Effects of Non-Heated and Heat Processed Krill and Squid Meal-Based Diet on Growth Performance and Biochemical Composition in Juvenile Pacific Bluefin Tuna Thunnus orientalis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Feeding Experiment and Sampling Schedule
2.3. Chemical Analyses
2.4. Statistical Analyses
3. Results
3.1. Survival and Growth of PBT Juveniles
3.2. Chemical Property of PBT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Food and Agriculture Organization, Year Book of Fishery Statistics Summary Tables; Sales and Marketing Group, Information Division, Food and Agriculture Organization: Rome, Italy, 2019; Available online: http://www.fao.org/3/ca5495t/CA5495T.pdf (accessed on 14 April 2021).
- MAFF (Ministry of Agriculture, Forestry and Fisheries). Statistics Division Annual Report of Fisheries and Aquaculture Industry Statistics; Chiyoda: Tokyo, Japan, 2019. (In Japanese) [Google Scholar]
- JFA (Japanese Fisheries Agency). Marino-Forum 21 General Incorporated Association. 2015. Available online: http://www.yousyokugyojyou.net/index4.htm (accessed on 14 April 2021).
- Bransden, M.P.; Carter, C.G.; Nichols, P.D. Replacement of Fish Oil with Sunflower Oil in Feeds for Atlantic Salmon (Salmo salar L.): Effect on Growth Performance, Tissue Fatty Acid Composition and Disease Resistance. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 135, 611–625. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization, The State of World Fisheries and Aquaculture; Contributing to Food Security and Nutrition for All, Food and Agriculture Organization: Rome, Italy, 2016; Available online: http://www.fao.org/3/i5555e/i5555e.pdf (accessed on 14 April 2021).
- Glencross, B.D.; Booth, M.; Allan, G.L. A Feed Is Only as Good as Its Ingredients—A Review of Ingredient Evaluation Strategies for Aquaculture Feeds. Aquacult. Nutr. 2007, 13, 17–34. [Google Scholar] [CrossRef]
- Storebakken, T.; Refstie, S.; Ruyter, B. Soy products as fat and protein sources in fish feeds for intensive aquaculture. In Soy in Animal Nutrition; Drackly, J.K., Ed.; Federation of Animal Science Societies Savoy: Champaign, IL, USA, 2000; pp. 127–170. [Google Scholar]
- Barrows, F.; Gaylord, T.; Stone, D.; Smith, C. Effect of Protein Source and Nutrient Density on Growth Efficiency, Histology and Plasma Amino Acid Concentration of Rainbow Trout (Oncorhynchus mykiss Walbaum). Aquacult. Res. 2007, 38, 1747–1758. [Google Scholar] [CrossRef]
- Gatlin, D.; Barrows, F.; Brown, P.; Dabrowski, K.; Gaylord, T.; Hardy, R.; Herman, E.; Hu, G.; Krogdahl, A.; Nelson, R.; et al. Expanding the Utilization of Sustainable Plant Products in Aquafeeds: A Review. Aquacult. Res. 2007, 38, 551–579. [Google Scholar] [CrossRef]
- Oliva-Teles, A.; Enes, P.; Peres, H. Replacing Fish Meal and Fish Oil in Industrial Aquafeed. In Feed and Feeding Practices in Aquaculture; Davis, A., Ed.; Woodhead Publishing Limited: Waltham, MA, USA, 2015; pp. 203–233. [Google Scholar]
- Wilson, R.P. Amino Acids and Proteins. In Fish Nutrition, 3rd ed.; Halver, J.E., Hardy, R.W., Eds.; Academic Press Inc.: San Diego, CA, USA, 2002; pp. 143–179. [Google Scholar] [CrossRef]
