Taste Attributes of the “June Hairy Crab” Juveniles of Chinese Mitten Crab (Eriocheir sinensis) in Yangcheng Lake, China—A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crab Materials
2.2. Sample Pretreatment
2.3. Taste Sensing System Testing
2.4. Data Analysis
3. Results
3.1. Taste-Active Values of June Hairy Crabs
3.2. Multivariate Statistical Analysis of the Taste Profiles
4. Discussion
4.1. Characteristics of Taste Profiles
4.2. Comparison of the Differences in the Taste of Male and Female June Hairy Crabs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xue, J.R.; Jiang, T.; Chen, X.B.; Liu, H.B.; Yang, J. Multi-mineral element profiles in genuine and “Bathing” cultured Chinese Mitten Crabs (Eriocheir sinensis) in Yangcheng Lake, China. Fishes 2022, 7, 11. [Google Scholar] [CrossRef]
- Xue, J.R.; Liu, H.B.; Jiang, T.; Chen, X.B.; Yang, J. Shape variation in the carapace of Chinese mitten crabs (Eriocheir sinensis H. Milne Edwards, 1853) in Yangcheng Lake during the year-long culture period. Eur. Zool. J. 2022, 89, 217–228. [Google Scholar] [CrossRef]
- Xue, J.R.; Jiang, T.; Chen, X.B.; Liu, H.B.; Yang, J. Multi-mineral fingerprinting analysis of the Chinese mitten crab (Eriocheir sinensis) in Yangcheng Lake during the year-round culture period. Food Chem. 2022, 390, 133167. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, X.G.; Tao, N.P.; Wang, X.C.; Zheng, Y. Nutritional and flavor quality analysis of Liu Yuehuang (Eriocheir sinensis). J. Chin. Inst. Food Sci. Technol. 2017, 17, 219–227. [Google Scholar] [CrossRef]
- Shao, L.; Wang, C.; He, J.; Wu, X.; Cheng, Y. Hepatopancreas and gonad quality of Chinese mitten crabs fattened with natural and formulated diets. J. Food Qual. 2013, 36, 217–227. [Google Scholar] [CrossRef]
- Zhang, L.; Tao, N.P.; Wu, X.G.; Wang, X.C. Metabolomics of the hepatopancreas in Chinese mitten crabs (Eriocheir sinensis). Food Res. Int. 2022, 152, 110914. [Google Scholar] [CrossRef]
- Wang, Y.C.; Ni, K.D.; Wang, Z.J.; Chen, H.H.; Liu, X.C.; Chen, X.W.; Wang, J.; Wang, C.H. Comparison on major nutritional quality in hepatopancreas tissues between the June-caught and adult Chinese mitten crab, Eriocheir sinensis. Fish. Sci. Technol. Inf. 2021, 48, 181–186. [Google Scholar] [CrossRef]
