Concentration of Metals in Native and Invasive Species of Fish in the Fluvial-Lagoon-Deltaic System of the Palizada River, Campeche
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
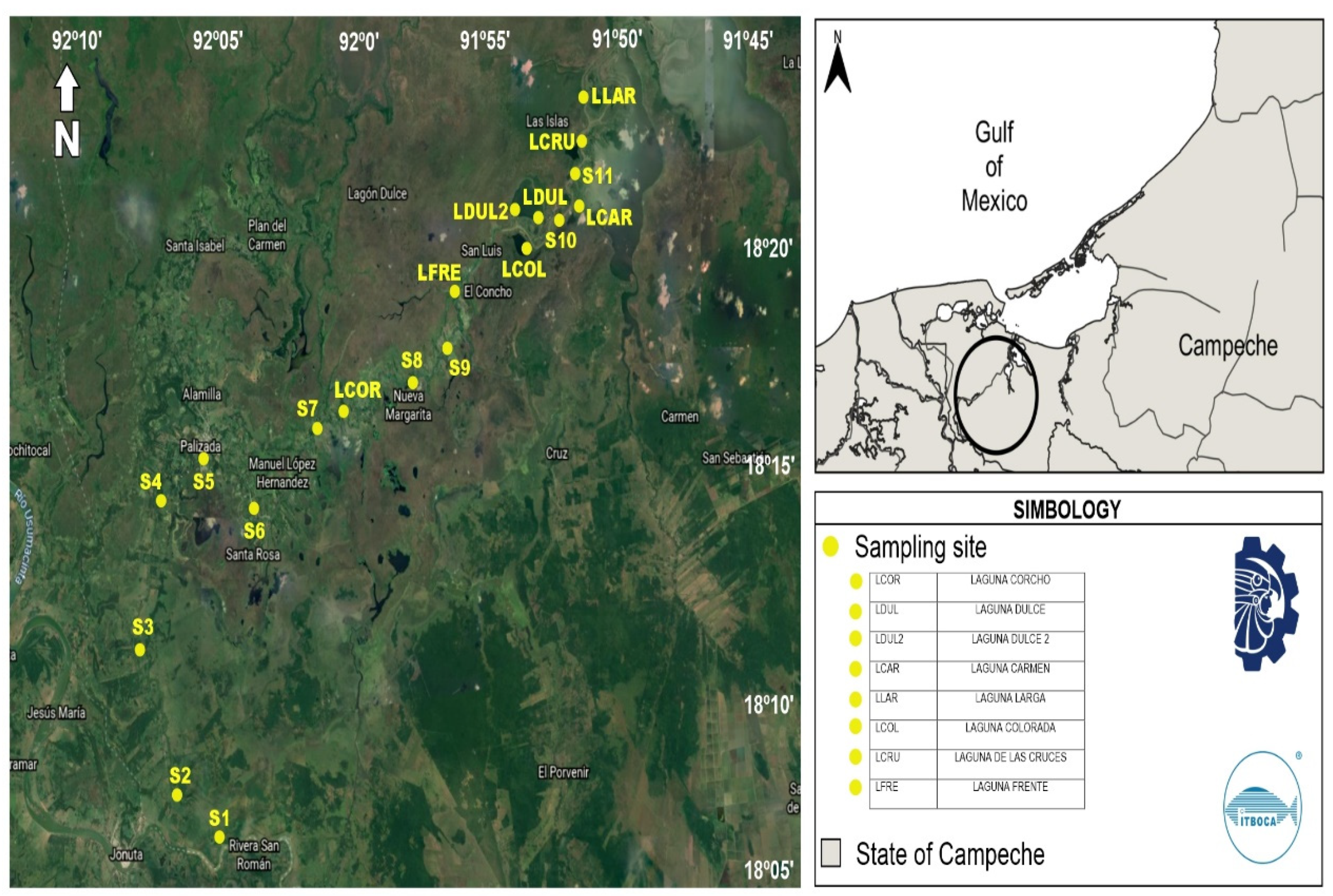
2.2. Sampling Sites and Collection of Specimens
2.3. Ethics and Animal Use
2.4. Sample Processing
2.5. Analytical Preparation and Microwave Acid Digestion
2.6. Quantification of Metals
2.7. Statistical Analysis
3. Results
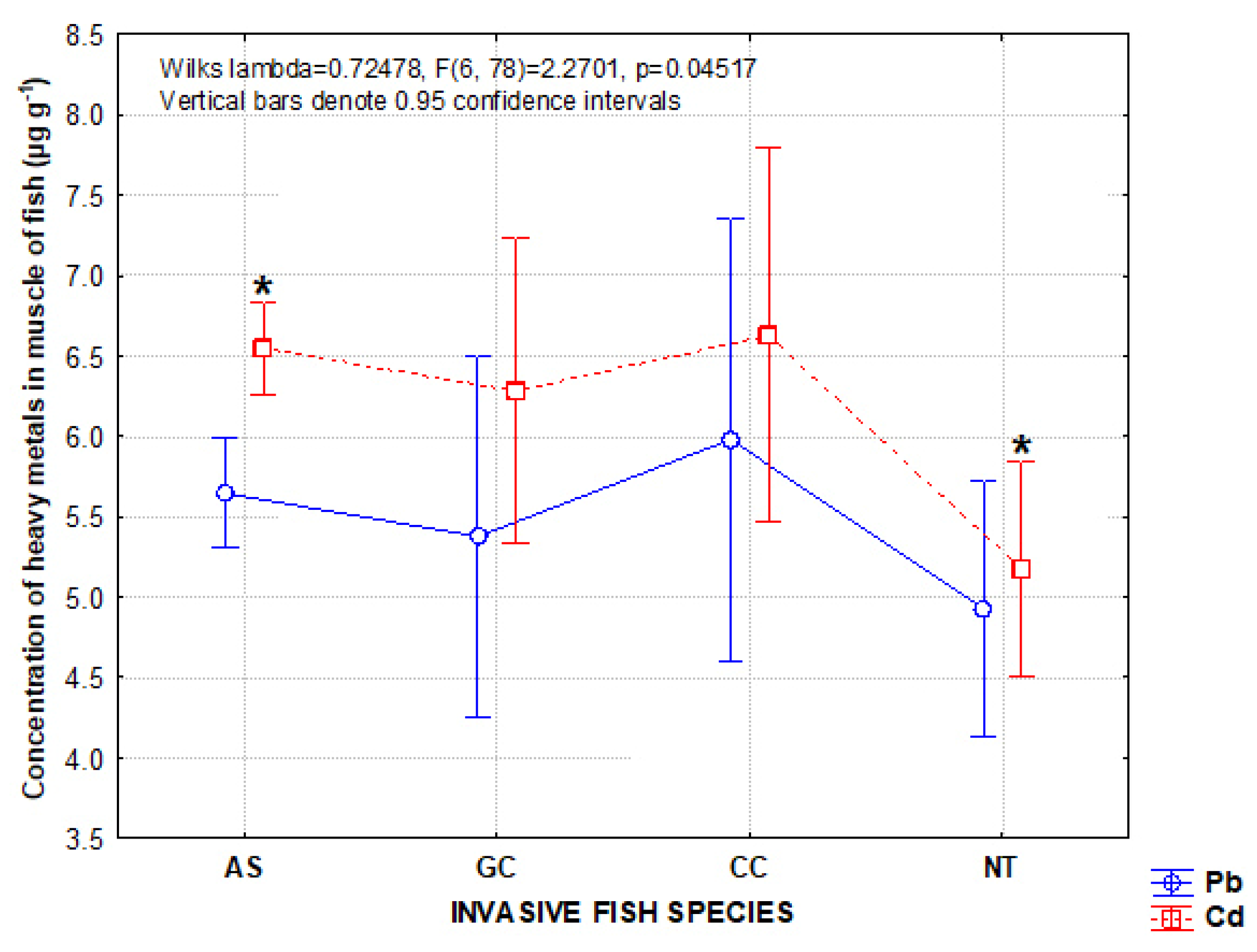
3.1. Metal Concentrations in Invasive Species
3.2. Metal Concentration in Native Species
4. Discussion
4.1. Concentration of Metals in Invasive Species
4.2. Concentration of Metals in Native Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Londoño-Franco, L.F.; Londoño-Muñoz, P.T.; Muñoz-García, F.G. Los riesgos de los metales pesados en la salud humana y animal. Biotecnol. Sect. Agropecu. Agroind. 2016, 14, 145–153. [Google Scholar]
- Annabi, A.; Saïd, K.; Messaoudi, I. Heavy metal levels in gonad and liver tissues-effects on the reproductive parameters of natural populations of Aphanius facsiatus. Environ. Sci. Pollut. Res. Int. 2013, 20, 7309–7319. [Google Scholar] [CrossRef]
- Páez-Osuna, F.; Osuna-Martínez, C.C. Bioavailability of cadmium, copper, mercury, lead, and zinc in subtropical coastal lagoons from the Southeast Gulf of California using mangrove oysters (Crassostrea corteziensis and Crassostrea palmula). Arch. Environ. Contam Toxicol. 2015, 68, 305–316. [Google Scholar] [CrossRef]
- Castañeda-Chávez, M.R.; Navarrete-Rodríguez, G.; Lango-Reynoso, F.; Galaviz-Villa, I.; Landeros-Sánchez, C. Heavy Metals in Oysters, Shrimps and Crabs from Lagoon Systems in the Southern Gulf of México. J. Agric. Sci. 2014, 6, 108–117. [Google Scholar] [CrossRef][Green Version]
- Castañeda-Chávez, M.R.; Lango-Reynoso, F.; Navarrete-Rodríguez, G. Heavy Metals in Sediment from Alvarado Lagoon System in Veracruz, México. Intern. J. Environ. Agric. Biotechnol. 2017, 2, 1209–1214. [Google Scholar] [CrossRef]