- Floreto, E.A.T.; Bayer, R.C.; Brown, P.B. The Effects of Soybean-Based Diets, With and Without Amino Acid Supplementation, on Growth and Biochemical Composition of Juvenile American Lobster, Homarus americanus. Aquaculture 2000, 189, 211–235. [Google Scholar] [CrossRef]
- Francis, G.; Makkar, H.P.S.; Becker, K. Antinutritional Factors Present in Plant-Derived Alternate Fish Feed Ingredients and Their Effects in Fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Ostaszewska, T.; Dabrowski, K.; Palacios, M.E.; Olejniczak, M.; Wieczorek, M. Growth and Morphological Changes in the Digestive Tract of Rainbow Trout (Oncorhynchus mykiss) and Pacu (Piaractus mesopotamicus) Due to Casein Replacement with Soybean Proteins. Aquaculture 2005, 245, 273–286. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Penn, M.; Thorsen, J.; Refstie, S.; Bakke, A.M. Important Antinutrients in Plant Feedstuffs for Aquaculture: An Update to Recent Findings Regarding Responses in Salmonids. Aquacult. Res. 2010, 41, 333–344. [Google Scholar] [CrossRef]
- Komaki, Y.; Matsue, Y. Ecological Studies on the Euphausia cea Distributed in the Japan Sea. Rep. Coop. Surv. Warm Tsushima Curr. Relat. Waters 1958, 2, 146–159. (In Japanese) [Google Scholar]
- Nicol, S.; Endo, Y. Krill Fisheries: Development, Management and Ecosystem Implications. Aquat. Living Resour. 1999, 12, 105–120. [Google Scholar] [CrossRef]
- Rehbein, H. Amino Acid Composition and Pepsin Digestibility of Krill Meal. J. Agric. Food Chem. 1981, 29, 682–684. [Google Scholar] [CrossRef]
- Van der Meeren, T.; Olsen, R.E.; Hamre, K.; Fyhn, H.J. Biochemical Composition of Copepods for Evaluation of Feed Quality in Production of Juvenile Marine Fish. Aquaculture 2008, 274, 375–397. [Google Scholar] [CrossRef]
- Everson, I. Krill: Biology, Ecology, and Fisheries; Blackwell Science Publishing: Oxford, UK, 2008. [Google Scholar]
- Hansen, J.Ø.; Shearer, K.D.; Øverland, M.; Penn, M.H.; Krogdahl, Å.; Mydland, L.T.; Storebakken, T. Replacement of LT Fish Meal with a Mixture of Partially Deshelled Krill Meal and Pea Protein Concentrates in Diets for Atlantic Salmon (Salmo salar). Aquaculture 2011, 315, 275–282. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Olsen, R.E.; Lall, S.P. Effects of Partial or Total Replacement of Fish Meal with Freeze-Dried Krill (Euphausia superba) on Growth and Nutrient Utilization of Juvenile Atlantic Cod (Gadus morhua) and Atlantic Halibut (Hippoglossus hippoglossus) Fed the Same Practical Diets. Aquac. Nutr. 2011, 17, 287–303. [Google Scholar] [CrossRef]
- Lu, H.F.S.; Bruheim, I.; Jacobsen, C. Oxidative Stability and Non-Enzymatic Browning Reactions in Antarctic Krill Oil (Euphausia superba). Lipid Technol. 2014, 26, 111–114. [Google Scholar] [CrossRef]
- Watanabe, T.; Arakawa, T.; Kitajima, C.; Fujita, S. Effect of Nutritional Quality of Broodstock Diets on Reproduction of Red Seabream. Nippon Suisan Gakkaishi 1984, 50, 495–501. [Google Scholar] [CrossRef]
- Watanabe, T.; Itoh, A.; Murakami, A.; Tsukashima, Y.; Kitajima, C.; Fujita, S. Effect of Nutritional Quality of Diets Given to Broodstock on the Verge of Spawning on Reproduction of Red Seabream. Nippon Suisan Gakkaishi 1984, 50, 1023–1028. [Google Scholar] [CrossRef]
