Growth Rates of Non-Native Bighead and Silver Carp in the Upper Mississippi River
Abstract
:1. Introduction
2. Materials and Methods
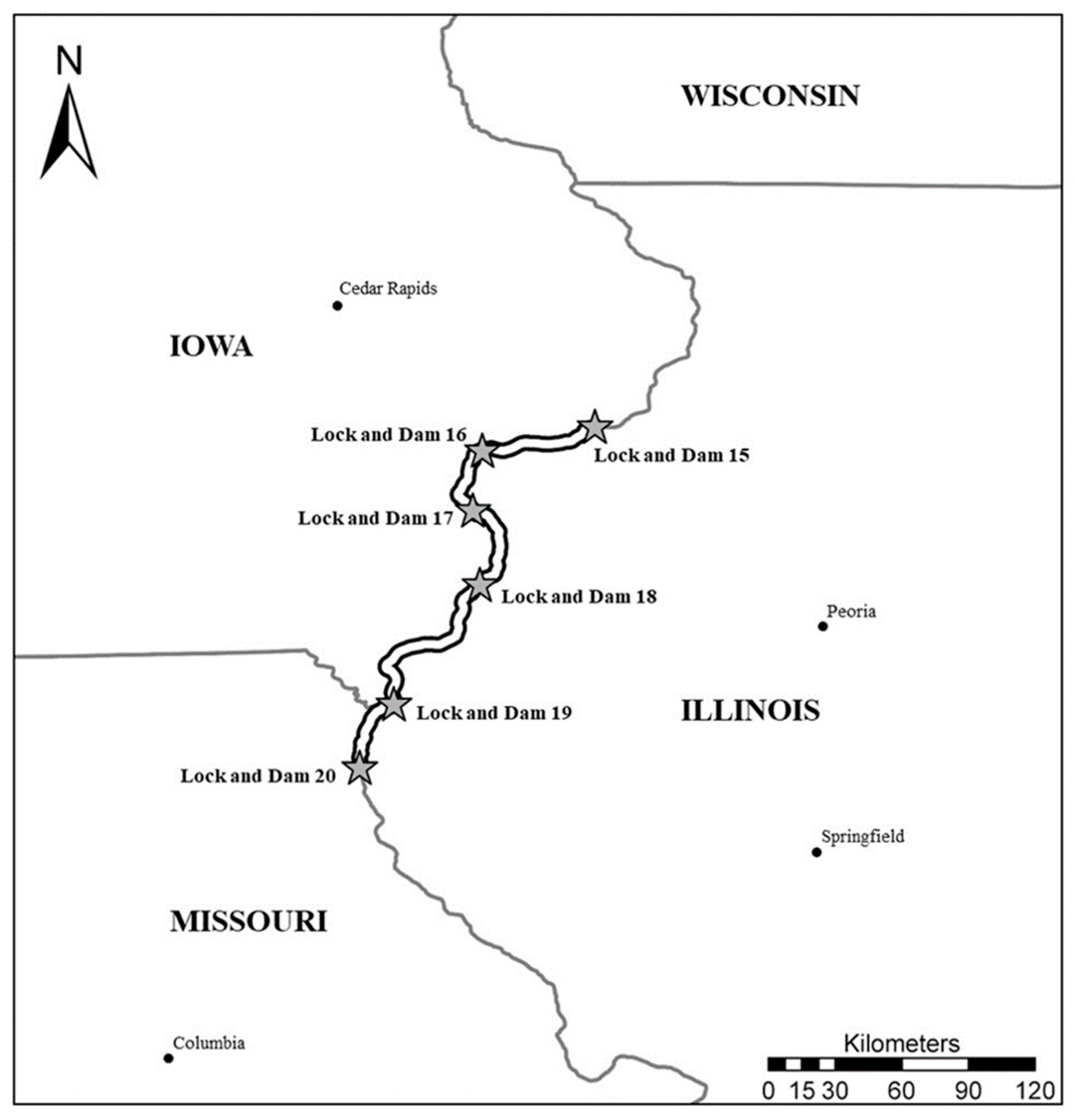
2.1. Fish Collection
2.2. Aging
2.3. Statistical Analysis
3. Results
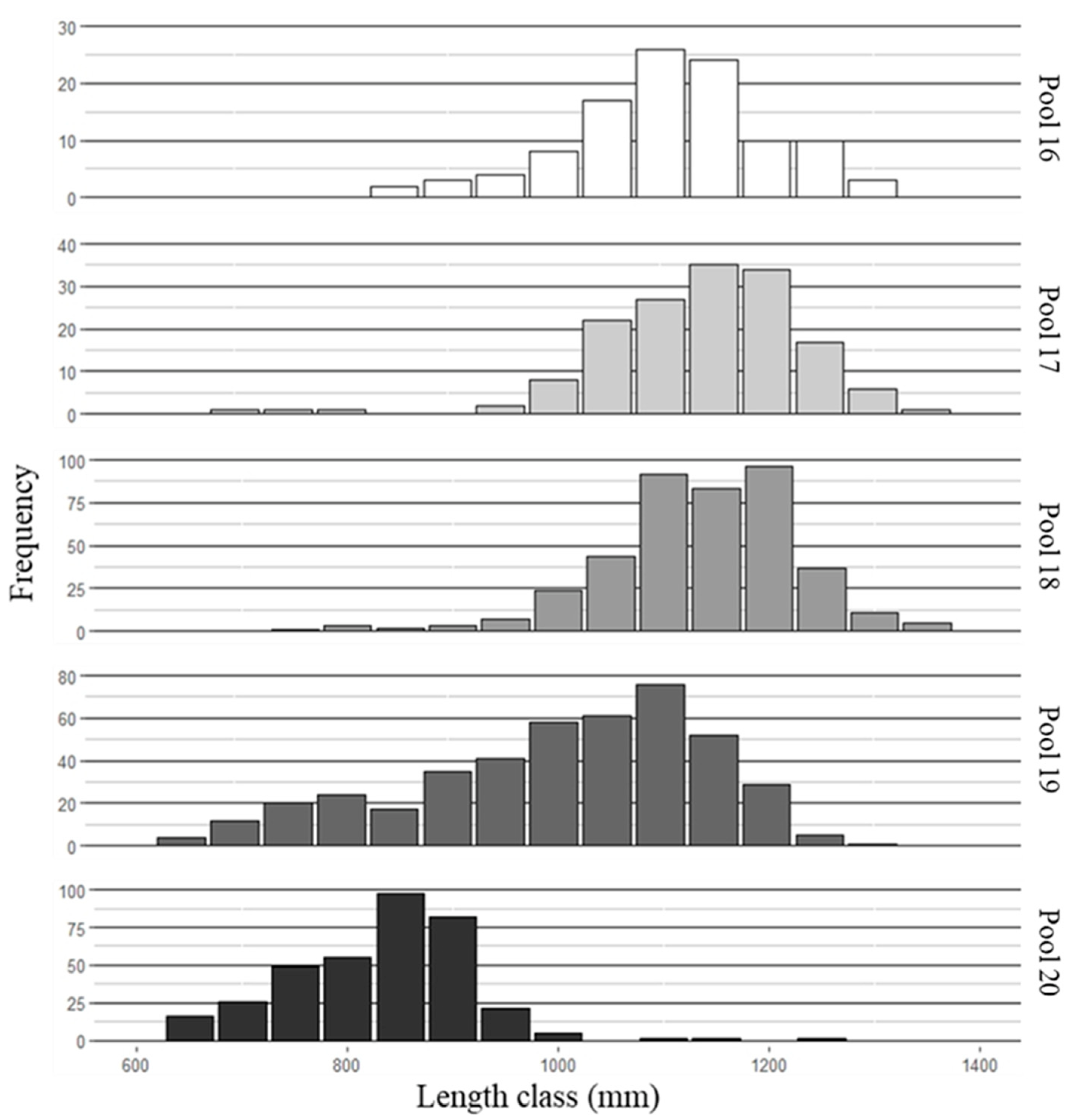
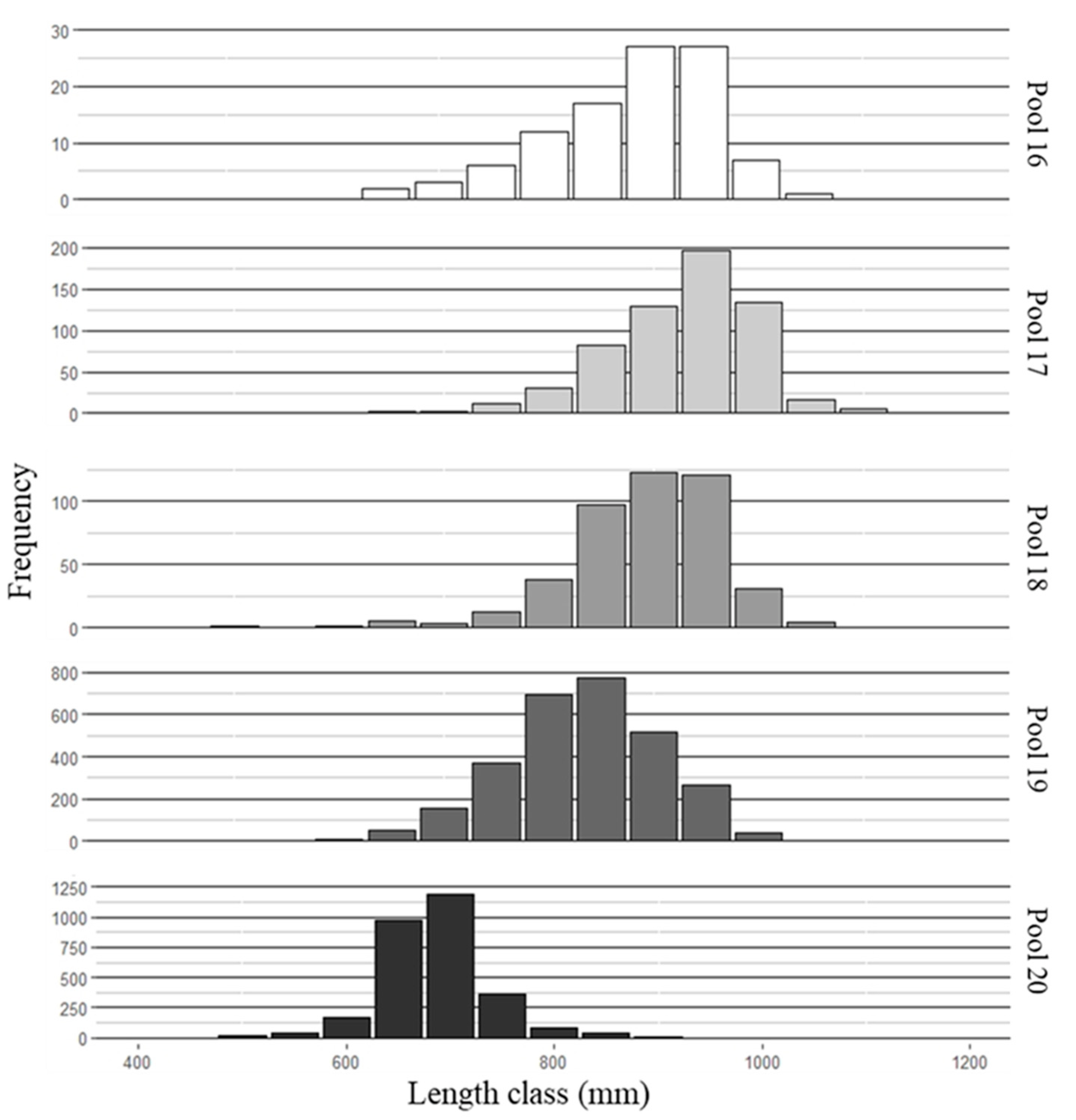
3.1. Fish Collection and Aging
3.2. Mean Length-at-Age
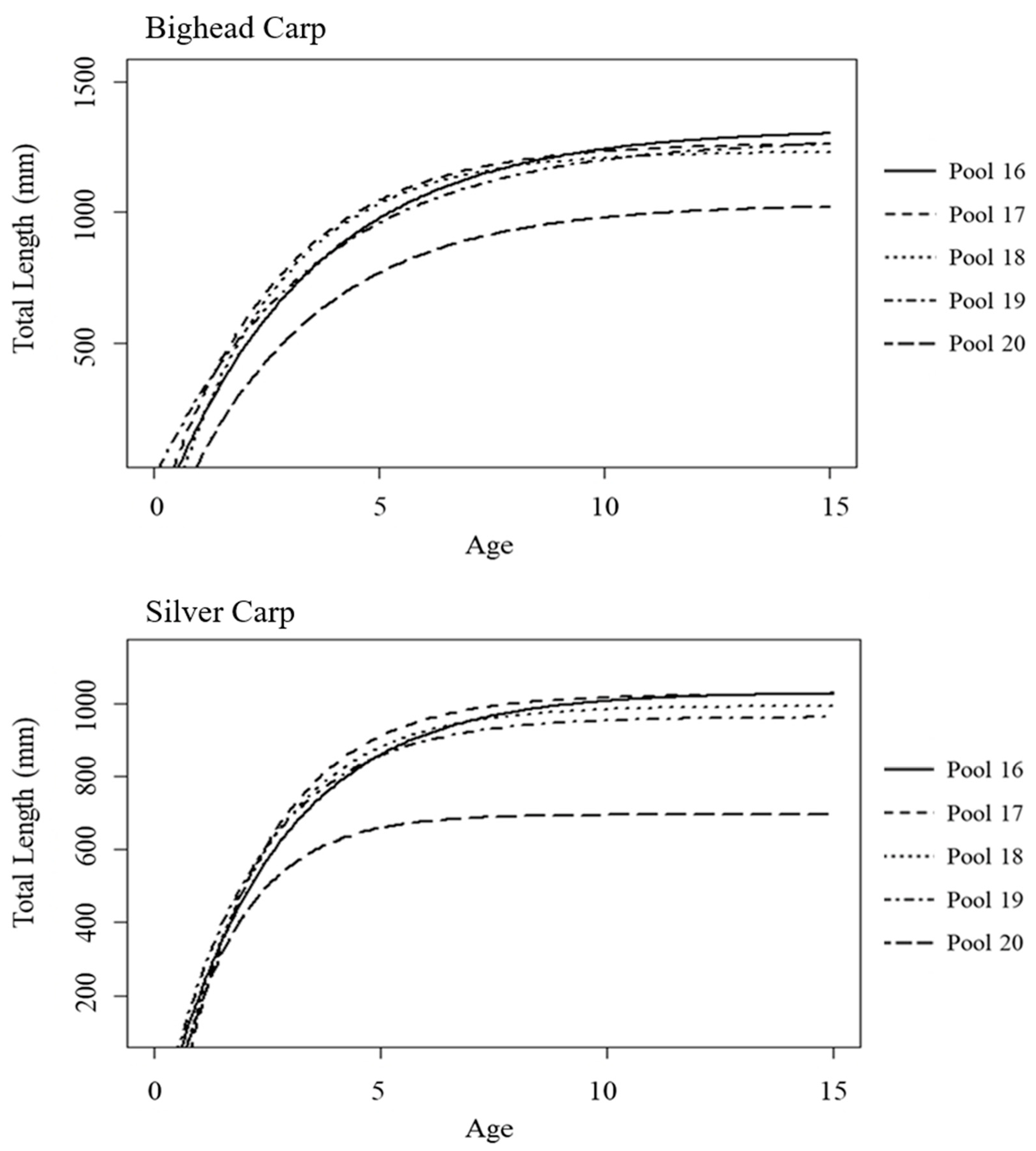
3.3. Von Bertalanffy Growth Curves
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Miranda, L.E.; Bettoli, P.W. Mortality. In Analysis and Interpretation of Freshwater Fisheries Data; Guy, C.S., Brown, M.L., Eds.; American Fisheries Society: Bethesda, MD, USA, 2007; pp. 229–278. [Google Scholar]
- Michaletz, P.H. Factors affecting abundance, growth, and survival of age-0 gizzard shad. Trans. Am. Fish. Soc. 1997, 126, 84–100. [Google Scholar] [CrossRef]
- Margenau, T.L.; Rasmussen, P.W.; Kampa, J.M. Factors affecting growth of northern pike in small northern Wisconsin lakes. N. Am. J. Fish. Manag. 1998, 18, 625–639. [Google Scholar] [CrossRef] [Green Version]
- Viadero, R.C. Factors affecting fish growth and production. Water Encycl. 2005, 3, 129–133. [Google Scholar]
