Abstract
A total of 756 yellowfin tuna (Thunnus albacares) caught by a Chinese drifting longliner in the tropical western and central Pacific Ocean (WCPO) from May 2018 to March 2019 were investigated to describe the reproductive biology of the species. Generalized linear model and polytomous logistic regression for the ordinal response model were employed to assess the effects of biometric and spatiotemporal factors (such as individual fork length (FL), fishing depth, dissolved oxygen, and month) on the reproductive traits of yellowfin tuna. The results showed that FLs ranged from 87 to 163 cm, averaging 115.8 cm (SD = ±14.2) for females and 121.8 cm (SD = ±16.8) for males. The proportion of males in the sampled fish was 0.61 (SD = ±0.29), and larger males (>130 cm) were proportionally predominant. Analyses based on the monthly variation of the gonadosomatic index and monthly proportion of sexual maturity stages of the gonads showed that the main spawning period of yellowfin tuna lasts from September to December. In addition, the 50% first maturity FLs of males and females were 111.96 cm (SD = ±1.04) and 119.64 cm (SD = ±1.30), respectively. This study provides new information on the reproductive development of T. albacares in the tropical WCPO region. These reproductive parameters reduce uncertainty in current stock assessment models, which will ultimately assist the fishery in becoming sustainable for future generations.
1. Introduction
The yellowfin tuna (Thunnus albacares) is a highly migratory fish that inhabits tropical and subtropical waters and mainly feeds on pelagic fish, cephalopods, and crustaceans [1]. After skipjack tuna (Katsuwonus pelamis), yellowfin tuna is the second most targeted species for the world tuna production [2]. From 1960 to 2019, the global fishing output of yellowfin tuna has increased from less than 550,000 tons to more than 1.5 million tons [3]. Being the sea area with the highest tuna production of all oceans, the western and central Pacific Ocean (WCPO) is under the jurisdiction of tuna regional fishery management organizations (RFMOs). One of these is the Western and Central Pacific Fisheries Management Committee (WCPFC), which conducts yellowfin tuna stock assessments across the regions [2]. The primary goal of tuna RFMOs is the maintenance of the stocks of both tuna and tuna-like species at levels enabling maximum sustainable yield. To achieve this overarching objective, management and conservation strategies typically include regulations such as catch quotas, time area closures, and fishing capacity limits, many of which rely on annual or multi-annual stock assessments. The assessment of yellowfin tuna conducted by the WCPFC in 2021 showed that the spawning potential of yellowfin tuna has stabilized over the past decade, showing a small increase in the mid to late 2010s [4]. However, the estimated spawning potential had decreased noticeably in the previous decades [2].
Understanding the reproductive biology of tunas and quantifying size-specific parameters provides the means for accurately predicting the effects of fishing on the reproductive potential of stock [5]. Reproductive parameters of yellowfin tuna, including sex ratio [6], size at first maturity [7], temporal and spatial distributions of spawning [8], fecundity [9], spawning frequency [10,11], spawning season [12], and occurrence and duration of spawning [13] had been assessed in different regions of the Atlantic space [14] and the Indian [6] and the Pacific Oceans [8]. In the WCPO, although several studies have provided considerable information on different aspects of the reproductive biology of yellowfin tuna [8,15,16,17], increasing fishing pressure [8] and the influence of environmental factors [5,18] (such as temperature and dissolved oxygen concentration) on reproductive biology have resulted in data that remain incomplete and inconsistent. Therefore, to improve the assessment and management of yellowfin tuna stocks in the tropical WCPO region, it is necessary to increase the quality and quantity of basic reproductive data used to estimate these reproductive parameters.
The aims of this study were to: (1) analyze reproductive characteristics such as sex ratio, gonadosomatic index, and sexual maturity, as well as (2) explore the influencing factors of these parameters. The results provide important data for improving management strategies on yellowfin tuna in the WCPO region.
2. Materials and Methods
2.1. Sampling Collection
Samples of yellowfin tuna were caught by an ultra-low temperature tuna longline fishing vessel with an overall length of 49.9 m and a gross tonnage of 560 t. The sampling campaign complied with the management requirements regarding fishing gear and fishing methods issued by the WCPFC and related organizations. Drift longline fishing was used with a distance between the main lines of 1200 m, the interval between the main lines of 40 m, and hooked branch lines (29 branch lines in total between the two buoys). The length of the buoy rope was 35.0 m and the length of the branch line was 25.0 m. Mackerels (Pneumatophorus japonicus) and sardines (Sardine sp.) were used as bait. It is well-known that the FL composition of tunas sampled by baited hooks differs from the FL composition of population because of the size-selectivity of hooks or baits [19] and the difference in feeding behavior with different sizes [20]. However, due to the difficulties in sampling oceanic species, most of the research on the reproductive biology of tunas, including yellowfin tuna, bigeye tuna, etc., depends on samples from the commercial fishing gear, such as drift longline [21,22,23] and purse seine [7].
During the daily operation of the fishing vessel, among the 29 branches of a main line, depth thermometers (DST centi-TD) were attached around the hooks of the 1st (i.e., the shallowest), 5th, 11th, and 15th (i.e., the deepest) branch lines to collect information on the operating depth and water temperature where hooks were deployed.