- Cho, S.H.; Lee, S.M.; Lee, S.M.; Park, B.H.; Park, I.S.; Choi, C.Y.; Min, B.H.; Hur, S.B.; Jo, J.Y. Effect of Partial Replacement of Fish Meal with Squid Liver meal™ in the Diet on Growth and Body Composition of Juvenile Olive Flounder (Paralichthys olivaceus) during Winter Season. Fish. Aquat. Sci. 2005, 8, 65–69. [Google Scholar] [CrossRef][Green Version]
- Seoka, M.; Bandou, T.; Suimon, M.; Sawada, Y. Toyama Squid Watasenia scintillans Meal as a Protein Source in Artificial Diet for Juvenile Pacific Bluefin Tuna Thunnus orientalis. Aquac. Sci. 2010, 58, 143–144. [Google Scholar] [CrossRef]
- Cho, J.H.; Haga, Y.; Masuda, R.; Satoh, S. Periodic Changes in the Growth Performance and Biochemical Composition of Juvenile Red Sea Bream Pagrus major Fed Non-Heated and Heated Squid and Krill Meal-Based Diets. Fish. Sci. 2018, 84, 699–713. [Google Scholar] [CrossRef]
- Tonheim, S.K.; Nordgreen, A.; Høgøy, I.; Hamre, K.; Rønnestad, I. In Vitro Digestibility of Water-Soluble and Water-Insoluble Protein Fractions of Some Common Fish Larvae Feeds and Feed Ingredients. Aquaculture 2007, 262, 426–435. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Izumi, T.; Kurata, M.; Okada, T. Effects of the Tank Wall Pattern on the Survival Rate, Bone Injury, and Stress Response of Pacific Bluefin Tuna, Thunnus orientalis. Aquac. Eng. 2013, 56, 13–17. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- De Schrijver, R.; Ollevier, F. Protein Digestion in Juvenile Turbot (Scophthalmus maximus) and Effects of Dietary Administration of Vibrio proteolyticus. Aquaculture 2000, 186, 107–116. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloan Stanley, G.H. A Simple Method for Isolation and Purification of Total Lipids from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of Fatty Acid Methyl Esters and Dimethylacetals from Lipids with Boron Fluoride-Methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Boonyoung, S.; Haga, Y.; Satoh, S. Preliminary Study on Effects of Methionine Hydroxyl Analog and Taurine Supplementation in a Soy Protein Concentrate-Based Diet on the Biological Performance and Amino Acid Composition of Rainbow Trout Oncorhynchus mykiss (Wallbaum). Aquac. Res. 2012, 44, 1339–1347. [Google Scholar] [CrossRef]
- Kvåle, A.; Harboe, T.; Mangor-jensen, A.; Hamre, K. Effects of Protein Hydrolysate in Weaning Diets for Atlantic Cod (Gadus morhua L.) and Atlantic Halibut (Hippoglossus hippoglossus L.). Aquac. Nutr. 2009, 15, 218–227. [Google Scholar] [CrossRef]
- Haga, Y.; Naiki, T.; Tazaki, Y.; Takebe, T.; Kumon, K.; Tanaka, Y.; Shiozawa, S.; Nakamura, T.; Ishida, S.; Ide, K.; et al. Effect of Feeding Microdiet and Yolk-Sac Larvae of Spangled Emperor Lethrinus nebulosus at Different Ages on Survival and Growth of Pacific Bluefin Tuna Thunnus orientalis Larvae. Aquac. Sci. 2010, 58, 491–499. [Google Scholar] [CrossRef]
- Cho, J.H.; Haga, Y.; Kamimura, Y.; Akawaza, A.; Itoh, A.; Satoh, S. Production Performance of Pacific Bluefin Tuna Thunnus orientalis Larvae and Juveniles Fed Commercial Diets and Effects of Switching Diets. Aquac. Sci. 2016, 64, 359–370. [Google Scholar] [CrossRef]
- Cho, J.H.; Haga, Y.; Kamimura, Y.; Itoh, A.; Satoh, S. Peruvian Fish Meal Has Comparative Potential to Enzyme-Treated Chilean Fish Meal as Protein Source of Diet for Larvae and Juvenile Pacific Bluefin Tuna Thunnus orientalis. Fish. Sci. 2022, 186, 107–116. [Google Scholar] [CrossRef]
