Conversion of Fishery Waste to Proteases by Streptomyces speibonae and Their Application in Antioxidant Preparation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Protease Production
2.3. Protease Activity
2.4. Zymogram
2.5. Effects of Temperature and pH
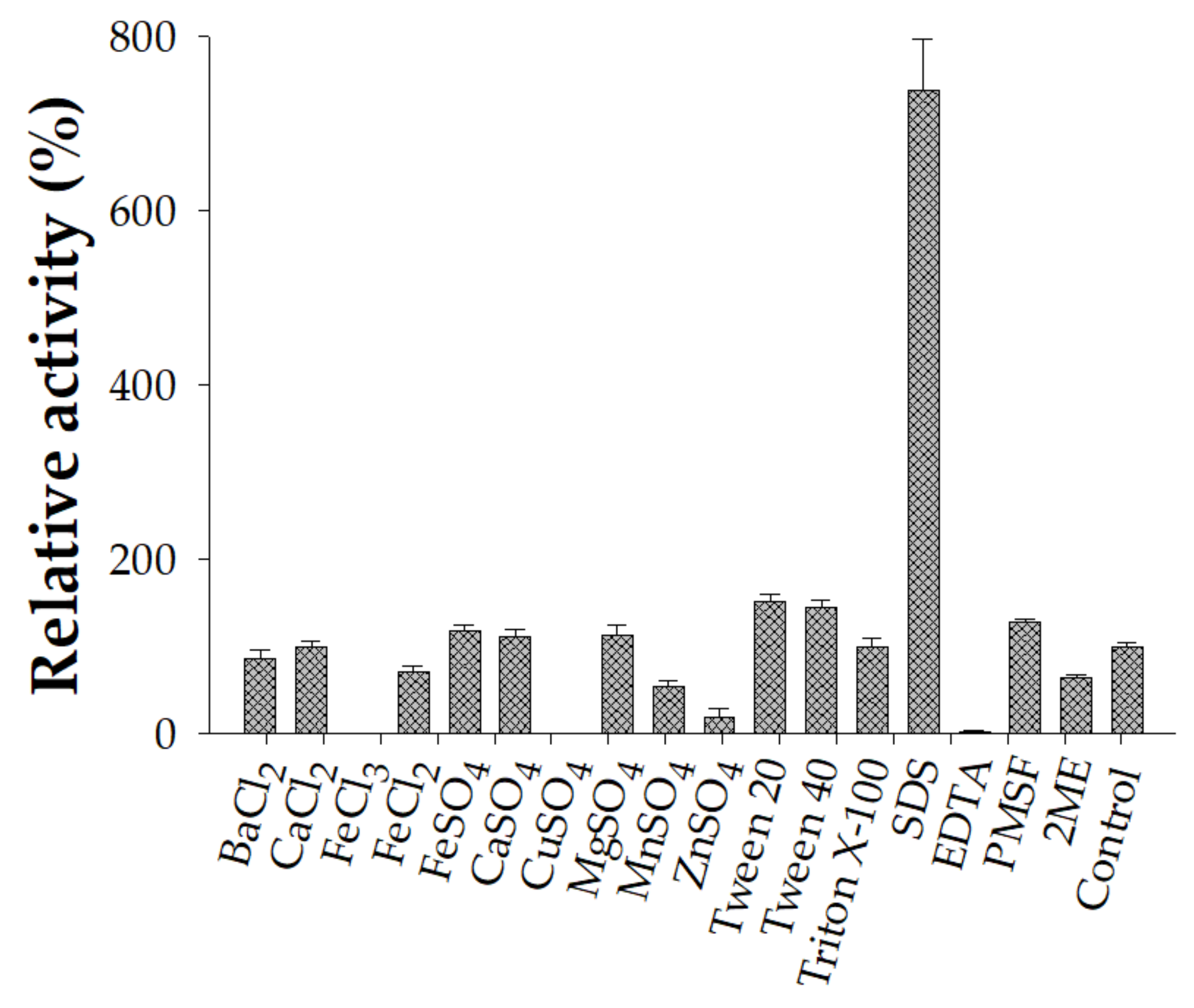
2.6. Effects of Chemicals
2.7. Substrate Specificity
2.8. Proteinaceous Material Hydrolysis
2.9. DPPH Radical Scavenging Activity
2.10. ABTS Radical Scavenging Activity
3. Results and Discussion
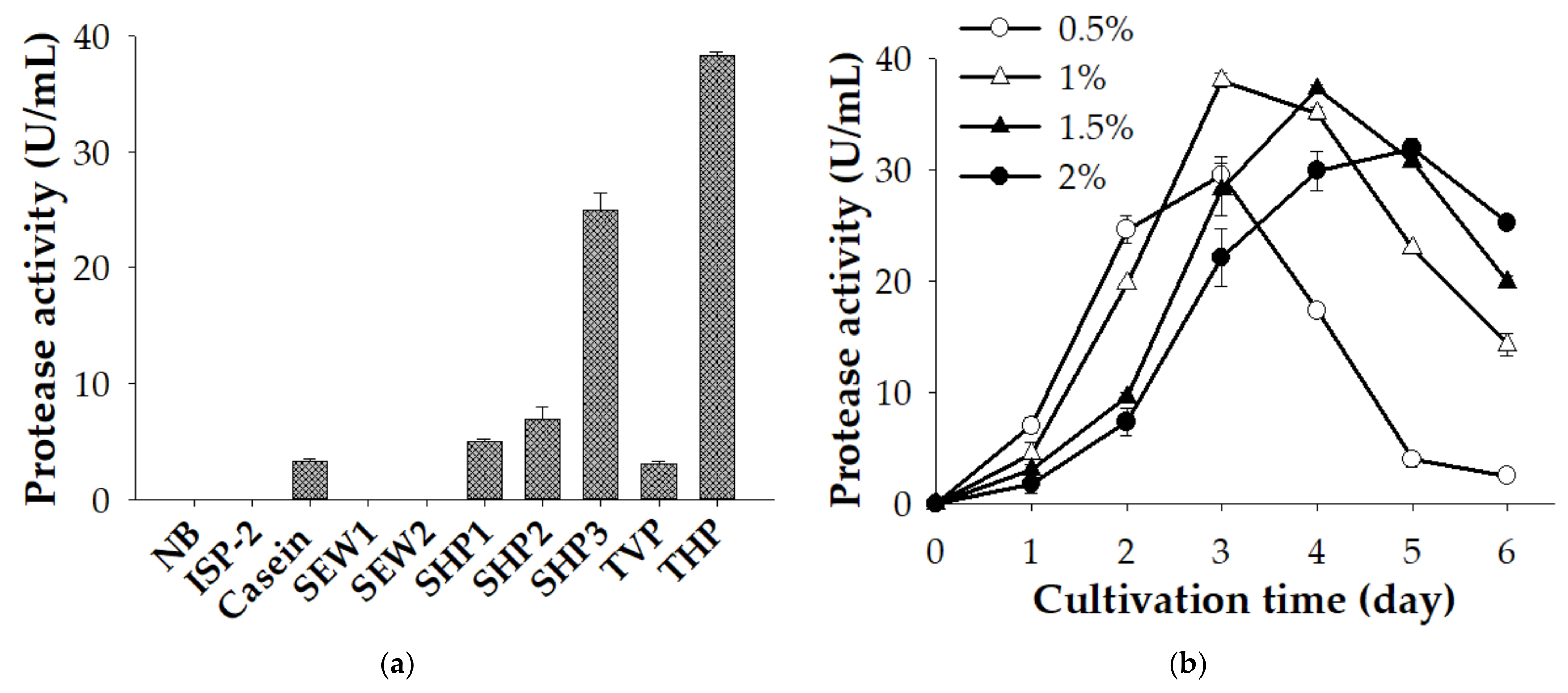
3.1. Protease Production of S. speibonae TKU048 on Different C/N Sources
3.2. Zymogram of the Enzymes Produced
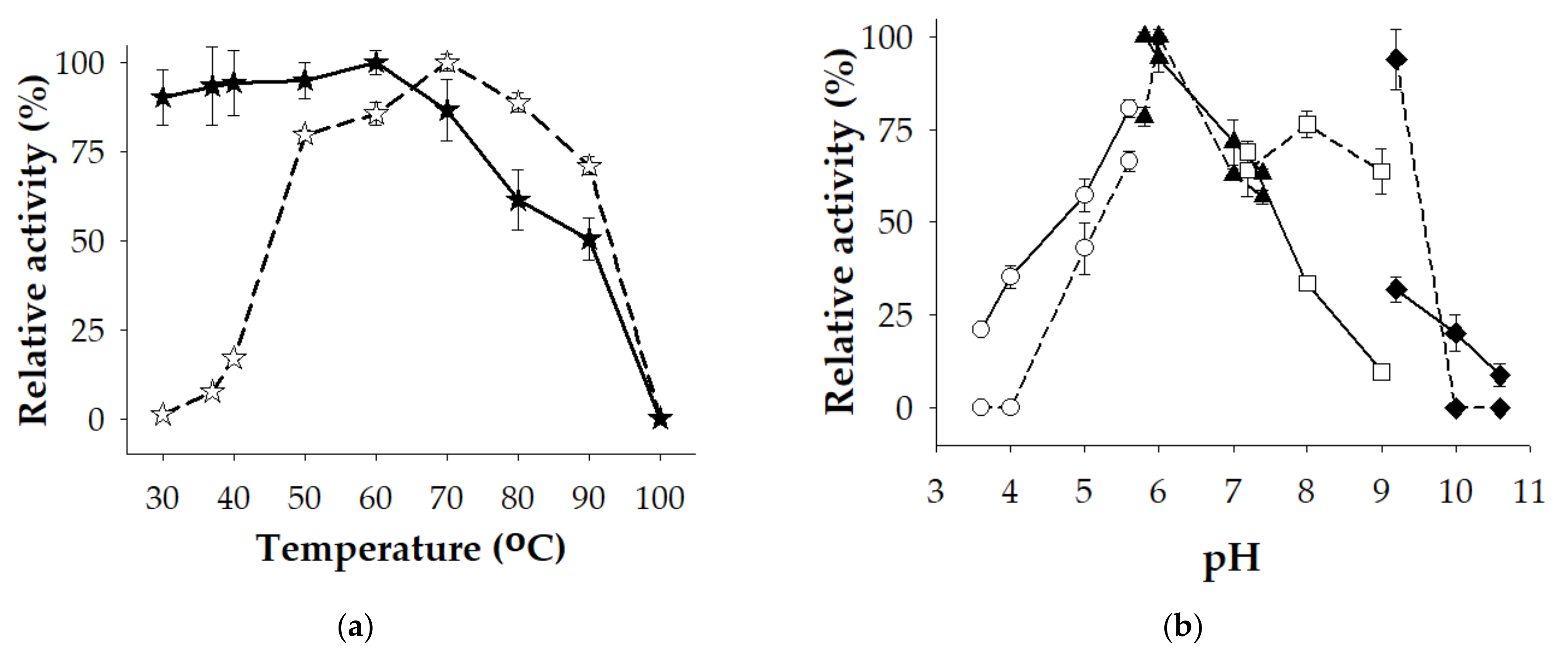
3.3. The Characterization of Crude-Enzyme Cocktail
3.4. Substrate Specificity of Protease
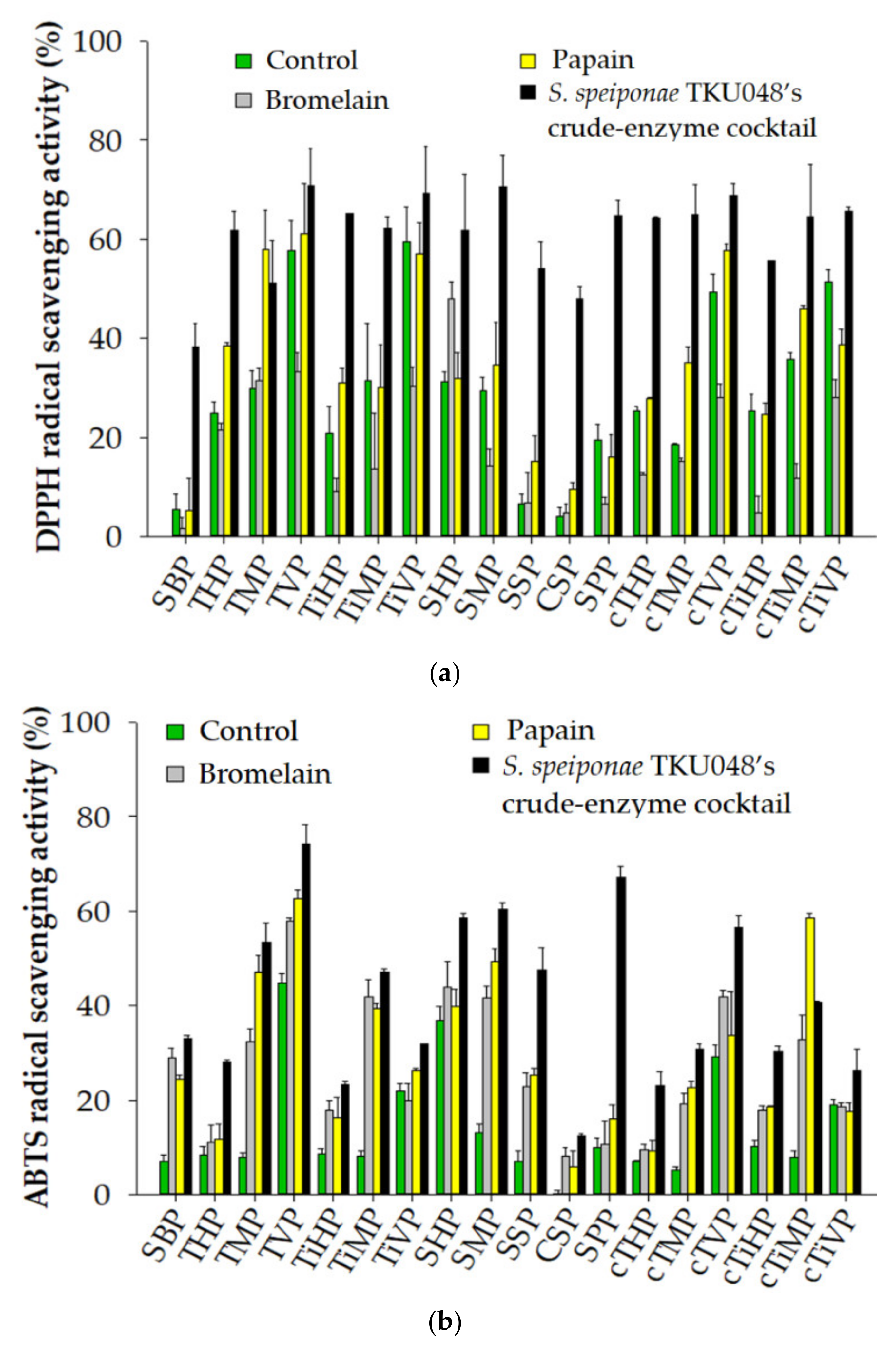
3.5. Antioxidant from the Hydrolysis of Fish Materials
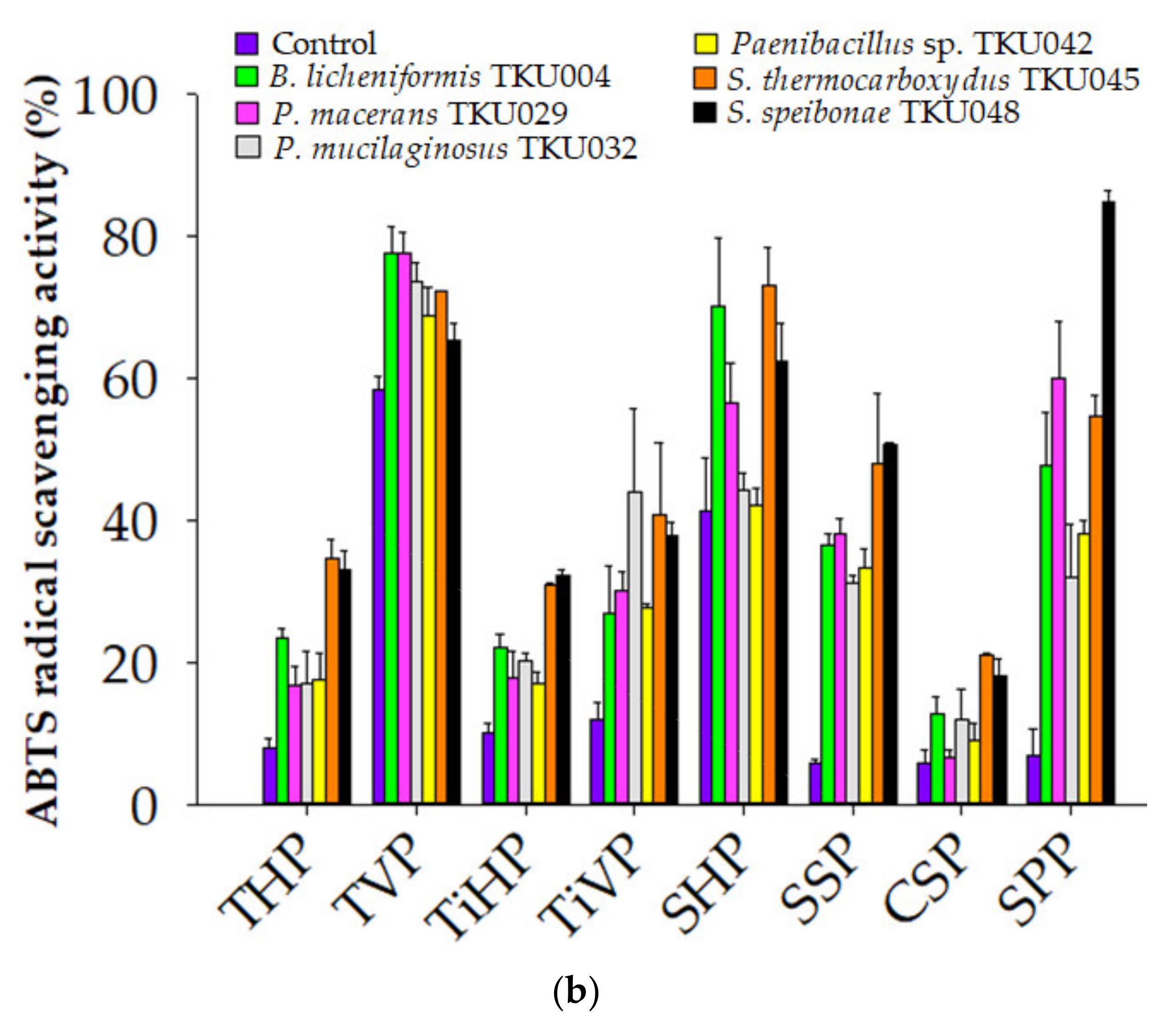
3.6. Comparison of the Antioxidant Activity of Proteinaceous Wastes Hydrolysates Catalyzed by Different Crude-Enzyme Cocktails
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Racioppo, A.; Speranza, B.; Campaniello, D.; Sinigaglia, M.; Corbo, M.R.; Bevilacqua, A. Fish loss/waste and low-value fish challenges: State of art, advances, and perspectives. Foods 2021, 10, 2725. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G. Fishery wastes and by-products: A resource to be valorised. J. Fish. Sci. 2015, 9, 80–83. [Google Scholar]
- Wang, C.H.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Reclamation of fishery processing waste: A mini-review. Molecules 2019, 24, 2234. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, B.; Pallarés, N.; Berrada, H.; Barba, F.J. Salmon (Salmo salar) Side streams as a bioresource to obtain potential antioxidant peptides after applying pressurized liquid extraction (PLE). Mar. Drugs 2021, 19, 323. [Google Scholar] [CrossRef]
