Abstract
Changes in nutritional constituents and enzyme activities were clarified in yellowfin tuna (YFT, Thunnus albacares) eggs during embryonic development, from eggs immediately after fertilization to hatching. The protein levels in the eggs gradually increased with development until the completion of hatching. In contrast, the triglyceride (TG) and free amino acid (FAA) levels in the eggs gradually declined with embryonic development until hatching was complete, although the energy composition of the FAAs was lower than that of the TGs throughout embryonic development. These results indicate that endogenous TGs are preferentially expended as an energy source during embryonic development. Overall, changes in the activities of aspartate aminotransferase, alanine aminotransferase, creatine kinase, and alkaline phosphatase showed similar patterns throughout development. First, the enzyme levels diminished; then, they remained at constant, low levels just before hatching, when they rapidly increased. This rapid increase was consistent with the protein content, suggesting that organ differentiation and functionalization were promoted during this period. These results will contribute to the establishment of mass-seeding production of YFT.
1. Introduction
Yellowfin tuna (YFT, Thunnus albacares) is one of the most-caught fish and is a commercially important species in Central and South American countries, including Panama, Mexico, Ecuador, and Venezuela [1]. The global demand for tuna species has increased with the growing popularity of raw tuna products, such as sashimi and sushi. Recently, concerns have arisen that overfishing may destroy tuna populations. In the eastern Pacific, the tuna catch increased from about 0.1 million tons in 1960 to over 0.4 million tons in 2003; since 2003, the catch has declined [2]. Thus, research on sustainable fisheries and the management of YFT stock is needed.
Various studies have reported on the captive propagation of YFT [3,4,5], the food selectivity of larvae [6,7] age validation and growth and survival [8,9], the enzyme activities and amino acid composition of early juveniles [10], and the optimum water-quality parameters for larval growth [11]. However, high mortality at the larval and early juvenile stages was shown by Lang et al. [12] and Kaji et al. [13,14] for both YFT and Pacific bluefin tuna (PBT, Thunnus orientalis) [15,16]. Therefore, it is important to clarify the cause of death by elucidating the early life history of YFT.
It is necessary to explore related aspects that can lead to the technical development of the mass production of yellowfin tuna seedlings. If we can obtain information on which nutrients have been utilized during the embryonic developmental stages, it may provide the opportunity to improve egg quality by enriching these nutrients in the eggs through broodstock diet manipulation.
Generally, cells rapidly divide during the ovum cleavage stage. From the gastrula stage, cells begin to differentiate and form organ placodes. In the prehatching stage, muscle function and enzyme synthesis are promoted. After hatching, the larvae rapidly develop the head skeleton and the gastrointestinal tract. A large amount of energy is required for these morphological alterations. Nutrient constituents and their usage processes differ among fish species [17]. Hence, the question arises: What nutritional component(s) do YFT eggs and newly hatched larvae use as a source of endogenous energy? To answer this question, the present study was conducted.
In this study, we examined the changes in nutritional constituents and enzyme activities of YFT eggs during embryonic development, from eggs immediately after fertilization to hatching.
2. Materials and Methods
2.1. Eggs and Larvae
The fertilized eggs of YFT were collected in June 2012 from naturally spawning, wild-caught broodstock maintained at the Inter-American Tropical Tuna Commission’s (IATTC) Achotines Laboratory in Los Santos Province, Republic of Panama. The broodstock management systems followed for the YFT were as previously described by Wexler et al. [5]. The eggs were collected immediately after spawning with a 100 μm mesh net into a 1 m3 collection basket and incubated with gentle aeration in three 0.3 m3 tanks. The eggs were sampled at the following developmental stages as described by Guillen et al. [18]: cells (2C), late cleavage (LC), early gastrula (EG), appearance of embryonic shield (E), appearance of Kupffer’s vesicle (K), beginning of heart beat (H), just before hatching (BH), 50% hatching (50H), and newly hatched larvae after completion of hatching (HC), to determine the chemical constituents and enzyme activities at each stage. Figure 1 shows the morphological alterations with the development of yellowfin tuna eggs and yolk sac larvae. The eggs used in this study were collected from one female fish that spawned in a single day. At each stage, 150–200 eggs and larvae were collected and weighed, and the average weight per individual was calculated. All the eggs and larvae used in this study were analyzed, including the chorion of the embryos. At the 50% hatching point, both unhatched and hatched embryos were analyzed. Water-quality parameters, including dissolved oxygen saturation (98–106%), temperature (29.1–29.5 °C), and salinity (29–30 ppt), were measured at each embryonic stage.
