Assessing the Stock Dynamics of Elasmobranchii off the Southern Coast of Sicily by Using Trawl Survey Data
Abstract
1. Introduction
2. Materials and Methods
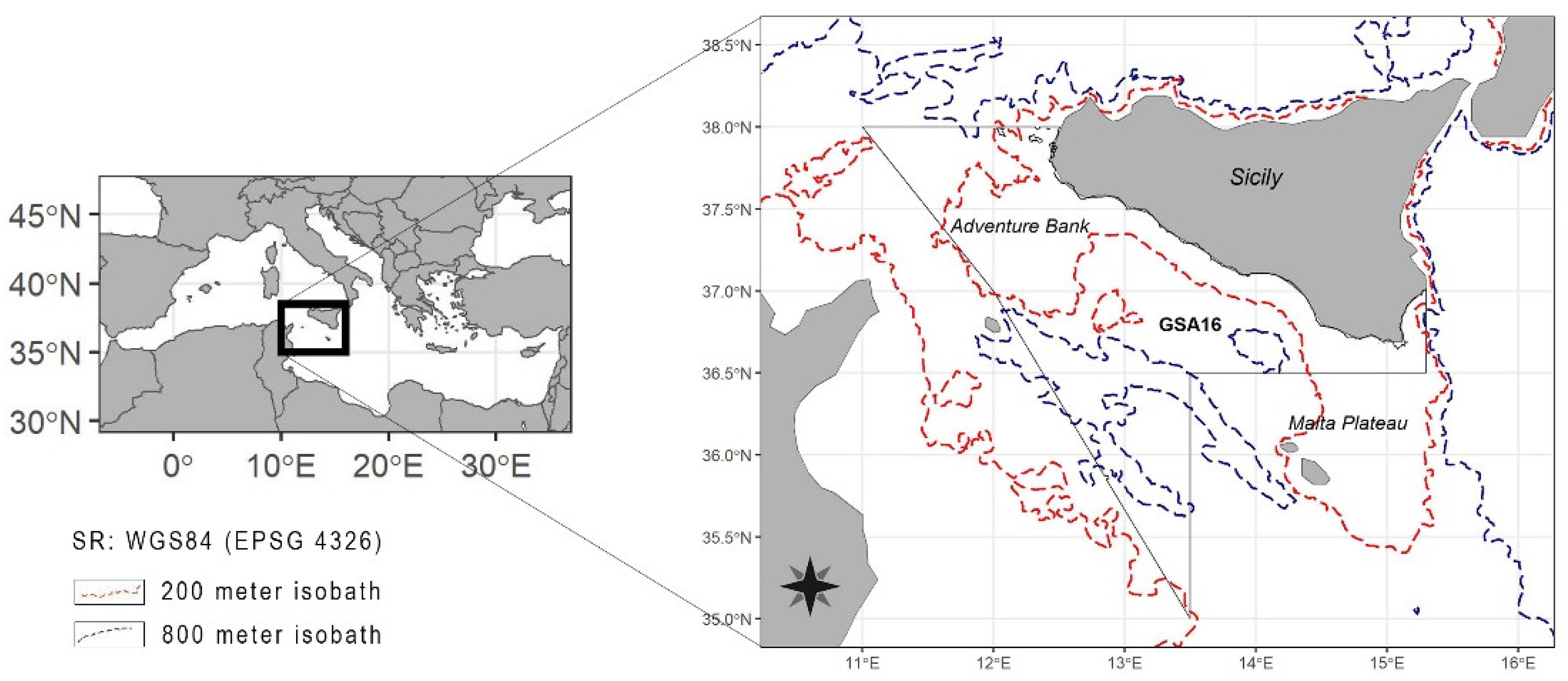
2.1. Study Area
2.2. Data Sources
2.3. Stock Assessment Models
2.4. Prior Selection
3. Results
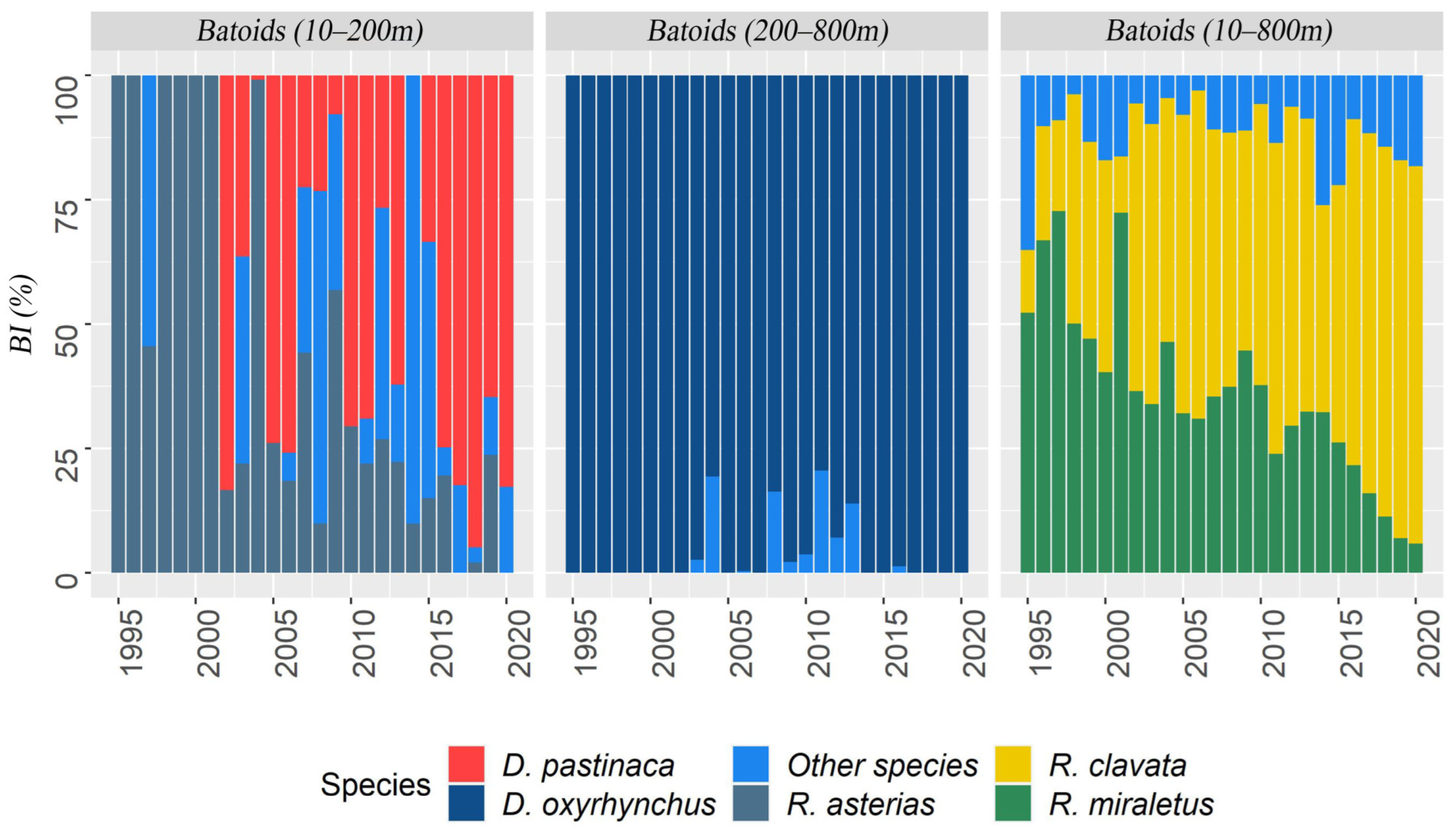
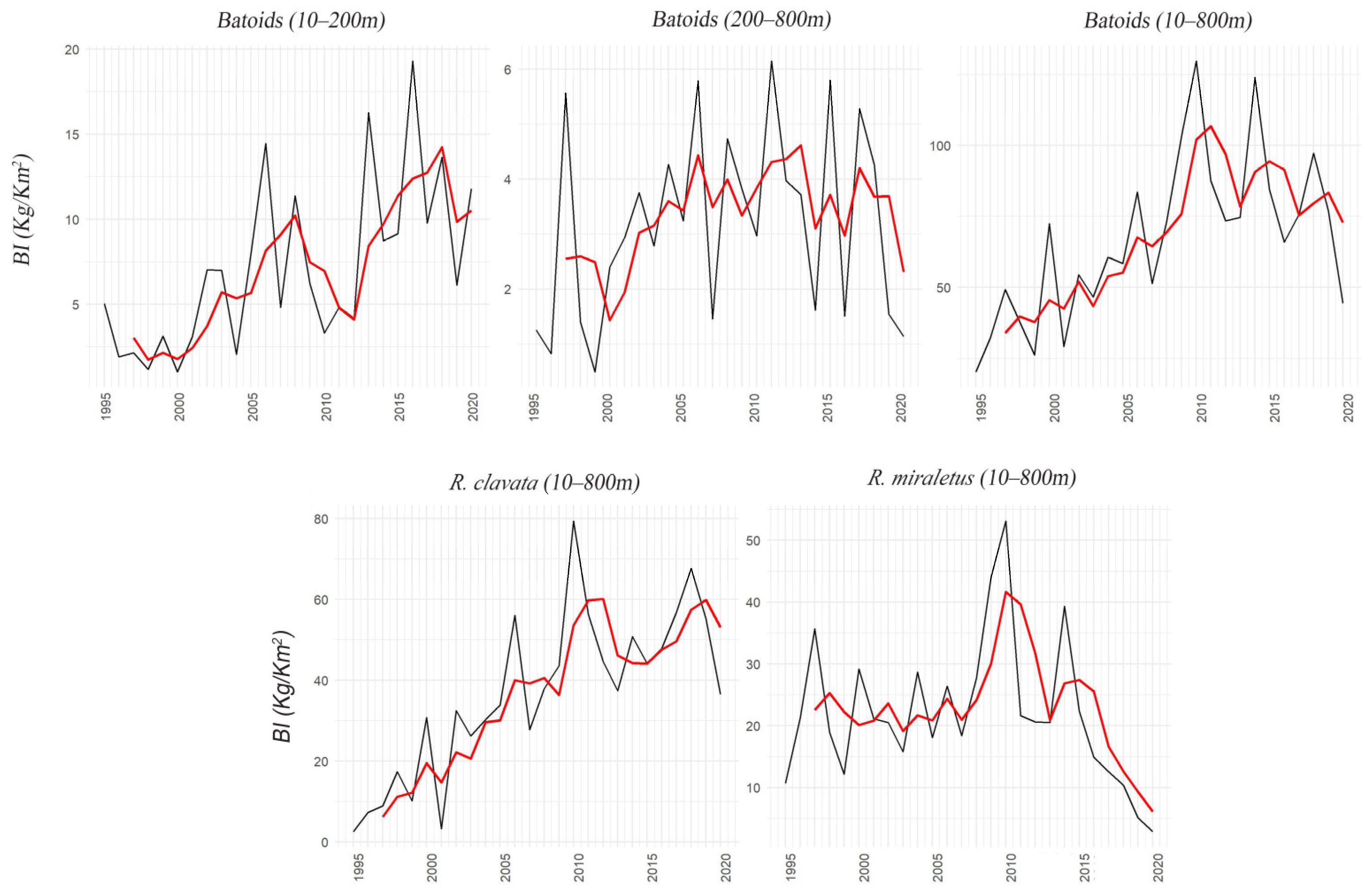
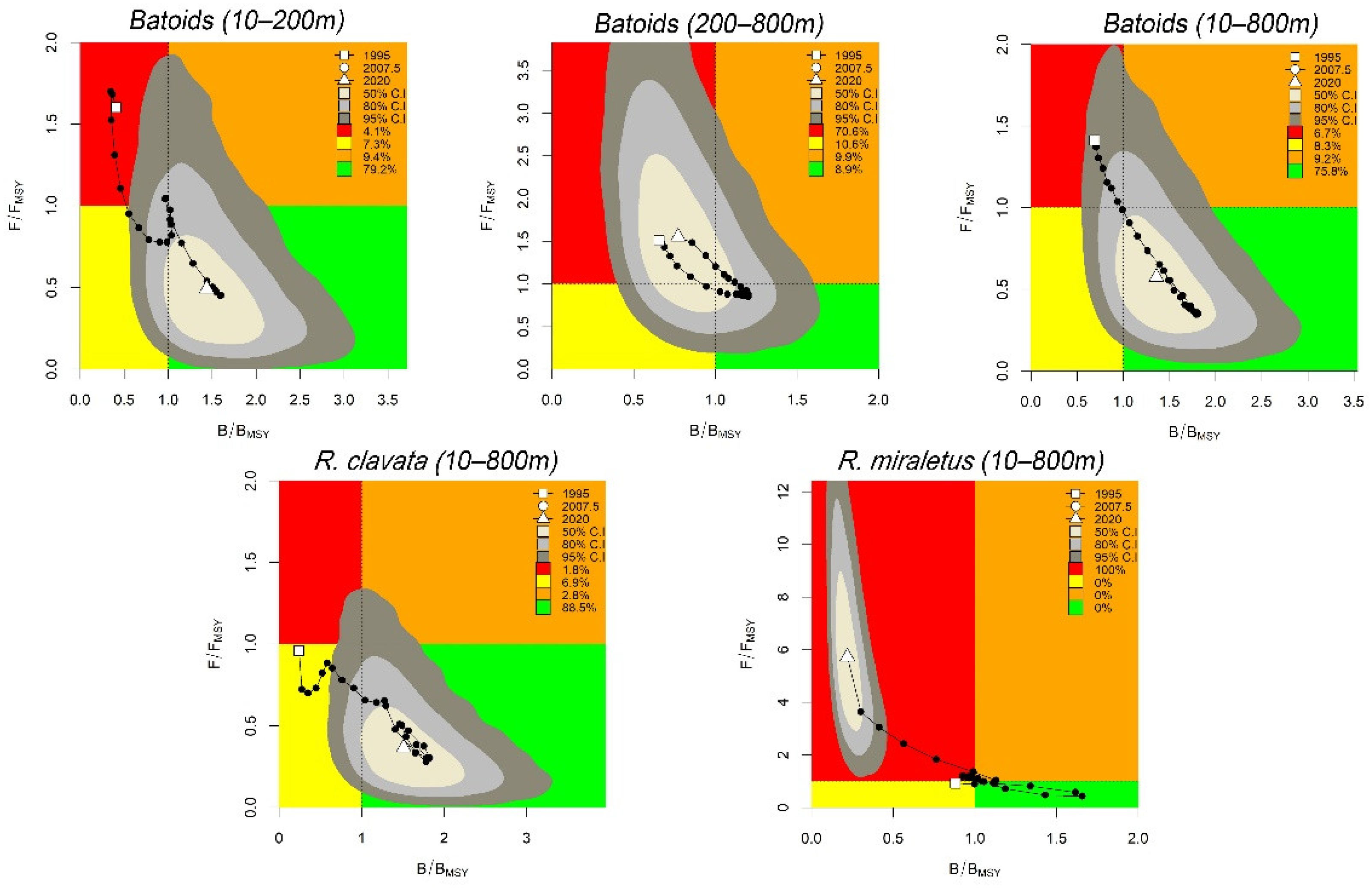
3.1. Batoids
3.2. Sharks
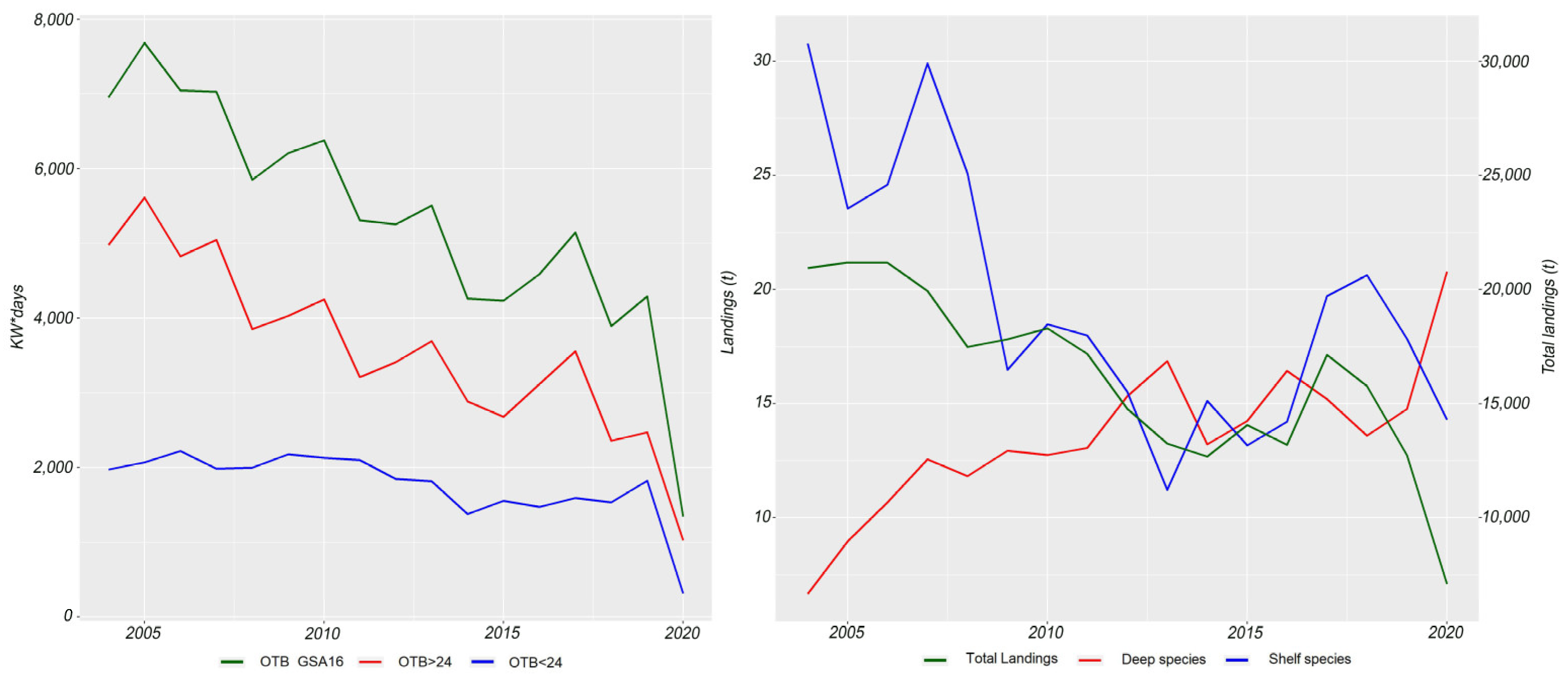
3.3. Trends in Fishing Efforts and Patterns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, J.D.; Bonfil, R.; Dulvy, N.K.; Walker, P.A. The effects of fishing on sharks, rays, and chimaeras (elasmobranchs), and the implications for marine ecosystems. ICES J. Mar. Sci. 2000, 57, 476–494. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Pacoureau, N.; Rigby, C.L.; Pollom, R.A.; Jabado, R.W.; Ebert, D.A.; Finucci, B.; Pollock, C.M.; Cheok, J.; Derrick, D.H. Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr. Biol. 2021, 31, 4773–4787. [Google Scholar] [CrossRef]
- Pacoureau, N.; Rigby, C.L.; Kyne, P.M.; Sherley, R.B.; Winker, H.; Carlson, J.K.; Fordham, S.V.; Barreto, R.; Fernando, D.; Francis, M.P.; et al. Half a century of global decline in oceanic sharks and rays. Nature 2021, 589, 567–571. [Google Scholar] [CrossRef]
- Cavanagh, R.D.; Gibson, C. Overview of the Conservation Status of Cartilaginous Fishes (Chrondrichthyans) in the Mediterranean Sea; The World Conservation Union (IUCN): Gland, Switzerland; Malaga, Spain, 2007; Volume 3, p. 42. [Google Scholar]
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.K.; Fordham, S.V.; Francis, M.P. Extinction risk and conservation of the world’s sharks and rays. eLife 2014, 3, e00590. [Google Scholar] [CrossRef]
- Ferretti, F.; Myers, R.A.; Serena, F.; Lotze, H.K. Loss of large predatory sharks from the Mediterranean Sea. Conserv. Biol. 2008, 22, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Bradai, M.N.; Saidi, B.; Enajjar, S. Overview on Mediterranean shark’s fisheries: Impact on the biodiversity. In Marine Ecology–Biotic and Abiotic Interactions; Turkoglu, M., Onal, U., Ismen, A., Eds.; IntechOpen: London, UK, 2018; pp. 211–230. [Google Scholar] [CrossRef]
- Cashion, M.S.; Bailly, N.; Pauly, D. Official catch data underrepresent shark and ray taxa caught in Mediterranean and Black Sea fisheries. Mar. Policy 2019, 105, 1–9. [Google Scholar] [CrossRef]
- Swift, D.G.; Portnoy, D.S. Identification and Delineation of Essential Habitat for Elasmobranchs in Estuaries on the Texas Coast. Estuar. Coasts 2021, 44, 788–800. [Google Scholar] [CrossRef]
- Espinoza, M.; Araya-Arce, T.; Chaves-Zamora, I.; Chinchilla, I.; Cambra, M. Monitoring elasmobranch assemblages in a data-poor country from the Eastern Tropical Pacific using baited remote underwater video stations. Sci. Rep. 2020, 10, 17175. [Google Scholar] [CrossRef]
