Incorporation of Fructooligosaccharides in Diets Influence Growth Performance, Digestive Enzyme Activity, and Expression of Intestinal Barrier Function Genes in Tropical Gar (Atractosteus tropicus) Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Larviculture
2.2. Experimental Diets
2.3. Experimental Design
2.4. Evaluation of Growth Indexes and Survival Rate
2.5. Biological Sampling
2.6. Enzyme Activities Quantification
2.7. RNA Extraction and Reverse Transcription
2.8. Gene Expression Analysis
2.9. Statistical Analysis
3. Results
3.1. Growth Indexes and Survival Rate
3.2. Digestive Enzyme Activities
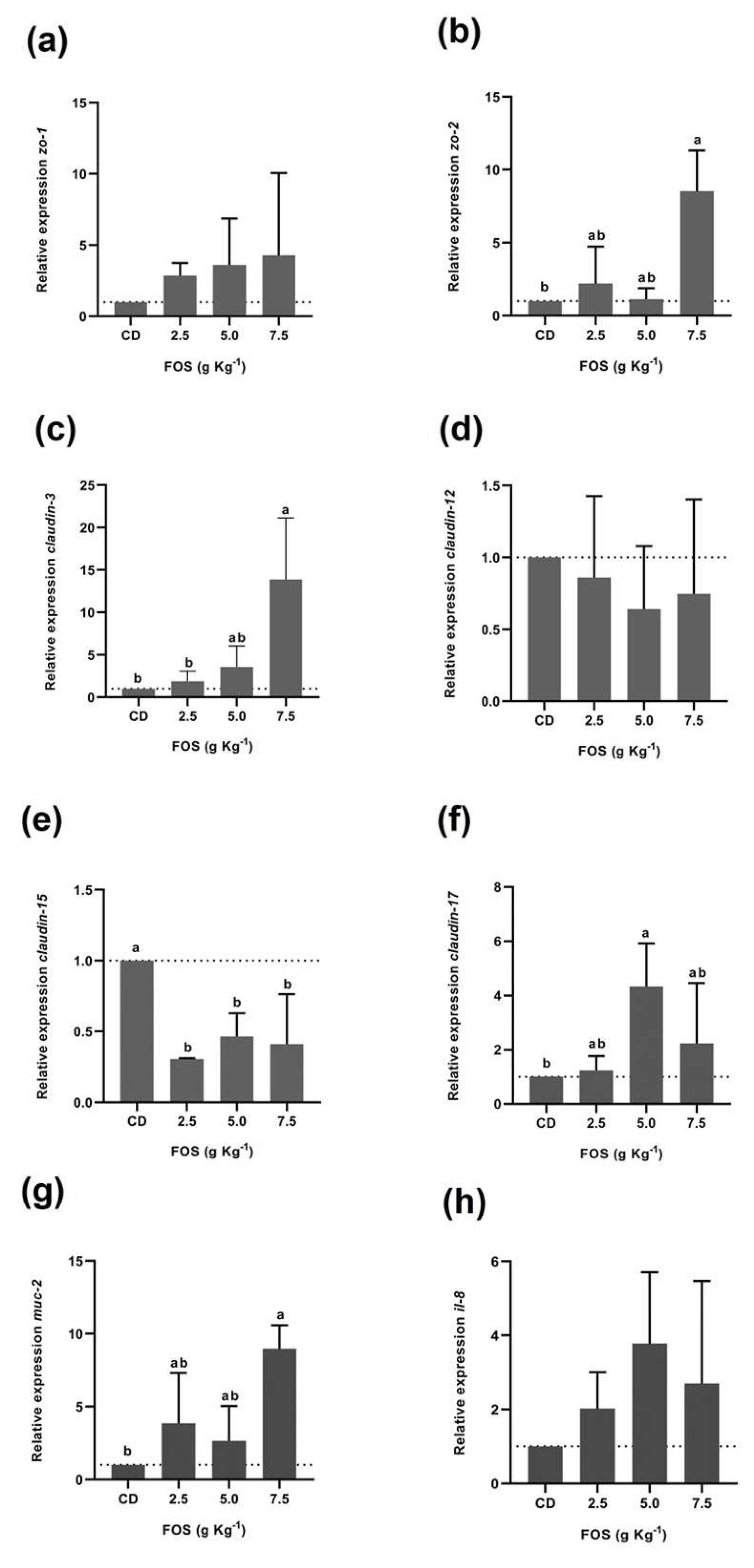
3.3. Intestinal Barrier Protein Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Márquez-Couturier, G.; Álvarez-González, C.A.; Contreras-Sánchez, W.M.; Hernández-Vidal, U.; Hernández-Franyutti, A.; Mendoza-Alfaro, R.; Goytortúa-Bores, E. Avances en la Alimentación y Nutrición del Pejelagarto Atractosteus tropicus; Avances En Nutrición Acuícola VIII; Universidad Autonóma de Nuevo León: Monterrey, Nuevo León, México, 2006; pp. 446–523. [Google Scholar]
- Frías-Quintana, C.; Domínguez-Lorenzo, J.; Álvarez-González, C.; Tovar-Ramírez, D.; Martínez-García, R. Using cornstarch in microparticulate diets for larvicultured tropical gar (Atractosteus tropicus). Fish Physiol. Biochem. 2016, 42, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Ortiz, M.; Álvarez-González, C.; Civera-Cerecedo, R.; Martínez-García, R.; Camarillo-Coop, S.; Goytortúa-Bores, E.; Peña, E.; Pérez-Morales, A. Optimum level of dietary lipids for growth, chemical composition and apparent digestibility of lipids for Atractosteus tropicus. Lat. Am. J. Aquat. Res. 2018, 46, 1073–1082. [Google Scholar] [CrossRef]
- Merrifield, D.; Dimitroglou, A.; Foey, A.; Davies, S.; Baker, R.; Bogwald, J.; Mathieu, C.; Ringo, E. The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 2010, 302, 1–18. [Google Scholar] [CrossRef]
- Ye, J.; Wang, K.; Li, F.; Sun, Y. Single or combined effects of fructo- and mannan oligosaccharide supplements and Bacillus clausii on the growth, feed utilization, body composition, digestive enzyme activity, innate immune response and lipid metabolism of the Japanese flounder Paralichthys olivaceus. Aquac. Nutr. 2011, 17, e902–e911. [Google Scholar] [CrossRef]
- Gibson, G.; Roberfroid, M. Dietary modulation of the human colonic microbiota-introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Ringo, E.; Olsen, R.; Gifstad, T.; Dalmo, R.; Amlund, H.; Hemre, G.; Bakke, A. Prebiotics in aquaculture: A review. Aquac. Nutr. 2010, 16, 117–136. [Google Scholar] [CrossRef]
- Kumar, C.; Sripada, S.; Poornachandra, Y. Status and future prospects of frutooligosaccharides as nutraceuticals. In Role of Materials Science in Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 451–503. [Google Scholar]
- Ortiz, L.; Rebolé, A.; Velasco, S.; Rodríguez, L.; Treviño, J.; Tejedor, J.; Alzueta, C. Effects of inulin and fructooligosaccharides on growth performance, body chemical composition and intestinal microbiota of farmed rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2012, 19, 475–482. [Google Scholar] [CrossRef]