- Watanabe, Y. Intracellular Digestion of Horseradish Peroxidase by the Intestinal Cells of Teleost Larvae and Juveniles. Bull. Jpn. Soc. Sci. Fish. 1982, 48, 37–42. [Google Scholar] [CrossRef][Green Version]
- Fyhn, H.J.; Serigstad, B. Free Amino Acids as Energy Substrate in Developing Eggs and Larvae of the Cod Gudas morhua. Mar. Biol. 1987, 96, 335–341. [Google Scholar] [CrossRef]
- Fyhn, H.J. First Feeding of Marine Fish Larvae: Are Free Amino Acids the Source of Energy? Aquaculture 1989, 80, 111–120. [Google Scholar] [CrossRef]
- Satoh, K. Studies on Improvement of Composed Diet for Yellowtail Culture. Bull. Oita Inst. Mar. Fish. Sci. 2005, 6, 19–77, (In Japanese with English Abstract). [Google Scholar]
- Kohbara, J.; Miyazaki, T.; Takii, K.; Hosokawa, H.; Ukawa, M.; Kumai, H. Gustatory Responses in Pacific Bluefin Tuna Thunnus orientalis (Temminck and Schlegel). Aquac. Res. 2006, 37, 847–854. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Fish Protein Hydrolysates: Production, Biochemical, and Functional Properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef]
- Dohmoto, N. Antarctic Krill and Its Function for Human Health. Nippon Suisan Gakkaishi 2002, 68, 714–718. [Google Scholar] [CrossRef]
- Sánchez, C.A.O.; Zavaleta, E.B.; García, G.R.U.; Solano, G.L.; Díaz, M.P.R. Krill Oil Microencapsulation: Antioxidant Activity, Astaxanthin Retention, Encapsulation Efficiency, Fatty Acids Profile, In Vitro Bioaccessibility and Storage Stability. LWT Food Sci. Technol. 2021, 147, 111476. [Google Scholar] [CrossRef]
- Huynh, M.D.; Kitts, D.D.; Hu, C.; Trites, A.W. Comparison of Fatty Acid Profiles of Spawning and Non-Spawning Pacific Herring, Clupea harengus pallasi. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 146, 504–511. [Google Scholar] [CrossRef]
- Zlatanos, S.; Laskaridis, K. Seasonal Variation in the Fatty Acid Composition of Three Mediterranean Fish—Sardine (Sardina pilchardus), Anchovy (Engraulis incrasicholus) and Picarel (Spicara smaris). Food Chem. 2007, 103, 725–728. [Google Scholar] [CrossRef]
- Bell, J.G.; McEvoy, L.A.; Estevez, A.; Shields, R.J.; Sargent, J.R. Optimising Lipid Nutrition in First-Feeding Flatfish Larvae. Aquaculture 2003, 227, 211–220. [Google Scholar] [CrossRef]
- Seoka, M.; Kurata, M.; Tamagawa, R.; Biswas, A.K.; Biswas, B.K.; Seok Kian Yong, A.S.K.; Kim, Y.-S.; Ji, S.-C.; Takii, K.; Kumai, H. Dietary Supplementation of Salmon Roe Phospholipid Enhances the Growth and Survival of Pacific Bluefin Tuna Thunnus orientalis Larvae and Juveniles. Aquaculture 2008, 275, 225–234. [Google Scholar] [CrossRef]
- Tocher, D.R.; Sargent, J.R. Analysis of Lipids and Fatty Acids in Ripe Roes from Some Northwest European Marine Fish. Lipids 1984, 19, 492–499. [Google Scholar] [CrossRef]
- Drillet, G.; Jørgensen, N.O.G.; Sørensen, T.F.; Ramløv, H.; Hansen, B.W. Biochemical and Technical Observations Supporting the Use of Copepods as Live Feed Organisms in Marine Larviculture. Aquac. Res. 2006, 37, 756–772. [Google Scholar] [CrossRef]

| Ingredients (%) 2 | Treatment 1 | ||||
|---|---|---|---|---|---|
| HS | NHS | HK | NHK | PF | |
| Heated squid meal | 51.4 | - | - | - | - |
| Non-heated squid meal | - | 51.4 | - | - | - |
| Heated krill meal | - | - | 51.4 | - | - |