- Delaval, A.; Wagner, C.I.; Schwanck, T.; Wood, F.R.; Jones, C.S.; Hoarau, G.; Noble, L.R. Endangered Coastal Elasmobranchs of the North-East Atlantic. In Reference Module in Earth Systems and Environmental Sciences; Elsevier Inc.: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Barausse, A.; Correale, V.; Curkovic, A.; Finotto, L.; Riginella, E.; Visentin, E.; Mazzoldi, C. The role of fisheries and the environment in driving the decline of elasmobranchs in the northern Adriatic Sea. ICES J. Mar. Sci. 2014, 71, 1593–1603. [Google Scholar] [CrossRef]
- Erguden, D.; Kabasakal, H.; Ayas, D. Fisheries bycatch and conservation priorities of young sharks (Chondrichthyes: Elasmobranchii) in the Eastern Mediterranean. Zool. Middle East 2022, 1–10. [Google Scholar] [CrossRef]
- Fakıoğlu, Y.E.; Özbilgin, H.; Gökçe, G.; Herrmann, B. Effect of ground gear modification on bycatch of rays in Mediterranean bottom trawl fishery. Ocean Coast. Manag. 2022, 223, 106134. [Google Scholar] [CrossRef]
- Colloca, F.; Cardinale, M.; Maynou, F.; Giannoulaki, M.; Scarcella, G.; Jenko, K.; Bellido, J.M.; Fiorentino, F. Rebuilding Mediterranean fisheries: A new paradigm for ecological sustainability. Fish Fish. 2013, 14, 89–109. [Google Scholar] [CrossRef]
- Fiorentino, F.; Vitale, S. How Can We Reduce the Overexploitation of the Mediterranean Resources? Front. Mar. Sci. 2021, 8, 674633. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Allen, D.J.; Ralph, G.M.; Walls, R.H. The Conservation Status of Sharks, Rays and Chimaeras in the Mediterranean Sea [Brochure]; IUCN Centre for Mediterranean Cooperation: Malaga, Spain, 2016; p. 14. [Google Scholar]
- FAO. The State of Mediterranean and Black Sea Fisheries 2020; General Fisheries Commission for the Mediterranean: Rome, Italy, 2020. [CrossRef]
- Froese, R.; Demirel, N.; Coro, G.; Kleisner, K.M.; Winker, H. Estimating fisheries reference points from catch and resilience. Fish Fish. 2017, 18, 506–526. [Google Scholar] [CrossRef]
- Dowling, N.A.; Smith, A.D.M.; Smith, D.C.; Parma, A.M.; Dichmont, C.M.; Sainsbury, K.; Wilson, J.; Dougherty, D.T.; Cope, J.M. Generic solutions for data-limited fishery assessments are not so simple. Fish Fish. 2018, 20, 174–188. [Google Scholar] [CrossRef]
- Bonfil, R. Fishery stock assessment models and their application to sharks. In Management Techniques for Elasmobranch Fisheries; Musick, J.A., Bonfil, R., Eds.; FAO Fisheries Technical Paper; Food and Agriculture Organization: Rome, Italy, 2005; Volume 474, pp. 154–181. [Google Scholar]
- Ferretti, F.; Myers, R.A. By-catch of sharks in the Mediterranean Sea: Available mitigation tools. In The Proceedings of the International Workshop on Mediterranean Cartilaginous Fish with Emphasis on Southern and Eastern Mediterranean; Başusta, N., Keskin, Ç., Serena, F., Seret, B., Eds.; Turkish Marine Research Foundation: Istanbul, Turkey, 2006; pp. 149–161. [Google Scholar]
- Marino, I.A.M.; Finotto, L.; Colloca, F.; Di Lorenzo, M.; Gristina, M.; Farrell, E.D.; Zane, L.; Mazzoldi, C. Resolving the ambiguities in the identification of two smooth-hound sharks (Mustelus mustelus and Mustelus punctulatus) using genetics and morphology. Mar. Biodivers. 2017, 48, 1551–1562. [Google Scholar] [CrossRef]