- Soleimani, N.; Hoseinifar, S.; Merrifield, D.; Barati, M.; Abadi, Z. Dietary supplementation of fructooligosaccharide (FOS) improves the innate immune response, stress resistance, digestive enzyme activities and growth performance of Caspian roach (Rutilus rutilus) fry. Fish Shellfish Immunol. 2012, 32, 316–321. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, W.; Li, H.; Xu, W.; He, J.; Li, X.; Jian, G. Effects of dietary supplementation of fructooligosaccharide on growth performance, body composition, intestinal enzymes activities and histology of blunt snout bream (Megalobrama amblycephala) fingerlings. Aquac. Nutr. 2013, 19, 886–894. [Google Scholar] [CrossRef]
- Akrami, R.; Iri, Y.; Rostami, H.; Mansour, M. Effect of dietary supplementation of frutooligosaccharide (FOS) on growth performance, survival, lactobacillus bacterial population and hemato-immunological parameters of stella sturgeon (Acipenser stellatus) juvenile. Fish Shellfish Immunol. 2013, 35, 1235–1239. [Google Scholar] [CrossRef]
- Sepúlveda-Quiroz, C.; Peña-Marín, E.; Pérez-Morales, A.; Martínez-García, R.; Alvarez-Villagomez, C.; Maytorena-Verdugo, C.; Camarillo-Coop, S.; Vissio, P.; Sirkin, D.; Tovar-Ramírez, D.; et al. Fructooligosaccharide supplementation in diets for tropical gar (Atractosteus tropicus) juveniles: Effects on morphophysiology and intestinal barrier function. Aquac. Res. 2020, 52, 37–50. [Google Scholar] [CrossRef]
- Niklasson, L.; Sundh, H.; Fridell, F.; Taranger, G.; Sundell, K. Disturbance of the intestinal mucosal immune system of farmed Atlantic salmon (Salmo salar), in response to long-term hypoxic conditions. Fish Shellfish Immunol. 2011, 31, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, W.; Liu, Y.; Jiang, W.; Kuang, S.; Jiang, J.; Tnag, L.; Wu, P.; Tang, W.; Zhang, Y.; et al. Dietary phenylalanine improved intestinal barrier health in young grass carp (Ctenopharyngodon idella) is associated with increased immune status and regulated gene expression of cytokines, tight junction proteins, antioxidant enzymes and related signalling molecules. Fish Shellfish Immunol. 2015, 45, 495–509. [Google Scholar] [CrossRef] [PubMed]
- González-Mariscal, L.; Betanzos, A.; Nava, P.; Jaramillo, B. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003, 81, 1–44. [Google Scholar] [CrossRef]
- Álvarez-González, C.A.; Ortiz-Galindo, J.; Dumas, S.; Martínez-Díaz, S.; Hernández-Ceballos, D.; Del Alamo, T.; Moreno-Legorreta, M.; Peña-Martínez, R. Effect of Stocking Density on the Growth and Survival of Spotted Sand Bass Paralabrax maculatofasciatus Larvae in a Closed Recirculating System. J. World Aquac. Soc. 2001, 32, 151–159. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2000. [Google Scholar]
- Ricker, W. Handbook of computations for biological statistics of fish populations. Bull. Fish Res. Board Can. 1958, 119, 300. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Chem. 1976, 72, 248–254. [Google Scholar]
