Abstract
Four members of the miR-200 family in Japanese flounder (Paralichthys olivaceus) have sex-biased expression patterns, but their target genes and how they work in the development of the gonads are rarely known. Anti-Müllerian hormone (AMH) can inhibit the development of Muller’s duct in female mammals and regulate the formation of gametes after sexual maturity. There is no Muller’s duct in teleosts, but the amh gene still exists. Knockout of amh results in sex reversal from male to female. Therefore, it is essential to explore the relationship between the miR-200 family and amh to clarify what role miR-200 plays in the development of the gonads. In Japanese flounder, the two binding sites for the miR-200 family in the 3′UTR of amh were found through bioinformatic prediction. Double luciferase and green fluorescent protein reporter experiments demonstrated amh to be directly targeted by miR-200a and miR-200b. Moreover, miR-200a and miR-200b reduced the expression of amh through site 1 rather than site 2. To explore the regulatory role of miR-200a in gonadal development, we further overexpressed miR-200a in the primary Sertoli cells of the testis. With the overexpression of miR-200a, the expression of amh decreased, while the expression of the other two male sex-related genes, dmrt1 (doublesex and mab-3 related transcription factor 1) and gsdf (diagonal soma driven factor), increased significantly. This result indicates that the miR-200 family regulates the gonadal differentiation and development by targeting amh in Japanese flounder.
1. Introduction
MicroRNAs (miRNAs) are single-stranded non-coding small RNAs that widely exist in the intracellular and extracellular fluids of eukaryotes [1]. Base matching of the heptamer “seed sequence” at the 5′-end with the 3′UTR region of the target mRNA induces mRNA degradation, instability of the target gene, or inhibits the expression of the target gene at the post-transcriptional level [2,3,4]. To clarify the functional roles of miRNAs, the screening and identification of target genes directly controlled by miRNAs are crucial. The miR-200 family in mammals has been identified and found to have five members (miR-429, miR-141, miR-200a, miR-200b, and miR-200c). The core sequences of the miR-200 family are remarkably conserved in vertebrates, which suggests that they are also functionally conserved across species [5]. Its research has mainly focused on tumors and cancer [6], with few studies on gonadal development. The miR-200 family has been found to directly regulate the epithelial-mesenchymal transformation genes zeb1, zeb2, and β-catenin in the NCI-60 cell line [7]. In mice, it was found that the female could not ovulate normally after miR-429 and miR-200b were knocked down [8]. High-throughput sequencing of miRNAs in Pelteobagrus fulvidraco showed that miR-200 family genes might be involved in spermatophore development and spermatogenesis. miR-141 and miR-429 were expressed at lower levels in the spermathecae of YY super-males compared with those of XY normal males. These were downregulated as the spermathecae developed, while the estradiol treatment upregulated the expression of five miR-200 family genes in the spermathecae [9]. After deleting six precursors of the miR200 family in the zebrafish genome using CRISPR/CAS9 gene editing, mutant males with miR-200 family members (miR-200a, miR-200b, and miR-429a) knocked out on chromosome 23 had significantly higher sperm motility than that of the WT zebrafish, while the miR-200 knockout females failed to reproduce normally. miR-200a, miR-200b, and miR-429a overexpression in adult zebrafish decreased amh and male sperm motility [10]. This evidence reveals that the miR-200 family can affect the function of gonads in vertebrates. However, the direct target genes of the miR-200 family in fish are hardly reported.
The anti-Müllerian hormone (AMH) mainly exists in mammalian gonadal somatic cells. It inhibits the development of Müllerian ducts in sex determination, facilitating embryos to develop into males. After human sexual maturity, the AMH content can be used as a biological index of ovarian function and a basis for judging sperm activity in male serum [10]. Like mammals, the testis and ovary of teleosts contain germ and somatic cells, in which germ cells carry the transmission of genetic information and somatic cells are responsible for secreting hormones to promote germ–cell differentiation and division [11,12]. Teleosts lack Müllerian ducts, but the amh gene can still determine fish gonad differentiation and development [13]. After the sexual maturation of many teleosts, amh is still expressed in the granulosa and Sertoli cells of gonads, which determines the formation and differentiation of gametes [14]. amh regulates downstream target genes by binding to its receptor amhr II [15]. Although amhr II does not exist in zebrafish, amh can still regulate gonadal development and differentiation [16]. Zebrafish with amh knockdown have a female-biased sex ratio and gonadal hypertrophy. In its male mutant, the expressions of supporting-cell marker genes including dmrt1 and gsdf were significantly upregulated; the expression of vasa, a spermatogonial marker gene, was also increased [17]. After knocking out amh in Nile tilapia, Oreochromis niloticus, XY male fishes will reverse to female individuals, and the ovarian function of female individuals after sexual reversal is the same as that of normal females [18]. Amh signaling in medaka acts on the supporting cells of the spermathecae to promote the proliferation of mitotically active spermatogonia, but not of resting germ cells, and blocking Amh signaling leads to partial meiosis of XY germ cells, which in turn leads to a shift of XY individuals toward females [19]. In Japanese flounder, Paralichthys olivaceus, the proliferation of germ cells was significantly inhibited by estradiol and 17α-methyltestosterone treatment, and amh and gsdf showed contrasting expression changes [20]. These indicate that amh affects the gonadal differentiation and development in teleosts.