- Das, S.; Roy, D.; Sen, R. Utilization of chitinaceous wastes for the production of chitinase. Adv. Food Nutr. Res. 2016, 78, 27–46. [Google Scholar]
- Nguyen, V.B.; Wang, S.L. Production of potent antidiabetic compounds from shrimp head powder via Paenibacillus conversion. Process Biochem. 2019, 76, 18–24. [Google Scholar] [CrossRef]
- Hsu, C.H.; Nguyen, A.D.; Chen, Y.W.; Wang, S.L. Tyrosinase inhibitors and insecticidal materials produced by Burkholderia cepacia using squid pen as the sole carbon and nitrogen source. Res. Chem. Intermed. 2014, 40, 2249–2258. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Wang, S.L.; Nguyen, D.N.; Nguyen, A.D.; Nguyen, T.H.; Doan, M.D.; Ngo, V.A.; Doan, C.T.; Kuo, Y.H.; Nguyen, V.B. Bioprocessing of Marine Chitinous Wastes for the Production of Bioactive Prodigiosin. Molecules 2021, 26, 3138. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.L. Chitin extraction from shrimp waste by liquid fermentation using an alkaline protease-producing strain, Brevibacillus parabrevis. Int. J. Biol. Macromol. 2019, 131, 706–715. [Google Scholar] [CrossRef]
- Liang, T.W.; Tseng, S.C.; Wang, S.L. Production and characterization of antioxidant properties of exopolysaccharides from Paenibacillus mucilaginosus TKU032. Mar. Drugs 2016, 14, 40. [Google Scholar] [CrossRef]
- Ben Elhoul, M.; Jaouadi, N.Z.; Rekik, H.; Bejar, W.; Touioui, S.B.; Hmidi, M.; Badis, A.; Bejar, S.; Jaouadi, B. A novel detergent-stable solvent-tolerant serine thiol alkaline protease from Streptomyces koyangensis TN650. Int. J. Biol. Macromol. 2015, 79, 871–882. [Google Scholar] [CrossRef]