- Walter, H. Proteinases: Methods with hemoglobin, casein and azocoll as substrates. In Methods of Enzymatic Analysis; Verlag Chemie: Weinheim, Germany, 1984; pp. 270–277. [Google Scholar]
- Erlanger, B.; Kokowsky, N.; Cohen, W. The preparation and properties of two new chromogenic substrates of trypsin. Arch. Biochem. Biophys. 1961, 95, 271–278. [Google Scholar] [CrossRef]
- Del Mar, E.; Largman, C.; Brodrick, J.; Geokas, M. A sensitive new substrate for chymotrypsin. Anal. Biochem. 1979, 99, 316–320. [Google Scholar] [CrossRef]
- Gjellesvik, D.; Lombardo, D.; Walther, B. Pancreatic bile salt dependent lipase from cod (Gadus morhua): Purification and properties. Biochim. Biophys. Acta 1992, 1124, 123–124. [Google Scholar] [CrossRef]
- Nieves-Rodríguez, K.; Álvarez-González, C.; Peña-Marín, E.; Vega-Villasante, F.; Martínez-García, R.; Camarillo-Coop, S. Effect of β-Glucans in diets on growth, survival, digestive enzyme activity, and immune system and intestinal barrier gene expression for Tropical Gar (Atractosteus tropicus) juveniles. Fishes 2018, 3, 27. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real- time quantitative PCR and the 2−ΔΔCT method. Method 2002, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Boonanuntanasarn, S.; Tiengtam, N.; Pitaksong, T.; Piromyou, P.; Teaumroong, N. Effects of inulin and Jerusalem artichoke (Helianthus tuberosus) on intestinal microbiota community and morphology of Nile tilapia (Oreochromis niloticus) fingerlings. Aquac. Nutr. 2017, 24, 712–722. [Google Scholar] [CrossRef]
- Nájera-Arzola, I.; Álvarez-González, C.; Frías-Quintana, C.; Peña, E.; Martínez-García, R.; Camarillo-Coop, S.; Méndez-Marín, O.; Gisbert, E. Evaluation of Mannan oligosaccharides (MOS) in balanced diets for tropical gar juveniles (Atractosteus Trop). Hidrobiológica 2018, 28, 239–246. [Google Scholar] [CrossRef]
- Abasubong, K.; Li, X.; Zhang, D.; Jia, E.; Xiang-Yang, Y.; Xu, C.; Liu, W. Dietary supplementation of xylooligosaccharides benefits the growth performance and lipid metabolism of common carp (Cyprinus carpio) fed hight-fat diets. Aquac. Nutr. 2018, 24, 1416–1424. [Google Scholar] [CrossRef]
- Hoseinifar, S.; Khalili, M.; Rostami, H.; Esteban, M. Dietary galactooligosaccharide affects intestinal microbiota, stress resistence, and performance of Caspian roach (Rutilus rutilus) fry. Fish Shellfish Immnunol. 2013, 35, 1416–1420. [Google Scholar] [CrossRef]
- Sunde, J.; Eiane, S.; Rustad, A.; Jensen, H.; Opstvedt, J.; Nygard, E.; Venturini, G.; Rungruangsak-Torrissen, K. Effect of fish feed processing conditions on digestive protease activities, free amino acid pools, feed conversion efficiency and growth in Atlantic salmon (Salmo salar L.). Aquac. Nutr. 2004, 10, 261–277. [Google Scholar] [CrossRef]
- Cahu, C.; Zambonino, J. Substitution of live food by formulated diets in marine fish larvae. Aquaculture 2001, 200, 61–181. [Google Scholar] [CrossRef] [Green Version]