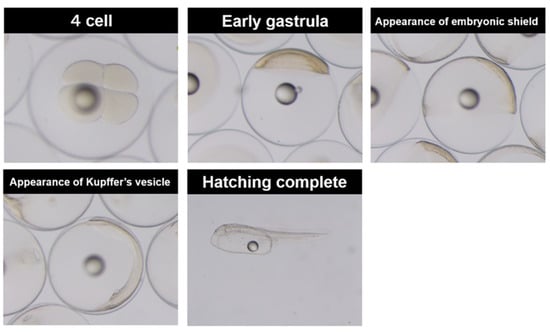
Figure 1.
The morphological alterations with the development of yellowfin tuna eggs and yolk sac larvae.
2.2. Assays of Nutritional Constituents
The moisture and total nitrogen (TN) content of the eggs and larvae were determined using the ambient-pressure-drying and Kjeldahl methods, respectively [19]. The protein content was determined using a modified Lowry Protein Assay Kit (Thermo Scientific, Rockford, IL, USA). Free amino acid (FAA) levels were quantified using the ninhydrin method [20] with l-leucine as a standard. The ammonia content of the eggs and larvae was measured using a commercial kit (Ammonia Test Wako, Wako Pure Chemicals, Osaka, Japan) after extraction with 6% trichloroacetic acid. Triglyceride (TG), phospholipid (PL), and free glucose contents were measured using commercial kits (Triglyceride G-Test Wako, Phospholipid C-Test Wako, and Glucose CII-Test Wako; Wako Pure Chemicals). Glycogen was precipitated by the addition of ethanol into a homogenized solution of the eggs and larvae with 30% KOH [21]. The glycogen content was quantified using the anthrone–sulfuric acid method [22].
2.3. Gross Energy Content
The gross energy of the eggs and larvae was calculated using the International Biological Program conversion factors [23]. The conversion factors for proteins (protein + FAA), fats (TG + PL), and carbohydrates (free glucose + glycogen) were 23.0, 39.8, and 17.2 J/mg, respectively. The molecular weight of FAAs was tentatively defined as 129.3, as previously reported [24].
2.4. Enzyme Activity Assays
The enzyme solution was prepared as follows: the eggs and larvae (0.1 g) were homogenized with RIPA buffer (Nacalai Tesque, Kyoto, Japan) in a microtube. The homogenate was centrifuged at 10,000× g for 10 min at 4 °C after incubation on ice for 30 min, and the supernatant was used for the enzyme assays. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities were determined using commercial kits (Transaminase C II-test Wako; Wako Pure Chemicals). Alkaline phosphatase (ALP) activity was colorimetrically assayed using disodium p-nitrophenyl phosphate as a substrate [25]. Creatine kinase (CK) activity was determined using a commercial kit (EnzyChrom™ Creatine Kinase Assay Kit, BioAssay Systems, Hayward, CA, USA). One unit was defined as the activity that hydrolyzed 1 μmol of substrate per minute at 37 °C.
2.5. Statistical Analysis
All experiments were performed using 0.1–1.0 g eggs or larvae. After weighing, the number of eggs or larvae was counted to calculate the measurements per egg or larva. Three replicates of each egg and larval assay were conducted for each growth stage. The data are presented as means ± standard deviation (SD). All the data were analyzed using Excel Toukei 2006 (SSRI, Tokyo, Japan). A one-way analysis of variance, followed by a post hoc Bonferroni correction, was used to examine the differences between the different stages. Statistical significance was set at p < 0.05.
3. Results
3.1. Changes in Dry Egg and Larval Weights with Embryonic Development of YFT Eggs and Larvae
Figure 2 shows the time elapsed at each embryonic stage, 2C, LC, EG, E, K, H, BH, 50H, and HC (0, 1.1, 3.6, 5.3, 7.3, 12.1, 16.3, 18.3, and 20.1 h, respectively); and changes in the dry egg and larval weights were recorded over the course of embryonic development. A hatching rate of >93% was observed. As development progressed, the dry weight per egg and larva gradually decreased.