| Non-heated krill meal | - | - | - | 51.4 | - |
| Porcine blood meal | 5.0 | 5.0 | 5.0 | 5.0 | - |
| Defatted horse mackerel meal | 12.0 | 12.0 | 12.0 | 12.0 | - |
| Chicken egg albumin | 6.6 | 6.6 | 6.6 | 6.6 | |
| Chicken egg lecithin | 3.2 | 3.2 | 3.2 | 3.2 | - |
| DHA70E 3 | 1.3 | 1.3 | 1.3 | 1.3 | - |
| Taurine | 1.0 | 1.0 | 1.0 | 1.0 | - |
| Fish oil 4 | 6.7 | 6.7 | 8.1 | 8.1 | - |
| α-Starch | 2.0 | 2.0 | 2.0 | 2.0 | - |
| Monobasic calcium phosphate | 1.9 | 1.9 | 1.9 | 1.9 | - |
| Vitamin E (50%) | 0.1 | 0.1 | 0.1 | 0.1 | - |
| Choline chloride | 0.8 | 0.8 | 0.8 | 0.8 | - |
| Sodium ascorbyl phosphate | 0.1 | 0.1 | 0.1 | 0.1 | - |
| Mineral mixture 5 | 1.5 | 1.5 | 1.5 | 1.5 | - |
| Vitamin mixture 6 | 3.0 | 3.0 | 3.0 | 3.0 | - |
| Bonito peptide 7 | 2.0 | 2.0 | 2.0 | 2.0 | - |
| Carboxymethyl cellulose | 2.0 | 2.0 | 2.0 | 2.0 | - |
| Cellulose | 1.4 | 1.4 | - | - | - |
| Proximate composition (%, dry-weight) | |||||
| Moisture | 7.6 | 6.6 | 6.3 | 7.6 | 91.8 |
| Crude protein | 57.2 | 58.4 | 56.8 | 56.2 | 63.7 |
| Water-soluble protein | 4.1 | 7.8 | 3.3 | 4.9 | - |
| Water-insoluble protein | 48.9 | 48.6 | 51.5 | 47.6 | - |
| Crude lipid | 22.4 | 24.8 | 21.2 | 21.8 | 22.4 |
| Crude ash | 8.3 | 8.0 | 13.2 | 14.1 | 12.3 |
| HS | NHS | HK | NHK | PF | |
|---|---|---|---|---|---|
| Essential amino acids | |||||
| Arginine | 2.25 | 2.91 | 2.68 | 2.83 | 3.06 |
| Lysine | 2.83 | 3.12 | 2.67 | 3.55 | 4.77 |
| Histidine | 1.86 | 1.00 | 0.92 | 1.15 | 2.71 |
| Phenylalanine | 1.93 | 2.11 | 1.99 | 1.99 | 2.27 |
| Leucine | 3.19 | 3.32 | 3.20 | 3.42 | 4.08 |
| Isoleucine | 1.27 | 1.42 | 1.32 | 1.30 | 1.39 |
| Methionine | 0.97 | 1.25 | 1.06 | 1.16 | 1.61 |
| Valine | 1.48 | 1.74 | 1.62 | 1.53 | 1.82 |
| Threonine | 1.99 | 2.02 | 1.87 | 1.82 | 2.82 |
| Tryptophan | 0.41 | 0.47 | 0.37 | 0.61 | 0.55 |
| Non-essential amino acids | |||||
| Taurine | 1.73 | 2.02 | 1.64 | 1.86 | 0.76 |
| Alanine | 2.86 | 2.89 | 2.83 | 2.68 | 2.96 |
| Tyrosine | 1.44 | 1.57 | 1.56 | 1.59 | 2.09 |
| Cystine | 0.43 | 0.50 | 0.33 | 0.39 | N/D |
| Cystathionine | 0.13 | 0.07 | 0.09 | 0.12 | 0.07 |
| Glycine | 2.18 | 2.40 | 2.76 | 2.91 | 2.14 |
| Glutamic acid | 6.09 | 6.82 | 6.40 | 6.33 | 6.69 |
| Serine | 2.25 | 2.37 | 2.22 | 2.22 | 3.67 |
| Aspartic acid | 4.42 | 4.70 | 4.73 | 4.67 | 4.26 |
| Proline | 1.79 | 2.56 | 1.73 | 2.29 | 2.35 |
| Total | 41.49 | 45.25 | 42.00 | 44.42 | 50.09 |
| HS | NHS | HK | NHK | PF | |
|---|---|---|---|---|---|
| Essential amino acids | |||||
| Arginine | 0.07 | 0.14 | 0.17 | 0.36 | 0.58 |
| Lysine | 0.05 | 0.12 | 0.06 | 0.22 | 0.65 |
| Histidine | 0.06 | 0.06 | 0.04 | 0.01 | 1.08 |
| Phenylalanine | 0.01 | 0.08 | 0.02 | 0.07 | 0.33 |
| Leucine | 0.03 | 0.19 | 0.04 | 0.14 | 0.57 |
| Isoleucine | 0.02 | 0.08 | 0.03 | 0.07 | 0.16 |
| Methionine | N/D | 0.03 | N/D | 0.03 | 0.19 |
| Valine | 0.03 | 0.09 | 0.04 | 0.10 | 0.20 |
| Threonine | 0.03 | 0.08 | 0.03 | 0.06 | 0.13 |
| Tryptophan | N/D | 0.01 | N/D | 0.01 | 0.13 |
| Non-essential amino acids | |||||
| Taurine | 1.29 | 1.62 | 1.20 | 1.46 | 0.53 |
| Alanine | 0.15 | 0.28 | 0.11 | 0.24 | 0.31 |
| Tyrosine | 0.02 | 0.10 | 0.02 | 0.08 | 0.27 |
| Cystine | 0.03 | 0.04 | N/D | N/D | N/D |
| Cystathionine | N/D | 0.01 | 0.01 | 0.01 | 0.04 |
| Glycine | 0.04 | 0.11 | 0.48 | 0.74 | 0.08 |