- Góngora-Gómez, A.M.; García-Ulloa, M.; Muñoz-Sevilla, N.P.; Domínguez-Orozco, A.L.; Villanueva-Fonseca, B.P.; Hernández-Sepúlveda, J.A.; Ortega Izaguirre, R. Heavy-metal contents in oysters (Crassostrea gigas) cultivated on the southeastern coast of the Gulf of California, Mexico. Hidrobiológica 2017, 27, 219–227. [Google Scholar] [CrossRef]
- Góngora-Gómez, A.M.; García-Ulloa, M.; Villanueva-Fonseca, B.P.; Domínguez-Orozco, A.L.; Hernández-Sepúlveda, J.A. Concentraciones de cobre y zinc en el ostión Crassostrea gigas cultivado en dos lagunas costeras del norte de Sinaloa, México. Adv. Investig. Agropecu. 2017, 21, 19–29. [Google Scholar]
- Góngora-Gómez, A.M.; Domínguez-Orozco, A.L.; Villanueva-Fonseca, B.P.; Muñoz-Sevilla, N.P.; García-Ulloa, M. Seasonal levels of heavy metals in soft tissue and muscle of the pen shell Atrina maura (sowerby, 1835) (Bivalvia: Pinnidae) from a farm in the southeastern coast of the Gulf of California, Mexico. Rev. Inter. Contam. Ambie 2018, 34, 57–68. [Google Scholar] [CrossRef]
- Frías-Espericueta, M.G.; Aguilar-Juárez, M.; Osuna-López, I.; Abad-Rosales, S.; Izaguirre-Fierro, G.; Voltolina, D. Los metales y la camaronicultura en México. Hidrobiológica 2011, 21, 217–228. [Google Scholar]
- Lango-Reynoso, F.; Castañeda-Chávez, M.R.; Landeros-Sánchez, C.; Galavíz-Villa, I.; Navarrete-Rodríguez, G.; Soto-Estrada, A. Cd, Cu, Hg AND Pb, and organochlorines pesticides in commercially important benthic organisms coastal lagoons SW Gulf of Mexico. Agric. Sci. 2013, 1, 63–80. [Google Scholar] [CrossRef]
- Páez-Osuna, F.; Osuna-Martínez, C.C. Biomonitores de la contaminación costera con referencia a las costas mexicanas: Una revisión sobre los organismos utilizados. Hidrobiológica 2011, 21, 229–238. [Google Scholar]
- Yancheva, V.; Velcheva, I.; Stoyanova, S.; Georgieva, E. Fish in Ecotoxicological Studies. Ecol. Balk. 2015, 7, 149–169. [Google Scholar]
- Velázquez-Velázquez, E.; Vega-Cendejas, M.E. Los peces como indicadores del estado de salud de los ecosistemas acuáticos. CONABIO. Biodiversitas 2004, 57, 12–15. [Google Scholar]
- del Ángel, L.E.A.; Wakida Kusunoki, A.T.; Guevara-Carrió, E.C.; Brito Pérez, R.; Cabrera-Rodríguez, P. Peces invasores de agua dulce en la región de la laguna de Términos, Campeche. Unacar Tecnociencia 2009, 3, 11–28. [Google Scholar]