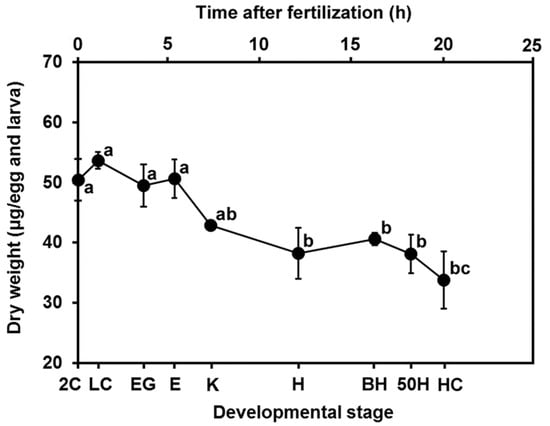
Figure 2.
Changes in the dry weight of eggs and larvae of yellowfin tuna. Values are presented as means ± SD. Letters next to values denote significant differences (p < 0.05). Abbreviations of developmental stages: 2C = 2 cells; LC = late cleavage; EG = early gastrula; E = appearance of embryonic shield; K = appearance of Kupffer’s vesicle; H = beginning of heart beat; BH = just before hatching; 50H = 50% hatching; and HC = hatching complete. The time elapsed at each embryonic stage, 2C, LC, EG, E, K, H, BH, 50H, and HC, is 0, 1.1, 3.6, 5.3, 7.3, 12.1, 16.3, 18.3, and 20.1 h, respectively.
3.2. Changes in Nutritional Constituents with the Development of Yellowfin Tuna Eggs and Larvae
To clarify the nutritional condition of the eggs and larvae during embryonic development, we measured protein, TN, FAA, ammonia, TG, PL, free glucose, and glycogen levels at each developmental stage. No significant changes were detected in TN throughout development (Figure 3a), although the protein content gradually increased with embryonic development (Figure 3a). FAA levels in the eggs and larvae gradually declined with embryonic development (Figure 3b), suggesting that FAAs, at least in part, were used as a material for protein synthesis. The TG content also significantly decreased as development progressed (Figure 3c). Ammonia (Figure 3b), free glucose (Figure 3d), and glycogen (Figure 3d) contents were maintained at very low levels throughout the developmental progression. The amounts of ammonia at the 2C, LC, EG, E, K, H, BH, 50H, and HC stages were 0.003 ± 0.002, 0.005 ± 0.003, 0.006 ± 0.003, 0.007 ± 0.001, 0.007 ± 0.001, 0.013 ± 0.005, 0.013 ± 0.003, 0.009 ± 0.001, and 0.010 ± 0.001 nmol/egg and larva, respectively. The free glucose levels in the eggs rapidly declined until the EG stage; then, the levels rapidly increased after the BH stage. The reverse tendency was observed in the glycogen levels; glycogen content rapidly decreased after hatching, followed by a gradual increase until the K stage.
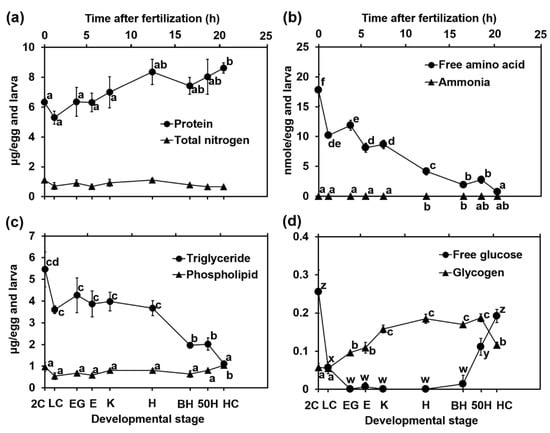
Figure 3.
Changes in (a) protein and total nitrogen, (b) free amino acid and ammonia, (c) triglyceride and phospholipid, (d) free glucose and glycogen with the development of yellowfin tuna eggs and larvae. Values are presented as means ± SD. Letters next to values denote significant differences (p < 0.05). Abbreviations are the same as those in Figure 2.