| Glutamic acid | 0.01 | 0.11 | 0.01 | 0.03 | 0.21 |
| Serine | 0.03 | 0.07 | 0.03 | 0.05 | 0.09 |
| Aspartic acid | 0.06 | 0.11 | 0.06 | 0.05 | 0.10 |
| Proline | 0.62 | 1.23 | 0.42 | 0.58 | 0.04 |
| Total | 2.55 | 4.57 | 2.76 | 4.31 | 5.69 |
| Fatty Acids | Experimental Diets | ||||
|---|---|---|---|---|---|
| HS | NHS | HK | NHK | PF | |
| 14:0 | 3.7 | 3.8 | 5.5 | 6.2 | 4.4 |
| 16:0 | 18.0 | 17.5 | 19.2 | 17.5 | 19.7 |
| 16:1n−7 | 5.5 | 5.0 | 5.3 | 6.0 | 4.2 |
| 18:0 | 4.6 | 4.1 | 3.4 | 2.9 | 5.2 |
| 18:1n−9 | 11.7 | 10.3 | 12.0 | 11.3 | 9.7 |
| 18:1n−7 | 3.5 | 3.3 | 3.7 | 3.8 | 2.0 |
| 18:2n−6 | 2.8 | 2.2 | 4.1 | 3.4 | 2.7 |
| 18:3n−3 | 0.4 | 0.5 | 0.5 | 0.5 | 0.7 |
| 18:4n−3 | 1.0 | 1.0 | 1.4 | 1.4 | 1.5 |
| 20:1n−9 | 3.2 | 2.8 | 1.7 | 1.9 | 0.5 |
| 20:1n−11 | 2.7 | 2.5 | 1.4 | 1.6 | 0.3 |
| 20:4n−6 | 1.5 | 1.6 | 1.5 | 1.3 | 1.7 |
| 20:4n−3 | 0.4 | 0.4 | 0.3 | 0.3 | 0.5 |
| 20:5n−3 | 8.2 | 10.7 | 11.7 | 11.5 | 6.3 |
| 22:1n−11 | 4.0 | 3.1 | 2.8 | 2.8 | 0.3 |
| 22:1n−13 | 0.7 | 0.6 | 0.7 | 0.6 | 0.1 |
| 22:5n−3 | 0.9 | 0.8 | 0.5 | 0.5 | 1.1 |
| 22:6n−3 | 15.0 | 18.3 | 11.5 | 11.7 | 25.0 |
| 22:6n−3/20:5n−3 | 1.8 | 1.7 | 1.0 | 1.0 | 4.0 |
| Others | 12.1 | 11.5 | 12.8 | 15.0 | 13.6 |
| Σn−3 LC−PUFA 1 | 25.5 | 31.2 | 25.4 | 25.4 | 34.4 |
| Average Total Length of Initial Fish (mm) 1 | 20.5 ± 0.2 |
| Average body weight of initial fish (mg) 1 | 74.1 ± 2.7 |
| Age of initial fish body (day post hatching) | 19 |
| Tank volume (L) | 500 |
| Number of fish (ind./tank) | 240 |
| Rearing period (days) | Weaning period 3 |
| Sole feeding of test diet period 6 | |
| Water temperature (°C) 2 | 27.8 ± 0.6 |
| pH2 | 8.1 ± 0.0 |
| Dissolved oxygen (mg/L) 2 | 11.0 ± 1.7 |
| Photoperiod | 11L (07:00–18:00):13D |
| Exchange rate of sea water (% tank volume/day) | 1200 |
| Aeration (mL/min) | 800 |
| Initial | Final (28 dph) | p | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (19 dph) | HS | NHS | HK | NHK | PF | I | H | I × H | |
| Growth performance | |||||||||
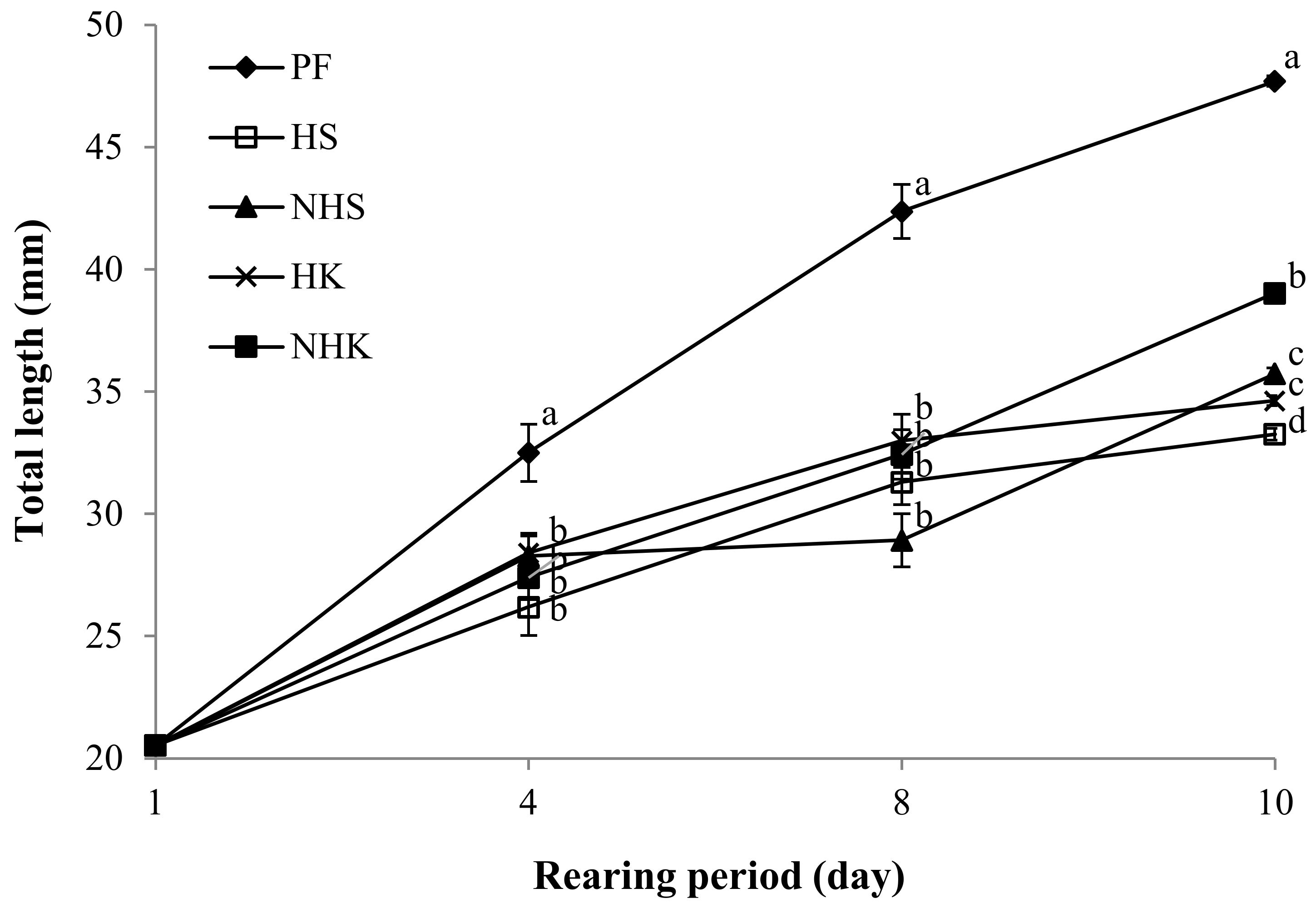
| Total length (mm) | 20.5 ± 0.2 | 33.2 ± 0.2 d | 35.7 ± 0.3 c | 34.6 ± 0.3 c | 39.0 ± 0.3 b | 47.7 ± 0.4 a | * | * | * |
| Body depth (mm) | 4.2 ± 0.0 | 6.4 ± 0.0 e | 7.1 ± 0.1 c | 6.7 ± 0.1 d | 7.4 ± 0.1 b | 8.6 ± 0.1 a | * | * | ns |
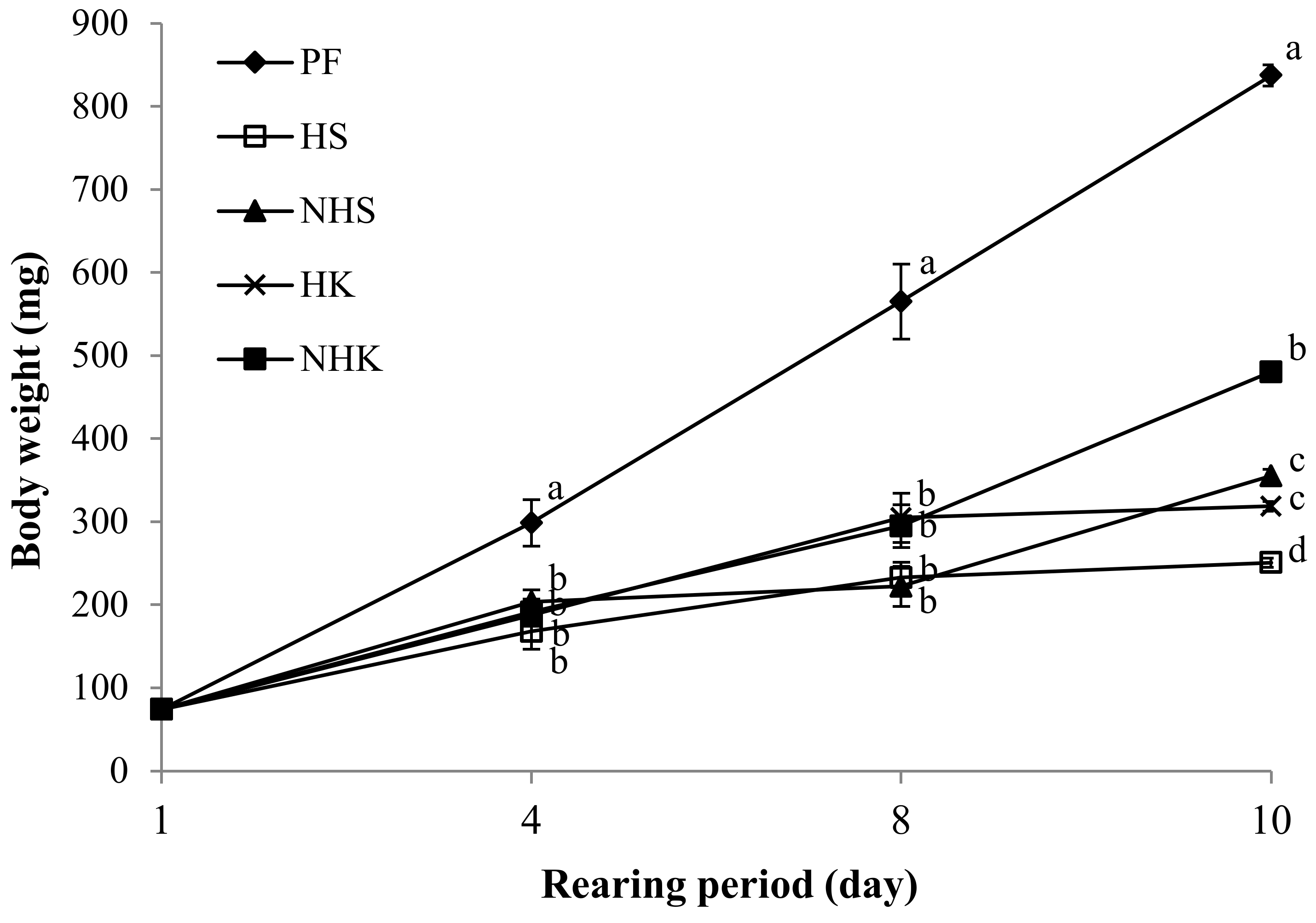
| Body weight (mg) | 74.1 ± 2.7 | 251.0 ± 5.6 d | 355.3 ± 10.7 c | 318.6 ± 8.3 c | 480.4 ± 12.1 b | 837.4 ± 21.3 a | * | * | * |
| CF | 0.8 ± 0.1 | 0.7 ± 0.1 c | 0.8 ± 0.1 b | 0.7 ± 0.1 b | 0.8 ± 0.1 a | 0.8 ± 0.1 b | * | * | * |
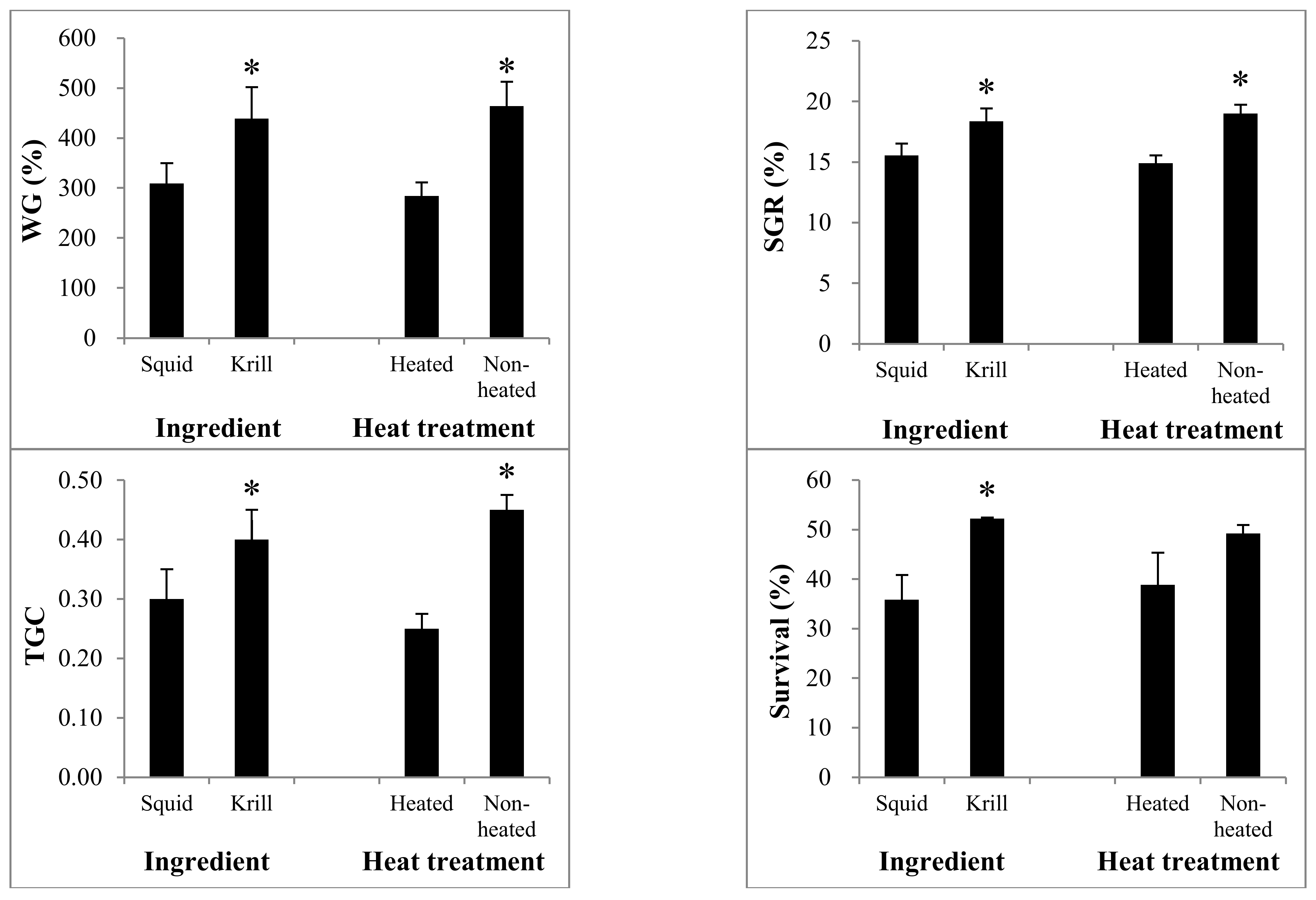
| WG (%) | 238.7 ± 3.1 c | 379.6 ±16.1 c | 330.0 ± 20.9 c | 548.3 ± 3.1 b | 1030.1 ± 76.4 a | * | * | ns | |
| SGR (%) | 13.6 ± 0.1 d | 17.5 ± 0.0 c | 16.2 ± 0.8 c | 20.5 ± 0.2 b | 27.4 ± 0.5 a | * | * | ns | |
| TGC | 0.2 ± 0.0 d | 0.4 ± 0.0 c | 0.3 ± 0.0 cd | 0.5 ± 0.0 b | 1.1 ± 0.1 a | * | * | ns | |
| Survival rate (%) | 25.8 ± 9.9 c | 45.9 ± 2.4 bc | 51.9 ± 3.3 b | 52.6 ± 5.2 b | 75.3 ± 2.5 a | * | ns | ns | |
| Proximate composition (%, wet-weight) | |||||||||
| Moisture | 83.5 ± 0.2 | 83.7 ± 0.2 ab | 82.9 ± 0.1 c | 84.1 ± 0.3 a | 83.3 ± 0.0 bc | 83.4 ± 0.2 bc | * | * | ns |
| Crude protein | 12.4 ± 0.0 | 11.2 ± 0.3 c | 11.9 ± 0.1 b | 11.6 ± 0.1 bc | 12.6 ± 0.0 a | 12.9 ± 0.1 a | * | * | ns |
