Abstract
Structural enrichment is considered a useful tool to improve the welfare conditions of captive fish by deliberately increasing the physical heterogeneity and complexity of captivity environments. However, the potential effects of structural enrichment on the stress response at the group level and on social interactions have not been well studied yet. In this study, we demonstrate that suspended vertical structures (U-shaped ropes) can reduce behavioural variability among fish groups (tank level) of European seabass (Dicentrarchus labrax) juveniles. Differences in behavioural responses during group risk-taking tests (e.g., number of passes per fish) between treatments were detected, and these responses in seabass in enriched captive conditions were more homogeneous among tanks compared to fish from non-enriched tanks. These results suggest a positive effect of the structural enrichment on social stabilisation and response to stressful events at the tank level in seabass. However, further research is still needed to improve the knowledge of the potential effects of structural enrichment on fish welfare and aquaculture management, considering different enrichment designs, intensities, and strategies according to farming conditions, biological needs, and preferences of the fish species and life-stage reared in captivity.
1. Introduction
Aquaculture is growing faster than other major food production sectors, and the increasing demand for cultured seafood requires further improvement of fish farming practices [1]. Many challenges must be overcome during the rearing process to obtain a final product with the desired characteristics suitable for sale [2]. Among these challenges, the welfare of captive fish can be severely affected by a wide number of stressors involved in routine husbandry [3]. In this sense, the application of environmental enrichment (EE) can help to improve the welfare of captive fish [4,5]. Among different EE strategies, the addition of physical structures in the rearing environment (i.e., structural enrichment) increases the heterogeneity and complexity of the rearing environment, providing diverse effects on the growth performance, physiology, behaviour, and health of farmed fish [6,7]. Vertically suspended plant-fibre ropes have been successfully used as an enrichment strategy for cultured gilthead seabream (Sparus aurata). Arechavala-Lopez et al. [8] reported positive effects of vertical ropes on juvenile seabream, such as aggressiveness reduction and modifications in spatial distribution, which led to fewer interactions with the experimental net-pen and better fin condition. In a similar study, Arechavala-Lopez et al. [9] demonstrated that vertical ropes could enhance seabream cognition, exploratory behaviour, and brain physiological functions in experimental rearing tanks. Adding suspended ropes to experimental sea cages of on-growing seabream increased the spatial use of fish in the net-pen [10]. Nevertheless, the existence of null or contradictory effects of structural enrichment on behaviour, physiology, and growth performance on other fish species of aquaculture interest highlights the need for further species-specific studies on the design and particular effects of different enrichment strategies [4,7].
Fish have the ability to modify behavioural and physiological traits to cope with stressful conditions at the individual level [11], so there are individuals within a population with different chances to succeed in the potentially unfavourable conditions of the rearing environment [12]. In this sense, two broad traits, the proactive and reactive stress coping styles, have been reported in fish [13]. Reactive fish are shier individuals that progressively adapt to stress conditions, whereas more aggressive and bolder individuals are considered proactive. Since the coping style is an inter-individual characteristic, reactive and proactive fish can cohabit in the same group. Moreover, farmed fish may establish a hierarchical system in which some individuals (dominants) may have total control over food, shelter, mates, and territory [14]. Indeed, dominant–subordinate relationships can affect physiological status and animal responsiveness [15], triggering social interactions and potential stress [16]. Social stress can be considered the result of physical contact between animals (e.g., high density, lack of space, and agonistic interaction) and psychological components, such as hierarchical instability and submission [16]. Therefore, the social environment can be a considerable source of stress, impairing the welfare of fish, but EE can help to stabilise hierarchies and social interactions within a group [17].
One way to evaluate the EE effects on the potential ability of fish species to cope with stressful conditions in their rearing conditions and to ensure good welfare conditions, is through the characterisation of risk-taking behaviour [18,19,20,21]. Therefore, the aim of this study was to assess the potential effects of suspended structural enrichment on risk-taking behavioural responses at the group level (as a proxy of social stabilisation) of European seabass (Dicentrarchus labrax) juveniles reared in experimental tanks. Seabass is one of the most important fishes in terms of aquaculture potential in Europe [22], and it is well known that the early life stages of this species are especially sensitive to acute stressors [23]. Increasing the knowledge on the possible effects of structures as EE will provide important tools to improve the welfare of captive fish, leading not only to ethical but also to economic benefits for fish farmers [4].