- Bargnesi, F.; Lucrezi, S.; Ferretti, F. Opportunities from citizen science for shark conservation, with a focus on the Mediterranean Sea. Eur. Zool. J. 2020, 87, 20–34. [Google Scholar] [CrossRef]
- Colloca, F.; Enea, M.; Ragonese, S.; Di Lorenzo, M. A century of fishery data documenting the collapse of smooth-hounds (Mustelus spp.) in the Mediterranean Sea. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 1145–1155. [Google Scholar] [CrossRef]
- Falsone, F.; Scannella, D.; Geraci, M.L.; Vitale, S.; Colloca, F.; Di Maio, F.; Milisenda, G.; Gancitano, V.; Bono, G.; Fiorentino, F. Identification and characterization of trammel net métiers: A case study from the southwestern Sicily (Central Mediterranean). Reg. Stud. Mar. Sci. 2020, 39, 101419. [Google Scholar] [CrossRef]
- GFCM. Report of the Workshop on Stock Assessment of Selected Species of Elasmobranchs in the GFCM Area; DGMARE: Brussels, Belgium, 2011; p. 32. Available online: https://www.academia.edu/8267058/Report_of_the_Workshop_on_Stock_Assessment_of_selected_species_of_Elasmobranchs_in_the_GFCM_area (accessed on 20 April 2022).
- GFCM. Fourth Meeting of the Subregional Group on Stock Assessment in the Black Sea (SGSABS); GFCM: Burgas, Bulgaria, 2016; p. 28. Available online: http://www.fao.org/gfcm/technical-meetings/detail/en/c/879801 (accessed on 23 April 2022).
- GFCM. Report of the Seventh Meeting of the Working Group on the Black Sea; GFCM: Burgas, Bulgaria, 2018; p. 114. Available online: http://www.fao.org/gfcm/technical-meetings/detail/en/c/1156493/ (accessed on 12 April 2022).
- Oliver, S.; Braccini, M.; Newman, S.J.; Harvey, E.S. Global patterns in the bycatch of sharks and rays. Mar. Policy 2015, 54, 86–97. [Google Scholar] [CrossRef]
- Tiralongo, F.; Messina, G.; Lombardo, B.M. Discards of elasmobranchs in a trammel net fishery targeting cuttlefish, Sepia officinalis Linnaeus, 1758, along the coast of Sicily (central Mediterranean Sea). Reg. Stud. Mar. Sci. 2018, 20, 60–63. [Google Scholar] [CrossRef]
- Tiralongo, F.; Mancini, E.; Ventura, D.; De Malerbe, S.; De Mendoza, F.; Sardone, M.; Arciprete, R.; Massi, D.; Marcelli, M.; Fiorentino, F.; et al. Commercial catches and discards composition in the central Tyrrhenian Sea: A multispecies quantitative and qualitative analysis from shallow and deep bottom trawling. Med. Mar. Sci. 2021, 22, 521–531. [Google Scholar] [CrossRef]
- Ragonese, S.; Vitale, S.; Dimech, M.; Mazzola, S. Abundances of demersal sharks and chimaera from 1994–2009 scientific surveys in the central Mediterranean Sea. PLoS ONE 2013, 8, e74865. [Google Scholar] [CrossRef]
- Serena, F.; Abella, A.J.; Bargnesi, F.; Barone, M.; Colloca, F.; Ferretti, F.; Fiorentino, F.; Jenrette, J.; Moro, S. Species diversity, taxonomy and distribution of Chondrichthyes in the Mediterranean and Black Sea. Eur. Zool. J. 2020, 87, 497–536. [Google Scholar] [CrossRef]