- García-Carreño, F.; Albuquerque-Cavalcanti, C.; Navarrete del Toro, M.; Zaniboni-Filho, E. Digestive proteinases of Brycon orbignyanus (Characidae, Teleostei): Characteristics and effects of protein quality. Comp. Biochem. Physiol. 2002, 132, 343–352. [Google Scholar] [CrossRef]
- Lemieux, H.; Blier, P.; Dutil, J. Do digestive enzymes set a physiological limit on growth rate and food conversion efficiency in the Atlantic cod (Gadus Morhua)? Fish Physiol. Biochem. 1991, 20, 293–303. [Google Scholar] [CrossRef]
- Hoseinifar, S.; Khalili, M.; Sun, Z. Intestinal histomorphology, autochthonous microbiota and growth performance of the oscar (Astronotus ocellatus Agassiz, 1831) following dietary administration of xylooligosaccharide. J. Appl. Ichthyol. 2015, 32, 1137–1141. [Google Scholar] [CrossRef]
- Anguiano, M.; Pohlenz, C.; Buentello, A.; Gatlin, D. The effects of prebiotics on the digestive enzymes and gut histomorphology of red drum (Sciaenops ocellatus) and hybrid striped bass (Morone chrysops × M. saxitilis). Br. J. Nutr. 2013, 109, 623–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerezuela, R.; Meseguer, J.; Esteban, M. Effects of dietary inulin, Bacillus subtilis and microalgae on intestinal gene expression in gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 2013, 34, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Refstie, S.; Bakke-McKellep, A.M.; Penn, M.H.; Sundby, A.; Shearer, K.D.; Krogdahl, S. Capacity for digestive hydrolysis and amino acid absorption in Atlantic salmon (Salmo salar) fed diets with soybean meal or inulin with or without addition of antibiotics. Aquaculture 2006, 26, 392–406. [Google Scholar] [CrossRef]
- Buddintong, R.; Diamond, J. Aristotle revisited: The function of pyloric caeca in fish. Proc. Natl. Acad. Sci. USA 1986, 83, 8012–8014. [Google Scholar] [CrossRef] [Green Version]
- Lee, S. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Feng, L.; Jiang, W.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.; Tang, L.; Zhang, Y.; Zhou, X. The impaired intestinal mucosal immune system by valine deficiency for young grass carp (Ctenopharyngodon idella) is associated with decreasing immune status and regulating tight junction proteins transcript abundance in the intestine. Fish Shellfish Immunol. 2014, 40, 197–207. [Google Scholar] [CrossRef]
- Chen, L.; Feng, L.; Jiang, W.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.; Tang, L.; Tang, W.; Zhang, Y.; et al. Intestinal immune function, antioxidant status and tight junction proteins mRNA expression in young grass carp (Ctenopharyngodon idella) fed riboflavin deficient diet. Fish Shellfish Immunol. 2015, 47, 470–484. [Google Scholar] [CrossRef]
- Wu, P.; Zheng, X.; Zhou, X.; Jiang, W.; Liu, Y.; Jiang, J.; Kuang, S.; Tang, L.; Zhang, Y.; Feng, L. Deficiency of dietary pyridoxine disturbed the intestinal physical barrier function of young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2018, 74, 459–473. [Google Scholar] [CrossRef]
- Patel, R.; Myers, L.; Kurundkar, A.; Maheshwari, A.; Nusrat, A.; Lin, P. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am. J. Pathol. 2012, 180, 626–635. [Google Scholar] [CrossRef] [Green Version]
