Complete Genome Sequences and Pathogenicity Analysis of Two Red Sea Bream Iridoviruses Isolated from Cultured Fish in Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viral Culture
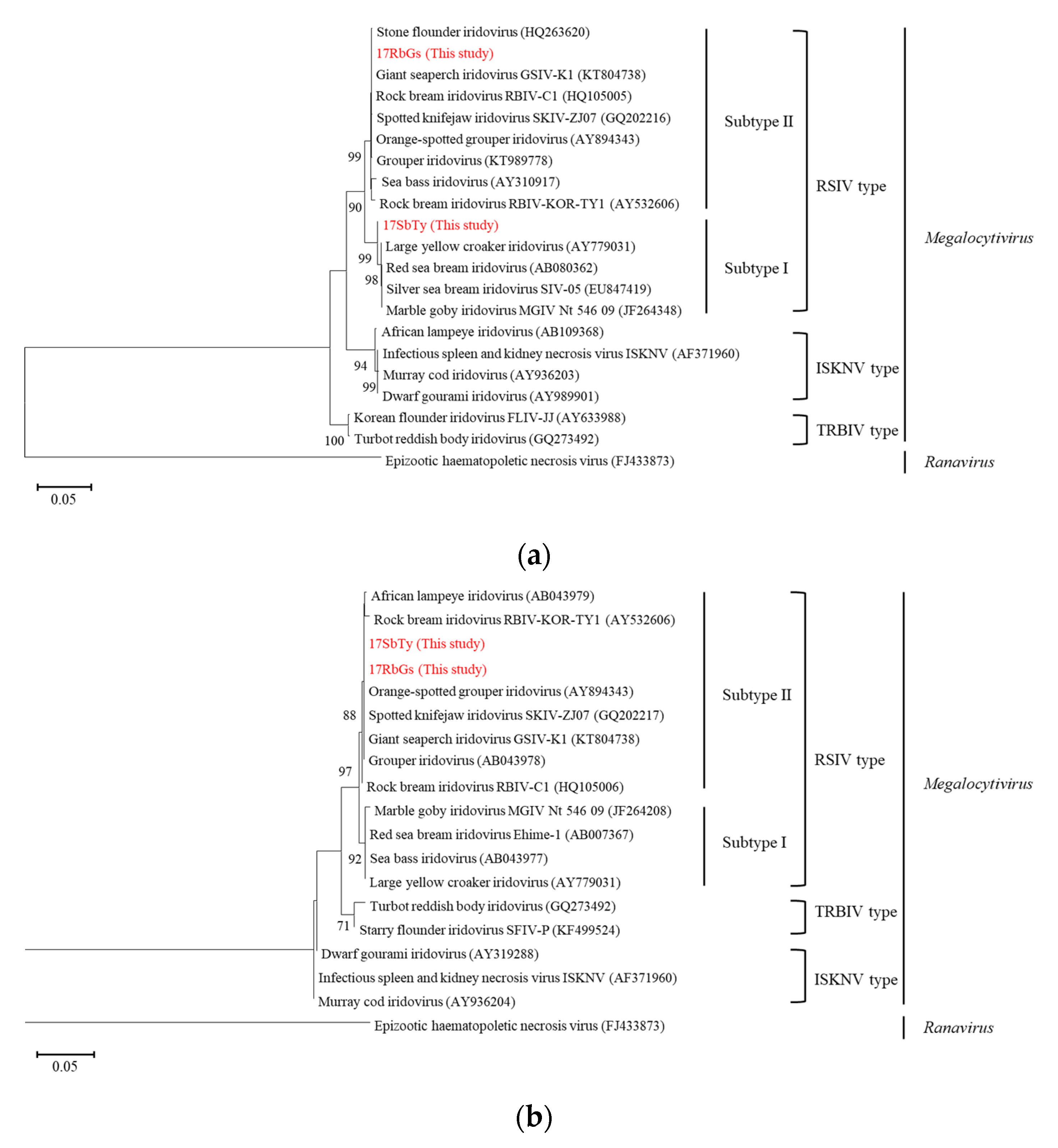
2.2. Phylogenetic Analysis
2.3. Determination of Complete Genome Sequences by Next-Generation Sequencing
2.4. Complete Genome Sequence Analysis
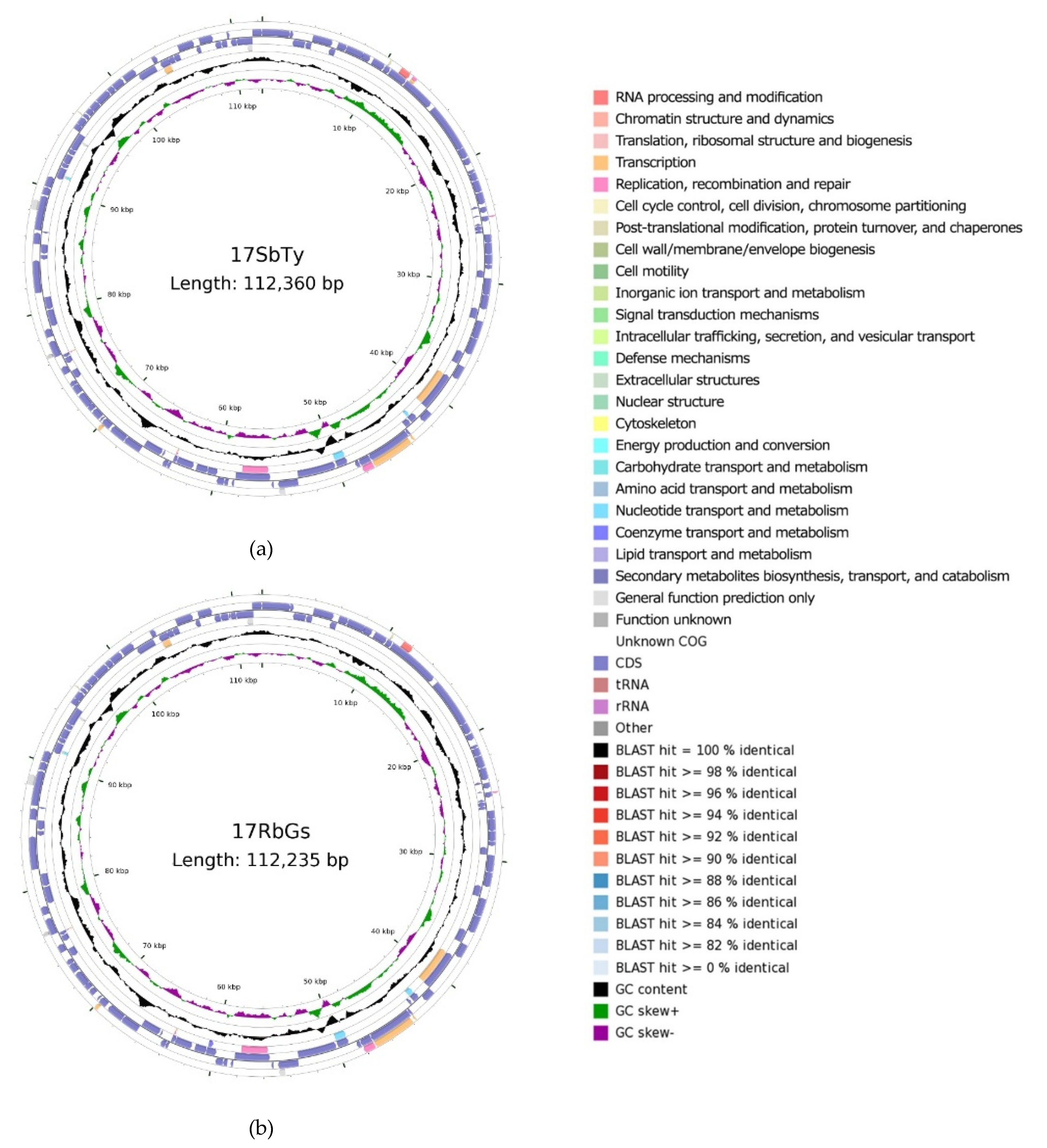
2.4.1. Construction of a Circular Map
2.4.2. Gene Annotation and Open Reading Frame (ORF) Analysis
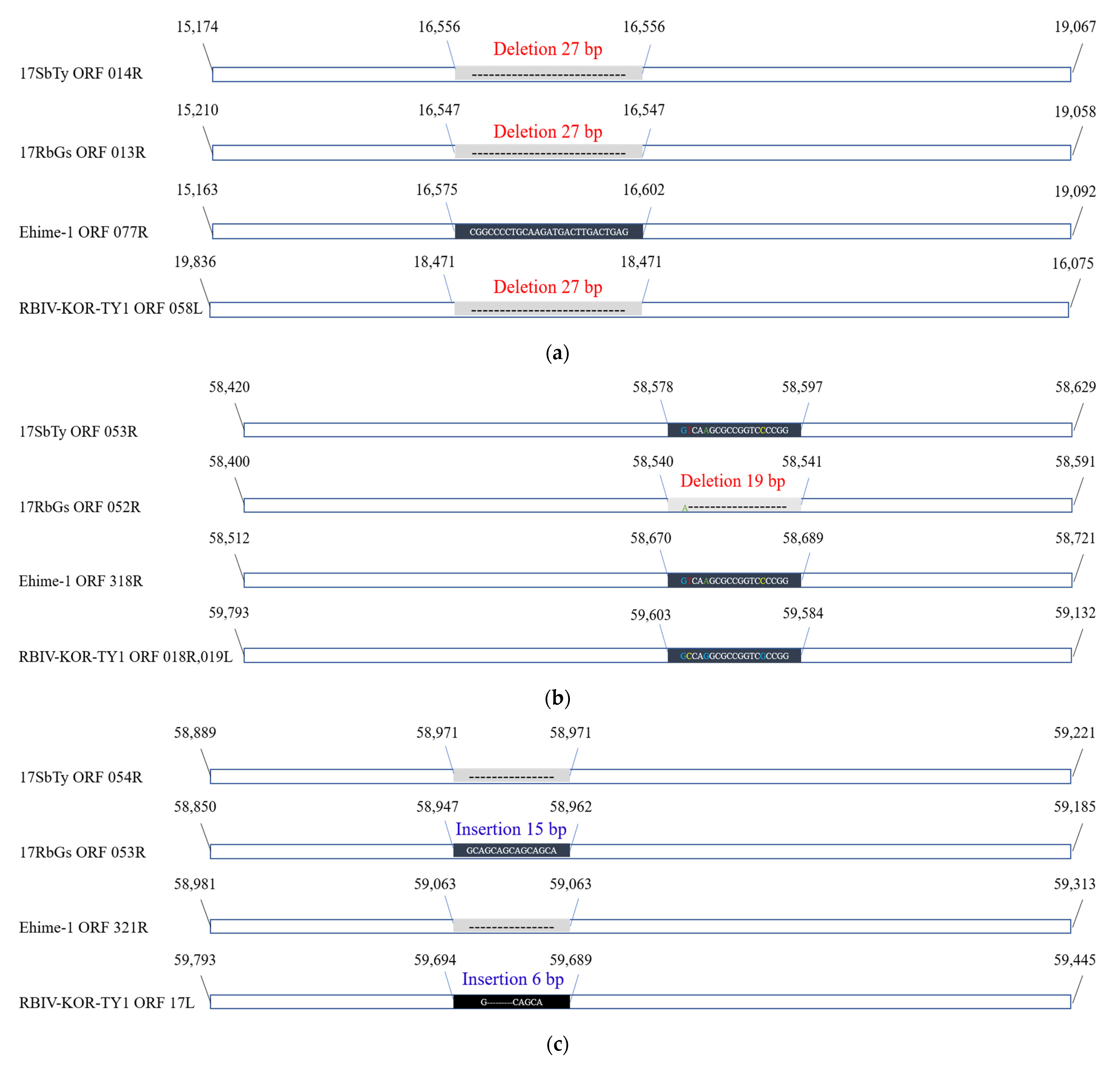
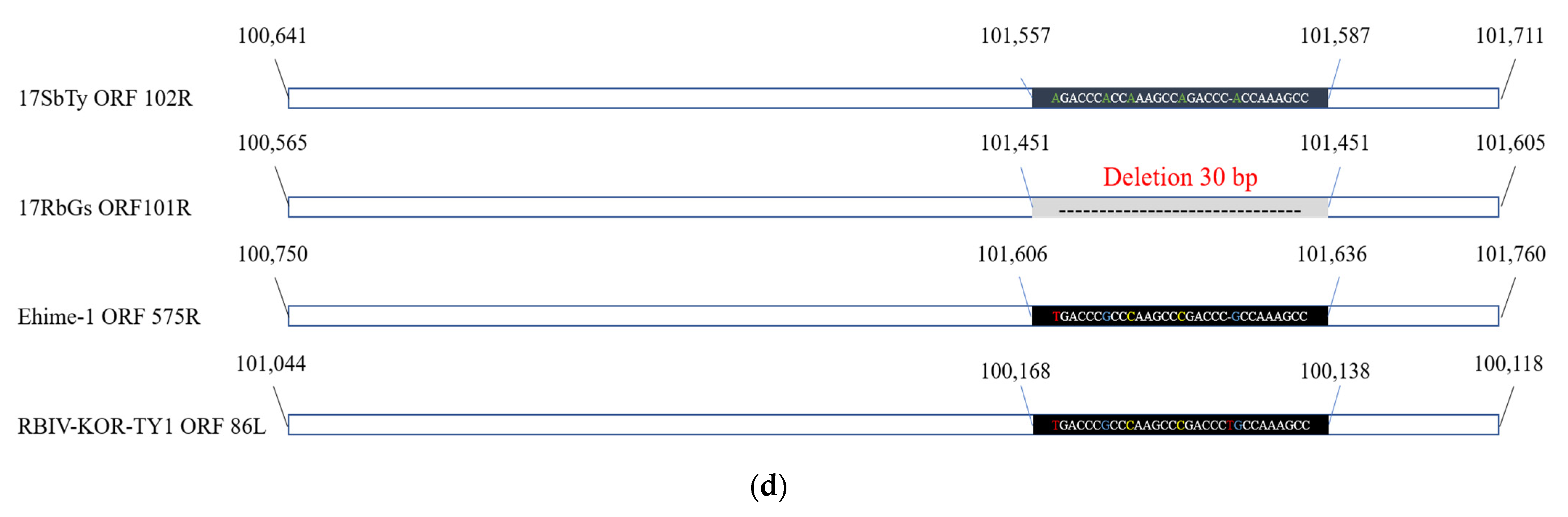
2.4.3. Analysis of InDels in RSIVs
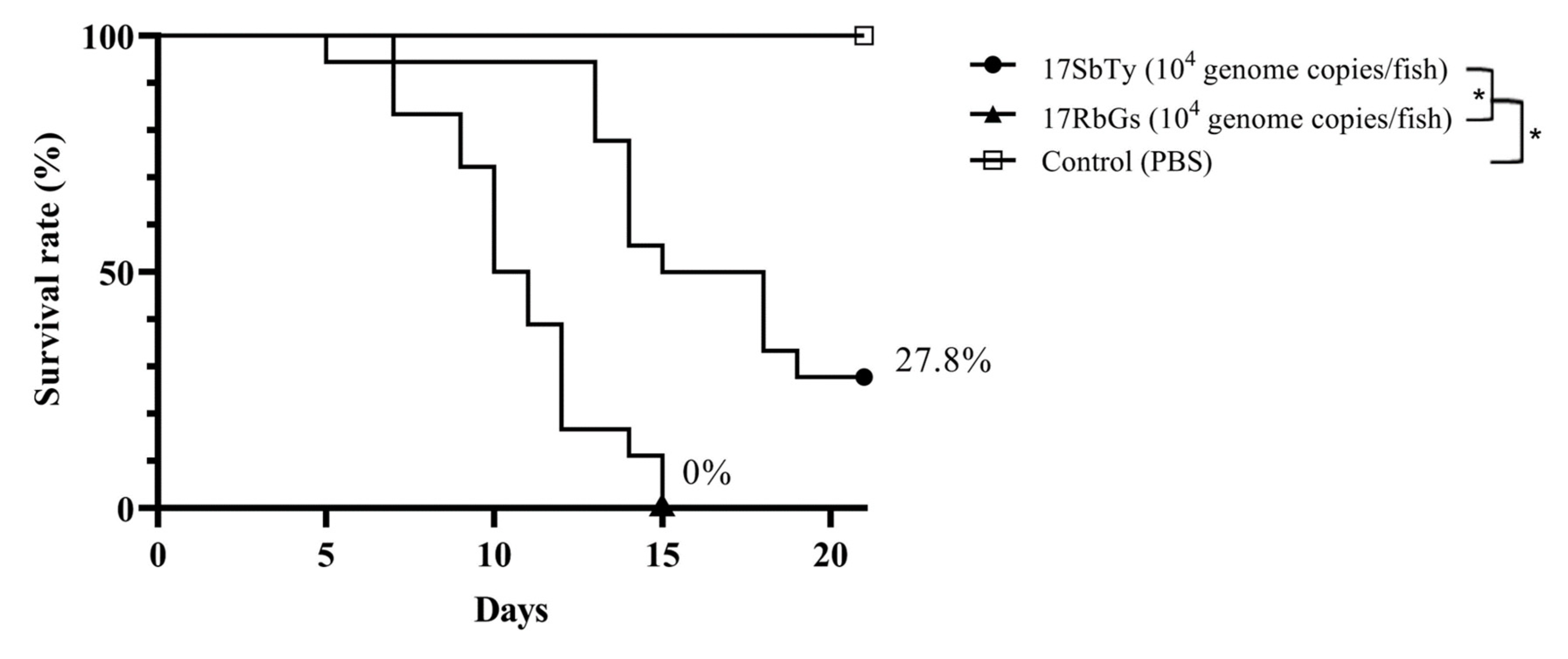
2.5. Pathogenicity of the Two RSIV Isolates in the Rock Bream
3. Results & Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Primer | Target | Sequence(5′-3′) | Reference |
|---|---|---|---|
| MCP 1F MCP 300R MCP 600F MCP 800R MCP 1015F MCP 1362R | Major capsid protein | ATG TCT GCR ATC TCA GGT GC CCA GCG RAT GTA GCT GTT CTC CAA GCT GCG GCG CTG GGA GG GGC GCC ACC TGR CAC TGY TC CTC ATT TTA CGA GAA CAC CC TYA CAG GAT AGG GAA GCC TGC | [29] |
| ATPase 1F ATPase 218R ATPase 529F ATPase 721R | ATPase | ATG GAA ATC MAA GAR TTG TCC YTG CAG TTR GGC AAY AGC TTG CT GGG GGY AAC ATA CCM AAG C CTT GCT TAC RCC ACG CCA G | This study |
| RSIV 1094F RSIV 1221R RSIV 1177 probe | Major capsid protein | CCA GCA TGC CTG AGA TGG A GTC CGA CAC CTT ACA TGA CAG G FAM-TAC GGC CGC CTG TCC AAC G-BHQ1 | [15] |
| 1-F 1-R | Pst I fragment | CTC AAA CAC TCT GGC TCA TC GCA CCA ACA CAT CTC CTA TC | [28] |
| 4-F 4-R | DNA polymerase gene | CGG GGG CAA TGA CGA CTA CA CCG CCT GTG CCT TTT CTG GA | |
| 14R-1F 14R-260R 14R-430F 14R-999R 14R-848F 14R-1202F 14R-1841R 14R-1620F 14R-2011F 14R-2630R 14R-2309F 14R-2740F 14R-3241R 14R-3190F 14R-3494F 14R-3849R | ORF 014R * | ATG AAG AAA TTT GAT TTT TGY RKA TGT C TCA TCC TCA GAG TCG CGG GCT CAG TTG TTC AAG ATG CC ATG CGT ATC ACA GTA CGC G CCA TAG AGG ATA ACA GCG C ACA AGC GGG ACC TAT GCA A TAC ATC GGC TCC TCA ACT G AGA ACT GGA GGA CTC ACA CAC CGT GAA CTG CGC ATC T GTC AGG TAT GTT TCC TGG TGT GTA TGA TCG AGG AGA TCG CA GAA CAC CGA GAG AGT GGA GAT G AGT AGT CTA CCA CAG TTG C TGT CAG CTA AAG GTC AGT GAT G GTA TGT TGG ACT ACA TCG ACC C TCA TTG ATT TTC ATT YAC ACC MAG | This study |
| 53R-1F 53R-RB-192R 53R-SB-210R | ORF 053R * | ATG CCA CAG CCY ATT ATC TTC CTA AGC GCG CCT GGC TGG CTA AGC AGC CCT GGC GGG | |
| ORF54-1F ORF54-348R | ORF 054R * | ATG CCG ACT ACC AAA CAC A TCA AAA CTC AAA GGC GCC G | |
| 102R-1F 102R-222R 102R-424F 102R-797F 102R-1071R | ORF 102R * | ATG AGT GCA ATA AAG GCA AAT G GTC CCG CAC GCC GTT GTT CGC GTG CAT GCA ATG TAT GCA ATG TCT GTC AGG TGG C CTA GGC AAA TGC AGC AAT AAC |
| Gene ID 17SbTy | Position | CDS Size (NT) | Predicted Structure and/or Function | Best-Match Homolog | Homolog to 17RbGs | Homolog to Ehime_1 (AB104413.1) | Homolog to ISKNV (AF371960) | Homolog to TRBIV (GQ273492) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Start | End | Genotype | Isolates | Identity (%) | ORF no. | Identity (%) | ORF no. | Identity (%) | ORF no. | Identity (%) | ORF no. | Identity (%) | |||
| ORF 001R | 111,584 | 2196 | 2973 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 OSGIV | 99.70% | ORF 001R | 99.70% | ORF 639R | 98.18% | 76L | 93.44% | 69L | 92.91% |
| ORF 002R | 2198 | 2467 | 270 | hypothetical protein | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV RSIV Ehime-1 | 100.00% | ORF 002R | 96.67% | ORF 010R | 100.00% | 75L | 91.30% | 68L | 87.26% |
| ORF 003L | 2476 | 3495 | 1020 | hypothetical protein | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV RSIV Ehime-1 | 100.00% | ORF 003L | 98.53% | ORF 016L | 100.00% | 74R | 93.63% | 67R | 93.94% |
| ORF 004L | 3544 | 4032 | 489 | hypothetical protein | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 LYCIV | 100.00% | ORF 004L | 95.09% | ORF 019L | 100.00% | 73R | 90.24% | 66R | 84.72% |
| ORF 005R | 4015 | 5625 | 1611 | hypothetical protein | RSIV subtype I | PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 | 100.00% | ORF 005R | 98.08% | ORF 018R | 100.00% | 71L | 93.61% | 65L | 93.42% |
| ORF 006L | 5528 | 6043 | 516 | hypothetical protein | RSIV subtype I | PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 | 100.00% | ORF 006L | 97.29% | ORF 026R | 100.00% | 70L | 95.20% | - | - |
| ORF 007R | 6065 | 6796 | 732 | hypothetical protein | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 | 100.00% | ORF 007R | 96.86% | ORF 029R | 100.00% | 69L | 86.07% | 64L | - |