- Amador-del Ángel, L.E.; Wakida-Kusunoki, A.T. Peces invasores en el sureste de México. In Especies Acuáticas Invasoras en México; Mendoza, R., Koleff, P., Coords, D.F., Eds.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Campeche, Mexico, 2014; pp. 425–433. [Google Scholar]
- del Ángel, L.E.A.; Guevara-Carrió, E.C.; Brito Pérez, R.; Endañú-Huerta, E. Aspectos Biológicos e Impacto Socio-Económico de los Plecos del Género Pterygoplichthys y dos Cíclidos no Nativos en el Sistema Fluvio Lagunar Deltaico Río Palizada, en el Área Natural Protegida Laguna de Términos, Campeche; Informe final SNIB-CONABIO. Ficha técnica tilapia Oreochromls niloticus, proyecto No. GN004; Universidad Autónoma del Carmen, Centro de Investigación de Ciencias Ambientales, Facultad de Ciencias Naturales: Campeche, Mexico, 2014. [Google Scholar]
- Mendoza, R.; Contreras, S.; Ramírez, C.; Koleff, P.; Álvarez, P.; Aguilar, V. Los peces diablo: Especies invasoras de alto impacto. CONABIO. Biodiversitas 2007, 70, 1–5. [Google Scholar]
- Mendoza, R.; Alfaro, E.; Cudmore, B.; Orr, R.; Fisher, J.P.; Contreras-Balderas, S.; Courtenay, W.R.; Koleff-Osorio, P.; Mandrak, N.; Álvarez-Torres, P.; et al. Trinational Risk Assessment Guidelines for Aquatic Alien Invasive Species. Test Cases for the Snakeheads (Channidae) and Armored Catfishes (Loricariidae) in North American Inland Waters; Commission for Environmental Cooperation Montreal: Quebec, QC, Canada, 2009. [Google Scholar]
- SAGARPA. Promueve CONAPESCA Consumo de Nuevas Especies y su Aprovechamiento Productivo. Comunicado de Prensa Num.601/11. 2011. Available online: http://www.sagarpa.gob.mx/saladeprensa/boletines2/2011/octubre/Documents/2011B601.pdf (accessed on 22 March 2021).
- Diario Oficial. Norma Oficial Mexicana. NOM-060-SAG/PESC-2016. Pesca Responsable en Cuerpos de Aguas Continentales Dulceacuícolas de Jurisdicción Federal de los Estados Unidos Mexicanos. Especificaciones Para el Aprovechamiento de los Recursos Pesqueros. 2016. Available online: http://www.dof.gob.mx/nota_detalle.php?codigo=5452927&fecha=19/09/2016 (accessed on 22 January 2021).