2. Materials and Methods
2.1. Experimental Design and Settings
A total of 420 seabass (mean standard length ± SD: 9.8 ± 1.1 cm; mean body weight ± SD: 16.5 ± 5.6 g) were obtained from a commercial hatchery (Aqüicultura Balear S.A.—Culmarex, Palma de Mallorca, Spain) and acclimated to laboratory conditions for one week at the Laboratory of Marine Research and Aquaculture (LIMIA) in Port d’Andratx, Spain. Then, seabass were randomly distributed in 6 circular tanks (water volume 150 L, Figure 1A) in groups of 70 and maintained at a temperature of 20 ± 1 °C with a light–dark (12:12 h) photoperiod. The salinity was 38 PSU, and dissolved oxygen was kept close to saturation by aeration through diffusion stones. The tanks were provided with mechanical filters, with a flow-through seawater system, UV sterilisation, and compressed air supply. Three tanks were enriched with 3 plant-fibre ropes hanging from one edge of the tank to the other, two parallel (130 cm) and one perpendicular larger (170 cm), all of them at different depths and similar distances among them (Figure 1B). The other three tanks did not present structural enrichment and were considered the control or non-enriched (NE) treatment. The choice of this type of enrichment was made based on previous studies on seabream [8,9,10] but also regarding the swimming behaviour of the species, given that seabass make vertical movements in the water column and the horizontal ropes might represent an obstacle/challenge. They were fed a commercial pelleted diet (sinking pellets; 2% of their body mass) specific for seabass (Skretting® 106 Perla MP, Stavanger, Norway) daily by hand at 13:00 h. All tanks were thoroughly cleaned daily by siphoning faeces and uneaten pellets. The seabass juveniles were maintained under these experimental conditions for 60 days.
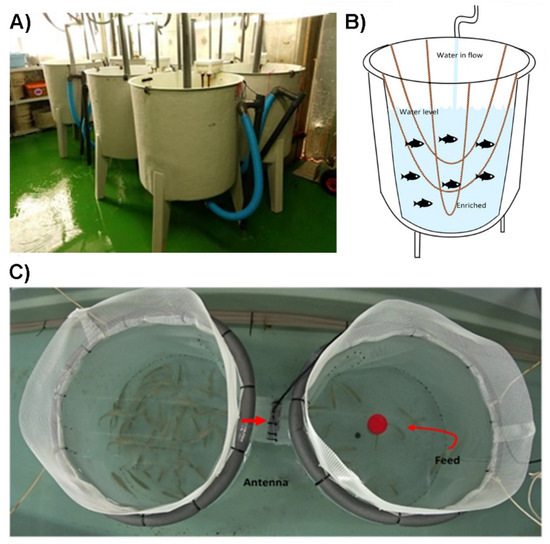
Figure 1.
Experimental settings: (A) six circular tanks (150 L each) in a flow-through system; (B) schematic representation of the suspended ropes used as structural enrichment; (C) top view of the group-based risk-taking test used in the present study.