| ORF 008R | 6808 | 8241 | 1434 | hypothetical protein | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 | 100.00% | ORF 008R | 97.63% | ORF 033R | 100.00% | 68L | 93.58% | 63L | 88.95% |
| ORF 009R | 8192 | 8860 | 669 | hypothetical protein | RSIV subtype I | LYCIV Zhoushan | 100.00% | ORF 009R | 98.06% | ORF 037R | 98.80% | 67L | 90.69% | 62L | 91.68% |
| ORF 010R | 9087 | 10,130 | 1044 | hypothetical protein | RSIV subtype II / ISKNV subtype I | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RSIV-Ku LYCIV Zhoushan OSGIV | 100.00% | ORF 010R | 99.81% | ORF 042R | 98.46% | 66L | 92.82% | 61L | 92.53% |
| ORF 011R | 10,181 | 10,651 | 471 | RING-finger-containing E3 ubiquitin ligase | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 RBIV-C1 LYCIV Zhoushan RSIV_121 17RbGs | 100.00% | ORF 011R | 100.00% | ORF 049R | 98.51% | 65L | 91.30% | 60L | 89.17% |
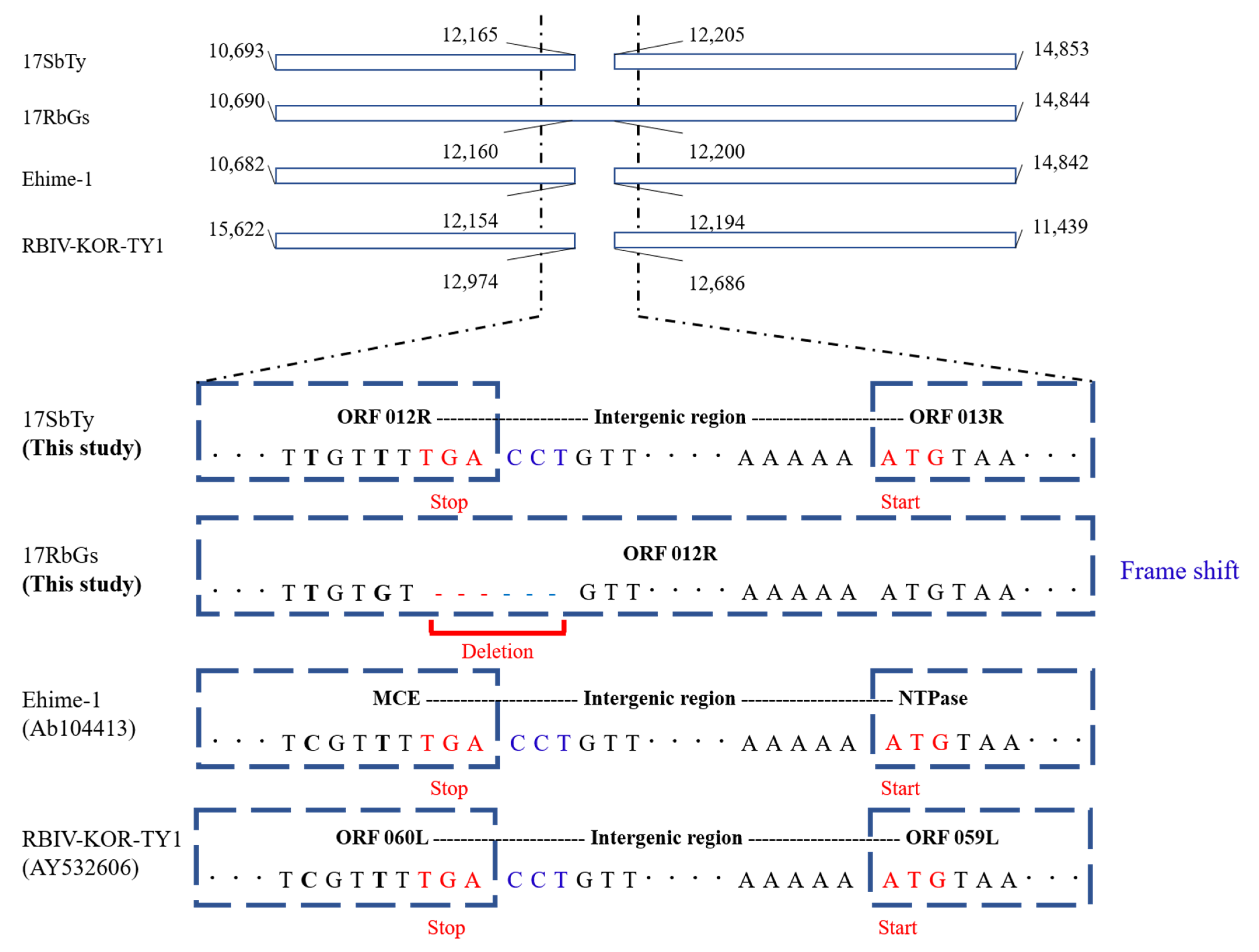
| ORF 012R | 10,693 | 12,165 | 1473 | mRNA capping enzyme | RSIV subtype II / ISKNV subtype I | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RSIV-Ku LYCIV Zhoushan OSGIV | 100.00% | ORF 012R | 99.93% | MCE | 97.49% | 64L | 93.36% | 59L | 93.28% |
| ORF 013R | 12,205 | 14,853 | 2649 | putative NTPase I | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 | 99.96% | - | - | NTPase | 97.92% | 63L | 93.36% | 58L | 93.42% |
| ORF 014R | 15,174 | 19,067 | 3849 | DNA-binding protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 | 100.00% | ORF 013R | 99.48% | ORF 077R | 96.78% | 62L | 91.81% | 57L | 93.08% |
| ORF 015R | 19,064 | 19,870 | 807 | putative replication factor and/or DNA binding-packing | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 OSGIV | 100.00% | ORF 014R | 92.94% | ORF 092R | 97.65% | 61L | 93.80% | 56L | 93.06% |
| ORF 016R | 19,934 | 20,446 | 513 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 GSIV-K1 OSGIV | 100.00% | ORF 015R | 89.35% | ORF 097R | 96.30% | 59L | 92.84% | 55L | 88.95% |
| ORF 017R | 20,918 | 21,178 | 261 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 SKIV RBIV-C1 RSIV_121 RBIV-KOR-TY1 OSGIV | 100.00% | ORF 016R | 95.40% | ORF 099R | 98.08% | 57L | 96.17% | 54L | 95.40% |
| ORF 018R | 21,185 | 21,832 | 648 | helicase family | RSIV subtype II | RSIV KagYT-96 GSIV-K1 OSGIV | 100.00% | ORF 017R | 99.23% | ORF 101R | 99.23% | 56L | 97.22% | 53L | 97.38% |
| ORF 019R | 21,843 | 22,784 | 942 | Serine-threonine protein kina | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 SKIV RBIV-C1 RSIV_121 OSGIV 17RbGs | 100.00% | ORF 018R | 100.00% | ORF 106R | 96.92% | 55L | 90.98% | 52L | 89.81% |
| ORF 020R | 22,807 | 23,751 | 945 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 GSIV-K1 SKIV RBIV-C1 RSIV_121 OSGIV 17RbGs | 100.00% | ORF 019R | 100.00% | ORF 111R | 97.67% | 54L | 90.08% | 51L | 90.48% |
| ORF 021L | 23,785 | 23,979 | 195 | hypothetical protein | RSIV subtype II | RSIV KagYT-96RSIV RIE12-1GSIV-K1SKIVRBIV-C1RSIV_121OSGIV17RbGs | 100.00% | ORF 020L | 100.00% | ORF 121L | 96.91% | 53R | 91.24% | 50R | - |
| ORF 022R | 23,981 | 24,433 | 453 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 SKIV RBIV-C1 RSIV_121 OSGIV 17RbGs | 100.00% | ORF 021R | 100.00% | ORF 122R | 96.47% | 52L | 88.91% | 49L | 88.21% |
| ORF 023L | 24,522 | 24,657 | 111 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 SKIV RBIV-C1 RSIV_121 RBIV-KOR-TY1 OSGIV 17RbGs | 100.00% | ORF 022L | 100.00% | ORF 127L | 93.86% | 51R | 91.46% | - | - |
| ORF 024R | 24,712 | 25,140 | 429 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-KOR-TY1 OSGIV | 100.00% | ORF 023R | 99.77% | ORF 128R | 98.37% | 50L | 93.24% | 48L | 91.61% |
| ORF 025L | 25,208 | 25,378 | 171 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 SKIV RBIV-C1 RSIV_121 RBIV-KOR-TY1 OSGIV 17RbGs | 100.00% | ORF 024L | 100.00% | ORF 134L | 97.66% | 49R | 94.74% | - | - |
| ORF 026L | 25,394 | 25,747 | 354 | PDGF/VEGF-like protein ORF 135L | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 OSGIV | 100.00% | ORF 025L | 99.72% | ORF 135L | 97.74% | 48R | 86.16% | 47R | 87.39% |
| ORF 027L | 25,744 | 26,007 | 264 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 SKIV RBIV-C1 RSIV_121 RBIV-KOR-TY1 OSGIV 17RbGs | 100.00% | ORF 026L | 100.00% | ORF 138L | 97.35% | 47R | 93.18% | 46R | 93.56% |
| ORF 028R | 26,167 | 26,850 | 684 | cytosine DNA methyltransferase | RSIV subtype I | PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 | 99.85% | ORF 027R | 97.95% | ORF 140R | 99.85% | 46L | 94.74% | 45L | 94.88% |