- Carugati, L.; Melis, R.; Cariani, A.; Cau, A.; Crobe, V.; Ferrari, A.; Follesa, M.C.; Geraci, M.L.; Iglésias, S.P.; Pesci, P.; et al. Combined COI barcode-based methods to avoid mislabelling of threatened species of deep-sea skates. Anim. Conserv. 2022, 25, 38–52. [Google Scholar] [CrossRef]
- Bellodi, A.; Benvenuto, A.; Melis, R.; Mulas, A.; Barone, M.; Barría, C.; Cariani, A.; Carugati, L.; Chatzispyrou, A.; Desrochers, M.; et al. Call me by my name: Unravelling the taxonomy of the gulper shark genus Centrophorus in the Mediterranean Sea through an integrated taxonomic approach. Zool. J. Linn. Soc. 2022, 20, 1–26. [Google Scholar] [CrossRef]
- Marino, I.A.; Riginella, E.; Cariani, A.; Tinti, F.; Farrell, E.D.; Mazzoldi, C.; Zane, L. New molecular tools for the identification of 2 endangered smooth-hound sharks, Mustelus mustelus and Mustelus punctulatus. J. Hered. 2015, 106, 123–130. [Google Scholar] [CrossRef]
- Frodella, N.; Cannas, R.; Velonà, A.; Carbonara, P.; Farrell, E.D.; Fiorentino, F.; Follesa, M.C.; Garofalo, G.; Hemida, F.; Mancusi, C.; et al. Population connectivity and phylogeography of the Mediterranean endemic skate Raja polystigma and evidence of its hybridization with the parapatric sibling R. montagui. Mar. Ecol. Prog. Ser. 2016, 554, 99–113. [Google Scholar] [CrossRef]
- Dell’Apa, A.; Pennino, M.G.; Bonzek, C.F. Modeling the habitat distribution of spiny dogfish (Squalus acanthias), by sex, in coastal waters of the northeastern United States. Fish. Bull. 2017, 115, 89. [Google Scholar] [CrossRef]
- Williams, T.; Helle, K.; Aschan, M. The distribution of chondrichthyans along the northern coast of Norway. ICES J. Mar. Sci. 2008, 65, 1161–1174. [Google Scholar] [CrossRef][Green Version]
- Winker, H.; Carvalho, F.; Kerwath, S. Age-structured biomass dynamics of north Atlantic shortfin mako with implications for the interpretation of surplus production models. Collect. Vol. Sci. Pap. ICCAT 2020, 76, 316–336. [Google Scholar]
- Papaconstantinou, C.; Farrugio, H. Fisheries in the Mediterranean. Med. Mar. Sci. 2000, 1, 5–18. [Google Scholar] [CrossRef]
- Di Maio, F.; Geraci, M.L.; Scannella, D.; Russo, T.; Fiorentino, F. Evaluation of the Economic Performance of Coastal Trawling off the Southern Coast of Sicily (Central Mediterranean Sea). Sustainability 2022, 14, 4743. [Google Scholar] [CrossRef]
- Levi, D.; Andreoli, M.G.; Giusto, G.B. An analysis based on trawl-survey data of the state of the ‘Italian’stock of Mullus barbatus in the Sicilian Channel, including management advice. Fish. Res. 1994, 17, 333–341. [Google Scholar] [CrossRef]
- Abella, A.; Belluscio, A.; Bertrand, J.; Carbonara, P.L.; Giordano, D.; Sbrana, M.; Zamboni, A. Use of MEDITS trawl survey data and commercial fleet information for the assessment of some Mediterranean demersal resources. Aquat. Living Resour. 1999, 12, 155–166. [Google Scholar] [CrossRef][Green Version]