2.2. Group-Based Risk-Taking Test
Fish were individually PIT-tagged (Trovan®, Aalten, The Netherlands) on the 30th day of the experiment and maintained in the same conditions (EE vs. NE) for another 30 days (totalling 60 days). Each fish was caught and anaesthetised by submersion in an aqueous solution of tricaine methane sulfonate (MS-222, 75 mg L-1, immersion period: 1–2 min). Then, a passive integrated transponder tag (PIT-tag) was implanted in the visceral cavity of each fish. Then, all 70 fish from each tank were exposed to a risk-taking test, a group-based test that consists of assessing the ability to explore a new risky area [24], which has been previously demonstrated to be a consistent and effective method for seabass [19,21]. Two circular cages (60 cm diameter × 50 cm depth) connected by a tubular passage (10 cm diameter × 20 cm length) were settled inside a bigger tank (10,000 L) with the same water conditions as the previous experimental period. One cage was provided with unattainable food to encourage passage, and it was considered the risky area (Figure 1C). A PIT-tag detection antenna (diameter 100/125 × 620 mm, Trovan®, The Netherlands) was located around the middle of the tunnel, which allowed for monitoring individual passages from one cage to the other. Each group of fish from each tank was left in the safe area (empty cage) for 1 h and 15 min. They were acclimated during the first period of 15 min, during which they were not allowed to pass through the tunnel. During the following 60 min, the number of movements of each individual between cages was determined through antenna detections. The test was repeated four times (every 4 days for 16 days) in order to assess the effects of EE on learning capabilities of fish from different treatments. The effects of EE on the heterogeneity of behavioural parameters were also assessed by estimation of the coefficient of variation (CV, %). In order to remove “false” passes (fish remaining motionless inside the tunnel and hence continuously detected by the antenna) [25], a segmented regression with an a priori unknown breakpoint was used to identify the time period that could be considered new “real” passes along the tunnel (breakpoint = 19.45 s). Therefore, real passes per individual were recorded in each test, together with the total number of individuals, which allowed us to estimate the total number of passes divided by the number of unique individuals crossing the antenna.
2.3. Statistical Analyses
A Bayesian approach was followed to fit generalised linear mixed-effects models (GLMMs, R library “MCMCglmm”) [26], which were used to test for differences in the number of fish passes through the tunnel among tanks, among weeks, and between treatments. The zero-inflated Poisson distribution was considered, accounting for the type of data that was being fitted. The GLMM included week and treatment as fixed effects and the identity of the fish and the tank as random intercept terms. In this model, we used the entire data set without considering differences in the size of the fish because it was previously tested, and no size effect was found on the number of passes through the antenna. The parameters, 97.5% credibility intervals, and p-values were estimated using a Bayesian Markov chain–Monte Carlo approach with uninformative priors. We set the initial iterations to 500,000 after discarding the initial 1000 iterations (burn-in period); 1 out of 100 of the remaining iterations were kept to prevent autocorrelation (thinning strategy), obtaining 4.990 posterior samples. The convergence of the MCMC chains was assessed by visual inspection of the chain trace plots. The adjusted repeatability (Adjusted-R) was estimated as the quotient of the between-individual variance (the variance across random intercepts attributed to the individuals: Vind) and the sum of Vind and the within-individual or residual variance (the variance associated with the tank and measurement error) for a given behavioural trait in accordance with previous studies [27].
2.4. Ethical Statement
All procedures with fish were approved by the Ethical Committee of Animal Experimentation (CEEA-UIB, Spain; Ref. 85/02/18) and carried out strictly by trained and competent personnel, in accordance with the European Directive (2010/63/UE) and Spanish Royal Decree (RD53/2013) to ensure good practices for animal care, health, and welfare.
3. Results
The developed model showed that trial (time) and treatment (EE or NE) had a significant effect on the total number of passes during the trials, whereas the interaction effect of both variables had no effect on the variable (Table 1). The mean repeatability per individual resulting from this model was 0.37 (the confidence interval ranged from 0.18 to 0.81). The total number of passes (counts) detected by the antenna for all tagged individuals increased over time, where some differences in the magnitude and the coefficient of variation (CV) could be observed between EE and NE fish during the experimental tests, being more notable during the third week’s test, but showing similar values at the other trials (Figure 2A,B). The mean number of tagged fish individuals detected by the antenna exploring the new area increased over time in consecutive tests in both treatments (Figure 2C). However, CV differed between treatments, remaining similar over time for EE fish groups and increasing in NE fish groups during the test (Figure 2D). In addition, the mean number of passes (counts) per fish in the EE tanks gradually increased over the experimental tests, whereas this pattern was not clear for NE fish tanks (Figure 2E), and the CVs in EE tanks were lower than the CVs of NE fish tanks, although in both cases, the variations showed a similar pattern over time (Figure 2F).