| Crude lipid | 2.0 ± 0.1 | 2.0 ± 0.0 c | 2.6 ± 0.1 a | 1.8 ± 0.1 c | 2.2 ± 0.0 b | 1.6 ± 0.0 d | * | * | * |
| Crude ash | 2.6 ± 0.1 | 3.2 ± 0.2 | 2.7 ± 0.2 | 2.9 ± 0.2 | 2.7 ± 0.1 | 2.8 ± 0.1 | ns | * | ns |
| Constitutional Amino Acid | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | Final (28 dph) | p | |||||||
| (19 dph) | HS | NHS | HK | NHK | PF | I | H | I × H | |
| Essential amino acid | |||||||||
| Arginine | 2.56 ± 0.27 | 1.67 ± 0.47 c | 2.31 ± 0.16 bc | 2.53 ± 0.16 ab | 2.65 ± 0.09 ab | 3.14 ± 0.07 a | * | * | ns |
| Lysine | 3.36 ± 0.38 | 2.21 ± 0.48 c | 3.06 ± 0.22 b | 3.30 ± 0.11 b | 3.52 ± 0.08 ab | 4.14 ± 0.04 a | * | * | ns |
| Histidine | 0.77 ± 0.15 | 0.53 ± 0.15 c | 0.76 ± 0.04 b | 0.82 ± 0.03 ab | 0.88 ± 0.02 ab | 1.01 ± 0.03 a | * | * | ns |
| Phenylalanine | 1.89 ± 0.25 | 1.42 ± 0.37 b | 1.84 ± 0.13 ab | 2.05 ± 0.04 a | 2.05 ± 0.10 a | 2.34 ± 0.07 a | * | * | ns |
| Leucine | 3.18 ± 0.40 | 2.35 ± 0.50 c | 3.08 ± 0.24 b | 3.34 ± 0.11 ab | 3.48 ± 0.09 ab | 3.82 ± 0.11 a | * | * | ns |
| Isoleucine | 1.25 ± 0.16 | 0.93 ± 0.19 b | 1.11 ± 0.21 ab | 1.21 ± 0.10 ab | 1.31 ± 0.05 a | 1.45 ± 0.03 a | * | ns | ns |
| Methionine | 0.92 ± 0.12 | 0.84 ± 0.29 b | 0.93 ± 0.14 b | 1.21 ± 0.08 ab | 1.18 ± 0.13 ab | 1.45 ± 0.10 a | * | ns | ns |
| Valine | 1.28 ± 0.18 | 0.98 ± 0.49 | 1.14 ± 0.20 | 1.28 ± 0.11 | 1.31 ± 0.03 | 1.53 ± 0.04 | ns | ns | ns |
| Threonine | 2.24 ± 0.25 | 1.66 ± 0.28 b | 2.14 ± 0.08 a | 2.21 ± 0.04 a | 2.39 ± 0.08 a | 2.50 ± 0.04 a | * | * | ns |
| Tryptophan | 0.39 ± 0.08 | 0.26 ± 0.13 | 0.41 ± 0.05 | 0.47 ± 0.07 | 0.36 ± 0.06 | 0.50 ± 0.11 | ns | ns | * |
| Free amino acid | p | ||||||||
| Essential amino acid | |||||||||
| Arginine | 0.39 ± 0.04 | 0.79 ± 0.20 a | 0.35 ± 0.25 bc | 0.29 ± 0.01 bc | 0.57 ± 0.02 ab | 0.15 ± 0.00 c | ns | ns | * |
| Lysine | 0.49 ± 0.01 | 0.84 ± 0.16 a | 0.43 ± 0.26 bc | 0.36 ± 0.01 bc | 0.61 ± 0.02 ab | 0.18 ± 0.00 c | ns | ns | * |
| Histidine | 0.69 ± 0.01 | 0.29 ± 0.04 d | 0.43 ± 0.05 c | 0.33 ± 0.00 d | 0.52 ± 0.01 b | 0.78 ± 0.01 a | * | * | ns |
| Phenylalanine | 0.32 ± 0.00 | 0.50 ± 0.16 a | 0.21 ± 0.17 b | 0.17 ± 0.01 b | 0.38 ± 0.01 ab | 0.11 ± 0.00 b | ns | ns | * |
| Leucine | 0.46 ± 0.01 | 0.75 ± 0.22 a | 0.31 ± 0.26 b | 0.26 ± 0.01 b | 0.52 ± 0.02 ab | 0.17 ± 0.00 b | ns | ns | * |
| Isoleucine | 0.23 ± 0.00 | 0.35 ± 0.09 a | 0.16 ± 0.13 b | 0.13 ± 0.00 b | 0.26 ± 0.01 ab | 0.10 ± 0.00 b | ns | ns | * |
| Methionine | 0.22 ± 0.00 | 0.32 ± 0.09 a | 0.15 ± 0.11 ab | 0.13 ± 0.00 b | 0.25 ± 0.01 ab | 0.08 ± 0.01 b | ns | ns | * |
| Valine | 0.37 ± 0.01 | 0.52 ± 0.12 a | 0.23 ± 0.19 b | 0.19 ± 0.00 b | 0.36 ± 0.01 ab | 0.17 ± 0.00 b | ns | ns | * |
| Threonine | 0.25 ± 0.00 | 0.35 ± 0.07 a | 0.19 ± 0.12 b | 0.15 ± 0.00 b | 0.25 ± 0.01 ab | 0.14 ± 0.00 b | ns | ns | * |
| Tryptophan | 0.05 ± 0.02 | 0.15 ± 0.07 a | 0.04 ± 0.04 b | 0.04 ± 0.00 b | 0.09 ± 0.01 ab | 0.02 ± 0.00 b | ns | ns | * |
| Initial | Final (28 dph) | p | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (19 dph) | HS | NHS | HK | NHK | PF | I | H | I × H | |
| 14:0 | 3.1 ± 0.6 | 1.6 ± 0.1 | 1.4 ± 0.2 | 1.6 ± 0.5 | 1.6 ± 0.1 | 0.9 ± 0.2 | ns | ns | ns |
| 16:0 | 25.4 ± 1.2 | 18.0 ± 0.7 b | 18.6 ± 0.8 b | 19.8 ± 0.9 b | 18.8 ± 2.2 b | 23.7 ± 0.4 a | ns | ns | ns |