- Relini, G. Demersal trawl surveys in Italian seas: A short review. Actes Colloq.-Ifremer 2000, 26, 46–75. [Google Scholar]
- Bertrand, J.A.; de Sola, L.G.; Papaconstantinou, C.; Relini, G.; Souplet, A. The general specifications of the MEDITS surveys. Sci. Mar. 2002, 66, 9–17. [Google Scholar] [CrossRef]
- Spedicato, M.T.; Massutí, E.; Mérigot, B.; Tserpes, G.; Jadaud, A.; Relini, G. The MEDITS trawl survey specifications in an ecosystem approach to fishery management. Sci. Mar. 2019, 83, 9–20. [Google Scholar] [CrossRef]
- D’iglio, C.; Albano, M.; Tiralongo, F.; Famulari, S.; Rinelli, P.; Savoca, S.; Spanò, N.; Capillo, G. Biological and ecological aspects of the blackmouth catshark (Galeus melastomus Rafinesque, 1810) in the southern Tyrrhenian sea. J. Mar. Sci. Eng. 2021, 9, 967. [Google Scholar] [CrossRef]
- Aldebert, Y. Demersal resources of the Gulf of Lions (NW Mediterranean). Impact of exploitation on fish diversity. Vie Milieu/Life Environ. 1997, 47, 275–284. [Google Scholar]
- Jukic-Peladic, S.; Vrgoc, N.; Krstulovic-Sifner, S.; Piccinetti, C.; Piccinetti-Manfrin, G.; Marano, G.; Ungaro, U. Long-term changes in demersal resources of the Adriatic Sea: Comparison between trawl surveys carried out in 1948 and 1998. Fish. Res. 2001, 53, 95–104. [Google Scholar] [CrossRef]
- Geraci, M.L.; Ragonese, S.; Norrito, G.; Scannella, D.; Falsone, F.; Vitale, S. A Tale on the Demersal and Bottom Dwelling Chondrichthyes in the South of Sicily South Sicily through 20 Years of Scientific Survey. In Chondrichthyes—Multidisciplinary Approach; Rodrigues-Filho, L.F., de Luna Sales, J.B., Eds.; IntechOpen: London, UK, 2017; pp. 13–37. [Google Scholar] [CrossRef]
- Marongiu, M.F.; Porcu, C.; Bellodi, A.; Cannas, R.; Cau, A.; Cuccu, D.; Cau, A.; Cuccu, D.; Mulas, A.; Follesa, M.C. Temporal dynamics of demersal chondrichthyan species in the central western Mediterranean Sea: The case study in Sardinia Island. Fish. Res. 2017, 193, 81–94. [Google Scholar] [CrossRef]
- Froese, R.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.C.; Dimarchopoulou, D.; Scarcella, G.; Palomares, M.L.D.; Dureuil, M.; Pauly, D. Estimating stock status from relative abundance and resilience. ICES J. Mar. Sci. 2020, 77, 527–538. [Google Scholar] [CrossRef]
- Jarboui, O.; Ceriola, L.; Fiorentino, F. Current fisheries management in the Strait of Sicily and progress towards an ecosystem approach. In Transition towards an Ecosystem Approach to Fisheries in the Mediterranean Sea—Lessons Learned through Selected Case Studies; Vasconcellos, M., Ünal, V., Eds.; FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2022; Volume 681, pp. 147–162. [Google Scholar]
- Di Lorenzo, M.; Sinerchia, M.; Colloca, F. The North sector of the Strait of Sicily: A priority area for conservation in the Mediterranean Sea. Hydrobiol. 2018, 821, 235–253. [Google Scholar] [CrossRef]
- GFCM. Establishment of Geographical Sub-Areas in the GFCM Area Amending the Resolution; GFCM Resolution RES-GFCM/33/2009/2; GFCM: Rome, Italy, 2009.