Table 1.
Resulting posterior mean, lower and upper 95% credibility intervals (CIs), effective sample size (out of 4990), pMCMC (MCMC p-value), and significance (asterisks) from the developed model.
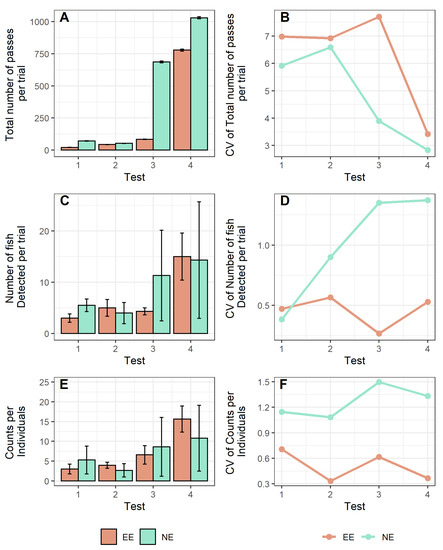
Figure 2.
Resulting values from the risk-taking test of European seabass reared in enriched (EE) and non-enriched (NE) tanks regarding: (A) sum of total passes (±SD: standard deviation) detected in each consecutive test and (B) their respective coefficients of variation; (C) mean values (±SD: standard deviation) of fish detected in each consecutive test and (D) their respective coefficients of variation (CVs); (E) mean values (±SD: standard deviation) of passes detected per fish in each consecutive test and (F) their respective coefficients of variation (CVs).
4. Discussion
Structural enrichment is considered a useful tool to improve the welfare conditions of captive fish by increasing the heterogeneity and complexity of captive environments [7]. The aim of this study was to assess the effects of suspended structural enrichment on the group risk-taking behaviour of European seabass juveniles in rearing tanks. This was approached by comparing fish reared in tanks with suspended ropes as enrichment and fish reared in non-enriched environments. We demonstrated that suspended vertical structures (U-shaped ropes) can positively affect the group risk-taking behaviour of captive seabass but also reduce the variations among groups. This behavioural homogeneity at the tank level indicates a more homogeneous group response to stressful situations, which might correspond to better adaptation or a more stable social structure within the fish group. Unstable social hierarchies can have consequences on fish welfare due to dominant/subordinate conflicts (territoriality, mating), competence for resources, and aggressive interactions, triggering social stress in fish groups and the impairment of fish welfare [15,16]. This can be seen particularly in Figure 2F, where the CV is much higher in the NE group, meaning that the number of counts in NE was due to a few individuals crossing very often. Conversely, in the EE group, the CV is very low, demonstrating that all individuals behaved similarly at the group or tank level.
Previous studies on seabream demonstrated that vertical structures had direct effects on fish behaviour in rearing conditions, reducing aggressiveness, increasing the effective space used, and promoting the spatial exploration and cognitive abilities of captive fish [8,9,10]. In addition, beneficial effects of the presence of a specific coloured substrate (as a mean of EE) were reported on the growth, behaviour, and stress response of Gilthead seabream [28,29,30,31]. The authors suggested that such positive effects may be related to altered social interactions, indicating the establishment of a less stressful social organisation in enriched-reared fish groups [29]. The relative lack of strong behavioural effects between the different treatments in our study could be related to the type or design of the EE strategy chosen. Some authors demonstrated that the level or intensity of physical structures (i.e., number of structures) influence the social stability and agonistic interactions of territorial species, such as black rockfish (Sebastes schlegelii) and fat greenling (Hexagrammos otakii) [32], Nile tilapia (Oreochromis niloticus) [33], redbreast tilapia (Tilapia rendalli) [34], and convict cichlid (Amatitlania nigrofasciata) [35]. The proper enrichment level can significantly accelerate the formation of social stability, whereas other specific enrichments may slow down this process [32]. In fact, some authors clearly pointed out the positive effects of environmental enrichment in reducing the maladaptive risk-taking behaviour of farmed fish [36,37]. Therefore, the effect of physical enrichment on risk-taking behaviour and social stability can be intensity-dependent, and the direction and magnitude may depend on resources, life stages, and rearing conditions.