- Lorenzen, K.; Enberg, K. Density-dependent growth as a key mechanism in the regulation of fish populations: Evidence from among-population comparisons. Proc. Royal Soc. Lond. B Biol. Sci. 2002, 269, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Brandner, J.; Cerwenka, A.F.; Schliewen, U.K.; Geist, J. Bigger is better: Characteristics of round gobies forming an invasion front in the Danube river. PLoS ONE 2013, 8, e73036. [Google Scholar] [CrossRef] [Green Version]
- Rehage, J.S.; Sih, A. Dispersal Behavior, Boldness, and the Link to Invasiveness: A Comparison of Four Gambusia Species. Biol. Invasions 2004, 6, 379–391. [Google Scholar] [CrossRef]
- Hilling, C.D.; Bunch, A.J.; Greenlee, R.S.; Orht, D.J.; Jiao, Y. Natural mortality and size structure of introduced blue catfish in Virginia Tidal Rivers. J. Southeast. Assoc. Fish Wildl. Agencies 2018, 5, 30–38. [Google Scholar]
- Irons, K.S.; Sass, G.G.; McClelland, M.A.; Stafford, J.D. Reduced condition factor of two native fish species coincident with invasion of non-native Asian carps in the Illinois River, USA. Is this evidence for competition and reduced fitness? J. Fish Biol. 2007, 71, 258–273. [Google Scholar] [CrossRef]