3.3. Changes in Gross Energy Content and Energy Composition with the Development of Yellowfin Tuna Eggs and Larvae
Figure 4 shows changes in the gross energy content and energy composition with the development of the YFT eggs and larvae. The gross energy content diminished with development; the value at the 2C and HC stages were approximately 0.46 J/egg and 0.29 J/larva, respectively, indicating that 37% of gross energy was consumed during development. The energy content of the TGs and FAAs showed a similar pattern to the gross energy content; however, the energy content of the FAAs was lower than that of the TGs. In contrast, the energy content of the proteins gradually increased until the HC stage. The energy contents of the FAAs, PLs, free glucose, and glycogen were markedly low throughout development.
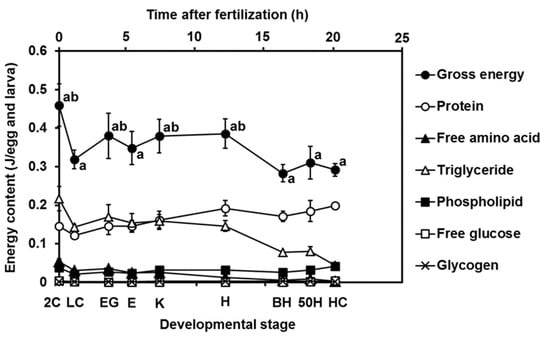
Figure 4.
Changes in gross energy contents and energy composition (protein, free amino acid, triglyceride, phospholipid, free glucose, and glycogen) with the development of yellowfin tuna eggs and larvae. Values were calculated using the International Biological Program conversion factors and are presented as means ± SD. Letters next to values denote significant differences (p < 0.05). Abbreviations are the same as those in Figure 2.
3.4. Changes in AST, ALT, ALP, and CK Activities with the Development of Yellowfin Tuna Eggs and Yolk Sac Larvae
The results of AST and ALT activities throughout development in the YFT eggs are shown in Figure 5a,b. AST activities declined from 0.2 × 10−3 units/egg at the 2C stage to 0.0 × 10−3 units/egg at the H stage. ALT activity decreased from the 2C stage (0.006 × 10−3 units/egg) to the LC stage (0.002 × 10−3 units/egg). Thereafter, the activities maintained constant levels (approximately 0.003 × 10−3 units/egg) from the LC stage to the H stage. Figure 5c,d illustrate ALP and CK activities according to the stage of embryonic development in YFT. The reduced pattern of ALP activity with development resembles that of AST activity; ALP activity declined from 0.95 × 10−3 units/egg at the 2C stage to 0.14 × 10−3 units/egg at the H stage. The decreasing pattern of CK activity was similar to that of ALT activity, where a decrease was observed from the 2C stage (3.3 × 10−3 units/egg) to the LC stage (2.1 × 10−3 units/egg). Thereafter, the activities remained constant (approximately 1.8 × 10−3 units/egg) from the LC stage to the H stage. All enzyme activities rapidly increased from the BH stage to the HC stage: AST, from 0.03 to 0.35; ALT, from 0.003 to 0.010; ALP, from 0.15 to 0.40; and CK, from 2.1 to 4.7 × 10−3 units/egg and larva.
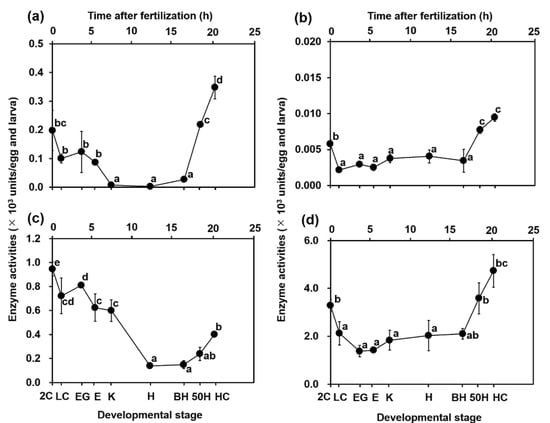
Figure 5.
Changes in (a) aspartate aminotransferase (AST), (b) alanine aminotransferase (ALT), (c) alkaline phosphatase (ALP), and (d) creatine kinase (CK) activities with the development of yellowfin tuna eggs and yolk sac larvae. Values are presented as means ± SD. Letters next to values denote significant differences (p < 0.05). Abbreviations are the same as those in Figure 2.