Further research is still needed to improve the knowledge of the potential benefits of structural enrichment on diverse aspects of fish welfare and aquaculture management, taking into consideration not only the farming conditions and systems but also the biological needs and preferences of fish species reared in captivity [4]. The structural enrichment used in this experiment can be easily implemented in rearing tanks and at other life stages, especially during the grow-out phase normally performed in open-sea cages, but different levels and designs must be explored. U-shaped suspended plant-fibre ropes have some extra benefits when compared with other environmental enrichment structures, namely, being affordable for aquaculture companies and biodegradable, with no negative impacts on the environmental footprint.
To conclude, our results suggest a positive effect of structural enrichment on group risk-taking behaviour, which might be influenced by social stabilisation, improving the response to stressful events at the tank level and thus increasing the welfare of seabass.
Author Contributions
Conceptualisation, P.A.-L.; methodology, P.A.-L.; formal analysis, S.N.-V., G.F.-B. and C.D.-G.; resources, P.A.-L.; data curation, S.N.-V. and C.D.-G.; writing—original draft preparation, P.A.-L. and S.N.-V.; writing—review and editing, P.A.-L., S.N.-V., G.F.-B., C.D.-G. and J.L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of the University of Balearic Islands (CEEA-UIB, Spain; Ref. 85/02/18; February 2018).
Data Availability Statement
Data are available on request from the authors.
Acknowledgments
This work is a contribution of the Joint Associated Unit IMEDEA-LIMIA. We would like to thank the staff at LIMIA for their help with maintenance and taking care of fish and tanks during the experimentation process, as well as Aqüicultura Balear S.A.U (Grupo Culmarex) for their support and interest in this project. This study (and corresponding author) was supported by Spanish Juan de la Cierva Incorporación (Ref. IJCI-2015-25595) and Ramón y Cajal (Ref. RYC2020-029629-I) postdoctoral grants and received Portuguese national funds from FCT—Foundation for Science and Technology through project UIDB/04326/2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bergqvist, J.; Gunnarsson, S. Finfish Aquaculture: Animal Welfare, the Environment, and Ethical Implications. J. Agric. Environ. Ethic 2011, 26, 75–99. [Google Scholar] [CrossRef]
- Huntingford, F.; Kadri, S.; Jobling, M. Introduction: Aquaculture and Behaviour; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 1–35. [Google Scholar] [CrossRef]
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Cabrera-Álvarez, M.J.; Maia, C.M.; Saraiva, J.L. Environmental enrichment in fish aquaculture: A review of fundamental and practical aspects. Rev. Aquac. 2021, 14, 704–728. [Google Scholar] [CrossRef]
- Brydges, N.; Braithwaite, V.A. Does environmental enrichment affect the behaviour of fish commonly used in laboratory work? Appl. Anim. Behav. Sci. 2009, 118, 137–143. [Google Scholar] [CrossRef]
- Jones, N.A.R.; Webster, M.M.; Salvanes, A.G.V. Physical enrichment research for captive fish: Time to focus on the DETAILS. J. Fish Biol. 2021, 99, 704–725. [Google Scholar] [CrossRef]