- Coulter, D.P.; MacNamara, R.; Glover, D.C.; Garvey, J.E. Possible unintended effects of management at an invasion front: Reduced prevalence corresponds with high condition of invasive bigheaded carps. Biol. Conserv. 2018, 221, 118–126. [Google Scholar] [CrossRef]
- DeVries, D.R.; Frie, R.V. Determination of Age and Growth. In Fisheries Techniques, 2nd ed.; Murphy, B.R., Willis, D.W., Eds.; American Fisheries Society: Bethesda, MD, USA, 1996; pp. 483–512. [Google Scholar]
- Pope, K.L.; Kruse, C.G. Condition. In Analysis and Interpretation of Freshwater Fisheries Data; Guy, C.S., Brown, M.L., Eds.; American Fisheries Society: Bethesda, MD, USA, 2007; pp. 423–471. [Google Scholar]
- Williamson, C.J.; Garvey, J.E. Growth, fecundity, and diets of newly established silver carp in the middle Mississippi River. Trans. Am. Fish. Soc. 2005, 134, 1423–1430. [Google Scholar] [CrossRef] [Green Version]
- Whitledge, G.W.; Knights, B.; Vallazza, J.; Larson, J.; Weber, M.J.; Lamer, J.T.; Phelps, Q.E.; Norman, J.D. Identification of bighead carp and silver carp early-life environments and inferring Lock and Dam 19 passage in the Upper Mississippi River: Insights from otolith chemistry. Biol. Invasions 2019, 21, 1007–1020. [Google Scholar] [CrossRef] [Green Version]