- Lorenzo-Márquez, H.; Torres-Dosal, A.; Barba-Macías, E.; Ilizaliturri HErnández, C.A.; Martínez-Salinas, R.I.; Morales López, J.J.; Sánchez-Moreno, I. Estimación de riesgo de exposición a metales pesados por consumo de plecos (Pterygoplichthys spp.) en infantes de comunidades ribereñas de los ríos Grijalva y Usumacinta, México. Rev. Int. Contam. Ambie 2016, 32, 153–164. [Google Scholar] [CrossRef]
- Villalobos-Zapata, G.J.; Mendoza-Vega, J. La Biodiversidad en Campeche: Estudio de Estado; Comisión Nacional para el Conocim22iento y Uso de la Biodiversidad (CONABIO), Gobierno del Estado de Campeche, Universidad Autónoma de Campeche, El Colegio de la Frontera Sur: Campeche, Mexico, 2010; p. 730. [Google Scholar]
- Wakida-Kusunoki, A.T.; del Ángel, L.E.A. Aspectos biológicos del pleco invasor Pterygoplichthys pardalis (Teleostei: Loricariidae) en el río Palizada, Campeche, México. Rev. Mex. Biodiv 2011, 82, 870–878. [Google Scholar] [CrossRef]
- Agraz Hernández, C.M.; Osti Sáenz, J.; Chan Keb, C.; Arriaga Martínez, V.; Acosta Velázquez, J.; Castillo Domínguez, S.; Gómez Ramírez, D.; Reyes-Castellanos, J.; Conde Medina, P.; Martínez Kumul, J. Grado de conservación del ecosistema de mangle en la laguna de Términos, Campeche. Propuesta de políticas ambientales y acciones de restauración. In Aspectos Socioambientales de la Región de la Laguna de Términos, Campeche; Ramos-Miranda, J., Villalobos-Zapata, G.J., Eds.; EPOMEX, Universidad Autónoma de Campeche: Campeche, Mexico, 2015; pp. 117–132. [Google Scholar]
- Miller, R.R.; Minckley, W.L.; Norris, S.M. Peces Dulceacuícolas de México; Conabio, Simac, Ecosur: Campeche, Mexico, 2009; 559p. [Google Scholar]
- Wakida Kusunoki, A.T.; del Ángel, L.E.A. Nuevos registros de los pIecos Pterygoplichthys pardalis (Castelnau, 1855) y P. disjunctivus (Weber, 1991) (Siluriformes: Loricariidae) en el Sureste de México. Hidrobiológica 2008, 18, 251–258. [Google Scholar]
- Diario Oficial, Norma Oficial Mexicana NOM-117-SSA1-1994. Bienes y servicios. método de prueba para la determinación de cadmio, arsénico, plomo, estaño, cobre, fierro, zinc y mercurio en alimentos, agua potable y agua purificada por espectrometría de absorción atómica. Diario Oficial de la Federación, 16 August 1995.