- Näslund, J.; Johnsson, J.I. Environmental enrichment for fish in captive environments: Effects of physical structures and substrates. Fish Fish. 2014, 17, 1–30. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Díaz-Gil, C.; Saraiva, J.L.; Moranta, D.; Castanheira, M.F.; Nuñez-Velázquez, S.; Ledesma-Corvi, S.; Mora-Ruiz, M.; Grau, A. Effects of structural environmental enrichment on welfare of juvenile seabream (Sparus aurata). Aquac. Rep. 2019, 15, 100224. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Caballero-Froilán, J.C.; Jiménez-García, M.; Capó, X.; Tejada, S.; Saraiva, J.; Sureda, A.; Moranta, D. Enriched environments enhance cognition, exploratory behaviour and brain physiological functions of Sparus aurata. Sci. Rep. 2020, 10, 11252. [Google Scholar] [CrossRef]
- Muñoz, L.; Aspillaga, E.; Palmer, M.; Saraiva, J.; Arechavala-Lopez, P. Acoustic Telemetry: A Tool to Monitor Fish Swimming Behavior in Sea-Cage Aquaculture. Front. Mar. Sci. 2020, 7, 645. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Korte, S.M.; De Boer, S.F.; Van Der Vegt, B.J.; Van Reenen, C.G.; Hopster, H.; De Jong, I.C.; Ruis, M.A.W.; Blokhuis, H.J. Coping styles in animals: Current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 1999, 23, 925–935. [Google Scholar] [CrossRef]
- Lee, J.S.F.; Berejikian, B.A. Effects of the rearing environment on average behaviour and behavioural variation in steelhead. J. Fish Biol. 2008, 72, 1736–1749. [Google Scholar] [CrossRef]
- Øverli, Ø.; Sørensen, C.; Pulman, K.G.; Pottinger, T.; Korzan, W.; Summers, C.H.; Nilsson, G.E. Evolutionary background for stress-coping styles: Relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates. Neurosci. Biobehav. Rev. 2007, 31, 396–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boscolo, C.N.P.; Morais, R.N.; de Freitas, E.G. Same-sized fish groups increase aggressive interaction of sex-reversed males Nile tilapia GIFT strain. Appl. Anim. Behav. Sci. 2011, 135, 154–159. [Google Scholar] [CrossRef]
- Dara, M.; Dioguardi, M.; Vazzana, M.; Vazzana, I.; Accardi, D.; Carbonara, P.; Alfonso, S.; Cammarata, M. Effects of Social Hierarchy Establishment on Stress Response and Cell Phagocytosis in Gilt-Head Sea Bream (Sparus aurata). Fishes 2022, 7, 75. [Google Scholar] [CrossRef]
- Maguire, S.M.; DeAngelis, R.; Dijkstra, P.D.; Jordan, A.; Hofmann, H.A. Social network dynamics predict hormone levels and behavior in a highly social cichlid fish. Horm. Behav. 2021, 132, 104994. [Google Scholar] [CrossRef]
- Galhardo, L.; Almeida, O.; Oliveira, R.F. Preference for the presence of substrate in male cichlid fish: Effects of social dominance and context. Appl. Anim. Behav. Sci. 2009, 120, 224–230. [Google Scholar] [CrossRef]
- Castanheira, M.F.; Conceição, L.E.; Millot, S.; Rey, S.; Bégout, M.-L.; Damsgård, B.; Kristiansen, T.; Höglund, E.; Øverli, Ø.; Martins, C.I. Coping styles in farmed fish: Consequences for aquaculture. Rev. Aquac. 2015, 9, 23–41. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, S.; Millot, S.; Leguay, D.; Chatain, B.; Bégout, M.-L. Consistency in European seabass coping styles: A life-history approach. Appl. Anim. Behav. Sci. 2015, 167, 74–88. [Google Scholar] [CrossRef] [Green Version]
- Huntingford, F.A.; Andrew, G.; Mackenzie, S.; Morera, D.; Coyle, S.M.; Pilarczyk, M.; Kadri, S. Coping strategies in a strongly schooling fish, the common carp Cyprinus carpio. J. Fish Biol. 2010, 76, 1576–1591. [Google Scholar] [CrossRef]
- Millot, S.; Bégout, M.-L.; Chatain, B. Risk-taking behaviour variation over time in sea bass Dicentrarchus labrax: Effects of day-night alternation, fish phenotypic characteristics and selection for growth. J. Fish Biol. 2009, 75, 1733–1749. [Google Scholar] [CrossRef] [Green Version]