- Haun, R.L. Comparison of Fish Community Composition and Structure among River Reaches of the Upper Mississippi River: Determining the Effects of Lock and Dam 19 in Structuring Fish Communities. Master’s Thesis, Western Illinois University, Macomb, IL, USA, 2015. [Google Scholar]
- Larson, J.H.; Knights, B.C.; McCalla, S.G.; Monroe, E.; Tuttle-Lau, M.; Chapman, D.C.; George, A.E.; Vallazza, J.M.; Amberg, J. Evidence of Asian Carp spawning upstream of a key choke point in the Mississippi River. N. Am. J. Fish. Manag. 2017, 37, 903–919. [Google Scholar] [CrossRef]
- Lamer, J.T.; Ruebush, B.C.; Arbieva, Z.H.; McClelland, M.A.; Epifanio, J.M.; Sass, G.G. Diagnostic SNPs reveal widespread introgressive hybridization between introduced bighead and silver carp in the Mississippi River Basin. Mol. Ecol. 2015, 24, 3931–3943. [Google Scholar] [CrossRef] [PubMed]
- Johal, M.S.; Esmaeili, H.R.; Tandon, K.K. Postcleithrum of silver carp, Hypophthalmichthys molitrix (Val. 1844), an authentic indicator for age determination. Curr. Sci. 2000, 79, 945. [Google Scholar]