- Lee, D.-H.; Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, C.-L.; Wang, S.-L. Proteases production and chitin preparation from the liquid fermentation of chitinous fishery by-products by Paenibacillus elgii. Mar. Drugs 2021, 19, 477. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Wen, I.-H.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.-L. Conversion of shrimp head waste for production of a thermotolerant, detergent-stable, alkaline protease by Paenibacillus sp. Catalysts 2019, 9, 798. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Wang, S.L. Production of thermophilic chitinase by Paenibacillus sp. TKU052 by bioprocessing of chitinous fishery wastes and its application in N-acetyl-D-glucosamine production. Polymers 2021, 13, 3048. [Google Scholar] [CrossRef]
- Shereena, E.K.; Nisha, M.K.; Gaayathiri Devi, E. Chitinase production by Aspergillus terreus from marine wastes and its efficacy in antifungal activity. Int. J. Adv. Res. 2020, 8, 1399–1404. [Google Scholar]
- Sánchez-Suárez, J.; Coy-Barrera, E.; Villamil, L.; Díaz, L. Streptomyces-derived metabolites with potential photoprotective properties—A systematic literature review and meta-analysis on the reported chemodiversity. Molecules 2020, 25, 3221. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, P.; Das, P.; Solanki, R.; Kapur, M.K. Potential applications of extracellular enzymes from Streptomyces spp. in various industries. Arch. Microbiol. 2020, 202, 1597–1615. [Google Scholar] [CrossRef]
- Tran, T.N.; Doan, C.T.; Wang, S.-L. Conversion of wheat bran to xylanases and dye adsorbent by Streptomyces thermocarboxydus. Polymers 2021, 13, 287. [Google Scholar] [CrossRef]
- Tran, T.N.; Doan, C.T.; Nguyen, M.T.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.L. An exochitinase with N-acetyl-β-glucosaminidase-like activity from shrimp head conversion by Streptomyces speibonae and its application in hydrolyzing β-chitin powder to produce N-acetyl-d-glucosamine. Polymers 2019, 11, 1600. [Google Scholar] [CrossRef]