Japanese flounder is an economically important flatfish species. The female is larger and grows rapidly. The weight of the female fish can be more than 1.8 times that of male individuals at the same age of 445 days after hatching. The economic value of female Japanese flounder is higher than that of males [21]. Research on sex determination and gonadal development in Japanese flounder has essential implications for sex control in aquaculture production. Our laboratory constructed an miRNA library from the testis and ovary of Japanese flounder and screened many miRNAs with different expression between males and females [22]. Four members (miR-429, miR-141, miR-200a, and miR-200b) of the miR-200 family have been obtained in Japanese flounder. Given the critical role that amh plays in mammalian and teleost gonadal development, we focused on the targeting role between the miR-200 family and amh, although there are certainly many targets for the miR-200 family to act genome-wide. Thereafter, the potential binding sites between the miR-200 family and amh were screened by bioinformatics, and the targeting relationship between the miR-200 family and amh was identified by the double luciferase and green fluorescent protein reporter gene system. Finally, the miR-200a was over-expressed in the primary Sertoli cells to detect the expression changes in the amh and male sex-related genes gsdf (diagonal soma driven factor) and dmrt1 (doublesex and mab-3 related transcription factor 1), and then we investigated the function of the miR-200 family and amh in the gonadal development of Japanese flounder.
2. Materials and Methods
2.1. Experimental Fish
The adult Japanese flounders (680 ± 10 g) used in this experiment were purchased from the Shanghai Jiangyang aquatic market. The development period of gonads is in Stage III–IV [23]. Before dissection, Japanese flounders were put into seawater with MS-222 (500 mg/L) as an anesthesia and euthanized. Then, tissue samples of the testis, ovary, brain, heart, liver, kidney, muscle, and intestine were obtained by aseptic dissection, washed with diethylpyrocarbonate treated water, and homogenized in Trizol (Invitrogen, Waltham, MA, USA) to extract the total RNA. The experimental fishes study protocol was approved by the Shanghai Ocean University Review Committee for the Use of Animal Subjects (Shou-DW-2018-010, 9 March 2018).
2.2. Real-Time PCR
Total RNAs were extracted by the Trizol method from the above-stated tissues. The integrity of RNA was detected by 1% agarose gel electrophoresis and then its concentration and quality were measured by a Nanodrop 2000. A total of 500 ng of total RNA was used for the synthesis of first cDNA strands. The first cDNA strands for the detection of miR-200 were reverse transcribed with an miRNA Synthesis Kit (Vazyme, Nanjing, China). The first cDNA strands for the quantification of other genes were synthesized by a Hiscript II Synthesis Kit (Vazyme, Nanjing, China). The mature sequences of each member of the miR-200 family from Japanese flounder were obtained from our previous miRNA libraries [22]. The downstream primers for real-time PCR were universal primers. The sequences of amh (XM_020080898.1), dmrt1 (XM_020080898.1), gsdf (KY123266.1), 5s (AB154836.1) and β-actin (HQ386788.1) were obtained from the NCBI archive. The quantitative PCR primers for miR-200 family genes, amh, dmrt1, gsdf, and 5S, β-actin as internal reference genes from Japanese flounder were designed by primer 5.0, and the universal primers downstream of miRNA were provided by the kit. The primer sequences of the above-mentioned genes are listed in Table 1.

Table 1.
The primers used in this experiment.
Using the SYBR qPCR Kit (Vazyme, Nanjing, China), real-time PCR was performed with a reaction system of 20 μL. The system included a cDNA template of 1 μL, 0.4 μL for the upstream and downstream primers (10 μM), respectively, 10 μL of SYBR Green, and 8.6 μL of non-enzymatic sterilized water. The PCR amplification procedure containing 94 °C, 30 s; 94 °C, 10 s; 60 °C, 30 s; and 40 cycles of reactions were carried out and the melting curve was developed. A standard curve of the target gene and internal reference gene was constructed by sequential 10-fold dilution of gonadal cDNA. The melting curve for each gene had only one specific peak, and the standard curve showed an R-value greater than 0.998, with a corresponding amplification efficiency (E) greater than 0.95. The same reaction system and conditions were used to quantify the target gene for all samples, and three replicates were set up. Relative quantifications of the miR-200 family and other genes in the male and female gonads were evaluated with the 2−ΔΔCT method [24]. The statistical significance between groups was analyzed by SPSS 24. One-way variance was performed and the differences were considered significant when p < 0.05. The figures were drawn by Sigmaplot software (12.5, Systat, San Jose, CA, USA).