| ORF 029R | 26,844 | 27,758 | 915 | hypothetical protein | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 | 100.00% | ORF 028R | 96.17% | ORF 145R | 100.00% | 45L | 88.74% | 44L | 89.84% |
| ORF 030R | 27,763 | 28,563 | 801 | hypothetical protein | RSIV subtype I | LYCIV Zhoushan RSIV Ehime-1 | 100.00% | ORF 029R | 97.50% | ORF 151R | 100.00% | 44L | 90.02% | 43L | 89.51% |
| ORF 031R | 28,570 | 28,932 | 363 | Erv1/Alr family | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 | 100.00% | ORF 030R | 97.80% | ORF 156R | 100.00% | 43L | 94.21% | 42L | 95.04% |
| ORF 032L | 29,016 | 29,615 | 600 | hypothetical protein | RSIV subtype I | PIV2010 LYCIV Zhoushan RSIV Ehime-1 | 100.00% | ORF 031L | 96.83% | ORF 161L | 100.00% | 42R | 89.33% | 41R | 91.01% |
| ORF 033R | 29,630 | 30,979 | 1350 | hypothetical protein | RSIV subtype I | LYCIV Zhoushan | 100.00% | ORF 032R | 97.04% | ORF 162R | 99.56% | 41L | 88.96% | 40L | 90.53% |
| ORF 034R | 30,981 | 32,129 | 1149 | hypothetical protein | RSIV subtype I | LYCIV Zhoushan | 100.00% | ORF 033R | 91.91% | ORF 171R | 91.22% | 40L | 89.65% | 39L | 98.43% |
| ORF 035L | 32,122 | 33,000 | 879 | hypothetical protein | RSIV subtype I | LYCIV Zhoushan RSIV Ehime-1 LYCIV | 100.00% | ORF 034L | 93.97% | ORF 179L | 100.00% | 39R | 90.22% | 38R | 90.90% |
| ORF 036R | 33,066 | 34,505 | 1440 | hypothetical protein | RSIV subtype I | PIV2016 PIV2014a PIV2010 RSIV Ehime-1 | 100.00% | ORF 035R | 93.75% | ORF 180R | 100.00% | 38L | 90.71% | 37L | 90.90% |
| ORF 037R | 34,514 | 35,863 | 1350 | hypothetical protein | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 | 99.93% | ORF 036R | 93.85% | ORF 186R | 99.93% | 37L | 90.11% | 36L | 90.96% |
| ORF 038L | 35,860 | 36,915 | 1056 | hypothetical protein | RSIV subtype I | PIV2010 LYCIV Zhoushan RSIV Ehime-1 LYCIV | 100.00% | ORF 037L | 95.17% | ORF 197L | 100.00% | 36R | 91.49% | 35R | 88.93% |
| ORF 039R | 36,909 | 38,048 | 1140 | hypothetical protein | RSIV subtype I | PIV2010 LYCIV Zhoushan | 100.00% | ORF 038R | 95.53% | ORF 198R | 99.91% | 35L | 88.64% | 34L | 88.88% |
| ORF 040L | 38,121 | 41,279 | 3159 | DNA dependent RNA polymerase second largest subunit | RSIV subtype I | LYCIV Zhoushan | 100.00% | ORF 039L | 96.52% | RPO-2 | 98.54% | 34R | 93.78% | 33R | 94.98% |
| ORF 041R | 41,362 | 42,264 | 903 | hypothetical protein | RSIV subtype I | LYCIV Zhoushan | 100.00% | ORF 040R | 95.90% | ORF 226R | 97.79% | 33L | 91.36% | 32L | 92.59% |
| ORF 042L | 42,327 | 42,943 | 582 | deoxyribonucleoside kinase | RSIV subtype I | LYCIV Zhoushan | 100.00% | ORF 041L | 88.87% | TK | 87.99% | 32R | 92.16% | 31R | 99.66% |
| ORF 043L | 43,008 | 43,535 | 243 | hypothetical protein | RSIV subtype I | PIV2016 PIV2014a PIV2010 RSIV Ehime-1 | 98.77% | ORF 042L | 95.47% | ORF 237L | 98.77% | 31.5L | 88.89% | 30R | 93.42% |
| ORF 044R | 43,603 | 43,824 | 222 | transcription elongation factor TFIIS | RSIV subtype I | PIV2016PIV2014aPIV2010LYCIV ZhoushanRSIV Ehime-1 | 100.00% | ORF 043R | 98.20% | ORF 238R | 100.00% | 29L | 96.40% | 29L | 97.06% |
| ORF 045R | 43,831 | 47,337 | 3507 | DNA dependent RNA polymerase largest subunit | RSIV subtype I | LYCIV Zhoushan PIV2016 PIV2014a PIV2010 | 99.94% | ORF 044R | 97.69% | RPO-1 | 99.37% | 28L | 94.66% | 28L | 95.30% |
| ORF 046R | 47,354 | 48,250 | 897 | probable XPG/RAD2 type nuclease | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 | 100.00% | ORF 045R | 98.33% | ORF 256R | 100.00% | 27L | 96.10% | 27L | 95.21% |
| ORF 047R | 48,272 | 48,595 | 324 | hypothetical protein | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 | 100.00% | ORF 046R | 97.53% | ORF 261R | 100.00% | 26L | 92.00% | 26L | 90.43% |
| ORF 048L | 49,064 | 50,002 | 939 | ribonucleotide diphosphate reductase small subunit | RSIV subtype I | PIV2016 PIV2014a PIV2010 RSIV Ehime-1 | 100.00% | ORF 047L | 98.08% | RR-2 | 100.00% | 24R | 94.68% | 25R | 95.21% |
| ORF 049L | 50,114 | 53,266 | 3153 | laminin-type epidermal growth factor | RSIV subtype I | PIV2010 RSIV Ehime-1 | 100.00% | ORF 048L | 93.77% | ORF 291L | 100.00% | 23R | 87.35% | 24R | 88.96% |
| ORF 050R | 53,339 | 54,934 | 1596 | LRP16 like protein macro domain-containing protein | RSIV subtype I | PIV2016 PIV2014a PIV2010 RSIV Ehime-1 | 100.00% | ORF 049R | 95.60% | ORF 292R | 100.00% | 22L | 93.41% | 23L | 93.52% |