- Xia, W.; Hu, M.; Pan, Y.; Wu, D.; Wu, J. Improved production of Streptomyces sp. FA1 xylanase in a dual-plasmid Pichia pastoris System. Curr. Issues Mol. Biol. 2021, 43, 2289–2304. [Google Scholar] [CrossRef]
- Tran, T.N.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. The isolation of chitinase from Streptomyces thermocarboxydus and its application in the preparation of chitin oligomers. Res. Chem. Intermed. 2019, 45, 727–742. [Google Scholar] [CrossRef]
- Nguyen, T.N.T.; Chataway, T.; Araujo, R.; Puri, M.; Franco, C.M.M. Purification and characterization of a novel alginate lyase from a marine Streptomyces species isolated from seaweed. Mar. Drugs 2021, 19, 590. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.K. Production, characterization and antioxidant potential of protease from Streptomyces sp. MAB18 using poultry wastes. BioMed. Res. Int. 2013, 2013, 496586. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Production of a thermostable chitosanase from shrimp heads via Paenibacillus mucilaginosus TKU032 conversion and its application in the preparation of bioactive chitosan oligosaccharides. Mar. Drugs 2019, 17, 217. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Dhamodharan, D.; Jemimah Naine, S.; Merlyn Keziah, S.; Subathra Devi, C. Novel fibrinolytic protease producing Streptomyces radiopugnans VITSD8 from marine sponges. Mar. Drugs 2019, 17, 164. [Google Scholar]
- Verma, P.; Chatterjee, S.; Merlyn Keziah, S.; Devi, S.C. Fibrinolytic protease from marine Streptomyces rubiginosus VITPSS1. Cardiovasc. Hematol. Agents Med. Chem. 2018, 16, 44–45. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Esmail, G.A.; Ghilan, A.M.; Arasu, M.V. Isolation and screening of Streptomyces sp. Al-Dhabi-49 from the environment of Saudi Arabia with concomitant production of lipase and protease in submerged fermentation. Saudi J. Biol. Sci. 2020, 27, 474–479. [Google Scholar] [CrossRef]