Based on the catenary theory [24], combined with the actual measured water depth of the fishing hook, the operating depth and corresponding temperature of other branch lines and fishing hooks were estimated. Because the depth of the hook varies with soaking time, the average water depth over the entire soaking time after the hook had completely settled was assumed as catch water depth. Dissolved oxygen data corresponding to the catch water depth was obtained from the Copernicus Marine Environmental Monitoring Service website http://marine.copernicus.eu (accessed on 20 November 2021).
2.2. Sample Collection
The samples were collected from May 2018 to March 2019, during a whole fishing trip of 267 days (244 effective sampling days) in the high seas of the tropical WCPO (2°03′ S to 11°17′ S, 163°14′ E to 173°35′ E, as shown in Figure 1). The number of hooks cast daily was 2684 (±290), and the hooking time ranged from 04:00 to 11:00 daily.
Figure 1.
Tropical western and central Pacific Ocean showing sampling locations of T. albacares (blue closed circles).
2.3. Data Analysis
All specimens were analyzed, and capture time, buoy number, and hook position were recorded and linked to the sample. Fish sampling included fork length (FL cm), eviscerated weight (EW, kg), sex, gonad weight (GW, g), and gonad maturity grade. Because it is not easy to measure total weight for each tuna, especially when the hooking ratio is high, the eviscerated weight of tuna recorded after being gilled and gutted is used as an alternative measurement more frequently and popularly in commercial longlining fisheries and research on biology of tunas [21,22,23,25]. The sexual maturity grade of fish was assessed according to Holden and Raitt [26]. For each individual, the developmental stage of the gonads was determined as: (I) undeveloped; (II) early developing; (III) later developing; (IV) mature; and (V) spawned.
The catches of yellowfin tuna of different sexes were counted in groups of 10 cm FL. The FL composition by sex and month, and the proportion of males in yellowfin tuna were analyzed. The Student’s t test was used to test for significant differences in FLs between sexes.
The FL–EW relationship was fitted using the power function as follows [25]:
where m and n are constants. EW is the eviscerated weight (kg) and FL is the individual fork length (cm).
The gonadosomatic index (GSI) was calculated according to Nootmorn [27]:
where GW is the wet mass of the gonads (g).
Individuals at sexual maturity stage III and above were recorded as mature [28]. A logistic curve is used to represent the probability PL (binomial distribution) of an individual to be sexually mature by length class [6]. The calculation formula is expressed as follows:
where a and b are the parameters of the logistic curve, and the 50% sexual maturity FL50 (i.e., the FL corresponding to individuals with a 50% probability of reaching sexual maturity) is calculated as follows:
A generalized linear model (GLM) with logit distribution was used to fit the logistic curve and to obtain the curve parameters and . The standard deviation of FL50 was estimated via the Delta method.
GLM was also used to explore the relationship between GSI, as well as biological factors and temporal factors. Circular statistical methods [29,30] were used to quantify monthly variation in the GSI value, and the following model was established:
where α0 represents the model intercept, α1 and α2 represent the regression coefficients of FL and the square of FL, represents the impact of the fishing month on GSI (where t represents the month), ω represents the angular frequency, and ε represents error item. The calculation formula is expressed as follows:
The polytomous logistic regression for the ordinal response model [31] was used to analyze the influence of temporal and biological factors on the sexual maturity level. A multivariate ordered logistic regression model was established as follows:
where Y represents the maturity level of sampled individuals (i represents the number of response variable categories); β0j represents the model intercept, and j = I − 1; β1, β2, and β3 represent coefficient of the FL, fishing depth (D), and dissolved oxygen (DO), respectively, and ɛ represents the error item. represents the monthly effect on the response variable.
The GLM model was fitted using the “glmer” function of the “lme4” package and the polytomous logistic regression for ordinal response model was fitted using the “polr” function of the “MASS” package in R-4.1.2 [32].
3. Results
3.1. Biometric Information
A total of 756 yellowfin tuna samples were obtained by a Chinese drifting longliner vessel, 423 of which were male, 321 were female, and 12 were indetermined. FL for male and female fish was 121.8 cm (SD = ±16.8 cm, range 89 to 163 cm) and 115.8 cm (SD = ±14.2 cm, range 87 to 152 cm), respectively. The proportion of males in the sampled fish was 0.61 (SD = ±0.29), showing a significant difference from the expected 1:1 ration (p < 0.01). Figure 2a shows the distribution of FLs for each sex. Male proportion noticeably increased from smaller to bigger FLs, and the percentage of males reached 0.73 when the FL was around 130 cm. The results of Student’s t tests showed that there was a significant difference in mean FL between male and female yellowfin tuna (p < 0.01). Regarding the monthly evolution of the sex ratio, the proportion of males in December 2018 was below 0.5, while in other months the male proportion was significantly higher than that of females, with a peak in October (0.77) (Figure 2b).