- Gibson-Reinemer, D.K.; Solomon, L.E.; Pendleton, R.M.; Chick, J.H.; Casper, A.F. Hydrology controls recruitment of two invasive cyprinids: Bigheaded carp reproduction in a navigable large river. PeerJ 2017, 5, e3641. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, C.J.; Weber, M.J.; Pierce, C.L.; Wahl, D.H.; Phelps, Q.E.; Camacho, C.A.; Colombo, R.E. Factors regulating year-class strength of silver carp throughout the Mississippi River basin. Trans. Am. Fish. Soc. 2018, 147, 541–553. [Google Scholar] [CrossRef] [Green Version]
- Francis, R. Back-calculation of fish length: A critical review. J. Fish Biol. 1990, 36, 883–902. [Google Scholar] [CrossRef]
- Lee, R.M. An investigation into the methods of growth determination in fishes by means of scales. J. Cons. Int. Pour L’exploration Mer. 1912, 1, 3–34. [Google Scholar] [CrossRef]
- Quist, M.C.; Pegg, M.A.; Devries, D.R. Age and Growth. In Fisheries Techniques, 3rd ed.; Zale, A.V., Parrish, D.L., Sutton, T.M., Eds.; American Fisheries Society: Bethesda, MD, USA, 2012; pp. 677–731. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 12 March 2019).
- Ogle, D.H.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis. R Package Version 0.8.25. 2019. Available online: https://github.com/droglenc/FSA (accessed on 12 March 2019).