| ORF 051R | 55,282 | 55,464 | 183 | hypothetical protein | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 LYCIV | 100.00% | ORF 050R | 97.27% | ORF 300R | 100.00% | 20L | 89.95% | 21L | 94.54% |
| ORF 052L | 55,511 | 58,354 | 2844 | DNA polymerase family B exonuclease | RSIV subtype I | PIV2010 LYCIV Zhoushan RSIV Ehime-1 | 100.00% | ORF 051L | 97.23% | DPO | 100.00% | 19R | 95.11% | 20R | 93.15% |
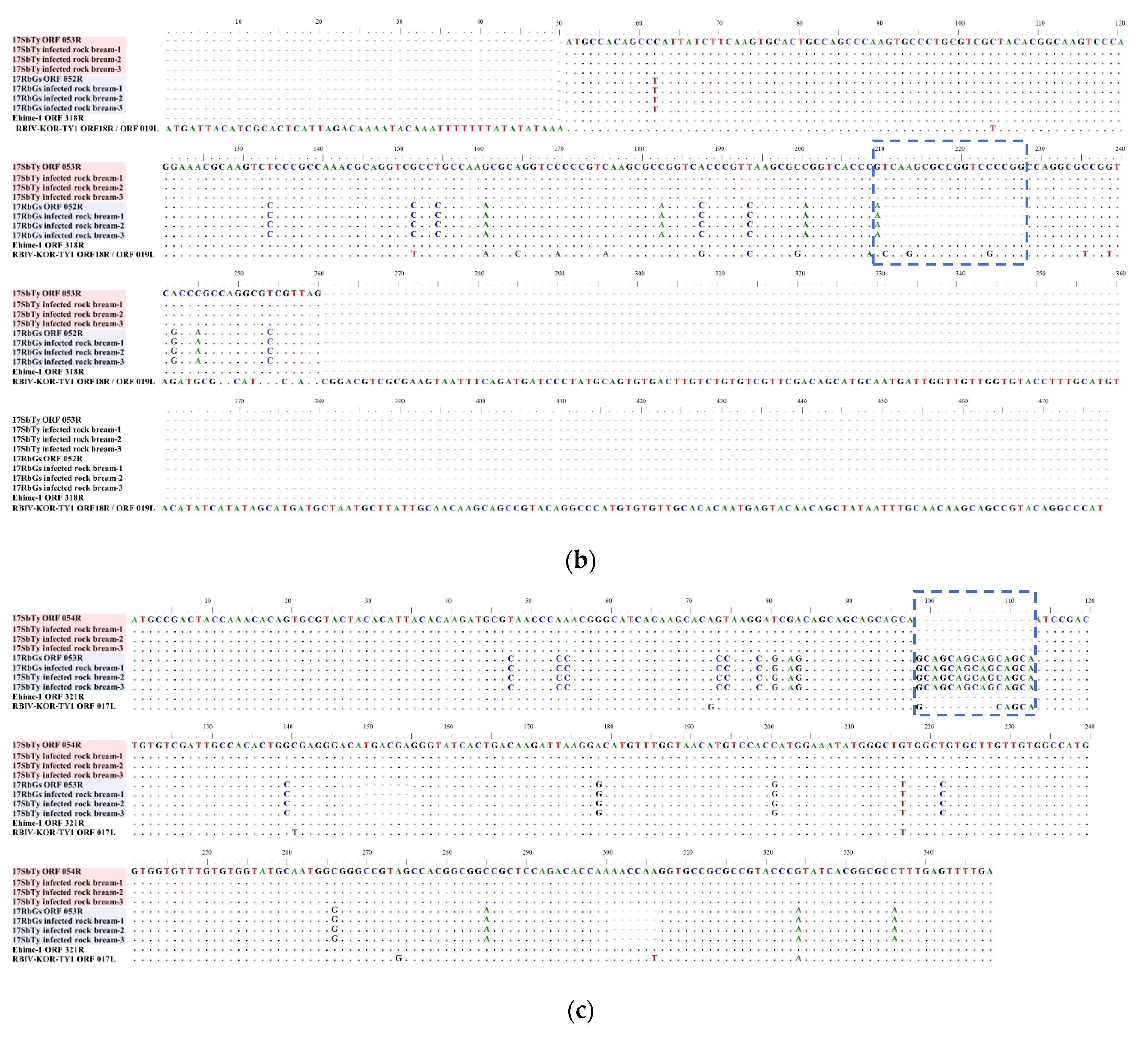
| ORF 053R | 58,420 | 58,629 | 210 | hypothetical protein | RSIV subtype I | PIV2010 LYCIV Zhoushan RSIV Ehime-1 | 100.00% | ORF 052R | 92.55% | ORF 318R | 100.00% | 18.5L | 89.89% | 19L | 91.76% |
| ORF 054R | 58,889 | 59,221 | 333 | hypothetical protein | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 | 100.00% | ORF 053R | 88.22% | ORF 321R | 100.00% | 17L | 92.81% | 17L | 89.47% |
| ORF 055R | 59,236 | 59,823 | 588 | hypothetical protein | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 | 99.66% | ORF 054R | 92.35% | ORF 324R | 99.66% | 16L | 91.50% | 16L | 92.35% |
| ORF 056L | 59,881 | 60,672 | 792 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 LYCIV Zhoushan RBIV-KOR-TY1 OSGIV | 92.12% | ORF 055L | 95.58% | ORF 333L | 98.86% | 15R | 94.44% | 15R | 93.43% |
| ORF 057L | 60,678 | 61,652 | 975 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 LYCIV Zhoushan OSGIV | 100.00% | ORF 056L | 99.90% | ORF 342L | 97.03% | 14R | 92.31% | 14R | 92.23% |
| ORF 058L | 61,907 | 63,304 | 1398 | serine/threonine protein kinase | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 OSGIV | 100.00% | ORF 057L | 99.93% | ORF 349L | 97.49% | 13R | 90.19% | 13R | 91.91% |
| ORF 059L | 63,311 | 63,643 | 333 | RING-finger-containing ubiquitin ligase | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 LYCIV Zhoushan RSIV_121 RBIV-KOR-TY1 OSGIV 17RbGs | 100.00% | ORF 058L | 100.00% | ORF 350L | 98.50% | 12R | 96.36% | 12R | 95.80% |
| ORF 060R | 63,662 | 63,922 | 261 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 LYCIV Zhoushan RSIV_121 OSGIV | 100.00% | ORF 059R | 98.04% | ORF 351R | 96.55% | 11L | 95.02% | 11L | 94.90% |
| ORF 061R | 63,919 | 64,311 | 393 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 RBIV-C1 RSIV_121 RBIV-KOR-TY1 | 100.00% | ORF 060R | 92.11% | ORF 353R | 97.96% | 10L | 92.62% | 10L | 92.11% |
| ORF 062L | 64,470 | 64,631 | 162 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 LYCIV Zhoushan RSIV_121 RBIV-KOR-TY1 OSGIV 17RbGs | 100.00% | ORF 061L | 100.00% | ORF 360L | 98.77% | 9R | 97.53% | 9R | 98.77% |
| ORF 063L | 64,727 | 66,274 | 1548 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 LYCIV Zhoushan RSIV_121 OSGIV 17RbGs | 100.00% | ORF 062L | 100.00% | ORF 373L | 96.13% | 8R | 91.88% | 8R | 91.68% |
| ORF 064R | 66,345 | 67,802 | 1458 | myristoylated membrane protein | RSIV subtype II | RSIV KagYT-96RSIV RIE12-1GSIV-K1LYCIV ZhoushanOSGIV | 100.00% | ORF 063R | 99.73% | ORF 374R | 97.46% | 7L | 94.51% | 7L | 94.65% |
| ORF 065R | 67,819 | 69,180 | 1362 | major capsid protein | RSIV subtype I | LYCIV Zhoushan | 100.00% | ORF 064R | 98.24% | MCP | 99.63% | 6L | 94.57% | 6L | 94.27% |
| ORF 066R | 69,326 | 70,090 | 765 | NIF-NLI interacting factor-like phosphatase | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 LYCIV | 100.00% | ORF 065R | 98.35% | ORF 385R | 100.00% | 5L | 95.17% | 5L | 92.82% |
| ORF 067R | 70,164 | 70,340 | 177 | hypothetical protein | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 LYCIV | 100.00% | ORF 066R | 99.44% | ORF 388R | 100.00% | 4L | 91.78% | 4L | 97.89% |
| ORF 068R | 70,413 | 71,196 | 486 | hypothetical protein | RSIV subtype I | LYCIV Zhoushan | 100.00% | ORF 067R | 96.30% | ORF 390R | 99.79% | 3L | 90.00% | 86.59% | |
| ORF 069R | 71,268 | 71,735 | 468 | DNA dependent RNA polymerase subunit H like protein | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 LYCIV | 100.00% | ORF 068R | 99.36% | RPOH | 100.00% | 2R | 93.83% | 2R | 94.25% |
| ORF 070R | 71,705 | 72,841 | 1137 | probable transmembrane amino acid transporter | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 LYCIV | 100.00% | ORF 069R | 97.89% | ORF 396R | 100.00% | 1L | 93.23% | 1L | 92.52% |
| ORF 071R | 72,956 | 73,672 | 717 | hypothetical protein | RSIV subtype II | RSIV RIE12-1 RSIV KagYT-96 GSIV-K1 OSGIV | 100.00% | ORF 070R | 99.86% | ORF 401R | 98.61% | 124L | 93.01% | 115L | 92.39% |
| ORF 072R | 73,681 | 74,061 | 381 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 OSGIV 17RbGs | 100.00% | ORF 071R | 100.00% | ORF 407R | 98.69% | 123R | 97.58% | 114R | 95.90% |