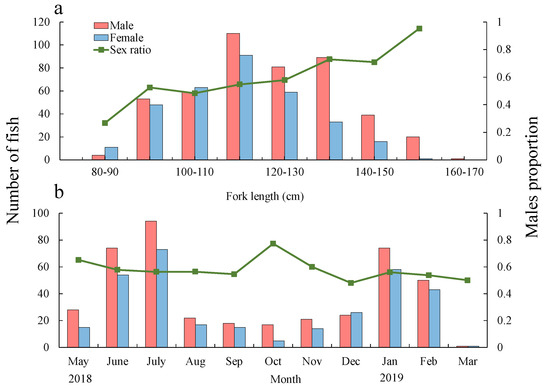
Figure 2.
Fork length (FL) composition columns by sex (a) and month (b) and the proportion of males (line) in T. albacares.
The relationship between the overall, the male, and the female individual FL and eviscerated weight (EW) is as follows (Figure 3): EW = 1.919 × 10−5 FL2.932 (R2 = 0.929), EW = 1.397 × 10−5 FL2.999 (R2 = 0.926), and EW = 3.475 × 10−5 FL2.806 (R2 = 0.926), respectively.
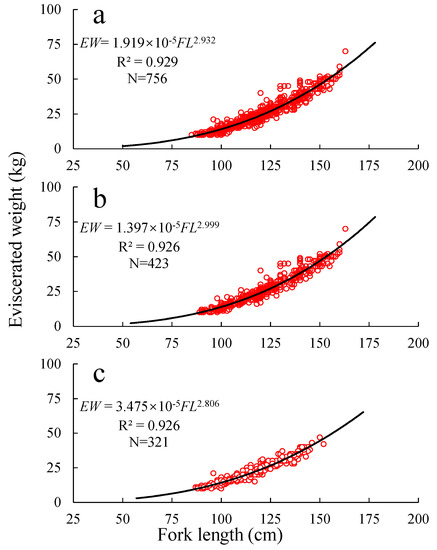
Figure 3.
Relationship between FL (mean = 118.87 cm) and EW (mean = 24.67 kg) of T. albacares ((a) both sexes; (b) male; (c) female).
3.2. Spawning Period
In females, the peak of ln(GSI + 1) occurs between 140 and 150 cm FL; the relationship between female ln(GSI + 1) and the FL was approximately linear, and the GSI of different sexes differed significantly (male: ln(GSI + 1) = 0.0386FL − 0.000122FL2 − 2.430; female: ln(GSI + 1) = 0.0187FL − 0.0000322FL2 − 1.162, Figure 4a,b). The results of the GLM showed that the relationship between the GSI of male individuals and FL and the quadratic power of FL was significant (p < 0.001), while the GSI of female individuals was significant and positively correlated with FL (p = 0.0328 < 0.05, Table 1). Significant differences in GSI among months were found for both sexes (Table 1, p < 0.05). Monthly variation showed that mean GSI values of females were significantly higher from September to December and peaked in September. For males, the overall annual variation was similar to that of females, although the peak was in November (Figure 4c,d). Thus, the main spawning period of female yellowfin tuna was between September and December.
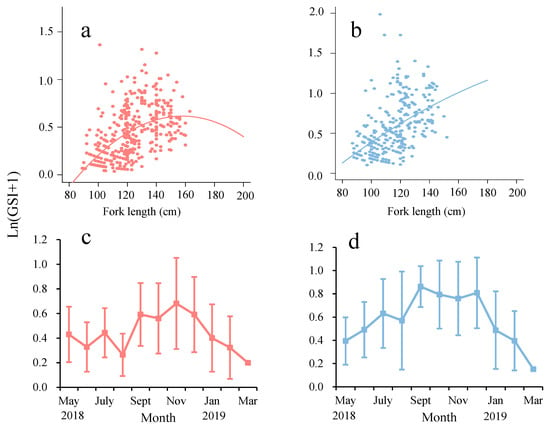
Figure 4.
ln(GSI + 1) by FL and month ((a,c) males; (b,d) females). Error bars represent the standard deviations.

Table 1.
Outputs of generalized linear model (GLM) fitting for gonadosomatic index (GSI) with biological and temporal factors. Significant p-values are formatted in bold (<0.05).
With increasing FL, the proportion of highly mature stages in yellowfin tuna gradually increased (Figure 5a,b). In the same FL group, the proportion of gonadal mature individuals was higher in females ( > 0 according to the multivariate ordinal logistic model; p < 0.001, Table 2).
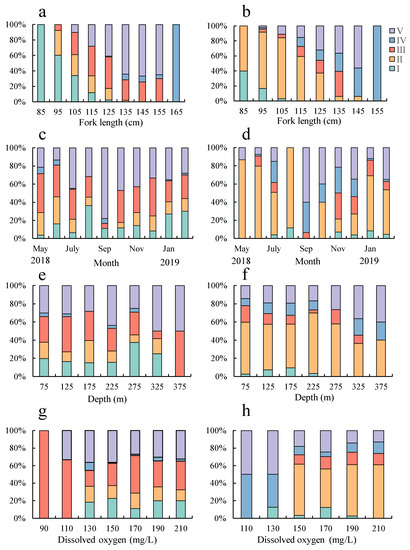
Figure 5.