2.3. Prediction of Target Genes of miR-200 Family by Bioinformatics
According to the mature sequences of the miR-200 family and the 3′UTR sequence of amh from the Japanese flounder in the NCBI, the binding sites between the 3′UTR of the candidate target gene amh and the seed sequence of the miR-200 family were predicted using RNAhybrid (https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid, accessed on 8 July 2019).
2.4. Construction of Wild and Mutant Recombinant Plasmid
According to the 3′UTR region of amh, primers (Table 1) were designed with primer 5.0, respectively, and the testis cDNA served as a template to amplify the 3′UTR of the amh gene by PCR. The reaction system was as follows: cDNA 1 μL, 2 × Taq PCR Master Mix enzyme 22 μL, and the upstream and downstream primer (10 μM), both 1 μL. The PCR reaction procedure consisted of pre-denaturation (94 °C, 3 min), denaturation (94 °C, 10 s), annealing (60 °C, 10 s), and extension (72 °C, 5 s), a total of 35 cycles. After the reaction was completed, the resulting amplified product was analyzed by 1% agarose gel electrophoresis. Electrophoretic bands of the PCR products were excised under UV transillumination and recovered with a gel extraction kit. The pmiRGLO and pEGFP vectors were digested with SacI restriction endonuclease (NEB, Ipswich, MA, USA) at 37 °C for 15 min. The PCR product of amh 3′UTR with a homologous arm was ligated with the digested pmiRGLO vector and the pEGFP vector using a seamless cloning kit (Beyotime, Shanghai, China), transformed into competent cells, sent to the Suzhou Jinweizhi Biological Company for sequencing verification, and then by using an omega kit (Omega, Norcross, GA, USA), the plasmids were extracted, and the successfully constructed wild recombinant plasmids were named pmiRGLO-amh and pEGFP-amh.
Using the recombinant plasmids pmiRGLO-amh and pEGFP-amh as templates, mutant primers (Table 1) were devised for PCR amplification. The reaction system was as follows: recombinant plasmid templates 1 ng and 2 × Max Buffer 25 μL, upstream and downstream primers both 1 μL, dNTP Mix 1 μL, Phanta Max DNA Polymerase 1 μL, then ddH2O was added to 50 μL. The reaction conditions were as follows: 30 s pre-denaturation at 94 °C, 15 s denaturation at 94 °C, 15 s annealing at 60 °C, 5 min extension at 72 °C, a total of 35 cycles, and complete extension at 72 °C for 5 min. The amplified product was digested with the DpnI enzyme at 37 °C for 1 h. The digested product was cyclized with Exnase II, and the reaction was as follows: the digested product was 200 ng, 5 × CEII Buffer 4 μL, Exnase II 2 μL, and ddH2O was added to 20 μL. The reaction mixture was maintained at 37 °C for 30 min and placed on ice for cooling. The recombinant mutant product was transformed into competent cells and sequenced to verify its correctness. The mutant plasmid was extracted and stored in a refrigerator at −20 °C.
2.5. Cell Transfection and Luciferase Assay
The mimics of the miR-200 family were purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China) and the 293T cells were derived from laboratory preservation. The above-predicted candidate target genes were identified by the dual-luciferase and green fluorescence protein reporter assay. The specific steps were as follows: 293T cells in good condition were cultured in 24-well plates containing DMEM with 10% FBS and 1% double-antibody and transfected when the cell density reached about 70%. The mixed solution containing 100 nmoL of the miR-200 mimics or negative control mimics, 100 ng of the pmirGLO or pEGFP recombinant plasmid, 1 μL of the lipofectamineTM 3000 reagent, and 0.75 μL of the P3000 transfection reagent was diluted to 150 μL with the cell culture medium opti-MEM. The mixture was thoroughly mixed, incubated (room temperature, 15 min), dispensed into the corresponding wells, and continued for 24 h at 37 °C and 5% CO2. The above experiments were set up in three replicates. According to the Dual-luciferase® Reporter Assay System (Promega, Madison, WI, USA) manual, the luciferase activity was analyzed by recording the ratio of the firefly luciferase to Renilla luciferase. The significant difference in the data in each group was tested by ANOVA. The detection of the green fluorescent protein was photographed and recorded by a fluorescence microscope (Olympus, Tokyo, Japan).
