.J.; Xu, W.J.; Su, H.C.; Wen, G.L.; Yang, K.; Cao, Y.C. Genetic diversity analysis of first filial generation of seven introduced Litopenaeus vannamei populations using microsatellite DNA markers. South China Fish. Sci. 2019, 15, 54–62. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, X.C.; Jia, Z.Y.; Li, C.T.; Shi, L.Y. Study on the genetic structure of F3 common carp in the quality breeding population. Genom. Appl. Biol. 2015, 34, 90–95. [Google Scholar]
- Leberg, P.L. Estimating allelic richness: Effects of sample size and bottlenecks. Mol. Ecol. 2002, 11, 2445–2449. [Google Scholar] [CrossRef]
- Quan, Y.C.; Sun, X.W.; Liang, L.Q. Genetic popymorphism of microsatellite DNA in two populations of nothern sheatfish (Silurus soldatovi). Acta Genet. Sin. 2006, 33, 908–916. (In Chinese) [Google Scholar] [CrossRef]
- Tang, L.X.; Xu, Z.Q.; Ge, J.C.; Chen, Y.R.; Zhao, M.Z.; Yang, J.X. Microsatellite analysis on the genetic structure of three breeding stocks of Eriocheir sinensis. J. Nanjing Norm. Univ. Nat. Sci. Ed. 2013, 36, 84–90. (In Chinese) [Google Scholar] [CrossRef]
- Wang, W.; Xu, C.; Zhang, W.B.; Ma, X.Z. Comparative studies on morphological differences among four local populations of mitten crab. Chin. Agric. Sci. Bull. 2007, 23, 648–653. (In Chinese) [Google Scholar] [CrossRef]

| Locus Name | Primer Sequences (5′–3′) | Core Sequence | Temperature | Allele Size Range |
|---|---|---|---|---|
| A9 | F: GCAATGAGACTCAACAGGAGA | (GA)7(GT)7 | 60 | 200–282 |
| R: AATGTGGCTCACCTGACGT | ||||
| Eril1 | F: GATAGACCGTAAATGAGACGGCTG | (GGA)9 | 65 | 151–174 |
| R: GGACGGAGAAAACTAGAGACCACA | ||||
| Eril2 | F: GGATTTACTTAAGTTGGGGCTCGT | (GAG)8 | 65 | 119–161 |
| R: CGACGCAGTTTTGTCTAGAGACCT | ||||
| Eril3 | F: CAGCGAAAAACAGGAAGCATTTAG | (AC)11 | 65 | 140–212 |
| R: GGAAAGGGAAAGTGAAGGATGAAT | ||||
| Eril4 | F: TTCTTTGAGCGACATGCAAAAGT | (TG)29 | 65 | 132–192 |
| R: AGACAGACAGACAAAAACGCTCCT | ||||
| Eril5 | F: TAGGGGGTTTTAGGTGTGGTGATA | (TGA)9 | 65 | 125–167 |
| R: ATTTATGTGGAGGGAATGGGAGAT | ||||
| Eril8 | F: TGTTGAGTGTGATGTTTGTGATGC | (GT)12 | 65 | 160–226 |
| R: TAATAGCGGCCAAACTTTGTTGAT | ||||
| Eril10 | F: TACCTTTTTCAGGGTGAGTGAAGG | (GT)23 | 65 | 138–208 |
| R: AAGGACAGGAGGGAAAATGAGAGT | ||||
| Eril12 | F: ACCCATCTCAAGTCCAGACTCATC | (CCT)8 | 65 | 152–170 |
| R: AGAGGATGCAAGGGAAATAAGGAG | ||||