Proportions of the sexual maturity stages found in T. albacares ((a) male, FL; (b) female, FL; (c) male, month; (d) female, month; (e) male, water depth; (f) female, water depth; (g) male, dissolved oxygen; (h) female, dissolved oxygen).

Table 2.
Fitting results of multiple ordered logistic model for sexual maturity grade and influencing factors. Significant p-values are formatted in bold (<0.05).
The monthly proportions of gonadal development stages for both sexes based on mature individuals is illustrated in Figure 5c,d. For both sexes, the peak of mature and spawned gonads appeared in September, and later developing gonads mainly occurred in November and December. The spawning of female yellowfin tuna peaked in November (in the multivariate ordinal logistic model, male: sine p = 0.027, cosine p < 0.001; female: sine p = 0.073 < 0.1, cosine p < 0.001, Table 2).
The proportions of female and male gonad maturity by depth are shown in Figure 5e,f. The proportion of individual gonad maturity of yellowfin tuna varied significantly between different water layers, and the proportion of high sexual maturity grades increased with increasing water depth ( > 0 in the multivariate ordered logistic model; p < 0.05, Table 2).
As shown in Figure 5g,h, the proportion of individual gonad maturity of yellowfin tuna varied significantly between different dissolved oxygen. Over 80% of the mature and spawned gonads for females and males occurred in 150–190 mg/L dissolved oxygen, the peak of mature and spawned was 190 mg/L for males and 150 mg/L for females (p = 0.051 for males; p = 0.095 for females in the multivariate ordered logistic model, with marginal significance, Table 2).
3.3. Size at Sexual Maturity
The observed minimum length at sexual maturity was around 96 cm for female and 95 cm for male, respectively. The sexual maturity curve (logistic curve) shows that the FL50 for males and females was estimated at 111.96 cm (SD = ±1.04) and 119.64 cm (SD = ±1.30), respectively (Figure 6).
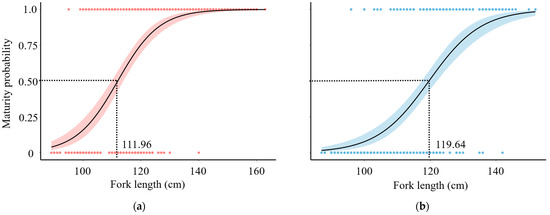
Figure 6.
Maturity ogive of (a) male and (b) female T. albacares. The shaded parts represent 95% confidence interval. The horizontal dotted line represents the proportion at which 50% of the population of T. albacares is mature and the vertical dotted line shows the corresponding FL.
4. Discussion
The present study provides useful information for assessors and managers about some reproductive traits of T. albacares. These reproductive parameters will reduce uncertainty in current stock assessment models, which will ultimately assist the fishery industry to become sustainable for future generations.
Stergiou [33] pointed out that under a lower fishing pressure, the growth rate of fish weight increased, expressed by greater values of the power index n. In comparison with other studies on FL–EW relationships, the n values in this study are slightly lower than those published for the Atlantic Ocean at 3.140 and 2.812 [21], and those published for the Indian Ocean at 3.197 and 3.244 [25], respectively. This could reflect an increment of the current fishing pressure for yellowfin tuna in the WCPO, which would be consistent with the latest assessment of the WCPFC stock results [2].
In FL lower than 110 cm, the proportion of males and females was balanced, while males became dominant in the sample at around an FL of 130 cm. The results of this study are similar to those obtained by Vincent et al. [34]. The positive correlation found here between FL and male predominance could be related to the higher mortality rates of mature females due to the physiological stresses related to spawning [35,36]. An alternative explanation may be a combination of this and different growth rates between males and females at older ages [37]. These differences in growth between male and female yellowfin tunas make the population assessment challenging and should be incorporated to obtain more accurate assessments.
The GSI is an important indicator to describe the reproductive traits and season of fish stocks worldwide [6]. Zhu et al. [6] suggested that yellowfin tuna undergo rapid development of gonads at an early age. When this age is exceeded, males and females start to have relatively different development speeds. Stéquert et al. [38] also found that in the western Indian Ocean, the GSI of male tuna is lower than that of females at the same age. This suggests that the ovaries of females develop faster and are heavier with higher GSI values than in males. This is similar to the results reported by Chen et al. [39] for yellowfin tuna in the south Pacific Ocean and by McPherson [10] for yellowfin tuna in the WCPO region.
Results of the monthly evolution of the GSI were consistent with research results for the western and central Indian Ocean [6], for the western Indian Ocean [24], for the south Pacific Ocean [40], for the western Pacific Ocean [41], and for the eastern Atlantic Ocean [14]. The GSI is higher at the end and beginning of the year and seasonal variations are evident. GSI is related to hormonal changes and environmental conditions (such as sea surface temperature, photoperiod, and monsoon) [38]. The reason for the seasonal reproductive peak may be that the northern monsoon weather in tropical waters affects yellowfin tuna. During this season, their reproductive activity is low [38] as low sea surface temperatures limit productivity and food availability, thereby inhibiting the reproductive activity of yellowfin tuna [8,12]. Furthermore, even a sudden small drop in sea surface temperature triggers a stress physiological response that reduces reproductive activity in yellowfin tuna [42]. In contrast to the asynchronous breeding peaks between male and female bigeye tuna, the monthly trend between male and female yellowfin tuna is consistent, as reported by Zudaire et al. [7] for the Indian Ocean. It is worth noting that the female GSI fitting curve is steeper than that of males, which further explains the earlier and faster development in female gonads compared with those in males.