2.6. Isolation and miRNA Overexpression of Primary Sertoli Cells from the Testis
After isolating the Japanese flounder testis tissue, we washed it with PBS for 0.5 h, and then transferred it to an L-15 medium with 10% tertiary antibody for washing for 10 min. We cut the tissue into small pieces with scissors, digested it with trypsin for 1 h, and filtered it with a 200-μm diameter sieve, subjected it to centrifugation (1000 rpm, 10 min), discarded the supernatant, added L-15 complete medium, resuspended it, and then transferred it to a cell culture bottle for culture at 26 °C. After 24 h, the stably adherent cells were Sertoli cells, and we collected the Sertoli cells for passage culture, and used them for the miRNA overexpression experiments at the seventh generation. The transfection method of the mimics was the same as above. After 24 h of transfection, the cells were collected for real-time PCR.
3. Results
3.1. Differential Expression of the miR-200 Family in Male and Female Gonads
As illustrated in Figure 1, our previous high-throughput sequencing results from the miRNA library of male and female gonads in Japanese flounder showed that the four members of the miR-200 family had obvious sex-biased expression patterns. The sequencing read numbers for miR-200a, miR-200b, miR-141, and miR-429 in the male/female gonads were 5378/16684, 975/6961, 4236/13621, and 939/3115, respectively [22]. In this study, the real-time PCR results showed that four miR-200 members had a relatively higher expression in the female gonads (ovaries) than in the male gonads (testes) (p < 0.05), which is consistent with our high-throughput sequencing results in Japanese flounder.
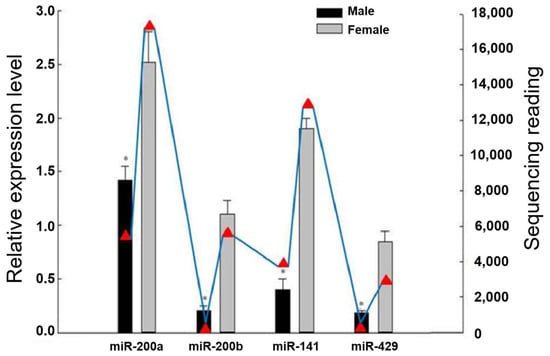
Figure 1.
The relative expression of the miR-200 family in the male and female gonads of Japanese flounder. Note: The blue line of the triangle indicates the results of the miRNA high-throughput sequencing, with its Y-axis on the right. The bar graph shows the results of the fluorescence quantification of each member of the miR-200 family, with its Y-axis on the left. * Indicates a statistically significant difference (p < 0.05).
3.2. Prediction of Target Sites between the miR-200 Family and amh
Two distinct sites that bind to each member of the miR-200 family were predicted in the 3’UTR of the candidate target gene amh, named site 1 and site 2, respectively. Among them, the seed sequences at positions 2 to 8 of miR-200a and miR-141 were entirely complementary to site 1, with one base was not complementary to site 2, and the seed sequences at positions 2 to 8 of miR-200b and miR-429 were not complementary to site 1, but entirely complementary to site 2 (Figure 2).

Figure 2.
The prediction of the target site between the miR-200 family and amh gene. Site 1 in the top square: the site where miR-200a and miR-141 are entirely complementary; site 2 in the bottom square: the site where miR-200b and miR-429 are entirely complementary. The squares represent complementary seed sequences at positions 2 to 8. The red letters are not complementary nucleotides.
3.3. Target Identification between the miR-200 Family and amh
Compared with the negative control (NC) group, the dual luciferase activities of the other three groups were significantly reduced except for the miR-429 mimics group (Figure 3). It preliminarily showed that miR-429 was not the target gene of amh, while the other three miRNAs had an apparent targeting relationship with amh. Furthermore, miR-200a and miR-200b had a more significant inhibitory effect than miR-141.
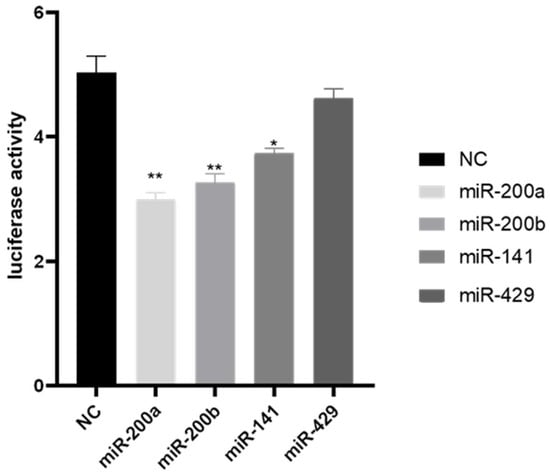
Figure 3.
The luciferase activity of wild recombinant reporter plasmid. * Indicates a statistically significant difference (p < 0.05); ** Indicates a very statistically significant difference (p < 0.01).