- Kammoun, M.; Soltana, H.; Nasri, M.; Hmidet, N. Streptomyces flavogriseus HS1: Isolation and characterization of extracellular proteases and their compatibility with laundry detergents. BioMed. Res. Int. 2014, 2014, 345980. [Google Scholar]
- Touioui, S.B.; Jaouadi, N.Z.; Boudjella, H.; Ferradji, F.Z.; Belhoul, M.; Rekik, H.; Badis, A.; Bejar, S.; Jaouadi, B. Purification and biochemical characterization of two detergent-stable serine alkaline proteases from Streptomyces sp. strain AH4. World J. Microbiol. Biotechnol. 2015, 31, 1079–1092. [Google Scholar] [CrossRef]
- Jaouadi, B.; Abdelmalek, B.; Fodil, D.; Ferradji, F.Z.; Rekik, H.; Zaraî, N.; Bejar, S. Purification and characterization of a thermostable keratinolytic serine alkaline proteinase from Streptomyces sp. strain AB1 with high stability in organic solvents. Bioresour. Technol. 2010, 101, 8361–8369. [Google Scholar] [CrossRef]
- Moormann, M.; Schlochtermeier, A.; Schrempf, H. Biochemical characterization of a protease involved in the processing of a Streptomyces reticuli cellulase (avicelase). Appl. Environ. Microbiol. 1993, 59, 1573–1578. [Google Scholar] [CrossRef]
- Sarkar, G.; Suthindhiran, K. Extraction and characterization of alkaline protease from Streptomyces sp. GS-1 and its ap-plication as dehairing agent. Biocatal. Agric. Biotechnol. 2020, 25, 101590. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Esmail, G.A.; Ghilan, A.M.; Arasu, M.V.; Duraipandiyan, V.; Ponmurugan, K. Characterization and fermentation optimization of novel thermo stable alkaline protease from Streptomyces sp. Al-Dhabi-82 from the Saudi Arabian environment for eco-friendly and industrial applications. J. King Saud Univ.-Sci. 2020, 32, 1258–1264. [Google Scholar] [CrossRef]