4. Discussion
In this study, the changes in nutritional constituents were clarified in YFT eggs and newly hatched larvae during embryonic development. As development progressed, the dry weight per egg and larva gradually decreased; the average dry weight from the 2C stage to the HC stage was approximately 42 μg. Similar dry weights have been reported in previous studies of YFT eggs [26]. The decrease in the dry weights of eggs is thought to be due to the utilization of the nutrients in the eggs for tissue development. The protein content gradually increased, although FAA levels in eggs and larvae gradually declined with morphological changes. These results suggest that FAAs, at least in part, are used as a material for protein synthesis.
The energy contents of the TGs and FAAs showed a pattern of temporal changes similar to the gross energy content; however, the energy content of the FAAs was lower than that of the TGs. Although TGs and FAAs are energy resources, TGs are more important because of their higher energy content. We hypothesized that increased β-oxidation and tricarboxylic acid cycle activities occurred in the mitochondria during this period. It would be reasonable to use TGs, the calorie content of which is greater than that of FAAs, as energy for accumulation and consumption in small YFT eggs (approximately 0.93 mm in diameter).
Similar to YFT eggs, PBT eggs have approximately the same size (0.97 mm diameter) and utilize TGs as an endogenous energy source during embryonic development [27]. In contrast, our previous studies demonstrated that TGs as well as FAAs were used as the main sources of endogenous energy in the embryonic development of the red seabream (Pagrus major), in which eggs are roughly 1.0 mm in diameter [28]. Furthermore, the main energy source in Atlantic cod (Gadus morhua) eggs (diameter <1.0 mm) was reportedly FAAs [29]. Thus, the endogenous energy source in eggs is not defined by egg size alone. Some studies suggest that nutritional demands change during embryonic development and depend on broodstock management and physiological events [29,30,31]. Accordingly, further studies are needed to better understand the changes in organ development during embryogenesis.
Tanaka reported that the digestive system of yellowfin tuna immediately after hatching was similar to that of most marine fish that hatched from small pelagic eggs [32]. We further measured enzyme activity to determine at what stage of egg development tissue differentiation progressed. AST and ALT activities were assayed as markers of liver development. ALT activities are more specific than those of AST in the liver, and AST activity has been detected not only in the liver, but also in the heart, skeletal muscle, and erythrocytes among others in mammals [33,34]. We also measured ALP and CK activity to better understand the development of organ function. In mammals, high levels of ALP activity are observed in the gut and placenta, followed by the skeleton and kidney. High CK activity, which is related to the metabolism of creatine phosphatase as a source of ATP, was detected in nerves and muscles. These four enzymes were detected until the completion of hatching. These enzyme activities also rapidly increased from the BH stage to the HC stage, suggesting that organ differentiation and function were rapidly promoted after hatching. This evidence is consistent with the increase in protein synthesis observed after hatching. In addition, Yamagami et al. described the synthesis of protein precursors in the egg envelope in the mother fish in some fish species [35]. These four enzyme levels were high during the first cleavage and decreased during the advanced development stages. This indicates that enzymes are not only markers of organ differentiation, but are also dependent on the health and nutritional status of the mother fish.
We are interested in what constitutes and determines internal egg quality. About 0.05 J/mg larva of TGs remained after the HC stage, although the levels were reduced with development. The quantity of TGs in YFT eggs was probably sufficient to accelerate embryonic development. Accordingly, although the quantity of TGs was sufficient to accelerate embryonic development, there are important factors other than TG content. Contrary to our expectations, phospholipid (a main constituent of cell membranes) content in the eggs remained at low levels until hatching. These results were consistent with the changes in PBT [27] and red seabream (P. major) eggs [28] during development. This result might have been due to the high energy demand for the synthesis of PLs. Considering active cell division and hypertrophy, we hypothesized that sufficient PL content in fertilized eggs is a critical factor for internal egg quality. This hypothesis has been previously proven in red seabream eggs [36].