To clarify whether miR-200a and miR-200b interacted with amh through site 1 or site 2, we mutated binding site 1 and site 2 on the wild plasmid, separately. In the dual-luciferase reporter assay, there was an obvious inhibitory effect between the wild-type and NC group after miR-200a mimics or miR-200b mimics transfection (p < 0.01). After the site 1 mutation, this inhibitory effect disappeared (Figure 4a). However, when site 2 was mutated, a significant difference remained between the mutant-type and NC group (Figure 4b).
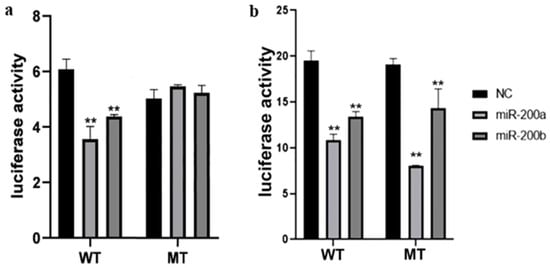
Figure 4.
The luciferase activity of the wild and mutant recombinant reporter plasmid (a) site 1, (b) site 2. ** Indicates a statistically significant difference (p < 0.01).
The fluorescence observation (Figure 5) further supported the interpretation that miR-200a and miR-200b interacted with amh and inhibited its expression via the predicted binding site 1, and not via binding site 2. In addition, miR-200a had the most obvious regulatory effect on amh (Figure 4 and Figure 5).
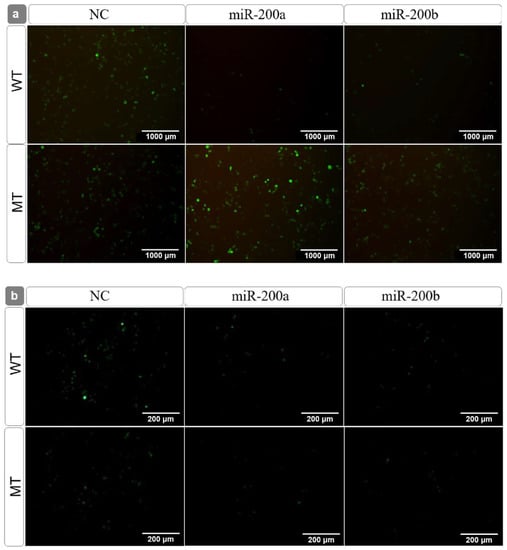
Figure 5.
The fluorescence observation after the wild and mutant amh-pEGFP plasmid transfection. Note: (a) site 1; (b) site 2.
3.4. Sex-Related Gene Expression Differences in Sertoli Cells and Germ Cells
The expression differences of several male sex-related genes, amh, dmrt1, vasa, and miR-200a in the Sertoli cells and germ cells were analyzed by real-time PCR, separately. Figure 6 shows that vasa was observed only in germ cells with no detectable vasa in the Sertoli cells, whereas the expression of amh, dmrt1, and miR-200a in the Sertoli cells showed much higher levels than in the germ cells.
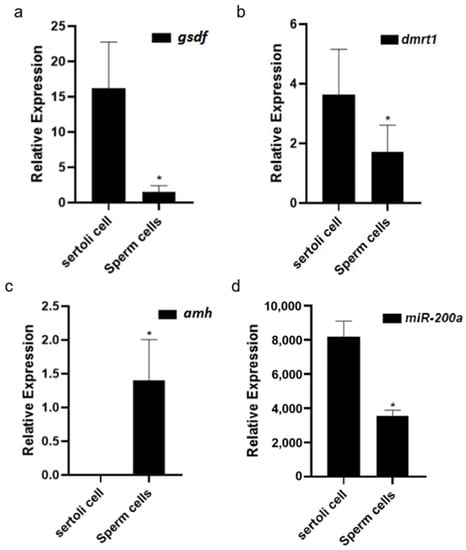
Figure 6.
The expression difference of (a) amh, (b) dmrt1, (c) vasa, and (d) miR200a in the Sertoli cells and germ cells. * Indicates that there is a statistically significant difference (p < 0.05).
3.5. Expression Changes of amh, dmrt1 and gsdf after Overexpression of miR-200a
The miR-200a mimics were transfected into the primary Sertoli cells of the Japanese flounder testis. The level of miR-200a increased significantly after 24 h of transfection (Figure 7a). As the miR-200a level increased, the expression of amh decreased significantly (Figure 7b). However, the expression of the other two male sex-related genes dmrt1 and gsdf increased significantly (Figure 7c,d).