- Bajaj, B.K.; Sharma, P. An alkali-thermotolerant extracellular protease from a newly isolated Streptomyces sp. DP2. New Biotechnol. 2011, 28, 725–732. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, P.; Cheng, G.; Zhang, B.; Dong, W.; Su, X.; Huang, Y.; Cui, Z.; Kong, Y. A fibrinolytic protease AfeE from Streptomyces sp. CC5, with potent thrombolytic activity in a mouse model. Int. J. Biol. Macromol. 2016, 85, 346–354. [Google Scholar] [CrossRef]
- Simkhada, J.R.; Mander, P.; Cho, S.S.; Yoo, J.C. A novel fibrinolytic protease from Streptomyces sp. CS684. Process. Biochem. 2010, 45, 88–93. [Google Scholar] [CrossRef]
- De Azevedo, L.A.I.; Freire, D.M.G.; Soares, R.M.A.; Leite, S.G.F.; Coelho, R.R.R. Production and partial characterization of thermophilic proteases from Streptomyces sp. isolated from Brazilian cerrado soil. Enzym. Microb. Technol. 2004, 34, 354–358. [Google Scholar] [CrossRef]
- Çorbacı, C.; Özcan, K. Streptomyces sp. K47 alkaline proteases: Partial purification and analysis by zymography. Acta Sci. Technol. 2021, 43, e51486. [Google Scholar] [CrossRef]
- Nagpure, A.; Choudhary, B.; Gupta, R.K. Mycolytic enzymes produced by Streptomyces violaceusniger and their role in antagonism towards wood-rotting fungi. J. Basic Microbiol. 2014, 54, 397–407. [Google Scholar] [CrossRef]
- Chi, W.J.; Kim, Y.H.; Kim, J.H.; Kang, D.K.; Kang, S.S.; Suh, J.W.; Hong, S.K. Streptomyces griseus trypsin has gelatinase activity and its proteolytic activity is enhanced manganese. J. Microbiol. 2003, 41, 289–294. [Google Scholar]
- Al-Askar, A.A.; Rashad, Y.M.; Hafez, E.E.; Abdulkhair, W.M.; Baka, Z.A.; Ghoneem, K.M. Characterization of alkaline protease produced by Streptomyces griseorubens E44G and its possibility for controlling Rhizoctonia root rot disease of corn. Biotechnol. Equip. 2015, 29, 457–462. [Google Scholar] [CrossRef]