- Mazerolle, M.J. AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c). R Package Version 2.2-2. 2019. Available online: https://cran.r-project.org/package=AICcmodavg (accessed on 12 March 2019).
- Von Bertalanffy, L. A quantitative theory of organic growth. Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Ogle, D.H. Individual Growth. In Introductory Fisheries Analysis with R; CRC Press: Boca Raton, FL, USA, 2016; pp. 222–248. [Google Scholar]
- Lester, N.P.; Shuter, B.J.; Abrams, P.A. Interpreting the von Bertalanffy model of somatic growth in fishes: The cost of reproduction. Proc. Royal Soc. B Biol. Sci. 2004, 271, 1625–1631. [Google Scholar] [CrossRef] [Green Version]
- Stauffer, J.R., Jr. Colonization Theory Relative to Introduced Populations. In Distribution, Biology, and Management of Exotic Fishes; Courtenay, W.R., Stauffer, J.R., Jr., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 1984; pp. 8–21. [Google Scholar]
- Lopez, D.P.; Jungman, A.A.; Rehage, J.S. Nonnative African jewelfish are more fit but not bolder at the invasion front: A trait comparison across an Everglades range expansion. Biol. Invasions 2012, 14, 2159–2174. [Google Scholar] [CrossRef]
- Phillips, B.L.; Brown, G.P.; Shine, R. Life-history evolution in range-shifting populations. Ecology 2010, 91, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- U. S. Fish and Wildlife Service. Juvenile Asian Carp Monitoring to Document Geographic Range of Recruitment of Asian Carp in the Upper Mississippi River. Unpublished Report. 2018. Available online: http://www.micrarivers.org/wp-content/uploads/2018/08/UMR-Monitoring-Pools-14-19.pdf (accessed on 10 December 2019).
- Ridgway, J.L.; Bettoli, P.W. Distribution, age structure, and growth of bigheaded carps in the lower Tennessee and Cumberland Rivers. Southeast. Nat. 2017, 16, 426–442. [Google Scholar]
- Tandon, K.K.; Johal, M.S.; Kukreja, T. Morphometry, age and growth of silver carp, Hypophthalmichthys molotrix (Valenciennes) from Gobindsagar, Himachal Pradesh, India. Res. Bull. Panjab Univ. 1993, 43, 117–128. [Google Scholar]
- Lamer, J.T.; Ruebush, B.C.; McClelland, M.A.; Epifanio, J.M.; Sass, G.G. Body condition (Wr) and reproductive potential of bighead and silver carp hybrids: Postzygotic selection in the Mississippi River basin. Ecol. Evol. 2019, 9, 8978–8986. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, D.B.; Stefanik, E.L.; Kelner, D.E.; Cornish, M.A.; Johnson, D.J.; Hodgins, I.J.; Zigler, S.J.; Johnson, B.L. Improving Fish Passage through Navigation Dams on the Upper Mississippi River System. ENV Report. 54. 2004. Available online: https://www.researchgate.net/publication/277009138 (accessed on 10 December 2019).
- U. S. Fish and Wildlife Service. Annual Report to Congress: Annual Summary of Activities and Expenditures to Manage the Threat of Asian Carp in the Upper Mississippi and Ohio River Basins. 2016. Available online: https://asiancarp.us/Documents/WRRDA2016.pdf (accessed on 10 December 2019).
- Anderson, R.L.; Anderson, C.A.; Larson, J.H.; Knights, B.; Vallazza, J.; Jenkins, S.E.; Lamer, J.T. Influence of a high-head dam as a dispersal barrier to fish community structure of the Upper Mississippi River. River Res. Appl. 2020, 36, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Jahn, L.A.; Anderson, R.V. The Ecology of Pool 19 and 20, Upper Mississippi River: A Community Profile; National Wetlands Research Center. Research and Development, Fish and Wildlife Service, US Department of the Interior: Washington, DC, USA, 1986. [Google Scholar]
- Pillard, D.A.; Anderson, R.V. Longitudinal Variation in Zooplankton Populations in Pool 19, Upper Mississippi River. J. Freshw. Ecol. 1993, 8, 127–132. [Google Scholar] [CrossRef]
- Anderson, C. Using Microchemistry and Stable Isotopes to Determine Early-Life Environment and Movement of the Emerging Bigheaded Carp Population in POOLS 16–19 of the Mississippi River. Master’s Thesis, Western Illinois University, Macomb, IN, USA, 2020. [Google Scholar]
- Lenaerts, A.W. Reproductive Potential of Bigheaded Carp in the Upper Mississippi River. Master’s Thesis, Western Illinois University, Macomb, IL, USA, 2019. [Google Scholar]
- Kamilov, B.G. Morphology of growth structures in silver carp, Hypophthalmichthys molitrix, in relation to estimation of age and growth rate. J. Ichthyol. 1985, 25, 49–59. [Google Scholar]
- Papoulias, D.M.; Chapman, D.; Tillitt, D.E. Reproductive condition and occurrence of intersex in bighead carp and silver carp in the Missouri River. Hydrobiologia 2006, 571, 355–360. [Google Scholar] [CrossRef]
- Barneche, D.R.; Robertson, D.R.; White, C.R.; Marshall, D.J. Fish reproductive-energy output increases disproportionately with body size. Science 2018, 360, 642–645. [Google Scholar] [CrossRef] [Green Version]
- Solomon, L.E.; Pendleton, R.M.; Chick, J.H.; Casper, A.F. Long-term changes in fish community structure in relation to the establishment of Asian carps in a large floodplain river. Biol. Invasions 2015, 18, 2883–2895. [Google Scholar] [CrossRef]
- Asian Carp Regional Coordination Committee (ACRCC). Asian Carp Monitoring and Response Plan. Available online: https://www.asiancarp.us/Documents/MRP2018.pdf (accessed on 10 December 2019).