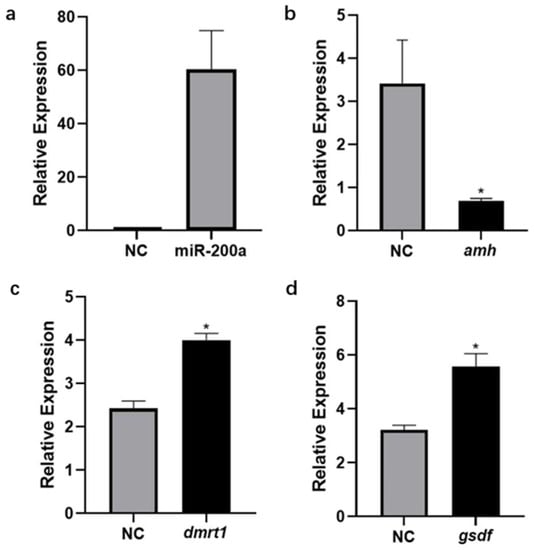
Figure 7.
The expression changes of (a) miR-200a, (b) amh, (c) gsdf, and (d) dmrt1 after miR-200a mimics transfection. NC, negative control. * Indicates that there is a statistically significant difference (p < 0.05).
4. Discussion
Our previous high-throughput sequencing results revealed that the miR-200 family exhibited a female sex-biased expression pattern in Japanese flounder [22]. In this study, four miRNAs in the ovary possessed far higher expression levels than in the testis. It has been suggested that amh is a male sex-determining gene and is involved in the differentiation and maintenance of the Japanese flounder testis [25]. Therefore, the miR-200 family and amh exhibited opposite sex-biased expression patterns, implying that amh and the miR-200 family may have a potential targeting relationship. This study verified their relationship through bioinformatic prediction and the dual-luciferase reporter assay, confirming that amh was indeed the direct target gene of miR-200a, miR-200b, and miR-141.
Generally, miRNA regulates the target gene, mainly by binding the seed sequence in the 3’UTR region of the target gene, thereby degrading the target mRNA or inhibiting the initiation of translation. The results of the bioinformatic prediction showed that there were two possible binding sites between the seed sequence of the miR-200 family and amh, namely site 1 and site 2. Because the role between miR-200a, miR-200b, and amh is more evident than that of miR-141, we mutated site 1 and site 2, separately. miR-200a and miR-200b acted directly on the target gene amh through site 1 rather than site 2, as shown through the dual luciferase and green fluorescent protein reporter experiments. For site 1, the seed sequence at positions 2~8 of miR-200a was entirely complementary to it, but miR-200b had a base that was not complementary to it. This observation indicates that miRNAs can regulate the target genes by combining with the 3’UTR region of the target gene by complete complementarity or incomplete complementarity [26,27]. Considering that in the target gene amh, site 1 lies in the 5’-upstream of site 2, we speculate that miRNA may preferentially select the upstream site to bind to the target gene.
Compared with miR-200b, miR-200a has a more apparent regulatory effect on the target gene amh at the cellular level. amh is an essential regulatory factor in mammalian gonadal development and function [28]. In teleost fishes with the XY chromosome type, amh shows early male-biased expression and promotes the differentiation of the gonad into testis [29]. High-throughput sequencing showed that miR-200a was more abundant in the gonads than the other miR-200 members in Japanese flounder. Therefore, it is indicated that miR-200a may serve a more critical role in regulating the expression of amh in the Japanese flounder gonads.
We found that unlike the vasa gene, which was specifically expressed in germ cells, amh, dmrt1, and miR-200a were mainly expressed in the Sertoli cells. To further examine the regulatory relationship between miR-200a and amh, we overexpressed miR-200a in the primary Sertoli cells of Japanese flounder testis. Two male sex-biased genes, dmrt1 and gsdf, were employed as the controls. When the expression level of miR-200a rose, the expression level of amh decreased significantly, but the expression of dmrt1 and gsdf increased significantly (p < 0.05).
amh and gsdf belong to the TGF-β family, but it is noteworthy that gsdf only exists in teleost fishes. gsdf was specifically expressed in the somatic cells around the germ cells of the Japanese flounder [20]. The deletion of the gsdf gene leads to the female-to-male sex reversal of medaka [30]. In Oreochromis niloticus, gsdf is the first gene to show a sexually dimorphic expression in the XY gonads during the critical period of gonadal differentiation and plays an vital role in male determination or differentiation [31]. Further knockdown of amh in tilapia resulted in the reversal of XY males to females, and the ovarian function in sex-reversed females was identical to that of the normal females [18]. In Japanese flounder, amh and gsdf showed an opposite expression trend after estradiol and 17 α-methyltestosterone treatment [20]. The phenotypes of amh and gsdf knockout individuals were very similar in Japanese medaka [30].
dmrt1 is one of the members of the DMRT family and is a known key target of the sex-determining gene sry [32]. In zebrafish, amh inhibits the excessive proliferation of spermatogonia and promotes the differentiation of germ cells to ensure the balance between proliferation and differentiation, while dmrt1 is responsible for the self-renewal, proliferation, and differentiation of male germ cells. Of note, the deletion of the amh gene results in a significant increase in the expression of dmrt1 and gsdf, which is accompanied by enlarging male gonads and germ cells staying in the proliferation stage without differentiation [17].