- Xin, Y.; Sun, Z.; Chen, Q.; Wang, J.; Wang, Y.; Luogong, L.; Li, S.; Dong, W.; Cui, Z.; Huang, Y. Purification and characterization of a novel extracellular thermostable slkaline protease from Streptomyces sp. M30. J. Microbiol. Biotechnol. 2015, 25, 1944–1953. [Google Scholar] [CrossRef]
- Kim, I.S.; Lee, K.J. Trypsin-like protease of Streptomyces exfoliatus SMF13, a potential agent in mycelial differentiation. Microbiology 1996, 142, 1797–1806. [Google Scholar] [CrossRef][Green Version]
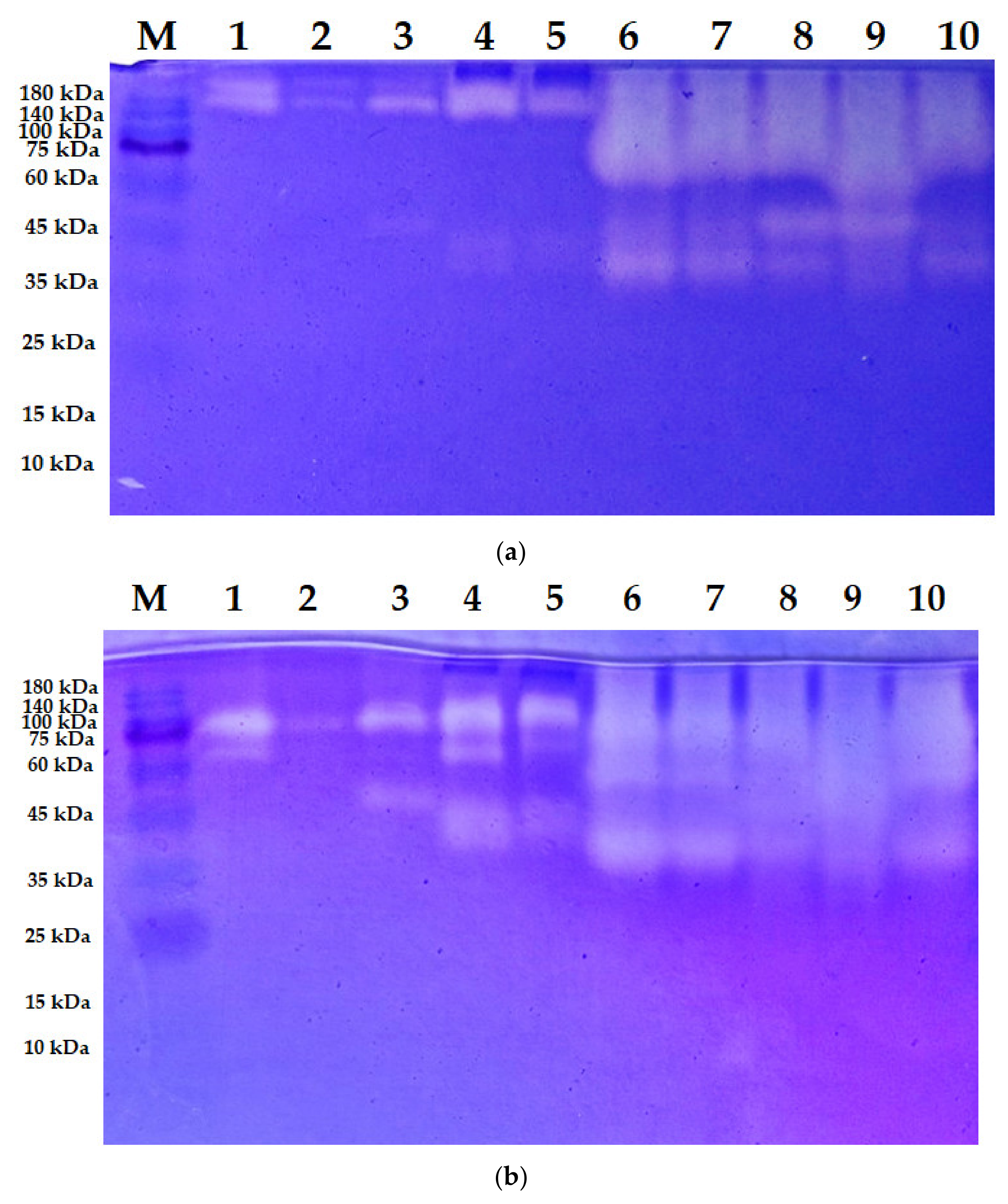
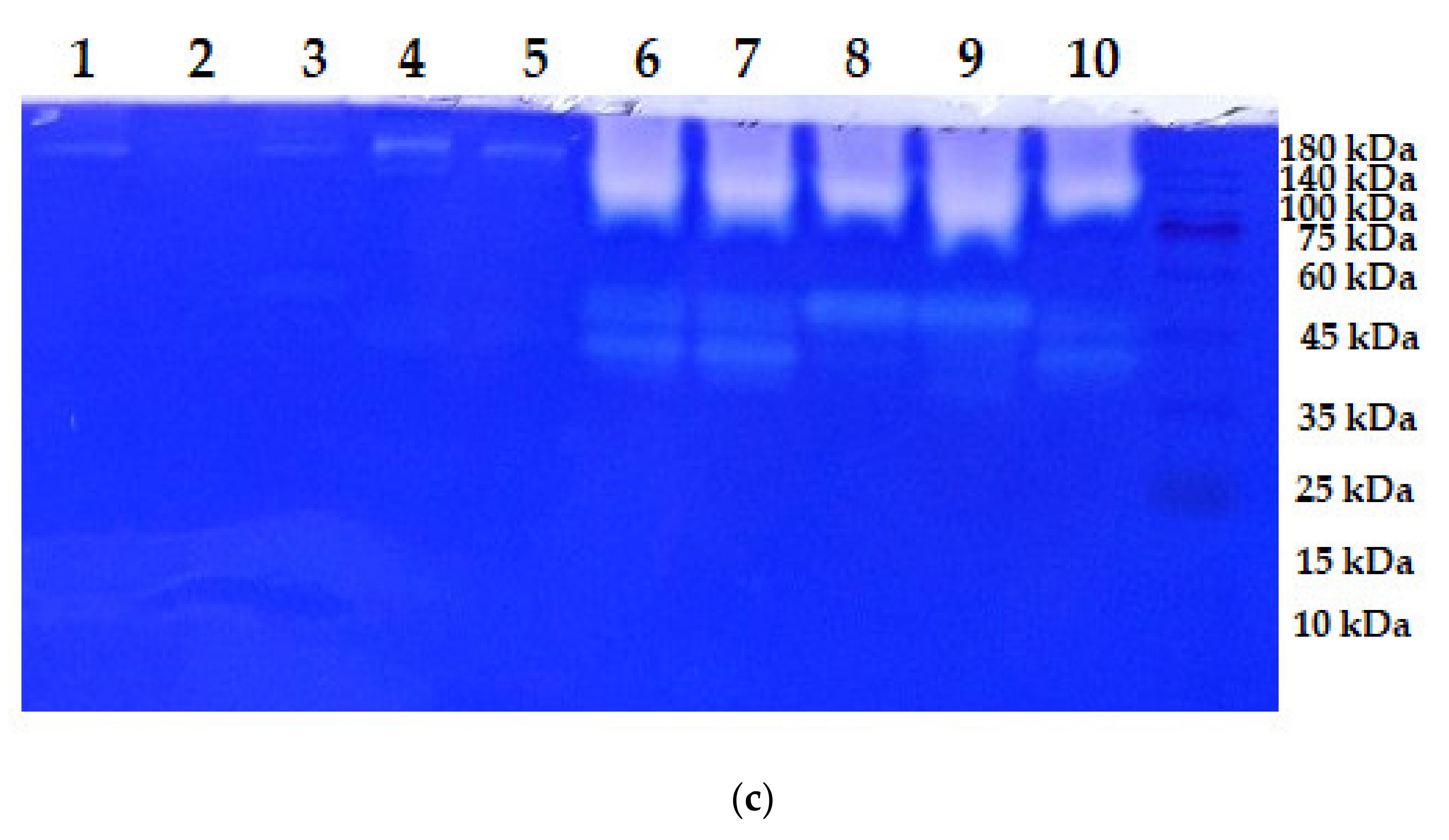


| Strains | MW (kDa) | C/N Source | Ref. |
|---|---|---|---|
| S. speibonae TKU048 | Three * (>140; >140; and 45) | ||
| Nine ** (138; 110; 73; 47; 45; 38; 25; 23; and <10) | Tuna head powder | This study | |
| Six *** (138; 88; 47; 38; 25; and <10) | |||
| S. radiopugnans VITSD8 | 38 | Maltose and peptone | [26] |
| S. rubiginosus VITPSS1 | 45 | Soybean meal | [27] |
| Streptomyces sp. Al-Dhabi-49 | Peptone | [28] | |
| S. flavogriseus HS1 **** | Dextrin and tryptone | [29] | |
| S. koyangensis TN650 | 45 | Casein, malt extract, and yeast extract | [11] |
| Streptomyces sp. AH4 | 36 and 21 | Casein, malt extract, and yeast extract | [30] |
| Streptomyces sp. AB1 | 30 | Chicken feather | [31] |
| Streptomyces sp. MAB18 | 43 | Chicken feather and peptone | [23] |
| Streptomyces reticuli | 36 | [32] | |
| Streptomyces sp. GS-1 | 30 | Wheat bran | [33] |
| Streptomyces sp. Al-Dhabi-82 | 37 | Maltose and yeast extract | [34] |
| Streptomyces sp. DP2 | Fructose and mustard cake | [35] | |
| Streptomyces sp. CC5 | 30 | Tryptone and yeast extract | [36] |
| Streptomyces sp. CS684 | 35 | Glucose and oatmeal | [37] |
| Streptomyces sp. 594 | 113.7; 63.8; and 49.5 | Casitone and molasses | [38] |