- Günzel, D.; Yu, A. Claudins and the Modulation of Tight Junction Permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulluwishewa, D.; Anderson, R.; McNabb, W.; Moughan, P.; Wells, J.; Roy, N. Regulation of Tight Junction Permeability by Intestinal Bacteria and Dietary Components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansson, G.; Johansson, M. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Gut Microbes. 2010, 1, 51–54. [Google Scholar] [CrossRef] [Green Version]
- Heazlewood, C.; Cook, M.; Eri, R.; Price, G.; Tauro, S.; Taupin, D.; Thornton, D.; Png, C.; Crockford, T.; Cornall, R.; et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008, 5, e54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.; Wei, B.; Wen, T.; Johansson, M.; Liu, X.; Bradford, E.; Thomsson, K.; McGee, S.; Mansour, L.; Tong, M.; et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J. Clin. Investig. 2011, 121, 1657–1666. [Google Scholar] [CrossRef] [Green Version]
- Hoseinifar, S.; Esteban, M.; Cuesta, A.; Sun, Y. Prebiotics and Fish Immune Response: A Review of Current Knowledge and Future Perspectives. Rev. Fish. Sci. Aquac. 2015, 23, 315–328. [Google Scholar] [CrossRef]

| FOS (g kg−1) | ||||
|---|---|---|---|---|
| Ingredients (g kg−1) | Control Diet | 2.5 | 5 | 7.5 |
| Fish Meal a | 305.4 | 305.4 | 305.4 | 305.4 |
| Poultry meal a | 150 | 150 | 150 | 150 |
| Pork meal a | 150 | 150 | 150 | 150 |
| Soybean meal a | 150 | 150 | 150 | 150 |
| Starch b | 123.7 | 121.2 | 118.7 | 116.2 |
| Soybean oil c | 79.9 | 79.9 | 79.9 | 79.9 |
| FOS d | 0 | 2.5 | 5 | 7.5 |
| Mineral premix e | 5 | 5 | 5 | 5 |
| Vitaminic premix e | 10 | 10 | 10 | 10 |
| Grenetin f | 20 | 20 | 20 | 20 |
| Vitamin C g | 5 | 5 | 5 | 5 |
| Vitamin E h | 1 | 1 | 1 | 1 |
| Proximate composition (g kg−1 of dry matter) | ||||
| Energy (kJ g−1) | 17.67 | 17.63 | 17.67 | 17.81 |
| Protein (%) | 43.58 | 44.51 | 43.28 | 43.79 |
| Ether extract (%) | 15.01 | 14.34 | 14.73 | 15.03 |
| Ash (%) | 15.09 | 14.74 | 14.21 | 15.18 |
| NFE 1 (%) | 26.32 | 26.41 | 27.78 | 26 |
| Target Gene | Primer Sequence (5′-3′) | Amplification Efficiency (%) | R2 | Amplicon Size (bp) | Reference |
|---|---|---|---|---|---|
| zo-1 | FW: TGTGCCTCAGATCACTCCAC RV: AAAGGCAGAGGGTTGGCTTC | 98.58 | 0.95 | 123 | This study |
| zo-2 | FW: TACCCATGGAAAATGTGCCTCA RV: CGGGGTCTCTTCACGGTAAT | 95.29 | 0.98 | 88 | This study |
| claudin-3 | FW: CCTGTATATCGGCTGGGCTG RV: TGCAAGCTAACGACTACGCA | 98.84 | 0.91 | 285 | This study |
| claudin-12 | FW: CGCAGGAAAAGGAGACCAATTT RV: CTGCTCAAAACAGCCTCCAAG | 96.06 | 0.93 | 105 | This study |
| claudin-15 | FW: ATCCCGGGACAAAGTACGAG RV:CAGATCGCTAGCAAGGCAGA | 97.63 | 0.93 | 70 | This study |
| claudin-17 | FW: GCAAACGGAATCATCCGAGC RV: TACAGCAGGAGGGCACAATG | 96.95 | 0.91 | 261 | This study |
| il-8 | FW: ATATTCACTGGTGGGCGGAG RV: GTGCGGCCTGAGATTGTTT | 94.18 | 0.96 | 369 | This study |
| muc-2 | FW: GGCCTCCTCAAGAGCACGGTG RV:TCTGCACGCTGGAGCACTCAATG | 90.94 | - | 100 | [24] |
| β-actin | FW: GGACTTTGAGCAGGAGATGG RV:GACGGAGTATTTACGCTCTGG | 89.91 | - | 355 | [24] |