- Garofalo, G.; Quattrocchi, F.; Bono, G.; Di Lorenzo, M.; Di Maio, F.; Falsone, F.; Gancitano, V.; Geraci, M.L.; Lauria, V.; Massi, D.; et al. What is in our seas? Assessing anthropogenic litter on the seafloor of the central Mediterranean Sea. Environ. Pollut. 2020, 266, 115213. [Google Scholar] [CrossRef] [PubMed]
- Milisenda, G.; Vitale, S.; Massi, D.; Enea, M.; Gancitano, V.; Giusto, G.B.; Badalucco, C.; Gristina, M.; Garofalo, G.; Fiorentino, F. Spatio-temporal composition of discard associated with the deep water rose shrimp fisheries (Parapenaeus longirostris, Lucas 1846) in the south-central Mediterranean Sea. Med. Mar. Sci. 2017, 18, 53–63. [Google Scholar] [CrossRef]
- Geraci, M.L.; Colloca, F.; Di Maio, F.; Falsone, F.; Fiorentino, F.; Sardo, G.; Scannella, D.; Gancitano, V.; Vitale, S. How is artificial lighting affecting the catches in deep water rose shrimp trawl fishery of the Central Mediterranean Sea? Ocean Coast. Manag. 2021, 215, 105970. [Google Scholar] [CrossRef]
- Pinello, D.; Gee, J.; Accadia, P.; Sabatella, E.C.; Vitale, S.; Polymeros, K.; Fiorentino, F. Efficiency of shallow- and deep-water trawling in the Mediterranean and its implications for discard reduction. Sci. Mar. 2018, 82, 97. [Google Scholar] [CrossRef]
- Geraci, M.L.; Ragonese, S.; Scannella, D.; Falsone, F.; Gancitano, V.; Mifsud, J.; Gambin, M.; Said, A.; Vitale, S. Batoid Abundances, Spatial Distribution and Life History Traits in the Strait of Sicily (Central Mediterranean Sea): Bridging a Knowledge Gap through Three Decades of Survey. Animals 2021, 11, 2189. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, M.J.; Overholtz, W.J.; Link, J.S. Aggregate surplus production models for demersal fishery resources of the Gulf of Maine. Mar. Ecol. Prog. Ser. 2021, 459, 247–258. [Google Scholar] [CrossRef]
- Demirel, N.; Zengin, M.; Ulman, A. First large-scale eastern Mediterranean and Black Sea stock assessment reveals a dramatic decline. Front. Mar. Sci. 2020, 7, 103. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. Available online: www.fishbase.org (accessed on 2 February 2022).
- Colloca, F.; Carrozzi, V.; Simonetti, A.; Di Lorenzo, M. Using local ecological knowledge of fishers to reconstruct abundance trends of elasmobranch populations in the Strait of Sicily. Front. Mar. Sci. 2020, 7, 508. [Google Scholar] [CrossRef]
- Barbato, M.; Barría, C.; Bellodi, A.; Bonanomi, S.; Borme, D.; Ćetković, I.; Colloca, F.; Colmenero, A.I.; Crocetta, F.; De Carlo, F.; et al. The use of fishers’ Local Ecological Knowledge to reconstruct fish behavioural traits and fishers’ perception of conservation relevance of elasmobranchs in the Mediterranean Sea. Mediterr. Mar. Sci. 2021, 22, 603. [Google Scholar] [CrossRef]
- General Fisheries Commission for the Mediterranean (GFCM) 2002. Deepwater Red Shrimp in the Eastern-Central Mediterranean Sea Week, Online Presentation 7–11 February 2002. Available online: https://www.fao.org/gfcm/meetings/info/en/c/1469548/ (accessed on 19 April 2022).
- Marsaglia, L.; Garofalo, G.; Vielmini, I.; Fiorentino, F. Preliminary analysis of bottom trawling fishing effort and abundance of European hake (Merluccius merluccius) and deep-water rose shrimp (Parapaeneus longirostris) inside and outside the East of Adventure Bank Fisheries Restricted Area. In Sub Regional Committee—Central Mediterranean; GFCM: Rome, Italy, 2022. [Google Scholar]
- Sguotti, C.; Lynam, C.P.; García-Carreras, B.; Ellis, J.R.; Engelhard, G.H. Distribution of skates and sharks in the North Sea: 112 years of change. Glob. Chang. Biol. 2016, 22, 2729–2743. [Google Scholar] [CrossRef]
- Pollom, R.; Cheok, J.; Pacoureau, N.; Gledhill, K.S.; Kyne, P.M.; Ebert, D.A.; Jabado, R.W.; Herman, K.B.; Bennett, R.H.; Silva, C.; et al. Overfishing and Climate Change Elevate Extinction Risk of Endemic Sharks and Rays in the Southwest Indian Ocean Hotspot. Res. Sq. Platf. 2021, in press. [Google Scholar] [CrossRef]