- Wu, X.; Tahara, Y.; Yatabe, R.; Toko, K. Taste sensor: Electronic tongue with lipid membranes. Anal. Sci. 2020, 36, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Ha, D.; Sun, Q.; Su, K.Q.; Wan, H.; Li, H.B.; Xu, N.; Sun, F.; Zhuang, L.J.; Hu, N.; Wang, P. Recent achievements in electronic tongue and bioelectronic tongue as taste sensors. Sens. Actuators B. 2015, 207, 1136–1146. [Google Scholar] [CrossRef]
- Liu, D.; Li, S.; Wang, N.; Deng, Y.J.; Sha, L.; Gai, S.M.; Liu, H.; Xu, X.L. Evolution of taste compounds of Dezhou-braised chicken during cooking evaluated by chemical analysis and an electronic tongue system. J. Food Sci. 2017, 82, 1076–1082. [Google Scholar] [CrossRef]
- Pattarapon, P.; Zhang, M.; Bhandari, B.; Gao, Z.X. Effect of vacuum storage on the freshness of grass carp (Ctenopharyngodon idella) fillet based on normal and electronic sensory measurement. J. Food Process. Preserv. 2018, 42, e13418. [Google Scholar] [CrossRef]
- Toko, K. Analysis of the Tastes on the Tip of the Tongue; China Quality Press: Beijing, China; China Standard Press: Beijing, China, 2013. [Google Scholar]
- Fan, L.C.; Xian, C.Y.; Tang, S.J.; Ding, W.; Xu, C.H.; Wang, X.C. Effect of frozen storage temperature on lipid stability of hepatopancreas of Eriocheir sinensis. LWT-Food Sci. Technol. 2022, 154, 112513. [Google Scholar] [CrossRef]
- Liu, H.B.; Jiang, T.; Xue, J.R.; Chen, X.B.; Xuan, Z.Y.; Yang, J. Taste profile characterization of Chinese mitten crab (Eriocheir sinensis) meat using electronic tongue analysis. Sens. Mater. 2021, 33, 2537–2547. [Google Scholar] [CrossRef]
- Wu, X.; Yuan, Y.; Tahara, Y.; Habara, M.; Ikezaki, H.; Toko, K. Reusability enhancement of taste sensor using lipid polymer membranes by surfactant cleaning treatment. IEEE Sens. J. 2020, 20, 4579–4586. [Google Scholar] [CrossRef]
- Yamaguchi, S. Basic properties of umami and effects on humans. Physiol. Behav. 1991, 49, 833–841. [Google Scholar] [CrossRef]
- Chen, D.W.; Zhang, M.; Shrestha, S. Compositional characteristics and nutritional quality of Chinese mitten crab (Eriocheir sinensis). Food Chem. 2007, 103, 1343–1349. [Google Scholar] [CrossRef]
- Sasaki, K.; Tani, F.; Sato, K.; Ikezaki, H.; Taniguchi, A.; Emori, T.; Iwaki, F.; Chikuni, K.; Mitsumoto, M. Analysis of pork extracts by taste sensing system and the relationship between umami substances and sensor output. Sens. Mater. 2005, 17, 397–404. Available online: https://sensors.myu-group.co.jp/sm_pdf/SM617.pdf (accessed on 22 March 2022).