| Pool 16 | Pool 17 | Pool 18 | Pool 19 | Pool 20 | Total | |
| Bighead Carp | ||||||
| Pectoral fin spines and postcleithra | 17 | 95 | 175 | 189 | 25 | 501 |
| Vertebrae | 3 | 28 | 14 | 21 | 3 | 69 |
| Silver Carp | ||||||
| Pectoral fin spines and postcleithra | 23 | 179 | 152 | 261 | 87 | 702 |
| Vertebrae | 7 | 25 | 12 | 7 | 10 | 61 |
| (a) Bighead Carp | ||||||||||||||||||||||||
| Age 1 | ±SE | n | Sig | Age 2 | ±SE | n | Sig | Age 3 | ±SE | n | Sig | Age 4 | ±SE | n | Sig | Age 5 | ±SE | n | Sig | Age 6 | ±SE | n | Sig | |
| Pool 16 | 244 a | ··· | 1 | a | 422 a | ··· | 1 | a | 665 a | ··· | 1 | a | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· | 1056 | 30 | 4 | a |
| Pool 17 | 263 a | 38 | 8 | a | 595 a | 53 | 8 | a | 728 a | 46 | 7 | a | 923 b | 38 | 8 | a | 1152 | 47 | 4 | a | 1088 | 19 | 16 | a |
| Pool 18 | 224 a | 33 | 4 | a | 441 a | 37 | 4 | a | 738 b | 85 | 4 | a | 969 b | 56 | 4 | a | 1070 | 37 | 9 | a,b | 1127 | 17 | 33 | a |
| Pool 19 | 298 a | 33 | 9 | a | 555 a | 53 | 8 | a | 705 b | 43 | 8 | a | 868 | 25 | 17 | a,b | 947 | 21 | 40 | b | 1031 | 17 | 53 | a |
| Pool 20 | 85 a | ··· | 1 | a | 312 a | ··· | 1 | a | 388 a | ··· | 1 | a | 690 | 48 | 4 | b | 782 | 37 | 6 | c | 847 | 26 | 9 | b |
| Age 7 | ±SE | n | Sig | Age 8 | ±SE | n | Sig | Age 9 | ±SE | n | Sig | Age 10 | ±SE | n | Sig | Age 11 | ±SE | n | Sig | Age 12 | ±SE | n | Sig | |
| Pool 16 | 1166 | 29 | 4 | a,b | 1200 | 37 | 5 | a | 1190 | 53 | 3 | a,b | ··· | ··· | ··· | ··· | 1190 | ··· | 1 | a | ··· | ··· | ··· | ··· |
| Pool 17 | 1178 | 18 | 30 | a | 1174 | 16 | 21 | a | 1266 | 10 | 10 | a | 1227 | 23 | 11 | a | 1169 | 30 | 2 | a | ··· | ··· | ··· | ··· |
| Pool 18 | 1124 | 14 | 30 | a,b | 1163 | 10 | 41 | a | 1193 | 12 | 29 | b | 1234 | 17 | 22 | a | 1196 | 37 | 4 | a | 1216 | 4 | 5 | a |
| Pool 19 | 1111 | 16 | 42 | b | 1171 | 16 | 20 | a | 1148 | 20 | 10 | b | 1157 | 37 | 3 | a | 1196 | 20 | 2 | a | 1101 | 0 | 1 | a |
| Pool 20 | 902 | 53 | 6 | c | 916 | 9 | 4 | b | 947 | ··· | 1 | c | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· |
| (b) Silver Carp | ||||||||||||||||||||||||
| Age 1 | ±SE | n | Sig | Age 2 | ±SE | n | Sig | Age 3 | ±SE | n | Sig | Age 4 | ±SE | n | Sig | Age 5 | ±SE | n | Sig | |||||
| Pool 16 | 237 a | 26 | 3 | a | 350 a | 63 | 2 | a | 705 a | 74 | 2 | a | 777 | 56 | 3 | a,b | 928 | 42 | 3 | a,b | ||||
| Pool 17 | 193 a | 17 | 10 | a | 396 b | 29 | 11 | a | 736 b | 51 | 10 | a | 863 | 17 | 12 | a | 939 | 12 | 44 | a | ||||