In this study, the sexual maturity of yellowfin tuna was studied using macroscopic classification. Macroscopical classification of mature stages may lead to misclassification of females as immature after spawning or in the resting state, or a misclassification of immature females as mature [43]. Consequently, it could lead to errors in the assessment of maturity and spawning season, thereby introducing uncertainty into assessments of maturity and reproductive potential [44]. The proportion of mature stages increased with FL (i.e., the regression coefficient β1 > 0 in the polytomous logistic regression for ordinal response model has a significant impact, Table 2). In tropical waters, yellowfin tuna reproduce year round but show seasonal reproductive peaks in the waters of the WCPO [8,45]. The results obtained in the present study show that the seasons with higher reproductive activity are winter and spring, which is consistent with the findings for the Indian Ocean [7], for the Atlantic Ocean [21], and for the WCPO [8]. Longhurst [46] suggested that, driven by the strongly reversed monsoon weather in the tropics, seasonal changes affect both eutrophic and oligotrophic biological systems in the waters, which in turn result in monthly changes in reproductive activity. Cole [47] suggested that the upwelling generated near oceanic islands or along the frontal boundary between the equatorial north–south current and the equatorial countercurrent leads to changes in the local ocean area.
The depth distribution of fish and environmental factors of suitable habitats are of great significance in ecological research on pelagic fish [48]. The study showed that female yellowfin tuna preferred deeper waters at maturity, while the effect of dissolved oxygen on gonad maturity was not significant. Therefore, the dissolved oxygen factor alone does not seem to play a key role in the gonad maturity of yellowfin tuna, but, rather, multiple factors interact, thus affecting gonad maturity. The dissolved oxygen in the Pacific Ocean is lower than in other oceans [49]. As a visual and opportunistic predator, tuna can heat their blood by vascular countercurrent heat exchangers, which greatly decreases oxygen affinity [50,51]. Their high tolerance to low to moderate dissolved oxygen levels allows them to dive into deeper waters for baiting [8,52]. Schaefer [5] pointed out that female tuna need more energy to reproduce than males. Therefore, females would attain deeper waters where they can obtain more food to support the storage of energy required for reproduction.
The size and age at which 50% (FL50) of a population of tunas reaches maturity and are thus capable of reproducing are important life history parameters used in fishery management [12]. In this study, the FL50 for yellowfin tuna was estimated at 111.96 cm (male) and 119.64 cm (female), which is smaller than the 120–129 cm FL reported by Yuen [53] for the Central Pacific. However, they are bigger than the 92 cm FL reported by Schaefer [12] for the eastern Pacific Ocean, the 104.95 and 109.69 cm FL reported by Nootmorn et al. [54] for the eastern Indian Ocean, and the 97–101 cm FL reported by Costa et al. [21] or the 99.2 cm FL reported by Diaha et al. [14] both for the Atlantic Ocean. This difference could be explained by the differences in latitudes and marine environments; the higher the latitude, the lower the sea surface temperature. High sustainable swimming speeds enable tunas to travel quickly between food patches and to search large volumes of water in the least amount of time. In addition, tunas have been shown to have very high rates of digestion [55], which is advantageous for species that must be able to fully exploit a food patch wherever one is found. Because the pelagic environment provides tuna no place to hide and rest while repaying an oxygen debt, the ability to quickly metabolize lactate is also advantageous [55]. High sustainable swimming speeds therefore allow tuna to rapidly repay an oxygen debt when one is accumulated, which also results in rapid growth rates and high fecundity of yellowfin tuna in the high seas of the tropical WCPO [9,56].
In addition, fluctuations in the breeding season can lead to delayed maturation [8]. The results of this study show that the sexual maturity ogive of male yellowfin tuna is steeper than that of females. When using the distance between 25% and 75% of sexual maturity FL (FL75–FL25) as an indicator, the result for males is 15.76 cm (SD = ±2.48), while that for females is 17.35 cm (SD = ±4.03), which is very similar to the results obtained by Zhu et al. [6] in the Indian Ocean. This indicates that the male gonad development in the same group may be more concentrated in time compared with the gonad development in females, and growth and gonad development are faster.
In conclusion, the reproductive parameters of tropical western and central Pacific yellowfin tuna can be described using months as the cyclic variable. The results indicated that female yellowfin tuna showed spawning behavior during most of the year. The monthly variation regularly showed that mean GSI values of both sexes were significantly higher from September to December. For both sexes, the peak of mature and spawned gonads appeared in September, and later developing gonads mainly occurred in November and December. This further indicates that female yellowfin tuna mainly spawn between September and December.
In this study, a first attempt was made to quantify the relationship between gonad maturity and driver factors in yellowfin tuna using polytomous logistic regression for the ordinal response model. Results are consistent with related studies, indicating that the model has good applicability.