The AST, ALT, and CK activities at the BH stage in YFT eggs were roughly 40-, 100-, and 10-fold lower than the respective activities in PBT eggs [27]. These results suggest delayed liver and muscle differentiation during the embryonic development of YFT. Similarly, 10-fold lower FAA contents at the BH stage were observed in YFT eggs compared to PBT eggs. Since amino acids are the source of proteins, we suggest that low amounts of amino acids contribute to delayed organ function. Therefore, we expect that the FAA content in fertilized eggs might play an important role as a material for morphological changes.
In conclusion, the present study showed that TGs play a central role as an energy source for the development of YFT eggs, and that embryonic cell differentiation is promoted after hatching with increased protein synthesis and enzyme activity. PLs and FAAs may be key factors in embryonic development and larval growth in YFT. We hope that these results on which nutrients are key components in embryonic development and early larval growth will provide an opportunity to improve egg quality through broodstock diet manipulation. It is expected that larval survival and growth may be improved if the egg quality can be manipulated. Therefore, the information from this study may support further initiatives to establish mass-seeding production of YFT.
Limitation
The data in this study may limit the wider interest of those working outside of tuna broodstock and hatchery management/research. However, we believe that the results of this study may also be applied to the hatchery management of other fish species similar to tuna for which the artificial production of seedlings is difficult.
Author Contributions
Conceptualization, T.T., T.H., A.B. and K.T.; methodology, T.T. and T.H.; investigation, T.T., and T.H.; writing—original draft preparation, T.T., T.H., Y.S., D.M., V.S., J.W., M.S., A.B. and K.T.; writing—review and editing, T.T. and T.H.; funding acquisition, Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the JST/JICA SATREPS project on “Spawning ecology, nutritional requirement, and early life history of yellowfin tuna”.
Institutional Review Board Statement
This study was conducted under the supervision of the steering committee of the Science and Technology Research Partnership for Sustainable Development Project, which was supported by the Japan Science and Technology Agency and the Japan International Cooperation Agency. In this project, all studies were conducted with the confirmation of the committees of animal experimentation of Kindai University, the Autoridad de los Recursos Acuáticos de Panamá, and the Inter-American Tropical Tuna Commission based on the Memorandum of Understanding exchanged among these organizations.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors thank the staff of the Achotines Laboratory in the Republic of Panama for their assistance during the trial. The authors would like to thank Toru Kobayashi (Faculty of Agriculture, Kindai University) for providing the photo of Figure 1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. Fishery and Aquaculture Statistic B-36; FAO: Rome, Italy, 2018. [Google Scholar]
- Aires-Da-Silva, A.; Maunder, M.N. Status of Yellowfin Tuna in the Eastern Pacific Ocean in 2010 and Outlook for the Future; Document SAC-02-06; Adopted at the 2nd Scientific Advisory Committee: La Jolla, CA, USA, 2011; p. 90. [Google Scholar]
- Harada, T.; Murata, O.; Oda, S. Rearing of and morphological changes in larvae and juveniles of yellowfin tuna. Boll. Fac. Agric. Kinki Univ. 1980, 13, 33–36. (In Japanese) [Google Scholar]