- Komiya, Y.; Mizunoya, W.; Kajiwara, K.; Yokoyama, I.; Ogasawara, H.; Arihara, K. Correlation between skeletal muscle fiber type and responses of a taste sensing system in various beef samples. Anim. Sci. J. 2020, 91, e13425. [Google Scholar] [CrossRef]
- Izawa, K.; Amino, Y.; Kohmura, M.; Ueda, Y.; Kuroda, M. Human-environment interactions-taste. In Comprehensive Natural Products II: Chemistry and Biology; Liu, H.W., Mander, L., Eds.; Elsevier Ltd.: London, UK, 2010; pp. 631–671. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Habara, M.; Ikezazki, H.; Chen, R.; Naito, Y.; Toko, K. Advanced taste sensors based on artificial lipids with global selectivity to basic taste qualities and high correlation to sensory scores. Sensors 2010, 10, 3411–3443. [Google Scholar] [CrossRef] [Green Version]
- Komata, Y. Umami taste of seafoods. Food Rev. Int. 1990, 6, 457–487. [Google Scholar] [CrossRef]
- Coupland, J.N.; Hayes, J.E. Physical approaches to masking bitter taste: Lessons from food and pharmaceuticals. Pharm. Res. 2014, 31, 2921–2939. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.B.; Jiang, T.; Luo, R.J.; Xue, J.R.; Chen, X.B.; Yang, J. Evaluation of the taste-active values of Chinese mitten crabs (Eriocheir sinensis) from different geographic origins using a taste sensing system. J. Food Sci. 2020, 41, 132–137. [Google Scholar] [CrossRef]
- Ogasawara, M.; Katsumata, T.; Egi, M. Taste properties of Maillard-reaction products prepared from 1000 to 5000 Da peptide. Food Chem. 2006, 99, 600–604. [Google Scholar] [CrossRef]
- Legin, A.; Rudnitskaya, A.; Di Natale, C.; Davide, F.; D′Amico, A. Tasting of beverages using an electronic tongue. Sens. Actuators B. 1997, 44, 291–296. [Google Scholar] [CrossRef]
- Ritthiruangdej, P.; Suwonsichon, T. Sensory properties of Thai fish sauces and their categorization. Kasetsart J.—Nat. Sci. 2006, 40, 181–191. [Google Scholar] [CrossRef]
- Méndez, M.L.R. Electronic Noses and Tongues in Food Science; Academic Press: London, UK; San Diego, CA, USA; Cambridge, UK; Oxford, UK, 2016. [Google Scholar]
- Zhang, H.; Zou, G.; Liu, X.; Xiao, Y.; Wang, W. Identification of Xinyang Maojian tea taste using electronic tongue. Sens. Mater. 2019, 31, 2347–2356. [Google Scholar] [CrossRef]