Author Contributions
Conceptualization, formal analysis, visualization, software, and writing—original draft preparation, X.S.; investigation, resources, X.W.; data curation, methodology, Y.W.; validation, review and editing, funding acquisition, J.Z.; project administration, J.Z. and C.L.; supervision, J.Z. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was jointly funded by Program on the Survey, Monitoring and Assessment of Global Fishery Resources sponsored by the Ministry of Agriculture and Rural Affairs and the National Key Research and Development Program of China (2020YFD0900803).
Data Availability Statement
The raw data which support this study are available from the corresponding author at reasonable request.
Acknowledgments
We would like to thank the crew of the fishing vessel “Huyu 927” for their help and support.
Conflicts of Interest
All authors declare no conflict of interest.
References
- Olson, R.J.; Young, J.W.; Menard, F.; Potier, M.; Allain, V.; Goni, N.; Logan, J.M.; Galvan-Magana, F. Bioenergetics, Trophic Ecology, and Niche Separation of Tunas. Adv. Mar. Biol. 2016, 74, 199–344. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Ducharme, B.N.; Hamer, P.; Hampton, J.; Williams, P.; Pilling, G. Stock Assessment of Yellowfin Tuna in the Western and Central Pacific Ocean; WCPFC: Kolonia, Federated States of Micronesia, 2020. [Google Scholar]
- FAO. FAOSTAT Statistics Database; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; pp. 280–290. [Google Scholar]
- Steven, H.; Graham, P. Updated WCPO Bigeye and Yellowfin TRP Evaluations; WCPFC: Kolonia, Federated States of Micronesia, 2021. [Google Scholar]
- Schaefer, K.M. Reproductive biology of tunas. Fish Physiol. 2001, 19, 225–270. [Google Scholar]
- Zhu, G.P.; Xu, L.X.; Zhou, Y.Q.; Song, L.M. Reproductive biology of yellowfin tuna (T. albacares) in the west-central Indian Ocean. J. Ocean. Univ. China 2008, 7, 327–332. (In Chinese) [Google Scholar] [CrossRef]
- Zudaire, I.; Murua, H.; Grande, M.; Bodin, N. Reproductive potential of yellowfin tuna (Thunnus albacares) in the western Indian Ocean. Fish. Bull. 2013, 111, 252–264. [Google Scholar] [CrossRef]
- Itano, D.G. The Reproductive Biology of Yellowfin Tuna (Thunnus albacares) in Hawaiian Waters and the Western Tropical Pacific Ocean: Project Summary; University of Hawaii, Joint Institute for Marine and Atmospheric Research: Honolulu, HI, USA, 2000. [Google Scholar]
- Schaefer, K.M. Spawning time, frequency, and batch fecundity of yellowfin tuna, Thunnus albacares near Clipperton Atoll in the eastern Pacific Ocean. Fish. Bull. 1996, 94, 98–112. [Google Scholar]
- McPherson, G. Reproductive biology of yellowfin tuna in the eastern Australian fishing zone, with special reference to the north-western Coral Sea. Mar. Freshw. Res. 1991, 42, 465–477. [Google Scholar] [CrossRef]
- Orange, C.J. Spawning of yellowfin tuna and skipjack in the eastern tropical Pacific, as inferred from studies of gonad development. Inter-Am. Trop. Tuna Comm. Bull. 1961, 5, 457–526. [Google Scholar]
- Schaefer, K.M. Reproductive biology of yellowfin tuna Thunnus albacares in the eastern Pacific Ocean. Inter-Am. Trop. Tuna Comm. Bull. 1998, 21, 205–272. [Google Scholar] [CrossRef][Green Version]
- Richardson, A.J.; Downes, K.J.; Nolan, E.T.; Brickle, P.; Brown, J.; Weber, N.; Weber, S.B. Residency and reproductive status of yellowfin tuna in a proposed large-scale pelagic marine protected area. Aquat. Conserv.: Mar. Freshw. Ecosyst. 2018, 28, 1308–1316. [Google Scholar] [CrossRef]
- Diaha, N.C.; Zudaire, I.; Chassot, E.; Barrigah, B.; Irié, Y.; Gbeazere, D.; Kouadio, D.; Pecoraro, C.; Romeo, M.; Murua, H. Annual monitoring of reproductive traits of female yellowfin tuna (Thunnus albacares) in the eastern Atlantic Ocean. Collect Vol. Sci. Pap. ICCAT 2016, 72, 534–548. [Google Scholar]
- McKechnie, S.; Tremblay, B.L.; Pilling, G. Background Analyses for the 2017 Stock Assessments of Bigeye and Yellowfin Tuna in the Western and Central Pacific Ocean; SC13-2017/SA-IP-06; WCPFC: Rarotonga, Cook Islands, 2017. [Google Scholar]
- Sun, C.L.; Wang, W.; Yeh, S. Reproductive Biology of Yellowfin Tuna in the Central and Western Pacific Ocean; SC1. BI WP-1; WCPFC: Kolonia, Federated States of Micronesia, 2005. [Google Scholar]