| ORF 073L | 74,033 | 74,752 | 720 | ATPase(adenosine triphosphatase) | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 OSGIV 17RbGs | 100.00% | ORF 072L | 100.00% | ORF 412L | 99.03% | 122R | 95.97% | 113R | 95.97% |
| ORF 074R | 74,762 | 75,397 | 636 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 OSGIV | 97.48% | ORF 073R | 97.48% | ORF 413R | 97.16% | 121L | 86.09% | 112L | 84.54% |
| ORF 075L | 75,418 | 75,924 | 507 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 GSIV-K1 RBIV-C1 RSIV_121 OSGIV 17RbGs | 100.00% | ORF 074L | 100.00% | ORF 420L | 97.24% | 120R | 93.53% | 111R | 92.28% |
| ORF 076L | 75,955 | 76,242 | 288 | probable transcriptional activator RING-finger domain-containing E3 protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 OSGIV 17RbGs | 100.00% | ORF 075L | 100.00% | ORF 423L | 98.96% | 119R | 93.71% | 110R | 92.01% |
| ORF 077R | 76,312 | 77,625 | 1314 | ankyrin repeat-containing protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 | 100.00% | ORF 076R | 99.77% | ORF 424R | 96.88% | 118L | 93.03% | 109L | 92.03% |
| ORF 078R | 77,958 | 78,632 | 675 | FV3 early 31KDa protein homolog | RSIV subtype II | RSIV KagYT-96 GSIV-K1 RSIV_121 OSGIV | 99.85% | ORF 077R | 99.85% | ORF 436R | 98.22% | 117L | 93.79% | 108L | 94.82% |
| ORF 079L | 78,686 | 80,062 | 1377 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 17RbGs | 100.00% | ORF 078L | 100.00% | ORF 450L | 96.27% | 116R | 86.68% | 107R | 85.92% |
| ORF 080L | 80,123 | 81,133 | 1011 | immediate-early protein ICP46 | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 17RbGs | 100.00% | ORF 079L | 100.00% | ORF 458L | 98.32% | 115R | 93.18% | 106R | 93.08% |
| ORF 081R | 81,568 | 84,150 | 2583 | putative tyrosine kinase | RSIV subtype II | GSIV-K1 | 100.00% | ORF 080R | 99.96% | ORF 463R | 97.99% | 114L | 93.69% | 105L | 93.26% |
| ORF 082L | 84,194 | 84,574 | 381 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 OSGIV | 100.00% | ORF 081L | 99.74% | ORF 483L | 97.38% | 113R | 92.66% | 104R | 92.89% |
| ORF 083L | 84,682 | 85,425 | 744 | proliferating cell nuclear antigen | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 OSGIV 17RbGs | 100.00% | ORF 082L | 100.00% | ORF 487L | 98.39% | 112R | 94.35% | 102R | 96.01% |
| ORF 084R | 85,445 | 86,341 | 897 | tumor necrosis factor recepter - assosiated factor-like protein | RSIV subtype II | RSIV KagYT-96RSIV RIE12-1GSIV-K1RBIV-C1RSIV_12117RbGs | 100.00% | ORF 083R | 100.00% | ORF 488R | 97.99% | 111L | 93.09% | 101L | 90.41% |
| ORF 085L | 86,338 | 86,493 | 156 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 RBIV-KOR-TY1 OSGIV 17RbGs | 100.00% | ORF 084L | 100.00% | ORF 492L | 96.79% | 110R | 90.38% | 100R | 91.03% |
| ORF 086R | 86,546 | 89,308 | 2763 | D5 family NTPase | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 OSGIV | 100.00% | ORF 085R | 99.96% | ORF 493R | 97.79% | 109L | 94.29% | 99L | 94.53% |
| ORF 087R | 89,389 | 90,018 | 630 | hypothetical protein | RSIV subtype II | RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 RBIV-KOR-TY1 OSGIV | 99.84% | ORF 086R | 99.84% | ORF 502R | 95.67% | 108.5L | 91.61% | 98L | 94.91% |
| ORF 088R | 90,058 | 90,930 | 873 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 OSGIV 17RbGs | 100.00% | ORF 087R | 100.00% | ORF 506R | 97.25% | - | - | 97L | 80.25% |
| ORF 089L | 90,937 | 91,901 | 888 | HIT-like protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 OSGIV | 99.89% | ORF 088L | 99.55% | ORF 515L | 96.83% | - | - | - | - |
| ORF 090L | 91,953 | 92,324 | 372 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 RBIV-KOR-TY1 OSGIV 17RbGs | 100.00% | ORF 089L | 100.00% | ORF 518L | 98.66% | 105R | 95.99% | 96R | 94.62% |
| ORF 091L | 92,326 | 93,102 | 777 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 OSGIV | 98.71% | ORF 090L | 98.71% | ORF 522L | 98.20% | 104R | 94.21% | 95R | 90.09% |
| ORF 092L | 93,164 | 93,577 | 414 | suppressor of cytokine signalling 1 homolog | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 | 100.00% | ORF 091L | 95.17% | ORF 524L | 100.00% | 103R | 88.38% | 94R | 88.22% |
| ORF 093L | 93,584 | 95,029 | 1446 | ankyrin repeat containing protein | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 | 100.00% | ORF 092L | 97.99% | ORF 534L | 100.00% | 102R | 91.46% | 93R | 92.39% |
| ORF 094R | 95,098 | 95,613 | 516 | hypothetical protein | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV Zhoushan RSIV Ehime-1 | 100.00% | ORF 093R | 97.29% | ORF 535R | 100.00% | 101L | 93.80% | 92L | 92.83% |