Our results were consistent with the above research in other teleosts. The overexpression of miR-200a downregulated the expression of amh, while this was accompanied by the upregulation of the expression of dmrt1 and gsdf. Indeed, dmrt1 and gsdf were not found to be potential target genes in the predicted target relationships with the miR-200 family members. This information suggests that miR-200a does not act directly on the dmrt1 and gsdf genes, but may be regulated in a similar way to zebrafish, with amh negatively regulating the expression of dmrt1. In response to miR-200a overexpression, amh expression was reduced, but dmrt1 expression was increased. Similarly, because amh and gsdf had the opposite expression trend after estradiol and 17α-methyltestosterone treatment in Japanese flounder [20], the expression of gsdf increased when the expression of amh decreased. Taken together, these observations strongly indicate that the miR-200 family, especially miR-200a, may directly regulate the expression of amh and then affect the gonadal development of Japanese flounder.
5. Conclusions
amh was identified to be a direct target gene of miR-200a and miR-200b within the miR-200 family in Japanese flounder. Overexpression of miR-200a in primary gonadal cells can inhibit the expression of amh, while also elevating the expression of two other male sex-related genes, dmrt1 and gsdf. This indicates that the miR-200 family was involved in the differentiation and development of gonads in Japanese flounder by targeting amh.
Author Contributions
Conceptualization, H.Z. and K.L.; Data curation, F.Z.; Formal analysis, F.Z. and J.W.; Funding acquisition, J.Z.; Investigation, H.Z. and K.L.; Supervision, J.Z.; Writing—original draft, H.Z. and K.L.; Writing—review & editing, J.W. and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (grant number 31972772).
Institutional Review Board Statement
The animal study protocol was approved by the Shanghai Ocean University Review Committee for the Use of Animal Subjects (Shou-DW-2018-010, 9 March 2018).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huntzinger, E.; Izaurralde, E. Gene silencing by microRNAs: Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011, 12, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Pasquinelli, A.E. MicroRNAs and their targets: Recognition, regulation and an emerging reciprocal relationship. Nat. Rev. Genet. 2012, 13, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Yates, L.; Norbury, C.; Gilbert, R.C. The long and short of microRNA. Cell 2013, 153, 516–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, J.; Xiong, S.; Li, Z.; Wu, J.; Zhou, L.; Gui, J.F.; Mei, J. A feedback regulatory loop involving p53/miR-200 and growth hormone endocrine axis controls embryo size of zebrafish. Sci. Rep. 2015, 5, 15906. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.L.; Sun, J.C.; Lu, Y.; Liu, Y.K.; Cao, H.Y.; Zhang, H.Y.; Calin, G.A. MiR-200 family and cancer: From a meta-analysis view. Mol. Asp. Med. 2019, 70, 57–71. [Google Scholar] [CrossRef]
- Park, S.M.; Gaur, A.B.; Lengyel, E.; Peter, M.E. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev 2008, 22, 894–907. [Google Scholar] [CrossRef] [Green Version]
- Hasuwa, H.; Ueda, J.; Ikawa, M.; Okabe, M. MiR-200b and miR-429 function in mouse ovulation and are essential for female fertility. Science 2013, 341, 71–73. [Google Scholar] [CrossRef]
- Jing, J.; Wu, J.; Wei, L.; Xiong, S.; Ma, W.; Jin, Z.; Wang, W.; Gui, J.F.; Jie, M. Sex-biased miRNAs in gonad and their potential roles for testis development in yellow catfish. PLoS ONE 2014, 9, e107946. [Google Scholar] [CrossRef]
- Xiong, S.; Ma, W.; Jing, J.; Zhang, J.; Dan, C.; Gui, J.F.; Mei, J. An miR-200 cluster on chromosome 23 regulates sperm motility in zebrafish. Endocrinology 2018, 159, 1982–1991. [Google Scholar] [CrossRef] [Green Version]
- De Siqueira-Silva, D.H.; da Silva Rodrigues, M.; Nobrega, R.H. Testis structure, spermatogonial niche and Sertoli cell efficiency in Neotropical fish. Gen. Comp. Endocrinol. 2019, 273, 218–226. [Google Scholar] [CrossRef]
- Yang, X.Y.; Jia, Z.W. The role of EGF-like factor signaling pathway in granulosa cells in regulation of oocyte maturation and development. Heredita 2019, 41, 137–145. [Google Scholar]