| FOS (g Kg−1) | ||||
|---|---|---|---|---|
| Control Diet | 2.5 | 5 | 7.5 | |
| initial weight (g) | 0.03 ± 0.006 | 0.03 ± 0.006 | 0.03 ± 0.006 | 0.03 ± 0.006 |
| final weight (g) | 0.12 ± 0.001 b | 0.10 ± 0.009 c | 0.09 ± 0.001 d | 0.13 ± 0.0005 a |
| initial lenght (cm) | 1.69 ± 0.12 | 1.69 ± 0.12 | 1.69 ± 0.12 | 1.69 ± 0.12 |
| final lenght (cm) | 3.13 ± 0.01 a | 2.78 ± 0.04 b | 2.79 ± 0.08 b | 3.11 ± 0.02 a |
| FI (g d−1) | 0.04 ±0.004 | 0.05 ± 0.14 | 0.04 ± 0.11 | 0.06 ± 0.016 |
| WG (%) | 302.4 ± 5.01 b | 253.01 ± 30.50 c | 208.2 ± 4.31 d | 355.35 ± 1.79 a |
| SGR (% d−1) | 9.73 ± 0.09 b | 8.76 ± 0.64 c | 7.79 ± 0.10 d | 10.63 ± 0.03 a |
| CF | 0.39 ± 0.002 b | 0.49 ± 0.064 a | 0.43 ± 0.041 ab | 0.45 ± 0.008 ab |
| FCE | 23.38 ± 3.88 | 23.29 ± 8.22 | 32.18 ± 4.44 | 25.97 ± 3.05 |
| PER | 0.09 ± 0.01 ab | 0.10 ± 0.03 a | 0.06 ± 0.009 b | 0.08 ± 0.01 ab |
| S (%) | 17.33 ± 0.94 b | 22.57 ± 1.07 ab | 17.39 ± 2.04 b | 24.18 ± 1.74 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Jiménez, G.M.; Peña-Marín, E.S.; Maytorena-Verdugo, C.I.; Sepúlveda-Quiroz, C.A.; Jiménez-Martínez, L.D.; De la Rosa-García, S.; Asencio-Alcudia, G.G.; Martínez, R.; Tovar-Ramírez, D.; Galaviz, M.A.; et al. Incorporation of Fructooligosaccharides in Diets Influence Growth Performance, Digestive Enzyme Activity, and Expression of Intestinal Barrier Function Genes in Tropical Gar (Atractosteus tropicus) Larvae. Fishes 2022, 7, 137. https://doi.org/10.3390/fishes7030137
Pérez-Jiménez GM, Peña-Marín ES, Maytorena-Verdugo CI, Sepúlveda-Quiroz CA, Jiménez-Martínez LD, De la Rosa-García S, Asencio-Alcudia GG, Martínez R, Tovar-Ramírez D, Galaviz MA, et al. Incorporation of Fructooligosaccharides in Diets Influence Growth Performance, Digestive Enzyme Activity, and Expression of Intestinal Barrier Function Genes in Tropical Gar (Atractosteus tropicus) Larvae. Fishes. 2022; 7(3):137. https://doi.org/10.3390/fishes7030137
Chicago/Turabian StylePérez-Jiménez, Graciela M., Emyr Saul Peña-Marín, Claudia I. Maytorena-Verdugo, Cesar Antonio Sepúlveda-Quiroz, Luis Daniel Jiménez-Martínez, Susana De la Rosa-García, Gloria Gertrudys Asencio-Alcudia, Rafael Martínez, Dariel Tovar-Ramírez, Mario A. Galaviz, and et al. 2022. "Incorporation of Fructooligosaccharides in Diets Influence Growth Performance, Digestive Enzyme Activity, and Expression of Intestinal Barrier Function Genes in Tropical Gar (Atractosteus tropicus) Larvae" Fishes 7, no. 3: 137. https://doi.org/10.3390/fishes7030137
APA StylePérez-Jiménez, G. M., Peña-Marín, E. S., Maytorena-Verdugo, C. I., Sepúlveda-Quiroz, C. A., Jiménez-Martínez, L. D., De la Rosa-García, S., Asencio-Alcudia, G. G., Martínez, R., Tovar-Ramírez, D., Galaviz, M. A., Martínez-Burguete, T., Alvarez-González, C. A., & Alvarez-Villagomez, C. S. (2022). Incorporation of Fructooligosaccharides in Diets Influence Growth Performance, Digestive Enzyme Activity, and Expression of Intestinal Barrier Function Genes in Tropical Gar (Atractosteus tropicus) Larvae. Fishes, 7(3), 137. https://doi.org/10.3390/fishes7030137