| ORF 095R | 95,588 | 96,229 | 642 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 OSGIV | 99.07% | ORF 094R | 98.75% | ORF 539R | 98.91% | 100L | 86.49% | 91L | 86.67% |
| ORF 096R | 96,283 | 96,606 | 324 | RING-finger-containing E3 ubiquitin ligase | RSIV subtype II | RSIV KagYT-96 GSIV-K1 RBIV-C1 RSIV_121 RBIV-KOR-TY1 OSGIV 17RbGs | 100.00% | ORF 095R | 100.00% | ORF 543R | 97.53% | 99L | 91.05% | 90L | 84.26% |
| ORF 097R | 96,655 | 97,146 | 492 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 OSGIV 17RbGs | 100.00% | ORF 096R | 100.00% | ORF 545R | 97.36% | 97.5L | 94.51% | 89L | 92.48% |
| ORF 098R | 97,137 | 97,888 | 738 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 OSGIV 17RbGs | 100.00% | ORF 097R | 100.00% | ORF 550R | 98.10% | 96L | 94.58% | 88L | 93.77% |
| ORF 099R | 97,896 | 99,059 | 1164 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 OSGIV | 100.00% | ORF 098R | 99.91% | ORF 554R | 96.91% | 95L | 91.21% | 87L | 91.02% |
| ORF 100R | 99,084 | 99,584 | 501 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 OSGIV 17RbGs | 100.00% | ORF 099R | 100.00% | ORF 562R | 98.60% | 94L | 95.41% | 86L | 93.01% |
| ORF 101R | 99,594 | 100,520 | 927 | probable RNA binding protein | RSIV subtype II | RSIV KagYT-96RSIV RIE12-1GSIV-K1SKIVRBIV-C1RSIV_121OSGIV17RbGs | 100.00% | ORF 100R | 100.00% | ORF 569R | 97.84% | 93L | 92.22% | 85L | 92.02% |
| ORF 102R | 100,641 | 101,711 | 1071 | myristoylated membrane protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 OSGIV | 98.62% | ORF 101R | 99.69% | ORF 575R | 95.94% | - | - | 83L | 91.36% |
| ORF 103L | 101,692 | 103,263 | 1572 | hypothetical protein | RSIV subtype I | PIV2016 PIV2014a PIV2010 LYCIV Zhoushan | 98.85% | ORF 102L | 98.54% | ORF 586L | 98.20% | 88R | 92.24% | 82R | 93.26% |
| ORF 104R | 103,311 | 103,724 | 414 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 OSGIV | 99.52% | ORF 103R | 99.52% | ORF 591R | 99.28% | 94.31% | 81R | 95.48% | |
| ORF 105L | 103,721 | 104,518 | 798 | RNase III-like ribonuclease | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 RBIV-C1 RSIV_121 17RbGs | 100.00% | ORF 104L | 100.00% | RNC | 97.99% | 87R | 94.16% | 80R | 94.86% |
| ORF 106L | 104,484 | 104,951 | 468 | Uvr/REP helicase | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 OSGIV | 100.00% | ORF 105L | 93.80% | ORF 600L | 97.44% | 86R | 92.55% | 79R | 92.95% |
| ORF 107L | 104,948 | 105,451 | 504 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 RBIV-KOR-TY1 OSGIV | 100.00% | ORF 106L | 92.86% | ORF 605L | 97.83% | 85R | 92.74% | 78R | 90.73% |
| ORF 108R | 105,565 | 106,869 | 1305 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 OSGIV | 100.00% | ORF 107R | 95.21% | ORF 606R | 97.70% | 84L | 93.16% | 77L | 91.58% |
| ORF 109L | 106,896 | 107,255 | 360 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 OSGIV | 100.00% | ORF 108L | 98.89% | ORF 617L | 98.33% | 83R | 93.46% | 76R | 92.48% |
| ORF 110R | 107,319 | 10,8425 | 1107 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 OSGIV | 100.00% | ORF 109R | 99.82% | ORF 618R | 97.92% | 82L | 93.59% | 75L | 93.22% |
| ORF 111L | 108,474 | 108,971 | 498 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 OSGIV 17RbGs | 100.00% | ORF 110L | 100.00% | ORF 628L | 97.99% | 81R | 95.78% | 74R | 94.32% |
| ORF 112L | 108,984 | 109,457 | 474 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RBIV-KOR-TY1 OSGIV 17RbGs | 100.00% | ORF 111L | 100.00% | ORF 632L | 95.81% | - | - | 73R | 85.56% |
| ORF 113R | 109,545 | 109,769 | 225 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 OSGIV 17RbGs | 100.00% | ORF 112L | 100.00% | ORF 634L | 92.06% | 79L | 93.78% | 72L | 92.27% |
| ORF 114L | 109,771 | 110,235 | 465 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 RSIV_121 OSGIV 17RbGs | 100.00% | ORF 113L | 100.00% | ORF 635L | 97.42% | 78R | 96.34% | 71R | 93.76% |
| ORF 115L | 110,232 | 111,566 | 1335 | hypothetical protein | RSIV subtype II | RSIV KagYT-96 RSIV RIE12-1 GSIV-K1 RBIV-C1 OSGIV | 99.93% | ORF 114L | 99.93% | ORF 641L | 96.55% | 77R | 90.95% | 70R | 90.42% |
| No. | Category | COG Function | COG Descrption | 17SbTy | 17RbGS |
|---|---|---|---|---|---|
| 1 | Metabolism | Amino acid transport metabolism | quinoprotein dehydrogenase-associated putative ABC transporter substrate-binding protein | ORF 093L | ORF 092L |