- Schaefer, K.M.; Fuller, D.W. Spatiotemporal variability in the reproductive biology of yellowfin tuna (Thunnus albacares) in the eastern Pacific Ocean. Fish. Res. 2022, 248, 215–225. [Google Scholar] [CrossRef]
- Nishida, T. Factors Affecting Distribution Of Adult Yellowfin Tuna (Thunnus albacares) and Its Reproductive Ecology in the Indian Ocean Based on Japanese Tuna Longline Fisheries and Survey Information; IOTC Proceedings No. 4; IOTC: Canberra, Australia, 2001; pp. 336–389. [Google Scholar]
- Gilman, E.; Chaloupka, M.; Musyl, M. Effects of pelagic longline hook size on species-and size-selectivity and survival. Rev. Fish Biol. Fish. 2018, 28, 417–433. [Google Scholar] [CrossRef]
- Bertrand, A.; Bard, F.-X.; Josse, E. Tuna food habits related to the micronekton distribution in French Polynesia. Mar. Biol. 2002, 140, 1023–1037. [Google Scholar]
- Costa, F.; Braga, F.; Amorim, A.; Arfelli, C. Fishery Biology of the Yellowfin Tuna, Thunnus albacares in Southern Brazil. Collect. Vol. Sci. Pap. Int. Comm. Conserv. Atl. Tunas 2005, 58, 309–349. [Google Scholar]
- Kai, M. Weight-length relationship of North Western Pacific bluefin tuna. In Proceedings of the Report of the Pacific Bluefin Tuna Working Group Workshop (ISC/07/PBFWG-3). In Proceedings of the International Scientific Committee for Tuna and Tuna-Like Species in the North Pacific Ocean, Shimizu, Japan, 11–18 December 2007; pp. 1–8. [Google Scholar]
- Hsu, C.; Liu, H.; Wu, C.; Huang, S.; Liao, H. New information on age composition and length-weight relationship of bluefin tuna, Thunnus thynnus, in the southwestern North Pacific. Fish. Sci. 2000, 66, 485–493. [Google Scholar] [CrossRef][Green Version]
- Rice, P.H. Use of Catenary Geometry to Estimate Hook Depth during Near-Surface Pelagic Longline Fishing: Theory versus Practice. N. Am. J. Fish. Manag. 2007, 27, 1148–1161. [Google Scholar] [CrossRef]
- Li, H.H.; Tian, S.Q. Study on reproductive biology of yellowfin tuna (Thunnus albacares) in the Central and Western Indian Ocean. J. Shanghai Ocean. Univ. 2015, 24, 594–602. (In Chinese) [Google Scholar]
- Holden, M.J.; Raitt, D.F.S. Manual of Fisheries Science. Part 2-Methods of Resource Investigation and Their Application; FAO Fish: Rome, Italy, 1974. [Google Scholar]
- Nootmorn, P. Reproductive Biology of Bigeye tuna in the Eastern Indian Ocean; IOTC: Canberra, Australia, 2004; pp. 1–5. [Google Scholar]
- Ashida, H. Spatial and temporal differences in the reproductive traits of skipjack tuna Katsuwonus pelamis between the subtropical and temperate western Pacific Ocean. Fish. Res. 2020, 221, 105352. [Google Scholar] [CrossRef]
- Lowry, M.; Williams, D.; Metti, Y. Lunar landings—Relationship between lunar phase and catch rates for an Australian gamefish-tournament fishery. Fish. Res. 2007, 88, 15–23. [Google Scholar] [CrossRef]
- Batschelet, E. Circular Statistics in Biology; Academic Press: Pittsburgh, PA, USA, 1981; pp. 371–375. [Google Scholar]
- Gao, G.; Zhang, M.Z. Polytomous Logistic regression for ordinal response and it’s appliance. J. Tongji Univ. 2003, 31, 1237–1241. (In Chinese) [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Stergiou, K.I.; Moutopoulos, D.K. A Review of Length-Weight Relationships of Fishes from Greek Marine Waters. Fishbyte 2001, 24, 23–39. [Google Scholar]
- Vincent, M.; Ducharme-Barth, N.; Hamer, P. Background Analyses for the 2020 Stock Assessments of Bigeye and Yellowfin Tuna: Technical Report SC16-SA-IP-06; WCPFC: Kolonia, Federated States of Micronesia, 2020. [Google Scholar]
- Fonteneau, A. Estimated Sex Ratio of Large Yellowfin Taken by Purse Seiners in the Indian Ocean: Comparison with Other Oceans; IOTC: Canberra, Australia, 2002; pp. 279–281. [Google Scholar]
- Wild, A. Growth of yellowfin tuna, Thunnus albacares, in the eastern Pacific Ocean based on otolith increments. Inter Am. Trop. Tuna Comm. Bull. 1986, 18, 421–482. [Google Scholar]
- Eveson, J.P.; Million, J.; Sardenne, F.; Le Croizier, G. Estimating growth of tropical tunas in the Indian Ocean using tag-recapture data and otolith-based age estimates. Fish. Res. 2015, 163, 58–68. [Google Scholar] [CrossRef]