- Leal, E.; Fernández-Durán, B.; Guillot, R.; Ríos, D.; Cerdá-Reverter, J.M. Stress-induced effects on feeding behavior and growth performance of the sea bass (Dicentrarchus labrax): A self-feeding approach. J. Comp. Physiol. B 2011, 181, 1035–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varsamos, S.; Flik, G.; Pepin, J.; Bonga, S.W.; Breuil, G. Husbandry stress during early life stages affects the stress response and health status of juvenile sea bass, Dicentrarchus labrax. Fish Shellfish. Immunol. 2006, 20, 83–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castanheira, M.F.; Herrera, M.; Costas, B.; Conceição, L.E.; Martins, C.I. Linking cortisol responsiveness and aggressive behaviour in gilthead seabream Sparus aurata: Indication of divergent coping styles. Appl. Anim. Behav. Sci. 2013, 143, 75–81. [Google Scholar] [CrossRef]
- Muggeo, V.M.R. Estimating regression models with unknown break-points. Stat. Med. 2003, 22, 3055–3071. [Google Scholar] [CrossRef]
- Hadfield, J.D. MCMC Methods for Multi-Response Generalized Linear Mixed Models: The MCMCglmm R Package. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, S.; Schielzeth, H. Repeatability for gaussian and non-gaussian data: A practical guide for biologists. Biol. Rev. 2010, 85, 935–956. [Google Scholar] [CrossRef]
- Batzina, A.; Dalla, C.; Papadopoulou-Daifoti, Z.; Karakatsouli, N. Effects of environmental enrichment on growth, aggressive behaviour and brain monoamines of gilthead seabream Sparus aurata reared under different social conditions. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2014, 169, 25–32. [Google Scholar] [CrossRef]
- Batzina, A.; Dalla, C.; Tsopelakos, A.; Papadopoulou-Daifoti, Z.; Karakatsouli, N. Environmental enrichment induces changes in brain monoamine levels in gilthead seabream Sparus aurata. Physiol. Behav. 2014, 130, 85–90. [Google Scholar] [CrossRef]
- Batzina, A.; Kalogiannis, D.; Dalla, C.; Papadopoulou-Daifoti, Z.; Chadio, S.; Karakatsouli, N. Blue substrate modifies the time course of stress response in gilthead seabream Sparus aurata. Aquaculture 2014, 420, 247–253. [Google Scholar] [CrossRef]
- Batzina, A.; Karakatsouli, N. The presence of substrate as a means of environmental enrichment in intensively reared gilthead seabream Sparus aurata: Growth and behavioral effects. Aquaculture 2012, 370, 54–60. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, Y.; Zhang, Z.; Zhang, X.; Chen, S. A Comparative Study on Two Territorial Fishes: The Influence of Physical Enrichment on Aggressive Behavior. Animals 2021, 11, 1868. [Google Scholar] [CrossRef] [PubMed]
- Barreto, R.E.; Carvalho, G.G.A.; Volpato, G.L. The aggressive behavior of Nile tilapia introduced into novel environments with variation in enrichment. Zoology 2011, 114, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Torrezani, C.S.; Pinho-Neto, C.F.; Miyai, C.A.; Sanches, F.H.C.; Barreto, R.E. Structural enrichment reduces aggression in Tilapia rendalli. Mar. Freshw. Behav. Physiol. 2013, 46, 183–190. [Google Scholar] [CrossRef]
- Barley, A.J.; Coleman, R.M. Habitat structure directly affects aggression in convict cichlids Archocentrus nigrofasciatus. Curr. Zool. 2010, 56, 52–56. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Li, Z. Effects of different levels of environmental enrichment on the sheltering behaviors, brain development and cortisol levels of black rockfish Sebastes schlegelii. Appl. Anim. Behav. Sci. 2019, 218, 104825. [Google Scholar] [CrossRef]
- Roberts, L.; Taylor, J.; de Leaniz, C.G. Environmental enrichment reduces maladaptive risk-taking behavior in salmon reared for conservation. Biol. Conserv. 2011, 144, 1972–1979. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).