| 2 | Nucleotide transport and metabolism | deoxynucleoside kinase | ORF 042L | ORF 041L | |
| 3 | ribonucleoside-diphosphate reductase | ORF 048L | ORF 047L | ||
| 4 | HIT domain-containing protein | ORF 089L | ORF 088L | ||
| 5 | Information storage and processing | Translation, ribosomal structure and biogenesis | O-acetyl-ADP-ribose deacetylase | ORF 050R | ORF 049R |
| 6 | Transcription | DNA-directed RNA polymerase subunit B | ORF 040L | ORF 039L | |
| 7 | transcription factor S | ORF 044R | ORF 043R | ||
| 8 | DNA-directed RNA polymerase subunit A&apos | ORF 045R | ORF 044R | ||
| 9 | phosphoprotein phosphatase | ORF 066R | ORF 065R | ||
| 10 | ribonuclease III | ORF 105L | ORF 104L | ||
| 11 | Replication, recombination and repair | DNA cytosine methyltransferase | ORF 028R | ORF 027R | |
| 12 | flap endonuclease-1 | ORF 046R | ORF 045R | ||
| 13 | DNA polymerase elongation subunit | ORF 052L | ORF 051L | ||
| 14 | Cellular process | Signal transduction mechanisms | protein-tyrosine-phosphatase | ORF 12R | ORF 012R |
| 15 | ankyrin repeat-containing protein | ORF 077R | ORF 076R | ||
| 16 | quinoprotein dehydrogenase-associated putative ABC transporter substrate-binding protein | ORF 093L | ORF 092L | ||
| 17 | ankyrin repeat-containing protein | ORF 115L | ORF 114L | ||
| 18 | Mobilome; prophages, transposons | hypothetical protein | ORF 086R | ORF 085R | |
| 19 | Poorly characterized | General function prediction only | HIT domain-containing protein | ORF 089L | ORF 088L |
| 20 | Function unknown | hypothetical protein | ORF 013R | ORF 012R |
| No. | Gene (GenBank Access. No.) | 17SbTy (OK042108) | 17RbGs (OK042109) | Ehime-1 (AB104413) | ISKNV (AF371960) | RBIV (AY532606) | TRBIV (GQ273492) |
|---|---|---|---|---|---|---|---|
| 1 | hypothetical protein | 001R | 001R | 639R | 76L | 72L | 69L |
| 2 | Putative NTPase I | 013R | 012R | NTPase | 63L | 59L | 58L |
| 3 | Putative replication factor and/or DNA binding-packing | 015R | 014R | 092R | 61L | 57L | 56L |
| 4 | Helicase family | 018R | 017R | 101R | 56L | 54L | 53L |
| 5 | Serine-threonine protein kinase | 019R | 018R | 106R | 55L | 53L | 52L |
| 6 | Erv1/Alr family | 031R | 030R | 156R | 43L | 43.5L | 42L |
| 7 | DNA dependent RNA polymerase second largest subunit | 040L | 039L | RPO-2 | 34R | 33R | 33R |
| 8 | Deoxynucleoside kinase | 042L | 041L | TK | 32R | 31R | 31R |
| 9 | Transcription elongation factor TFIIS | 044R | 043R | 238R | 29L | 29.5Lb | 29L |
| 10 | DNA dependent RNA polymerase largest subunit | 045R | 044R | RPO-1 | 28L | 29L | 26L |
| 11 | Putative XPPG-RAD2-type nuclease | 046R | 045R | 256R | 27L | 28L | 27L |
| 12 | Ribonucleotide reductase small subunit | 048L | 047L | RR-2 | 24R | 26R | 25R |
| 13 | DNA pol Family B exonuclease | 052L | 051L | DPO | 19R | 20R | 20R |
| 14 | Serine-threonine protein kinase | 058L | 057L | 349L | 13R | 13R | 13R |
| 15 | Myristoylated membrane protein | 064R | 063R | 374R | 7L | 8L | 7L |
| 16 | Major capsid protein | 065R | 064R | MCP | 6L | 7L | 6L |
| 17 | NIF-NLI interacting factor | 066R | 065R | 385R | 5L | 6L | 5L |
| 18 | ATPase(adenosine triphosphatase) | 073L | 072L | 412L | 122R | 116R | 113R |
| 19 | Immediate early protein ICP-46 | 080L | 079L | 458L | 115R | 108.5R | 106R |
| 20 | Putative tyrosin kinase/lipopolysaccharide modifying enzyme | 081R | 080R | 463R | 61L, 114L | 57L, 106Lb | 105L |
| 21 | Proliferating cell nuclear antigen | 083L | 082L | 487L | 112R | 103Rb | 102R |
| 22 | D5 family NTPase involved in DNA replication | 086R | 085R | 493R | 109L | 101L | 99L |
| 23 | hypothetical protein | 098R | 097R | 550R | 96L | 89.5Lb | 88L |
| 24 | Myristoylated membrane protein | 102R | 101R | 575R | 90.5L | 85L | 83R |
| 25 | RNase III-like ribonuclease | 105L | 104L | RNC | 87R | 83R | 80R |
| 26 | Uvr/REP helicase | 106L | 105L | 600L | 86R | 82.5R | 79R |
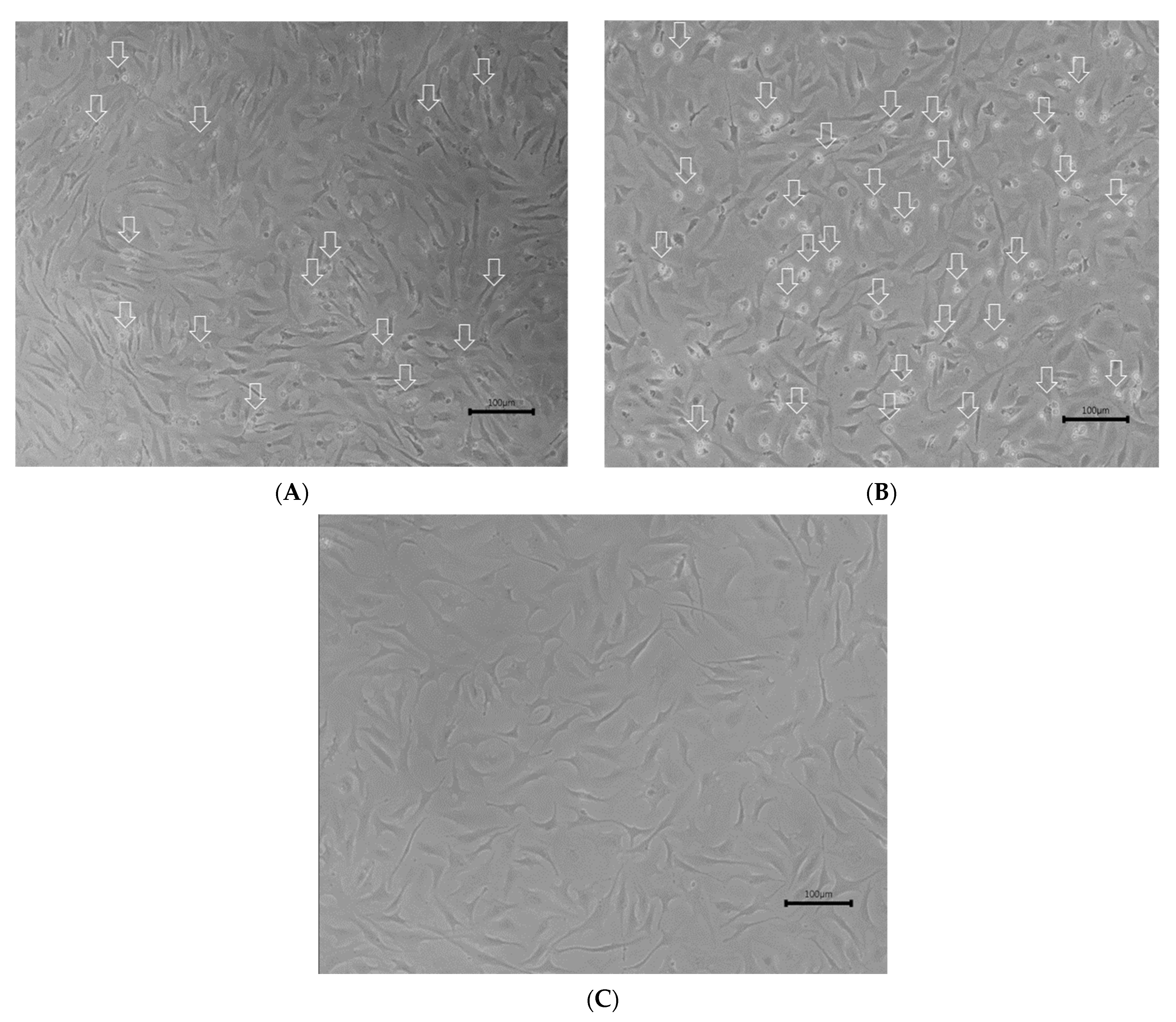
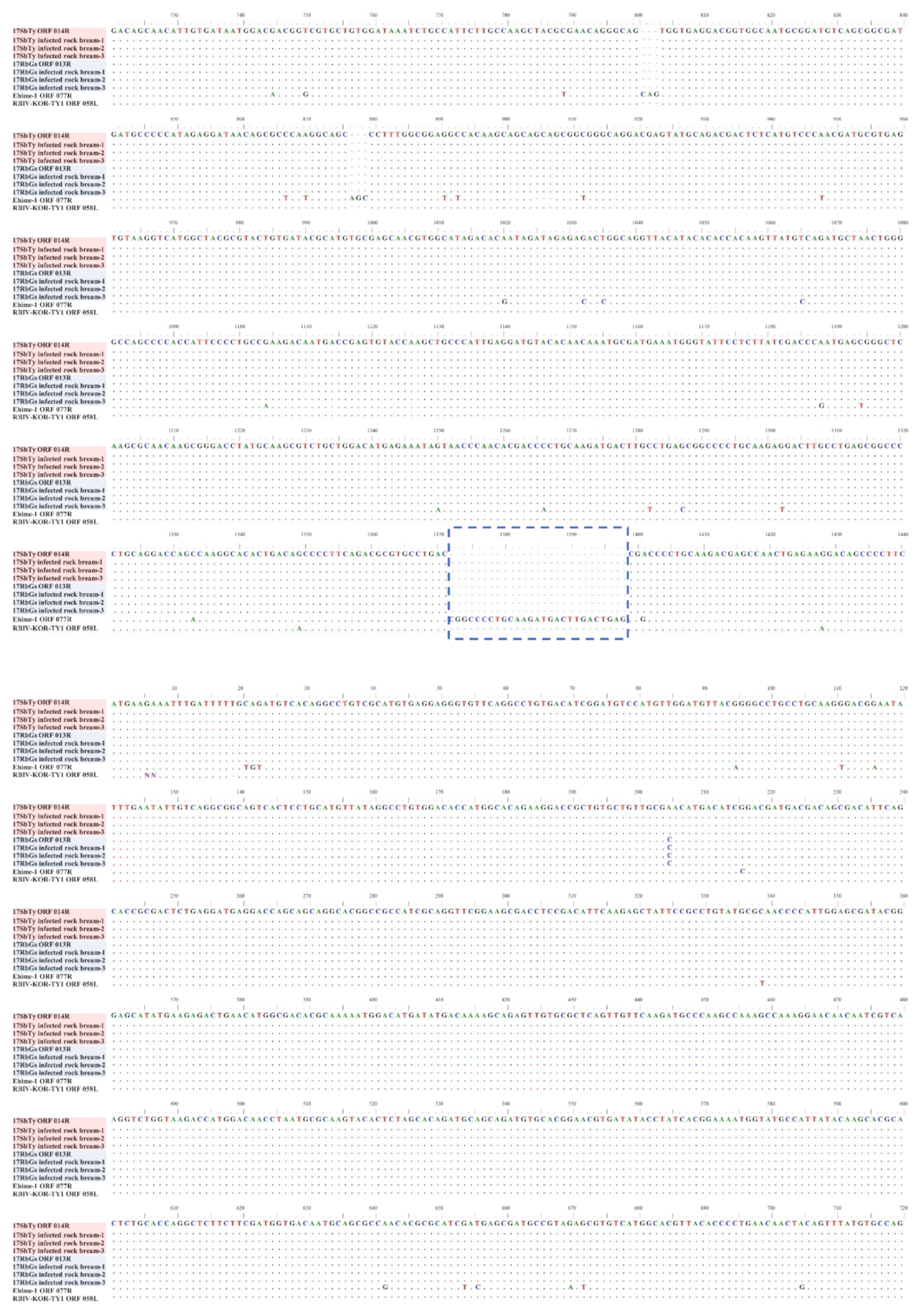
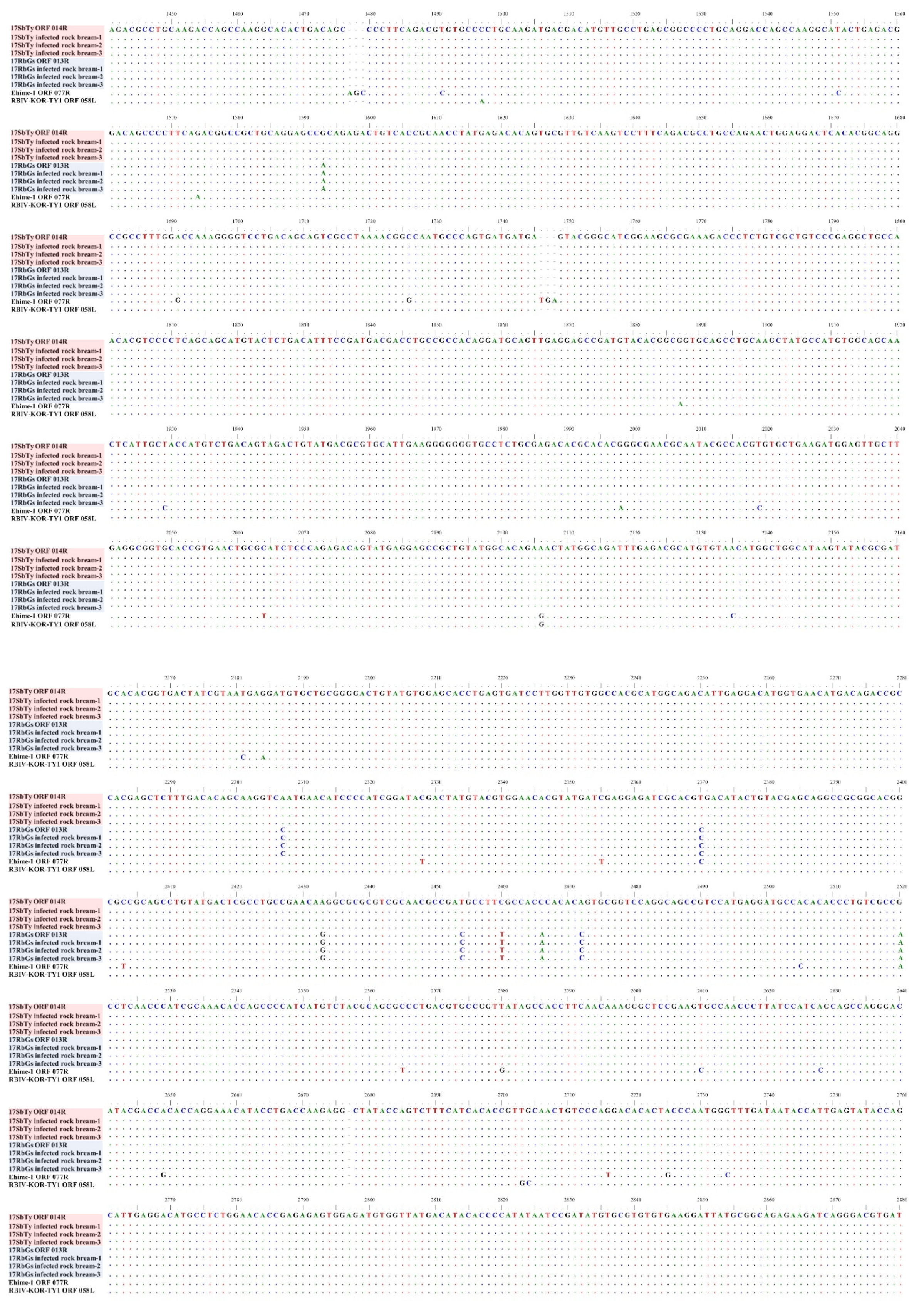
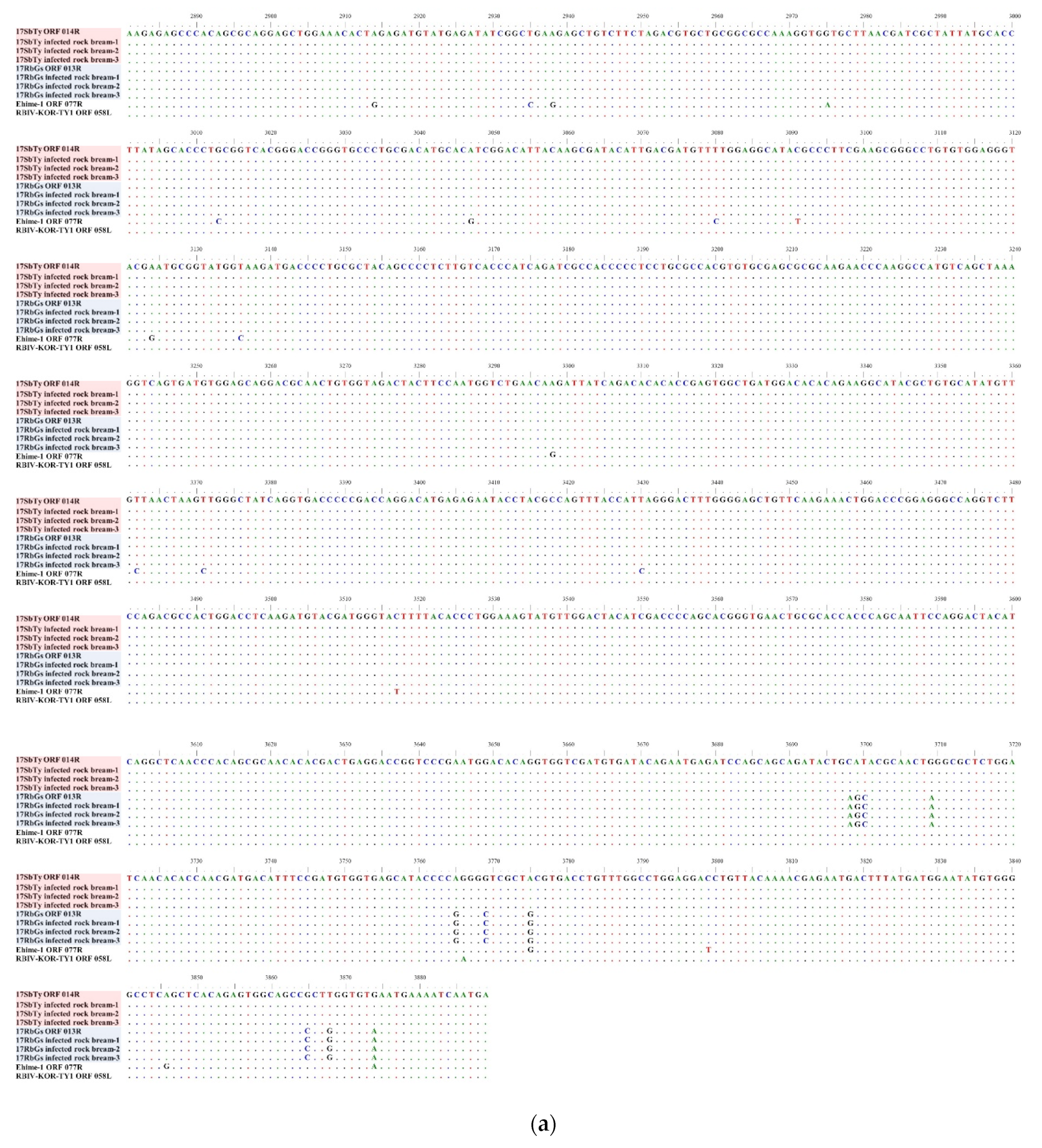

References
- Chinchar, V.G.; Hick, P.; Ince, I.A.; Jancovich, J.K.; Marschang, R.; Qin, Q.; Subramaniam, K.; Waltzek, T.B.; Whittington, R.; Williams, T.; et al. ICTV virus taxonomy profile: Iridoviridae. J. Gen. Virol. 2017, 98, 890–891. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health (OIE). Manual of Diagnostic Tests for Aquatic Animal. 2021. Available online: http://www.oie.int/standard-setting/aquatic-manual/access-online (accessed on 11 November 2021).