| Eril13 | F: AAGGAAGGCAGTTAGGAGGGTATG | (AC)11 | 65 | 189–259 |
| R: TTATTATTGTGGCGACGAAGGGT | ||||
| Eril16 | F: TCCTCCCTATGCTCTTTGTAGGTG | (AC)11 | 65 | 150–192 |
| R: AAGGCCCAGGAGTATGGTGAAC | ||||
| Esin18 | F: CACCGTAAGGTTCCGTAA | (GT)26 | 58 | 170–225 |
| R: AAGCACCCATAAGTCAATGTA | ||||
| Esin42 | F: GCACCGCAGTGATAATGTAGTGG | (AC)19 | 53 | 235–275 |
| R: GATCCTCGTGTGGGCGTGCTTAC | ||||
| Esin67 | F: TTTGGGATTCACCTTGTCAACTT | (GT)11 | 53 | 105–170 |
| R: CGACGCACGACAGAGGAGAGG | ||||
| Scaffold256287_157596 | F: CACGAAAGGGTAAAAGGGGTACAT | (AGG)9 | 63 | 102–126 |
| R: TCTTCTGTTTCATCCTCGTCTTCC | ||||
| Scaffold16450_13154 | F: TATTTTCTCCCCCTCCATCCTTTA | (GGA)8 | 55 | 147–189 |
| R: GCATCGCCTACACCGTTTATTATC | ||||
| Scaffold387247_202848 | F: TCCTCCTCCTCATCCTCATCA | (AG)40 | 55 | 134–173 |
| R: CTCGTGACTGGAAAATGCGAC | ||||
| Scaffold101834_74240 | F: TGTTCCTCTTGTTCTGTTTTGTTCC | (CTA)10 | 53 | 112–235 |
| R: GGTTGCTTAATGGTGGCTTATGAA | ||||
| Scaffold242247_151216 | F: TCCATAACAGTCCTCTGAGTTCCC | (CTC)8 | 56 | 133–175 |
| R: AGGAGAGAGTGTTGGCATGGTAAG | ||||
| Scaffold306931_218734 | F: CAATTTGCGCTAAATAGATGTCCC | (AGT)9 | 63 | 235–301 |
| R: CCTCGAAGTTTGGGGTTAAAGGTA |
| Locus | Na | Ne | Ho | He | I | PIC | FIS | FIT | FST | Nm | EWTN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A9 | 34.00 | 20.34 | 0.85 | 0.95 | 3.20 | 0.95 | 0.08 | 0.10 | 0.02 | 12.01 | 0.09 |
| Eril1 | 12.00 | 3.98 | 0.74 ** | 0.75 | 1.71 | 0.71 | −0.01 | 0.02 | 0.03 | 7.97 | 0.28 |
| Eril2 | 17.00 | 6.98 | 0.54 ** | 0.86 | 2.32 | 0.85 | 0.34 | 0.37 | 0.04 | 6.38 | 0.20 |
| Eril3 | 46.00 | 26.62 | 0.99 ** | 0.97 | 3.49 | 0.96 | −0.04 | −0.02 | 0.02 | 14.30 | 0.06 |
| Eril4 | 39.00 | 23.52 | 0.69 ** | 0.96 | 3.35 | 0.96 | 0.26 | 0.27 | 0.02 | 12.27 | 0.08 |
| Eril5 | 12.00 | 3.15 | 0.69 ** | 0.68 | 1.63 | 0.66 | −0.05 | −0.01 | 0.04 | 5.98 | 0.29 |
| Eril8 | 28.00 | 17.82 | 0.84 ** | 0.95 | 3.09 | 0.94 | 0.08 | 0.11 | 0.03 | 7.23 | 0.12 |
| Eril10 | 26.00 | 15.38 | 0.91 | 0.94 | 2.91 | 0.93 | 0.01 | 0.03 | 0.02 | 10.05 | 0.13 |
| Eril12 | 10.00 | 5.20 | 0.73 ** | 0.81 | 1.86 | 0.78 | 0.07 | 0.09 | 0.02 | 10.97 | 0.34 |
| Eril13 | 29.00 | 17.53 | 0.94 | 0.95 | 3.04 | 0.94 | −0.02 | 0.01 | 0.03 | 9.61 | 0.11 |
| Eril16 | 24.00 | 7.17 | 0.78 ** | 0.86 | 2.44 | 0.85 | 0.06 | 0.09 | 0.03 | 9.18 | 0.14 |