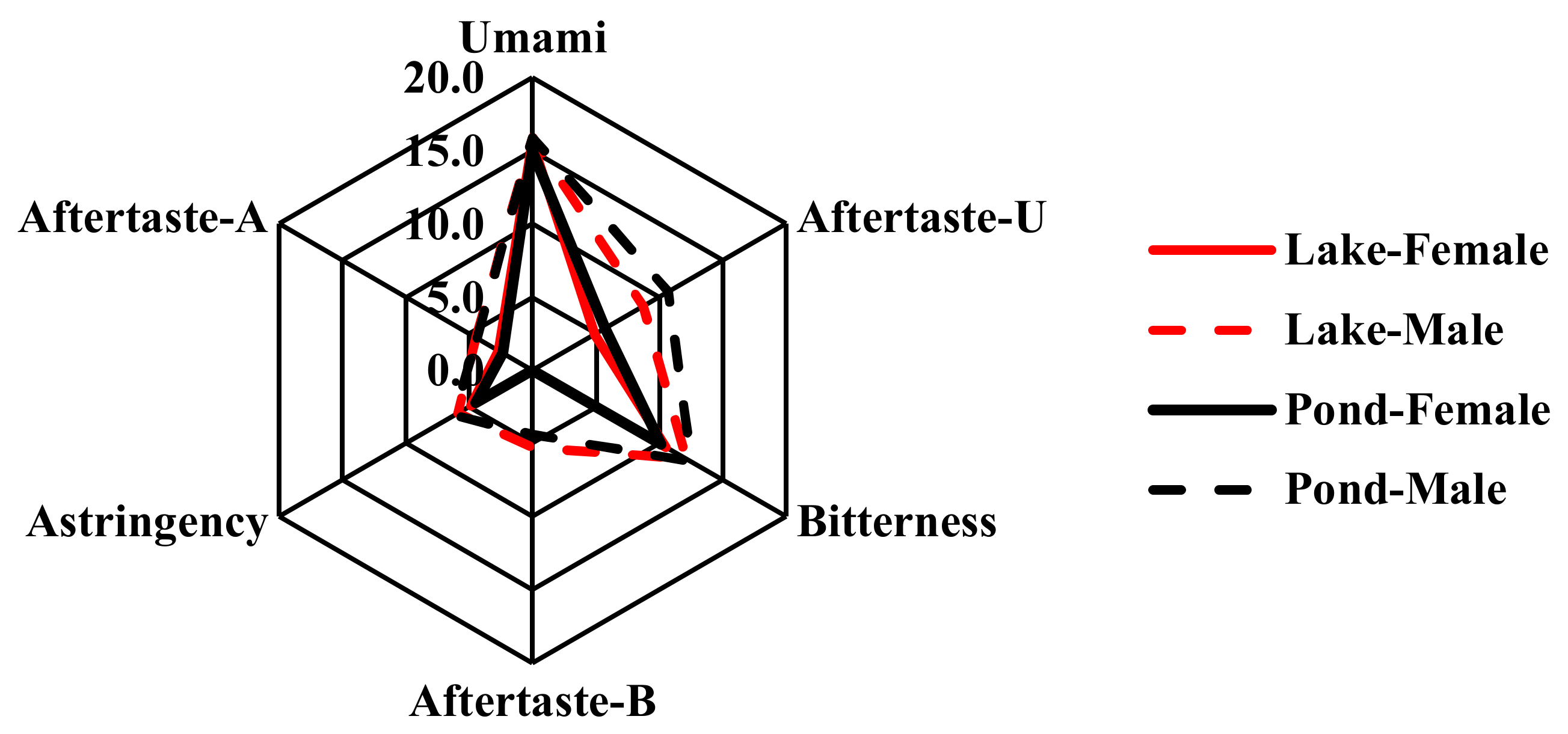


| Sampling Site | Sample Group Code | Sample Code | Umami | Aftertaste -U | Bitterness | Aftertaste -B | Astringency | Aftertaste-A |
|---|---|---|---|---|---|---|---|---|
| Yangcheng Lake | Lake—Female | Lake— Female 1 | 15.5 | 5.6 | 10.3 | 0.0 | 4.6 | 2.8 |
| Lake— Female 2 | 15.1 | 4.3 | 10.4 | 0.0 | 4.8 | 2.6 | ||
| Lake— Female 3 | 16.2 | 5.2 | 10.8 | 0.0 | 5.3 | 2.9 | ||
| Lake— Female 4 | 15.5 | 4.4 | 10.5 | 0.0 | 5.1 | 2.5 | ||
| (Mean ± SD) | 15.6 ± 0.4 | 4.9 ± 0.6 | 10.5 ± 0.2 | 0.0 ± 0.0 | 4.9 ± 0.2 | 2.7 ± 0.2 | ||
| Lake—Male | Lake— Male 1 | 15.9 | 9.8 | 12.4 | 5.3 | 6.1 | 4.6 | |
| Lake— Male 2 | 15.4 | 9.6 | 12.0 | 4.6 | 5.9 | 4.4 | ||
| Lake— Male 3 | 16.6 | 8.2 | 11.8 | 7.3 | 5.3 | 4.9 | ||
| Lake— Male 4 | 15.7 | 7.5 | 12.0 | 4.2 | 6.1 | 4.1 | ||
| Lake— Male 5 | 15.4 | 8.4 | 12.3 | 5.0 | 6.0 | 4.4 | ||
| (Mean ± SD) | 15.9 ± 0.5 | 8.7 ± 0.9 | 12.1 ± 0.2 | 5.3 ± 1.1 | 5.9 ± 0.3 | 4.5 ± 0.3 | ||
| Pond by Yangcheng Lake | Pond—Female | Pond— Female 1 | 15.1 | 6.4 | 10.3 | 0.0 | 4.6 | 2.7 |
| Pond— Female 2 | 15.7 | 5.9 | 10.3 | 0.0 | 4.5 | 2.8 | ||
| Pond— Female 3 | 14.7 | 5.9 | 10.0 | 0.0 | 4.5 | 2.4 | ||
| Pond— Female 4 | 14.7 | 5.6 | 9.9 | 0.0 | 4.5 | 2.3 | ||
| Pond— Female 5 | 14.7 | 4.7 | 9.9 | 0.0 | 4.2 | 2.1 | ||
| (Mean ± SD) | 15.0 ± 0.4 | 5.7 ± 0.5 | 10.1 ± 0.2 | 0.0 ± 0.0 | 4.5 ± 0.1 | 2.4 ± 0.2 | ||
| Pond—Male | Pond— Male 1 | 15.8 | 8.3 | 12.0 | 3.9 | 6.0 | 4.1 | |
| Pond— Male 2 | 16.2 | 12.2 | 12.5 | 4.9 | 6.1 | 4.7 | ||
| Pond— Male 3 | 15.8 | 10.3 | 12.6 | 4.6 | 6.1 | 4.4 | ||
| Pond— Male 4 | 16.2 | 10.6 | 12.7 | 4.4 | 6.3 | 4.5 | ||
| Pond— Male 5 | 15.5 | 12.2 | 12.5 | 4.0 | 6.3 | 4.2 | ||
| (Mean ± SD) | 15.9 ± 0.3 | 10.7 ± 1.5 | 12.5 ± 0.2 | 4.4 ± 0.4 | 6.2 ± 0.1 | 4.4 ± 0.2 |
| Umami | Aftertaste-U | Bitterness | Aftertaste-B | Astringency | Aftertaste-A | ||
|---|---|---|---|---|---|---|---|
| Lake crab | Umami | - | ** | ** | ** | ** | ** |
| Aftertaste-U | * | - | ** | ** | ** | ** | |
| Bitterness | * | * | - | ** | ** | ** | |
| Aftertaste-B | * | * | * | - | N.S. | N.S. | |
| Astringency | * | N.S. | * | * | - | ** | |
| Aftertaste-A | * | * | * | * | * | - | |
| Pond crab | Umami | - | ** | ** | ** | ** | ** |