| Streptomyces sp. K47 | Glucose and yeast extract | [39] | |
| S. violaceusniger MTCC3959 | 22.8; 62.5; 74.6; and 120.5 | Yeast extract and colloidal chitin | [40] |
| S. griseus IFO13350 | 28 and 30 | [41] | |
| S. griseorubens E44G | 35 | Glucose, peptone, yeast extract, beef extract, and wheat bran | [42] |
| Streptomyces sp. M30 | 37.1 | Soluble starch and KNO3 | [43] |
| S. exfoliatus SMF13 | 31.8 | Glucose and sodium caseinate | [44] |
| Substrates | Relative Activity (%) |
|---|---|
| Keratin | 9.95 ± 0.73 |
| HSA | 48.20 ± 4.92 |
| BSA | 62.29 ± 2.81 |
| Fibrinogen | 46.6 ± 3.98 |
| Myoglobulin | 41.37 ± 1.72 |
| Elastin | 4.87 ± 0.33 |
| Gelatin | 9.62 ± 1.15 |
| Collagen | 2.11 ± 0.33 |
| Hemoglobulin | 54.14 ± 4.00 |
| Casein | 100 ± 3.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.N.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.-L. Conversion of Fishery Waste to Proteases by Streptomyces speibonae and Their Application in Antioxidant Preparation. Fishes 2022, 7, 140. https://doi.org/10.3390/fishes7030140
Tran TN, Doan CT, Nguyen VB, Nguyen AD, Wang S-L. Conversion of Fishery Waste to Proteases by Streptomyces speibonae and Their Application in Antioxidant Preparation. Fishes. 2022; 7(3):140. https://doi.org/10.3390/fishes7030140
Chicago/Turabian StyleTran, Thi Ngoc, Chien Thang Doan, Van Bon Nguyen, Anh Dzung Nguyen, and San-Lang Wang. 2022. "Conversion of Fishery Waste to Proteases by Streptomyces speibonae and Their Application in Antioxidant Preparation" Fishes 7, no. 3: 140. https://doi.org/10.3390/fishes7030140
APA StyleTran, T. N., Doan, C. T., Nguyen, V. B., Nguyen, A. D., & Wang, S.-L. (2022). Conversion of Fishery Waste to Proteases by Streptomyces speibonae and Their Application in Antioxidant Preparation. Fishes, 7(3), 140. https://doi.org/10.3390/fishes7030140