- Masuma, S.N.; Tezuka, K.; Teruya, M.; Oka, M.K.; Nikaido, H. Maturation and Spawning of Reared Yellowfin Tunas at Yaeyama. In Proceedings of the Annual Meeting of the Japanese Society of Scientific Fisheries, Tokyo, Japan, 2–5 April 1993. [Google Scholar]
- Wexler, J.B.; Scholey, V.P.; Olson, R.J.; Margulies, D.; Nakazawa, A.; Suter, J.M. Tank culture of yellowfin tuna, Thunnus albacares: Developing a spawning population for research purposes. Aquaculture 2003, 220, 327–353. [Google Scholar] [CrossRef]
- Margulies, D.; Wexler, J.B.; Bentler, K.T.; Suter, J.M.; Masuma, S.; Tezuka, N.; Teruya, K.; Oka, M.; Kanematsu, M.; Nikaido, H. Food selection of yellowfin tuna, Thunnus albacares, larvae reared in the laboratory. Bull. Inter-Am. Trop Tuna. Commis. 2001, 21, 9–51. [Google Scholar]
- Stein, M.S.; Margulies, D.; Wexler, J.B.; Scholey, V.P.; Ryo, K.; Honryo, T.; Sasaki, T.; Guillen, A.; Agawa, Y.; Sawada, Y. A comparison of the effects of two prey enrichment media on growth and survival of Pacific bluefin tuna, Thunnus orientalis, larvae. J. World Aquacult. Soc. 2018, 49, 240–255. [Google Scholar] [CrossRef]
- Partridge, G.J.; Benetti, D.D.; Stieglitz, J.D.; Hutapea, J.; McIntyre, A.; Chen, B.; Hutchinson, W.; Scholey, V.P. The effect of a 24-hour photoperiod on the survival, growth and swim bladder inflation of pre-flexion yellowfin tuna (Thunnus albacares) larvae. Aquaculture 2011, 318, 471–474. [Google Scholar] [CrossRef]
- Honryo, T.; Tanaka, T.; Guillen, A.; Wexler, J.B.; Cano, A.; Margulies, D.; Scholey, V.P.; Stein, M.S.; Sawada, Y. Effect of water surface condition on survival, growth and swim bladder inflation of yellowfin tuna, Thunnus Albacares (Temminck and Schlegel), larvae. Aquacult. Res. 2016, 47, 1832–1840. [Google Scholar] [CrossRef]
- Buentello, J.A.; Pohlenz, C.; Margulies, D.; Scholey, V.P.; Wexler, J.B.; Tovar-Ramírez, D.; Neill, W.H.; Hinojosa-Baltazar, P.; Gatlin, D.M., III. A preliminary study of digestive enzyme activities and amino acid composition of early juvenile yellowfin tuna (Thunnus albacares). Aquaculture 2011, 312, 205–211. [Google Scholar] [CrossRef]
- Wexler, J.B.; Margulies, D.; Scholey, V.P. Temperature and dissolved oxygen requirements for survival of yellowfin tuna, Thunnus albacares, larvae. J. Exp. Mar. Biol. Ecol. 2011, 404, 63–72. [Google Scholar] [CrossRef]
- Lang, K.L.; Grimes, C.B.; Shaw, R.F. Variations in the age and growth of yellowfin tuna larvae, Thunnus albacares, collected about the Mississippi River plume. Environ. Biol. Fish. 1994, 39, 259–270. [Google Scholar] [CrossRef]
- Kaji, T.; Oka, M.; Takeuchi, H.; Hirokawa, J.; Tanaka, M. Development of growth hormone cells of laboratory reared yellowfin tuna Thunnus albacares larvae and early juveniles. Fish. Sci. 1991, 65, 583–587. [Google Scholar] [CrossRef][Green Version]
- Kaji, T.; Tanaka, M.; Oka, M.; Takeuchi, H.; Ohsumi, S.; Teruya, K.; Hirokawa, J. Growth and morphological development of laboratory-reared yellowfin tuna Thunnus albacares larvae and early juveniles, with special emphasis on the digestive system. Fish. Sci. 1999, 65, 700–707. [Google Scholar] [CrossRef]
- Miyashita, S.; Sawada, Y.; Okada, T.; Murata, O.; Kumai, H. Morphological development and growth of laboratory-reared larval and juvenile Thunnus thynnus (Pisces: Scombridae). Fish. Bull. 2001, 99, 601–616. [Google Scholar]
- Sawada, Y.; Okada, T.; Miyashita, S.; Murata, O.; Kumai, H. Completion of the Pacific bluefin tuna Thunnus orientalis (Temminck et Schlegel) life cycle. Aquac. Res. 2005, 36, 413–421. [Google Scholar] [CrossRef]
- Mourente, G.; Vázquez, R. Changes in the content of total lipid, lipid classes and their fatty acids of developing eggs and unfed larvae of the Senegal sole, Solea senegalensis Kaup. Fish Physiol. Biochem. 1996, 15, 221–235. [Google Scholar] [CrossRef]