- Yan, Y.L.; Batzel, P.; Titus, T.; Sydes, J.; Desvignes, T.; BreMiller, R.; Draper, B.; Postlethwait, J.H. A hormone that lost its receptor: Anti-Mullerian hormone (AMH) in zebrafish gonad development and sex determination. Genetics 2019, 213, 529–553. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mari, A.; Yan, Y.L.; Bremiller, R.A.; Wilson, C.; Canestro, C.; Postlethwait, J.H. Characterization and expression pattern of zebrafish anti-Mullerian hormone (Amh) relative to sox9a, sox9b, and cyp19a1a, during gonad development. Gene Expr. Patterns 2005, 5, 655–667. [Google Scholar] [CrossRef]
- Yan, X.; Xiong, X.; Chen, Y.G. Feedback regulation of TGF-beta signaling. Acta Biochim. Biophys. Sin. 2018, 50, 37–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Marca, A.; Ferraretti, A.P.; Palermo, R.; Ubaldi, F.M. The use of ovarian reserve markers in IVF clinical practice: A national consensus. Gynecol. Endocrinol. 2016, 32, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Mei, J.; Li, Z.; Zhang, X.; Zhou, L.; Gui, J.F. Distinct and cooperative roles of amh and dmrt1 in self-renewal and differentiation of male germ cells in zebrafish. Genetics 2017, 207, 1007–1022. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Sun, Y.; Zhao, J.; Shi, H.; Zeng, S.; Ye, K.; Jiang, D.; Zhou, L.; Sun, L.; Tao, W.; et al. A tandem duplicate of anti-Mullerian hormone with a missense SNP on the Y chromosome is essential for male sex determination in Nile Tilapia, Oreochromis niloticus. PLoS Genet. 2015, 11, e1005678. [Google Scholar] [CrossRef] [Green Version]
- Shuhei, N.; Ikuko, W.; Toshiya, N.; Jean-Yves, P.; Atsushi, T.; Yoshihito, T.; Nathalie, D.C.; Minoru, T. Hyperproliferation of mitotically active germ cells due to defective anti-Müllerian hormone signaling mediates sex reversal in medaka. Development 2012, 139, 2283–2287. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liu, Q.H.; Xiao, Y.S.; Xu, S.H.; Wang, X.Y.; Yang, J.K.; Song, Z.C.; You, F.; Li, J. Effects of environmental stress (sex steroids and heat) during sex differentiation in Japanese flounder (Paralichthys olivaceus): Insight from germ cell proliferation and gsdf-amh-cyp19a1a expression. Aquaculture 2020, 515, 734536. [Google Scholar] [CrossRef]
- Eiichi, Y. Studies on sex-manipulation and production of cloned populations in hirame, Paralichthys olivaceus (Temminck et Schlegel). Aquaculture 1999, 173, 235–246. [Google Scholar]
- Gu, Y.; Zhang, L.; Chen, X. Differential expression analysis of Paralichthys olivaceus microRNAs in adult ovary and testis by deep sequencing. Gen. Comp. Endocrinol. 2014, 204, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.H.; Liu, H.J.; Si, F.; Wang, Y.F.; Jiang, X.F. Histological observation of gonadal differentiation in cultured Japanese flounder Paralichthys olivaceus. J. Dalian Fish. Univ. 2008, 23, 451–454. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liang, S.; Zou, Y.; Wu, Z.; Wang, L.; Liu, Y.; You, F. Amh dominant expression in Sertoli cells during the testicular differentiation and development stages in the olive flounder Paralichthys olivaceus. Gene 2020, 755, 144906. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.M.; Donahoe, P.K. Mullerian Inhibiting Substance: A gonadal hormone with multiple functions. Endocr. Rev. 1993, 14, 152–164. [Google Scholar]
- Pfennig, F.; Standke, A.; Gutzeit, H.O. The role of Amh signaling in teleost fish Multiple functions not restricted to the gonads. Gen. Comp. Endocrinol. 2015, 223, 87–107. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, G.; Li, M.; Zhu, F.; Liu, Q.; Naruse, K.; Herpin, A.; Nagahama, Y.; Li, J.; Hong, Y. Autosomal gsdf acts as a male sex initiator in the fish medaka. Sci. Rep. 2016, 6, 19738. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, H.; Ijiri, S.; Kobayashi, T.; Izumi, H.; Kuramochi, Y.; Wang, D.-S.; Mizuno, S.; Nagahama, Y. Gonadal soma-derived factor (gsdf), a TGF-beta superfamily gene, induces testis differentiation in the teleost fish Oreochromis niloticus. Mol. Cell. Endocrinol. 2015, 415, 87–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raymond, C.S.; Murphy, M.W.; O’Sullivan, M.G.; Bardwell, V.J.; Zarkower, D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 2000, 14, 2587–2595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).