| Aftertaste-U | ** | - | * | ** | ** | ** | |
| Bitterness | ** | ** | - | ** | ** | ** | |
| Aftertaste-B | ** | ** | ** | - | ** | N.S. | |
| Astringency | ** | ** | ** | ** | - | ** | |
| Aftertaste-A | ** | ** | ** | ** | ** | - | |
| Lake—Female vs. Pond—Male | N.S. | * | * | * | * | * | |
| Lake—Female vs. Pond—Female | N.S. | N.S. | * | N.S. | * | N.S. | |
| Lake—Female vs. Pond—Male | N.S. | * | * | * | * | * | |
| Lake—Male vs. Pond—Female | * | ** | ** | ** | ** | ** | |
| Lake—Male vs. Pond—Male | N.S. | * | * | N.S. | N.S. | N.S. | |
| Pond—Female vs. Pond—Male | * | ** | ** | ** | ** | ** | |
| Total females vs. total males | * | ** | ** | ** | ** | ** | |
| Total lake carbs vs. Total pond carbs | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | |
| . | PC1 | PC2 | ||
|---|---|---|---|---|
| Loading | Eigenvector | Loading | Eigenvector | |
| Umami | 0.728 | 0.323 | 0.678 | 0.904 |
| Aftertaste-U | 0.907 | 0.402 | −0.269 | −0.359 |
| Bitterness | 0.986 | 0.437 | −0.097 | −0.129 |
| Aftertaste-B | 0.954 | 0.423 | −0.064 | 0.085 |
| Astringency | 0.943 | 0.418 | −0.127 | −0.17 |
| Aftertaste-A | 0.983 | 0.436 | 0.027 | 0.036 |
| Cumulative contribution (%) | 84.81% | 9.37% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Xue, J.; Tang, J.; Jiang, T.; Chen, X.; Yang, J. Taste Attributes of the “June Hairy Crab” Juveniles of Chinese Mitten Crab (Eriocheir sinensis) in Yangcheng Lake, China—A Pilot Study. Fishes 2022, 7, 128. https://doi.org/10.3390/fishes7030128
Liu H, Xue J, Tang J, Jiang T, Chen X, Yang J. Taste Attributes of the “June Hairy Crab” Juveniles of Chinese Mitten Crab (Eriocheir sinensis) in Yangcheng Lake, China—A Pilot Study. Fishes. 2022; 7(3):128. https://doi.org/10.3390/fishes7030128
Chicago/Turabian StyleLiu, Hongbo, Junren Xue, Jing Tang, Tao Jiang, Xiubao Chen, and Jian Yang. 2022. "Taste Attributes of the “June Hairy Crab” Juveniles of Chinese Mitten Crab (Eriocheir sinensis) in Yangcheng Lake, China—A Pilot Study" Fishes 7, no. 3: 128. https://doi.org/10.3390/fishes7030128
APA StyleLiu, H., Xue, J., Tang, J., Jiang, T., Chen, X., & Yang, J. (2022). Taste Attributes of the “June Hairy Crab” Juveniles of Chinese Mitten Crab (Eriocheir sinensis) in Yangcheng Lake, China—A Pilot Study. Fishes, 7(3), 128. https://doi.org/10.3390/fishes7030128