- Kurita, J.; Nakajima, K. Megalocytiviruses. Viruses 2012, 4, 521–538. [Google Scholar] [CrossRef] [Green Version]
- Inouye, K.; Yamano, K.; Maeno, Y.; Nakajima, K.; Matsuoka, M.; Wada, Y.; Sorimachi, M. Iridovirus infection of cultured red sea bream, Pagrus major. Fish. Pathol. 1992, 27, 19–27. [Google Scholar] [CrossRef]
- Kim, K.I.; Lee, E.S.; Do, J.W.; Hwang, S.D.; Cho, M.; Jung, S.H.; Jee, B.Y.; Kwon, W.J.; Jeong, H.D. Genetic diversity of Meglaocytivirus from cultured fish in Korea. Aquaculture 2019, 509, 16–22. [Google Scholar] [CrossRef]
- Kawakami, H.; Nakajima, K. Cultured fish species affected by red sea bream iridoviral disease from 1996 to 2000. Fish Pathol. 2002, 37, 45–47. [Google Scholar] [CrossRef]
- Jeong, J.B.; Jun, J.L.; Yoo, M.H.; Kim, M.S.; Komisar, J.L.; Jeong, H.D. Characterization of the DNA nucleotide sequences in the genome of red sea bream iridoviruses isolated in Korea. Aquaculture 2003, 220, 119–133. [Google Scholar] [CrossRef]
- He, J.G.; Deng, M.; Weng, S.P.; Li, Z.; Zhou, S.Y.; Long, Q.X.; Chan, S.M. Complete genome analysis of the mandarin fish infectious spleen and kidney necrosis iridovirus. Virology 2001, 291, 126–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.G.; Zeng, K.; Weng, S.P.; Chan, S.M. Experimental transmission, pathogenicity and physical-chemical properties of infectious spleen and kidney necrosis virus (ISKNV). Aquaculture 2002, 204, 11–24. [Google Scholar] [CrossRef]
- Shi, C.Y.; Wang, Y.G.; Yang, S.L.; Huang, J.; Wang, Q.Y. The first report of an iridovirus-like agent infection in farmed turbot, Scophthalmus maximus, in China. Aquaculture 2004, 236, 11–25. [Google Scholar] [CrossRef]
- Oh, M.J.; Jung, S.J.; Kim, Y.J. Detection of RSIV (red sea bream iridovirus) in the cultured marine fish by the polymerase chain reaction. Fish Pathol. 1999, 12, 66–69. [Google Scholar]
- Do, J.W.; Cha, S.J.; Kim, J.S.; An, E.J.; Lee, N.S.; Choi, H.J.; Lee, C.H.; Park, M.S.; Kim, J.W.; Kim, Y.C.; et al. Phylogenetic analysis of the major capsid protein gene of iridovirus isolates from cultured flounders Paralichthys olivaceus in Korea. Dis. Aquat. Organ. 2005, 64, 193–200. [Google Scholar] [CrossRef]
- Shiu, J.Y.; Hong, J.R.; Ku, C.C.; Wen, C.M. Complete genome sequence and phylogenetic analysis of megalocytivirus RSIV-Ku: A natural recombination infectious spleen and kidney necrosis virus. Arch. Virol. 2018, 163, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Cho, M.; Min, E.Y.; Jung, S.H.; Kim, K.I. Novel peptide nucleic acid-based real-time PCR assay for detection and genotyping of megalocytivirus. Aquaculture 2020, 518, 734818. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, M.J.; Choi, H.J.; Koo, M.J.; Kim, M.J.; Min, J.G.; Kim, K.I. Evaluation of a novel TaqMan probe-based real-time PCR assay for detection and quantification of red sea bream iridovirus. Fish Aquat Sci. 2021, 24, 351–359. [Google Scholar] [CrossRef]
- Andrews, S. Babraham Bioinformatics-FastQC a Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 11 November 2021).
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [Green Version]
- Hackl, T.; Hedrich, R.; Schultz, J.; Förster, F. Proovread: Large-scale high-accuracy PacBio correction through iterative short read consensus. Bioinformatics 2014, 30, 3004–3011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, J.R.; Arantes, A.S.; Stothard, P. Comparing thousands of circular genomes using the CGView Comparison Tool. BMC Genom. 2012, 13, 202. [Google Scholar] [CrossRef] [Green Version]
- Tatusov, R.L.; Koonin, E.V.; Lipman, D.J. A genomic perspective on protein families. Science 1997, 278, 631–637. [Google Scholar] [CrossRef] [Green Version]
- Tatusov, R.L.; Natale, D.A.; Garkavtsev, I.V.; Tatusova, T.A.; Shankavaram, U.T.; Rao, B.S.; Kiryutin, B.; Galperin, M.Y.; Fedorova, N.D.; Koonin, E.V. The COG database: New developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Res. 2001, 29, 22–28. [Google Scholar] [CrossRef]
- Kurita, J.; Nakajima, K.; Hirono, I.; Aoki, T. Complete genome sequencing of red sea bream iridovirus (RSIV). Fish. Sci. 2002, 68, 1113–1115. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.Y.; Jia, K.T.; Yang, B.; Huang, J. Complete genome sequence of a Megalocytivirus (family Iridoviridae) associated with turbot mortality in China. Virol. J. 2010, 7, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eaton, H.E.; Metcalf, J.; Penny, E.; Tcherepanov, V.; Upton, C.; Brunetti, C.R. Comparative genomic analysis of the family Iridoviridae: Re-annotating and defining the core set of iridovirus genes. Virol. J. 2007, 4, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eaton, H.E.; Ring, B.A.; Brunetti, C.R. The genomic diversity and phylogenetic relationship in the family Iridoviridae. Viruses 2010, 2, 1458–1475. [Google Scholar] [CrossRef] [Green Version]
- İnce, İ.A.; Özcan, O.; Ilter-Akulke, A.Z.; Scully, E.D.; Özgen, A. Invertebrate iridoviruses: A glance over the last decade. Viruses 2018, 10, 161. [Google Scholar] [CrossRef] [Green Version]
- Do, J.W.; Moon, C.H.; Kim, H.J.; Ko, M.S.; Kim, S.B.; Son, J.H.; Park, J.W. Complete genomic DNA sequence of rock bream iridovirus. Virology 2004, 325, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Kurita, J.; Nakajima, K.; Hirono, I.; Aoki, T. Polymerase chain reaction (PCR) amplification of DNA of red sea bream iridovirus (RSIV). Fish Pathol. 1998, 33, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.I.; Hwang, S.D.; Cho, M.Y.; Jung, S.H.; Kim, Y.C.; Jeong, H.D. A natural infection by the red sea bream iridovirus-type Megalocytivirus in the golden mandarin fish Siniperca scherzeri. J. Fish. Dis. 2018, 41, 1229–1233. [Google Scholar] [CrossRef]
- Xiang, Z.; Weng, S.; Qi, H.; He, J.; Dong, C. Identification and characterization of a novel FstK-like protein from spotted knifejaw iridovirus (genus Megalocytivirus). Gene 2014, 545, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wan, Q.; Huang, Y.; Huang, X.; Cao, J.; Ye, L.; Qin, Q. Proteomic analysis of Singapore grouper iridovirus envelope proteins and characterization of a novel envelope protein VP088. Proteomics 2011, 11, 2236–2248. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, M.-A.; Jeong, Y.-J.; Kim, K.-I. Complete Genome Sequences and Pathogenicity Analysis of Two Red Sea Bream Iridoviruses Isolated from Cultured Fish in Korea. Fishes 2021, 6, 82. https://doi.org/10.3390/fishes6040082
Jeong M-A, Jeong Y-J, Kim K-I. Complete Genome Sequences and Pathogenicity Analysis of Two Red Sea Bream Iridoviruses Isolated from Cultured Fish in Korea. Fishes. 2021; 6(4):82. https://doi.org/10.3390/fishes6040082
Chicago/Turabian StyleJeong, Min-A, Ye-Jin Jeong, and Kwang-Il Kim. 2021. "Complete Genome Sequences and Pathogenicity Analysis of Two Red Sea Bream Iridoviruses Isolated from Cultured Fish in Korea" Fishes 6, no. 4: 82. https://doi.org/10.3390/fishes6040082
APA StyleJeong, M.-A., Jeong, Y.-J., & Kim, K.-I. (2021). Complete Genome Sequences and Pathogenicity Analysis of Two Red Sea Bream Iridoviruses Isolated from Cultured Fish in Korea. Fishes, 6(4), 82. https://doi.org/10.3390/fishes6040082