| Pool 18 | 211 a | 32 | 4 | a | 440 a | 38 | 4 | a | 627 b | 53 | 5 | a,b | 826 | 15 | 40 | a | 888 | 14 | 30 | a,b | ||||
| Pool 19 | 213 a | 20 | 4 | a | 453 a | 83 | 3 | a | 749 | 13 | 19 | a | 798 | 8 | 65 | a | 831 | 12 | 96 | b | ||||
| Pool 20 | 180 a | 21 | 5 | a | 431 b | 30 | 4 | a | 449 b | 26 | 4 | b | 656 | 21 | 12 | b | 676 | 9 | 42 | c | ||||
| Age 6 | ±SE | n | Sig | Age 7 | ±SE | n | Sig | Age 8 | ±SE | n | Sig | Age 9 | ±SE | n | Sig | Age 10 | ±SE | n | Sig | |||||
| Pool 16 | 913 | 17 | 12 | a,b | 936 | 16 | 5 | a | 961 | ··· | 1 | a | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ||||
| Pool 17 | 951 | 8 | 72 | a | 971 | 12 | 40 | a | 987 | 10 | 5 | a | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ||||
| Pool 18 | 918 | 8 | 67 | a,b | 952 | 14 | 24 | a | 977 | 52 | 5 | a | 959 | ··· | 1 | a | ··· | ··· | ··· | ··· | ||||
| Pool 19 | 906 | 14 | 55 | b | 949 | 20 | 24 | a | 1046 | 11 | 3 | a | 1004 | ··· | 1 | a | 878 | 0 | 1 | ··· | ||||
| Pool 20 | 671 | 14 | 26 | c | 642 | 45 | 11 | b | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ||||
| (a.) Bighead Carp | ||||||
| L∞ | K | t0 | ||||
| Pool 16 | 1318.503 | a | 0.299 | a | 0.471 | a |
| Pool 17 | 1266.387 | a | 0.379 | a | 0.380 | a |
| Pool 18 | 1233.584 | a | 0.418 | a | 0.633 | a |
| Pool 19 | 1280.488 | a | 0.279 | a | 0.046 | b |
| Pool 20 | 1030.707 | b | 0.331 | a | 0.848 | c |
| (b.) Silver Carp | ||||||
| L∞ | K | t0 | ||||
| Pool 16 | 1030.720 | a | 0.395 | a | 0.440 | a |
| Pool 17 | 1025.834 | a | 0.509 | a | 0.689 | a |
| Pool 18 | 993.133 | a | 0.494 | a | 0.593 | a |
| Pool 19 | 963.133 | a | 0.480 | a | 0.396 | a |
| Pool 20 | 695.449 | b | 0.669 | a | 0.590 | a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broaddus, C.L.; Lamer, J.T. Growth Rates of Non-Native Bighead and Silver Carp in the Upper Mississippi River. Fishes 2022, 7, 73. https://doi.org/10.3390/fishes7020073
Broaddus CL, Lamer JT. Growth Rates of Non-Native Bighead and Silver Carp in the Upper Mississippi River. Fishes. 2022; 7(2):73. https://doi.org/10.3390/fishes7020073
Chicago/Turabian StyleBroaddus, Cortney Lynn, and James T. Lamer. 2022. "Growth Rates of Non-Native Bighead and Silver Carp in the Upper Mississippi River" Fishes 7, no. 2: 73. https://doi.org/10.3390/fishes7020073
APA StyleBroaddus, C. L., & Lamer, J. T. (2022). Growth Rates of Non-Native Bighead and Silver Carp in the Upper Mississippi River. Fishes, 7(2), 73. https://doi.org/10.3390/fishes7020073