- Guillén, A.; Honryo, T.; Ibarra, J.; Cano, A.; Margulies, D.; Scholey, V.P.; Wexler, J.B.; Stein, M.S.; Kobayashi, T.; Sawada, Y. Effect of water temperature on embryonic development of yellowfin tuna Thunnus Albacares inhabiting the Eastern Pacific Ocean. Aquacult. Sci. 2014, 62, 319–322. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 14th ed.; AOAC: Arlington, VA, USA, 1984; Volume 1141, p. 1. [Google Scholar]
- Tokyo University. Experiment of Agricultural Chemistry; Asakura Shoten: Tokyo, Japan, 1992; p. 320. (In Japanese) [Google Scholar]
- Fujii, N. Experimental Method for Biochemistry; Nankodo: Tokyo, Japan, 1949. (In Japanese) [Google Scholar]
- Sekine, T. Photoelectric Colorimetry in Biochemistry; Nankodo: Tokyo, Japan, 1958; pp. 36–39. (In Japanese) [Google Scholar]
- Winberg, G.G. Symbols, Units and Conversion Factors in Studies of Fresh Water Productivity; IBP Central Office: London, UK, 1971; pp. 1–23. [Google Scholar]
- Rønnestad, I.; Koven, W.M.; Tandler, A.; Harel, M.; Fyhn, H.J. Energy metabolism during development of eggs and larvae of gilthead sea bream (Sparus aurata). Mar. Biol. 1994, 120, 187–196. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takahashi, Y.; Tabata, Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials 2003, 24, 4375–4383. [Google Scholar] [CrossRef]
- Margulies, D.; Suter, J.M.; Hunt, S.L.; Olson, R.J.; Scholey, V.P.; Wexler, J.B.; Nakazawa, A. Spawning and early development of captive yellowfin tuna (Thunnus albacares). Fish. Bull. 2007, 105, 249–265. [Google Scholar]
- Takii, K.; Miyashita, S.; Seoka, M.; Tanaka, Y.; Kubo, Y.; Kumai, H. Changes in chemical contents and enzyme activities during embryonic development of bluefin tuna. Fish. Sci. 1997, 63, 1014–1018. [Google Scholar] [CrossRef][Green Version]
- Seoka, M.; Takii, K.; Takaoka, O.; Nakamura, M.; Kumai, H. Biochemical phases in embryonic Red Sea bream development. Fish. Sci. 1997, 63, 122–127. [Google Scholar] [CrossRef]
- Fyhn, H.J.; Serigstad, B. Free amino acids as energy substrate in developing eggs and larvae of the cod Gadus morhua. Mar. Biol. 1987, 96, 335–341. [Google Scholar] [CrossRef]
- Almansa, E.; Pérez, M.J.; Cejas, J.R.; Badía, P.; Villamandos, J.E.; Lorenzo, A. Influence of broodstock giltehead seabream (Sparus aurata L.) dietary fatty acids on egg quality and egg fatty acid composition throughout the spawning season. Aquaculture 1999, 170, 323–336. [Google Scholar] [CrossRef]
- Rainuzzo, J.R.; Reitan, K.I.; Olsen, Y. The significance of lipids at early stages of marine fish: A review. Aquaculture 1997, 155, 103–115. [Google Scholar] [CrossRef]
- Tanaka, M. Stusies on structure and function of the digestive system in teleost larvae I: Development of the digestive system during prelarval stage. Jpn. J. Ichthyol. 1969, 16, 1–9. [Google Scholar]
- Horio, Y.; Tanaka, T.; Taketoshi, M.; Uno, T.; Wada, H. Rat cytosolic aspartate aminotransferase: Regulation of its mRNA and contribution to gluconeogenesis. J. Biochem. 1988, 103, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.D.; Woolford, L.; O’Hara, A.J.; Nicholls, P.K.; Warren, K.S. Clinical chemistry values and tissue enzyme activities in western barred bandicoots (Perameles bougainville). Vet. Clin. Pathol. 2008, 37, 221–224. [Google Scholar] [CrossRef]
- Yamagami, K. Studies on the Hatching Enzyme (Choriolysin) and its Substrate, Egg Envelope, Constructed of the Precursors (Choriogenins) in Oryzias latipes: A Sequel to the Information in 1991/1992. Zoolog. Sci. 1996, 13, 331–340. [Google Scholar] [CrossRef]
- Seoka, M.; Takii, K.; Takaoka, O.; Furuta, S.; Nakamura, M.; Kumai, H. Chemical and enzyme comparison between Red Sea bream Eggs with high and low hatchability. Suisanzoshoku 1997, 45, 103–108. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).