| 16:1n−7 | 3.2 ± 0.5 | 2.5 ± 0.1 bc | 3.4 ± 0.2 ab | 3.2 ± 0.5 ab | 4.1 ± 0.2 a | 1.8 ± 0.3 c | * | * | ns |
| 18:0 | 11.3 ± 2.1 | 11.0 ± 0.9 a | 6.2 ± 0.3 c | 7.5 ± 0.2 bc | 6.1 ± 0.5 c | 8.9 ± 0.5 b | * | * | * |
| 18:1n−9 | 8.1 ± 1.0 | 8.3 ± 0.2 c | 10.6 ± 0.4 b | 9.9 ± 0.2 bc | 13.0 ± 1.3 a | 9.3 ± 0.6 bc | * | * | ns |
| 18:1n−7 | 2.0 ± 0.2 | 2.2 ± 0.0 c | 3.0 ± 0.1 b | 2.8 ± 0.1 bc | 3.7 ± 0.4 a | 2.2 ± 0.1 c | * | * | ns |
| 18:2n−6 | 2.2 ± 0.2 | 1.9 ± 0.2 c | 2.6 ± 0.1 b | 3.9 ± 0.3 a | 3.9 ± 0.2 a | 1.9 ± 0.1 c | * | ns | * |
| 18:3n−3 | 0.4 ± 0.1 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | ns | * | ns |
| 18:4n−3 | 0.7 ± 0.1 | 0.8 ± 0.3 | 0.6 ± 0.0 | 0.6 ± 0.2 | 0.8 ± 0.1 | 0.3 ± 0.1 | ns | ns | ns |
| 20:1n−9 | 0.5 ± 0.1 | 1.8 ± 0.2 a | 1.8 ± 0.1 a | 1.2 ± 0.3 b | 1.5 ± 0.1 ab | 0.3 ± 0.0 c | * | ns | ns |
| 20:1n−11 | 0.4 ± 0.1 | 1.4 ± 0.1 ab | 1.5 ± 0.1 a | 0.9 ± 0.2 c | 1.2 ± 0.1 bc | 0.3 ± 0.0 d | * | * | ns |
| 20:2n−6 | 0.1 ± 0.0 | 0.2 ± 0.0 b | 0.2 ± 0.0 a | 0.1 ± 0.0 c | 0.1 ± 0.0 c | 0.1 ± 0.0 c | * | * | * |
| 20:4n−6 | 3.0 ± 0.4 | 2.7 ± 0.4 b | 3.3 ± 0.2 b | 4.4 ± 0.6 a | 3.5 ± 0.2 ab | 3.7 ± 0.1 ab | * | ns | * |
| 20:4n−3 | 0.3 ± 0.1 | 0.2 ± 0.0 b | 0.3 ± 0.0 a | 0.2 ± 0.0 ab | 0.2 ± 0.0 ab | 0.3 ± 0.0 ab | ns | * | ns |
| 20:5n−3 | 5.6 ± 1.0 | 7.1 ± 0.4 b | 8.6 ± 0.4 ab | 8.2 ± 0.3 ab | 9.4 ± 0.8 a | 5.0 ± 0.3 c | * | * | ns |
| 22:5n−6 | 0.9 ± 0.1 | 0.6 ± 0.1 b | 0.9 ± 0.1 ab | 0.8 ± 0.1 ab | 0.9 ± 0.0 ab | 1.1 ± 0.2 a | ns | * | ns |
| 22:6n−3 | 22.8 ± 3.5 | 21.8 ± 3.9 ab | 26.0 ± 2.2 ab | 24.5 ± 3.5 ab | 21.1 ± 2.0 b | 30.5 ± 0.2 a | ns | ns | ns |
| 22:6n−3/20:5n−3 | 4.2 ± 0.6 | 3.1 ± 0.5 b | 3.0 ± 0.2 b | 3.0 ± 0.5 b | 2.3 ± 0.1 b | 6.1 ± 0.4 a | ns | ns | ns |
| Others | 9.8 ± 1.5 | 16.3 ± 5.4 | 8.8 ± 4.3 | 8.3 ± 2.1 | 7.7 ± 2.0 | 9.3 ± 1.8 | ns | ns | ns |
| Σn−3 LC−PUFA 1 | 30.7 ± 4.5 | 30.7 ± 4.0 | 36.6 ± 2.5 | 34.6 ± 3.2 | 32.7 ± 2.9 | 37.4 ± 0.2 | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.-H.; Kurimoto, T.; Haga, Y.; Kamimura, Y.; Itoh, A.; Satoh, S. Effects of Non-Heated and Heat Processed Krill and Squid Meal-Based Diet on Growth Performance and Biochemical Composition in Juvenile Pacific Bluefin Tuna Thunnus orientalis. Fishes 2022, 7, 83. https://doi.org/10.3390/fishes7020083
Cho J-H, Kurimoto T, Haga Y, Kamimura Y, Itoh A, Satoh S. Effects of Non-Heated and Heat Processed Krill and Squid Meal-Based Diet on Growth Performance and Biochemical Composition in Juvenile Pacific Bluefin Tuna Thunnus orientalis. Fishes. 2022; 7(2):83. https://doi.org/10.3390/fishes7020083
Chicago/Turabian StyleCho, Jeong-Hyeon, Takayuki Kurimoto, Yutaka Haga, Yuji Kamimura, Akira Itoh, and Shuichi Satoh. 2022. "Effects of Non-Heated and Heat Processed Krill and Squid Meal-Based Diet on Growth Performance and Biochemical Composition in Juvenile Pacific Bluefin Tuna Thunnus orientalis" Fishes 7, no. 2: 83. https://doi.org/10.3390/fishes7020083
APA StyleCho, J.-H., Kurimoto, T., Haga, Y., Kamimura, Y., Itoh, A., & Satoh, S. (2022). Effects of Non-Heated and Heat Processed Krill and Squid Meal-Based Diet on Growth Performance and Biochemical Composition in Juvenile Pacific Bluefin Tuna Thunnus orientalis. Fishes, 7(2), 83. https://doi.org/10.3390/fishes7020083