- Ordines, F.; Massutí, E.; Moranta, J.; Quetglas, A.; Guijarro, B.; Fliti, K. Balearic Islands vs. Algeria: Two nearby western Mediterranean elasmobranch assemblages with different oceanographic scenarios and fishing histories. Sci. Mar. 2011, 15, 707–717. [Google Scholar] [CrossRef]
- Ligas, A.; Osio, G.C.; Sartor, P.; Sbrana, M.; De Ranieri, S. Long-term trajectory of some elasmobranch species off the Tuscany coasts (NW Mediterranean) from 50 years of catch data. Sci. Mar. 2013, 77, 119–127. [Google Scholar] [CrossRef]
- Tsikliras, A.C.; Touloumis, K.; Pardalou, A.; Adamidou, A.; Keramidas, I.; Orfanidis, G.A.; Dimarchopoulou, D.; Koutrakis, M. Status and Exploitation of 74 Un-Assessed Demersal Fish and Invertebrate Stocks in the Aegean Sea (Greece) Using Abundance and Resilience. Front. Mar. Sci. 2021, 7, 1210. [Google Scholar] [CrossRef]
- Gristina, M.; Bahri, T.; Fiorentino, F.; Garofalo, G. Comparison of demersal fish assemblages in three areas of the Strait of Sicily under different trawling pressure. Fish. Res. 2006, 81, 60–71. [Google Scholar] [CrossRef]
- Massuti, E.; Moranta, J. Demersal assemblages and depth distribution of elasmobranchs from the continental shelf and slope off the Balearic Islands (western Mediterranean). ICES J. Mar. Sci. 2003, 60, 753–766. [Google Scholar] [CrossRef]
- Rey, J.; de Sola, L.G.; Massutı’, E. Distribution and biology of the blackmouth catshark Galeus melastomus in the Alboran Sea (Southwestern Mediterranean). J. Northwest Atl. Fish. Sci. 2005, 35, 215–223. [Google Scholar] [CrossRef]
- García-Rodríguez, M.; Abelló, P.; Fernández, A.; Esteban, A. Demersal Assemblages on the Soft Bottoms off the Catalan-Levante Coast of the Spanish Mediterranean. J. Mar. Biol. 2011, 2011, 976396. [Google Scholar] [CrossRef][Green Version]
- Gouraguine, A.; Hidalgo, M.; Moranta, J.; Bailey, D.M.; Ordines, F.; Guijarro, B.; Valls, M.; Barberá, C.; De Mesa, A. Elasmobranch spatial segregation in the western Mediterranean. Sci. Mar. 2011, 75, 653–664. [Google Scholar] [CrossRef]
- Follesa, M.C.; Marongiu, M.F.; Zupa, W.; Bellodi, A.; Cau, A.; Cannas, R.; Colloca, F.; Djurovic, M.; Isajlovic, I.; Jadaud, A.; et al. Spatial variability of Chondrichthyes in the northern Mediterranean. Sci. Mar. 2019, 83, 81–100. [Google Scholar] [CrossRef]
- Osgood, G.J.; White, E.R.; Baum, J.K. Effects of climate-change-driven gradual and acute temperature changes on shark and ray species. J. Anim. Ecol. 2021, 90, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Hilborn, R.; Walters, C.J. Stock and recruitment. In Quantitative Fisheries Stock Assessment; Springer: Boston, MA, USA, 1992; pp. 241–296. [Google Scholar] [CrossRef]
- Bundy, A.; Bohaboy, E.C.; Hjermann, D.O.; Mueter, F.J.; Fu, C.; Link, J.S. Common patterns, common drivers: Comparative analysis of aggregate surplus production across ecosystems. Mar. Ecol. Prog. Ser. 2012, 459, 203–218. [Google Scholar] [CrossRef]
- Brčić, J.; Herrmann, B.; De Carlo, F.; Sala, A. Selective characteristics of a shark-excluding grid device in a Mediterranean trawl. Fish. Res. 2015, 172, 352–360. [Google Scholar] [CrossRef]
- Chosid, D.M.; Pol, M.; Szymanski, M.; Mirarchi, F.; Mirarchi, A. Development and observations of a spiny dogfish Squalus acanthias reduction device in a raised footrope silver hake Merluccius bilinearis trawl. Fish. Res. 2012, 114, 66–75. [Google Scholar] [CrossRef]
- Janse, M.; Firchau, B.E.T.H.; Mohan, P.J. Elasmobranch nutrition, food handling, and feeding techniques. In The Elasmobranch Husbandry Manual: Captive Care of Sharks, Rays and their Relatives’; Smith, M., Warmolts, D., Thoney, D., Hueter, R., Eds.; Ohio Biological Survey, Inc.: Columbus, OH, USA, 2004; pp. 183–200. [Google Scholar]
- Poisson, F.; Séret, B.; Vernet, A.L.; Goujon, M.; Dagorn, L. Collaborative research: Development of a manual on elasmobranch handling and release best practices in tropical tuna purse-seine fisheries. Mar. Policy 2014, 44, 312–320. [Google Scholar] [CrossRef]