| Esin18 | 29.00 | 18.56 | 0.68 ** | 0.95 | 3.07 | 0.94 | 0.26 | 0.28 | 0.02 | 16.15 | 0.11 |
| Esin42 | 18.00 | 10.34 | 0.86 | 0.91 | 2.52 | 0.90 | 0.02 | 0.05 | 0.03 | 8.16 | 0.19 |
| Esin67 | 25.00 | 12.98 | 0.83 ** | 0.93 | 2.79 | 0.92 | 0.06 | 0.10 | 0.04 | 6.42 | 0.13 |
| Scaffold256287_157596 | 9.00 | 4.61 | 0.63 ** | 0.79 | 1.75 | 0.76 | 0.19 | 0.20 | 0.01 | 20.80 | 0.36 |
| Scaffold16450_13154 | 12.00 | 6.78 | 0.80 | 0.86 | 2.07 | 0.84 | 0.04 | 0.06 | 0.02 | 10.97 | 0.28 |
| Scaffold101834_74240 | 34.00 | 19.67 | 0.84 ** | 0.95 | 3.20 | 0.95 | 0.09 | 0.12 | 0.03 | 7.83 | 0.09 |
| Scaffold242247_151216 | 12.00 | 3.73 | 0.46 ** | 0.73 | 1.62 | 0.69 | 0.23 | 0.37 | 0.19 | 1.08 | 0.28 |
| Scaffold306931_218734 | 18.00 | 7.70 | 0.75 | 0.87 | 2.31 | 0.86 | 0.11 | 0.13 | 0.03 | 9.73 | 0.19 |
| Average | 22.84 | 12.21 | 0.77 | 0.88 | 2.55 | 0.86 | 0.09 | 0.12 | 0.03 | 7.31 | 0.18 |
| Populations | SFH | LH | NLJ |
|---|---|---|---|
| SFH | —— | 0.0352 ** | 0.0409 ** |
| LH | 0.3310 | —— | 0.0385 ** |
| NLJ | 0.3144 | 0.3292 | —— |
| Source of Variation | d. f. | Sum of Squares | Variance Components | Percentage of Variation (%) | FST | p |
|---|---|---|---|---|---|---|
| Among populations | 2 | 77.080 | 0.31807 Va | 3.82 | 0.03822 | 0.00000 |
| Within populations | 285 | 2281.438 | 8.00504 Vb | 96.18 | ||
| Total | 287 | 2358.517 | 8.32312 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Luo, L.; Zhang, R.; Guo, K.; Xu, W.; Zhao, Z. Population Genetic Diversity and Differentiation of Mitten Crab, Genus Eriocheir, Based on Microsatellite Markers. Fishes 2022, 7, 182. https://doi.org/10.3390/fishes7040182
Wang S, Luo L, Zhang R, Guo K, Xu W, Zhao Z. Population Genetic Diversity and Differentiation of Mitten Crab, Genus Eriocheir, Based on Microsatellite Markers. Fishes. 2022; 7(4):182. https://doi.org/10.3390/fishes7040182
Chicago/Turabian StyleWang, Shihui, Liang Luo, Rui Zhang, Kun Guo, Wei Xu, and Zhigang Zhao. 2022. "Population Genetic Diversity and Differentiation of Mitten Crab, Genus Eriocheir, Based on Microsatellite Markers" Fishes 7, no. 4: 182. https://doi.org/10.3390/fishes7040182
APA StyleWang, S., Luo, L., Zhang, R., Guo, K., Xu, W., & Zhao, Z. (2022). Population Genetic Diversity and Differentiation of Mitten Crab, Genus Eriocheir, Based on Microsatellite Markers. Fishes, 7(4), 182. https://doi.org/10.3390/fishes7040182