- Stéquert, B.; Rodriguez, J.N.; Cuisset, B.; Le Menn, F. Gonadosomatic index and seasonal variations of plasma sex steroids in skipjack tuna (Katsuwonus pelamis) and yellowfin tuna (Thunnus albacares) from the western Indian Ocean. Aquat. Living Resour. 2001, 14, 313–318. [Google Scholar] [CrossRef]
- Chen, F.; Guo, A.; Zhu, W.B.; Zhou, Y.D.; Xu, H.X.; Zhang, L. Study on reproductive biology of yellowfin tuna (Thunnus albacares) in the waters of the Solomon Islands of the South Pacific Ocean. J. Trop. Oceanogr. 2014, 33, 45–51. (In Chinese) [Google Scholar]
- Mallawa, A.; Zainuddin, M. Population dynamic indicator of the yellowfin tuna Thunnus albacares and its stock condition in the Banda Sea, Indonesia. Aquac. Aquar. Conserv. Legis. 2018, 11, 1323–1333. [Google Scholar]
- Kikawa, S. The distribution of maturing bigeye and yellowfin and an evaluation of their spawning potential in different areas in the tuna longline grounds in the Pacific. Bull. Nankai Reg. Fish. Res. Lab. 1966, 23, 131–208. [Google Scholar]
- Yamanaka, K.L. Age, Growth, and Spawning of Yellowfin Tuna, (Thunnus albacares) Bonnaterre 1788, in the Southern Philippines; University of British Columbia: Vancouver, BC, Canada, 1989. [Google Scholar]
- Farley, J.H.; Clear, N.P.; Leroy, B.; Davis, T.L.O.; McPherson, G. Age, growth and preliminary estimates of maturity of bigeye tuna (Thunnus obesus) in the Australian region. Mar. Freshw. Res. 2006, 57, 713–724. [Google Scholar] [CrossRef]
- Sun, C.L.; Chang, Y.J.; Chung, T.C.; Zan, Y.S. Reproductive biology of blue marlin (Makaira nigricans) in the western Pacific Ocean. Fish. Bull. 2009, 107, 420–432. [Google Scholar]
- Kikawa, S. Studies on the Spawning Activity of Pacific tunas, Parathunnus mebachi and Neothunnus macropterus, by the Gonad Index Examination; Occasional Report of the Nankai Regional Fisheries Research Laboratory; Nankai: Osaka, Japan, 1962; Volume 1, pp. 43–56. [Google Scholar]
- Longhurst, A. Seasonal cycles of pelagic production and consumption. Prog. Oceanogr. 1995, 36, 77–167. [Google Scholar] [CrossRef]
- Cole, J.S. Synopsis of biological data on the yellowfin tuna, Thunnus albacares (Bonnaterre, 1788), in the Pacific Ocean. Synop. Biol. Data Eight Species Scombrids 1980, 2, 71–150. [Google Scholar]
- Hampton, J. A Summary of Current Information on the Biology, Fisheries and Stock Assessment of Bigeye Tuna (Thunnus Obesus) in the Pacific Ocean, with Recommendations for Data Requirement and Future Research; The Secretariat of the Pacific Community: Noumea, New Caledonia, 1998; pp. 1–46. [Google Scholar]
- Gallo, N.D.; Levin, L.A. Fish Ecology and Evolution in the World’s Oxygen Minimum Zones and Implications of Ocean Deoxygenation. Adv. Mar. Biol. 2016, 74, 117–198. [Google Scholar] [PubMed]
- Neill, W.H.; Chang, R.K.; Dizon, A.E. Magnitude and ecological implications of thermal inertia in skipjack tuna, Katsuwonus pelamis (Linnaeus). Environ. Biol. Fishes 1976, 1, 61–80. [Google Scholar] [CrossRef]
- Brill, R.W.; Bigelow, K.A.; Musyl, M.K.; Fritsches, K.A.; Warrant, E.J. Bigeye tuna (Thunnus obesus) behavior and physiology and their relevance to stock assessments and fishery biology. Collect. Vol. Sci. Pap. ICCAT 2005, 57, 142–161. [Google Scholar]
- Helly, J.J.; Levin, L.A. Global distribution of naturally occurring marine hypoxia on continental margins. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2004, 51, 1159–1168. [Google Scholar] [CrossRef]
- Yuen, H.S.; June, F.C. Yellowfin Tuna Spawning in the Central Equatorial Pacific; US Fish and Wildlife Service: Washington, DC, USA, 1957; Volume 57, pp. 251–264. [Google Scholar]
- Nootmorn, P.; Yakoh, A.; Kawises, K. Reproductive Biology of Yellowfin Tuna in the Eastern Indian Ocean; IOTC-WPTT: Canberra, Australia, 2005; Volume 14, pp. 379–385. [Google Scholar]
- Brill, R.W. On the standard metabolic rates of tropical tunas, including the effects of body size and temperature change. Fish. Bull. Natl. Ocean. Atmos. Adm. 1987, 85, 25–35. [Google Scholar]
- Brill, R.W. Selective advantages conferred by the high performance physiology of tunas, billfishes, and dolphin fish. Comp. Biochem. Physiol. Part A Physiol. 1996, 113, 3–15. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).