- Geraci, M.L.; Di Lorenzo, M.; Falsone, F.; Scannella, D.; Di Maio, F.; Colloca, F.; Vitale, S.; Serena, F. The occurrence of Norwegian skate, Dipturus nidarosiensis (Elasmobranchii: Rajiformes: Rajidae), in the Strait of Sicily, central Mediterranean. Acta Ichthyol. Piscat. 2019, 49, 203–208. [Google Scholar] [CrossRef]
- Scannella, D.; Geraci, M.L.; Falsone, F.; Colloca, F.; Zava, B.; Serena, F.; Vitale, S. A new record of a great white shark, Carcharodon carcharias (Chondrichthyes: Lamnidae) in the Strait of Sicily, Central Mediterranean Sea. Acta Adriat. 2020, 61, 181–186. [Google Scholar] [CrossRef]
- Ward-Paige, C.A.; Keith, D.M.; Worm, B.; Lotze, H.K. Recovery potential and conservation options for elasmobranchs. J. Fish Biol. 2012, 80, 1844–1869. [Google Scholar] [CrossRef]
- Heupel, M.R.; Kanno, S.; Martins, A.P.B.; Simpfendorfer, C.A. Advances in understanding the roles and benefits of nursery areas for elasmobranch populations. Mar. Freshw. Res. 2018, 70, 897. [Google Scholar] [CrossRef]

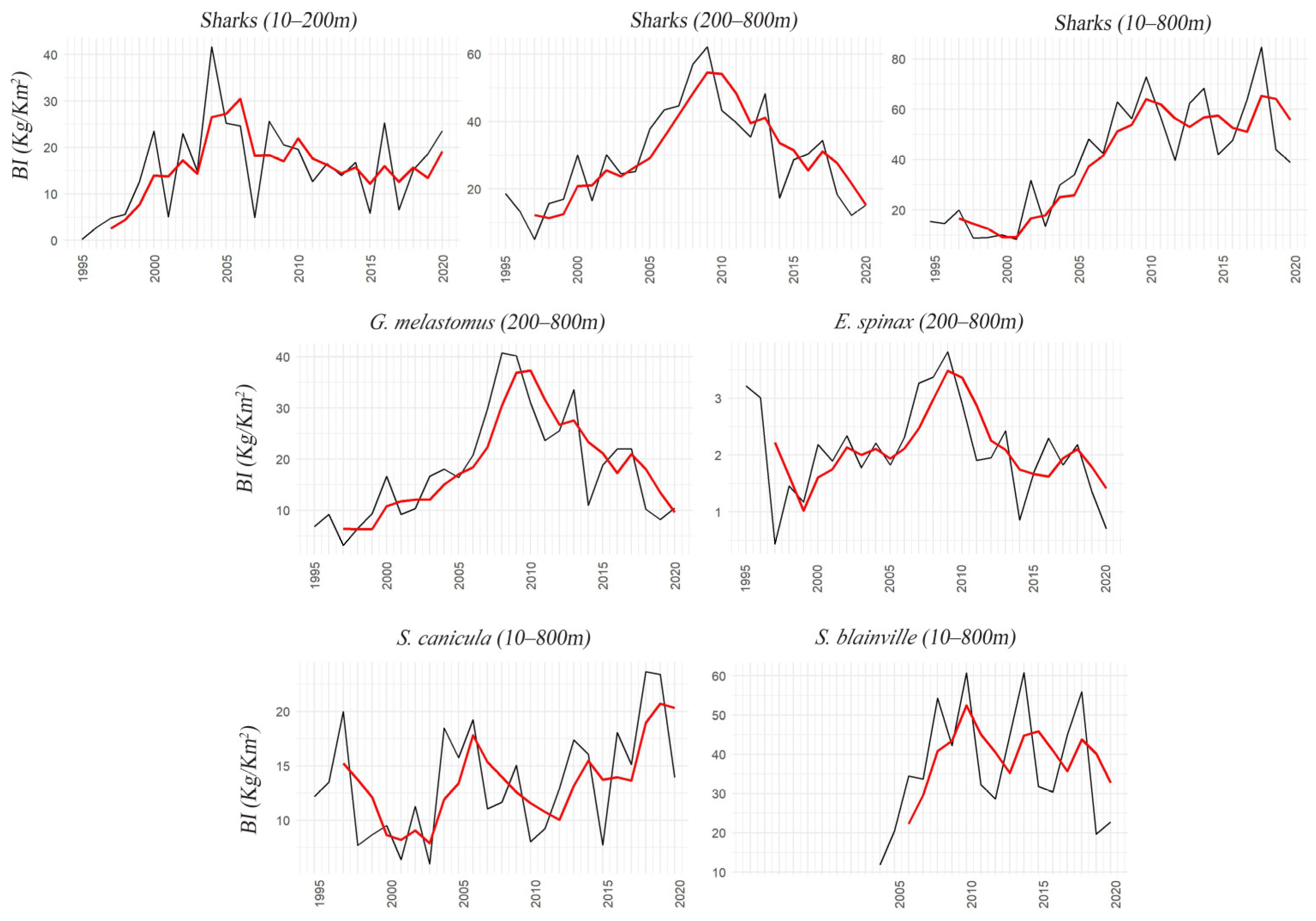
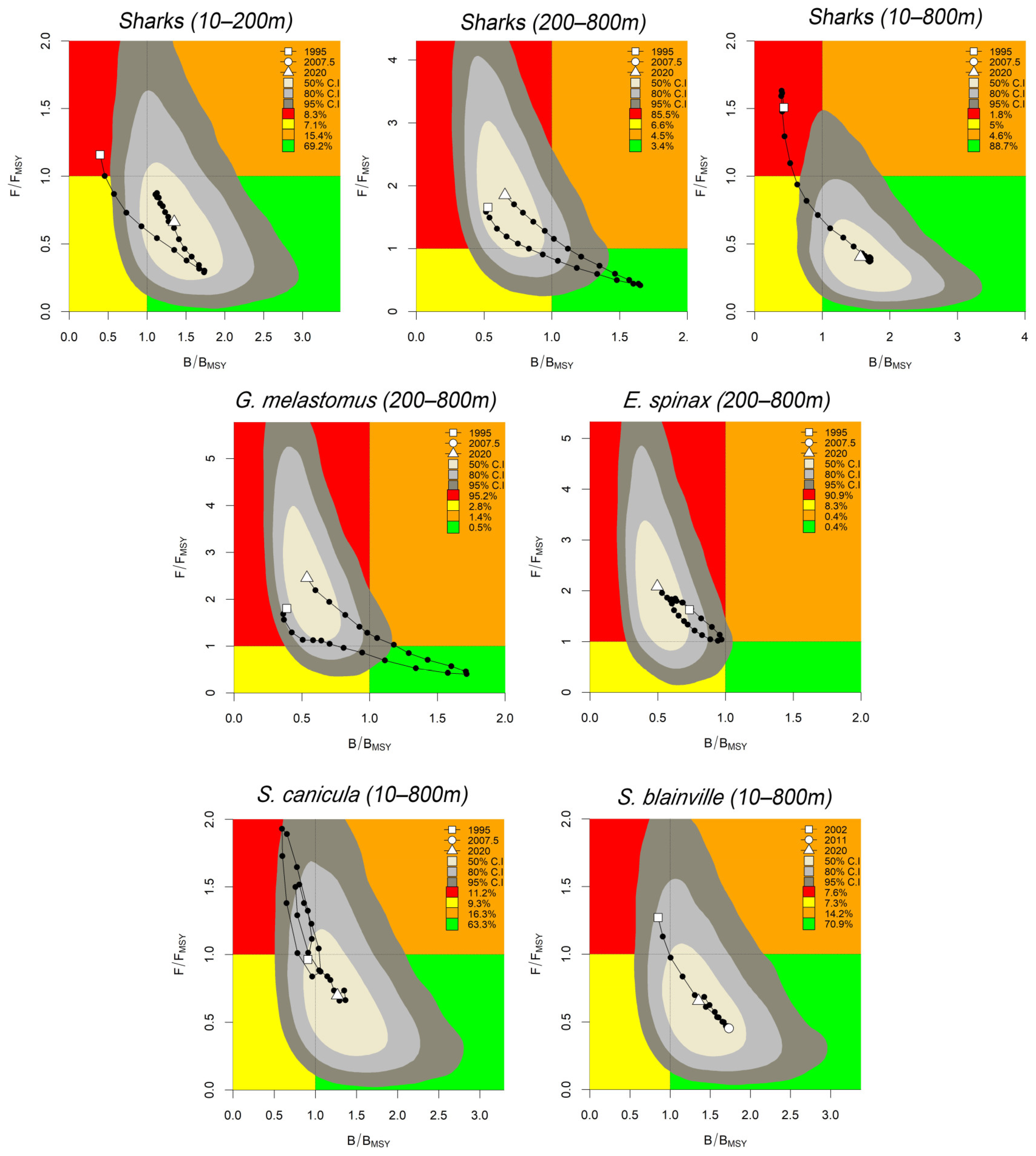
| Stock | r Prior (CI) | B/K Prior (CI) | r.est | K.est | K.est (CI) | B/BMSY | B/BMSY (CI) | F/FMSY | F/FMSY (CI) | Stock Status |
|---|---|---|---|---|---|---|---|---|---|---|
| Batoids | ||||||||||
| Batoids (10–200 m) | 0.015–0.8 | 0.01–0.4 | 0.70 | 14.9 | 12.0–20.5 | 1.43 | 0.79–2.61 | 0.50 | 0.05–1.16 | H |
| Batoids (200–800 m) | 0.015–0.8 | 0.2–0.6 | 0.55 | 6.7 | 5.7–7.9 | 0.77 | 0.43–1.38 | 1.48 | 0.44–2.93 | O |
| Batoids (10–800 m) | 0.015–0.8 | 0.2–0.6 | 0.63 | 105.1 | 92.8–120.8 | 1.35 | 0.75–2.48 | 0.61 | 0.10–1.25 | H |
| R. clavata | 0.05–0.8 | 0.01–0.6 | 0.74 | 64.1 | 56.6–74.5 | 1.50 | 0.82–2.72 | 0.38 | 0.05–0.84 | H |
| R. miraletus | 0.05–0.8 | 0.2–0.6 | 0.56 | 45.9 | 36.1–58.2 | 0.21 | 0.12–0.39 | 5.8 | 1.72–6.65 | C |
| Sharks | ||||||||||
| Sharks (10–200 m) | 0.015–0.8 | 0.01–0.4 | 0.83 | 26.4 | 22.5–32.6 | 1.34 | 0.75–2.41 | 0.66 | 0.10–1.74 | H |
| Sharks (200–800 m) | 0.015–0.8 | 0.01–0.6 | 0.59 | 58.3 | 46.5–73.4 | 0.65 | 0.36–1.17 | 1.70 | 0.55–3.3 | O |
| G. melastomus | 0.05–0.5 | 0.01–0.4 | 0.45 | 40.2 | 31.9–54.2 | 0.53 | 0.29–0.94 | 2.19 | 0.73–4.03 | O |
| E. spinax | 0.015–0.8 | 0.01–0.6 | 0.46 | 5.9 | 3.9–8.6 | 0.49 | 0.27–0.89 | 1.95 | 0.45–4.18 | C |
| Sharks (10–800 m) | 0.015–0.8 | 0.01–0.6 | 0.64 | 68.9 | 60.4–81.8 | 1.56 | 0.87–2.85 | 0.40 | 0.04–0.95 | H |
| S. blainville | 0.015–0.5 | 0.2–0.6 | 0.43 | 50.7 | 43.1–61.0 | 1.35 | 0.77–2.48 | 0.68 | 0.08–1.53 | H |
| S. canicula | 0.05–0.8 | 0.2–0.6 | 0.51 | 29.7 | 21.7–44.6 | 1.26 | 0.69–2.27 | 0.73 | 0.09–1.71 | H |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falsone, F.; Gancitano, V.; Geraci, M.L.; Sardo, G.; Scannella, D.; Serena, F.; Vitale, S.; Fiorentino, F. Assessing the Stock Dynamics of Elasmobranchii off the Southern Coast of Sicily by Using Trawl Survey Data. Fishes 2022, 7, 136. https://doi.org/10.3390/fishes7030136
Falsone F, Gancitano V, Geraci ML, Sardo G, Scannella D, Serena F, Vitale S, Fiorentino F. Assessing the Stock Dynamics of Elasmobranchii off the Southern Coast of Sicily by Using Trawl Survey Data. Fishes. 2022; 7(3):136. https://doi.org/10.3390/fishes7030136
Chicago/Turabian StyleFalsone, Fabio, Vita Gancitano, Michele Luca Geraci, Giacomo Sardo, Danilo Scannella, Fabrizio Serena, Sergio Vitale, and Fabio Fiorentino. 2022. "Assessing the Stock Dynamics of Elasmobranchii off the Southern Coast of Sicily by Using Trawl Survey Data" Fishes 7, no. 3: 136. https://doi.org/10.3390/fishes7030136
APA StyleFalsone, F., Gancitano, V., Geraci, M. L., Sardo, G., Scannella, D., Serena, F., Vitale, S., & Fiorentino, F. (2022). Assessing the Stock Dynamics of Elasmobranchii off the Southern Coast of Sicily by Using Trawl Survey Data. Fishes, 7(3), 136. https://doi.org/10.3390/fishes7030136