- Nakayama, S.M.; Ikenaka, Y.; Muzandu, K.; Choongo, K.; Oroszlany, B.; Teraoka, H.; Ishizuka, M. Heavy metal accumulation in lake sediments, fish (Oreochromis niloticus and Serranochromis thumbergi), and crayfish (Cherax quadricarinatus) in Lake Itezhi-tezhi and Lake Kariba, Zambia. Arch. Environ. Contam Toxicol 2010, 59, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Botello, V.A.; Villanueva, S.; Martínez, E. Metales y metaloides en praderas de pastos marinos de la laguna de Términos, Campeche. In Aspectos Socioambientales de la Región de la Laguna de Términos, Campeche; Ramos-Miranda, J., Villalobos-Zapata, G.J., Eds.; Universidad Autónoma de Campeche: Campeche, Mexico, 2015; pp. 133–143. [Google Scholar]
- Akoto, O.; Bismark-Eshun, F.; Darko, G.; Adei, E. Concentrations and Health Risk Assessments of Heavy Metals in Fish from the Fosu Lagoon. Int. J. Environ. Res. 2014, 8, 403–410. [Google Scholar]
- Zuluaga-Rodríguez, J.; Gallego-Ríos, S.E.; Ramírez-Botero, C.M. Content of Hg, Cd, Pb and As in fish species: A review. VITAE Rev. Fac. Cienc. Farm. Aliment. 2015, 22, 148–159. [Google Scholar] [CrossRef]
- Loganathan, P.; Hedley, M.J.; Grace, N.D. Pasture soils contaminated with fertilizer-derived cadmium and fluorine: Livestock effects. Rev. Environ. Contamin Toxicol. 2008, 192, 29–66. [Google Scholar]
- Frías-Espericueta, M.G.; Zamora-Sarabia, F.K.G.; Osuna-López, J.I.; Muy-Rangel, M.D.; Rubio-Carrasco, W.; Aguilar-Juárez, M.; Voltolina, D. Cadmium, Copper, Lead, and Zinc Contents of Fish Marketed in NW Mexico. Sci. World J. 2014, 1, 1–4. [Google Scholar] [CrossRef]
- Maldonado-Enríquez, E.J.; López-Noverola, U.; Salinas-Hernández, R.M.; González-Cortés, N.; Cuenca-Soria, C.A.; Jiménez-Vera, R.; Hernández-Juárez, J.L. Contenido de metales pesados en músculo de pez diablo Pterygoplichthys pardalis. ReIbCi 2015, 2, 67–73. [Google Scholar]
- Ernawati, Y. The analysis of the concentration of heavy metals cadmium, mercury and lead in the flesh of suckermouth catfish (Pterygoplichthys pardalis) in Ciliwung River, Indonesia. AACL Bioflux 2014, 7, 33–42. [Google Scholar]
- Elfidasari, D.; Nurul-Ismi, L.; Putri-Shabira, A.; Sugoro, I. The Correlation Between Heavy Metal and Nutrient Content in Plecostomus (Pterygoplichthys pardalis) from Ciliwung River in Jakarta. Biosaintifika J. Biol. Biol. Educ. 2018, 10, 597–604. [Google Scholar] [CrossRef][Green Version]
- Elfidasari, D.; Nuril-Ismi, L.; Sugoro, I. Heavy metal concentration in water, sediment, and Pterygoplichthys pardalis in the Ciliwung River, Indonesia. AACL Bioflux. 2020, 13, 1764–1778. [Google Scholar]
- Jinadasa, B.K.K.K.; Ariyarathne, D.S.; Ahmad, S.B.N. Trace Metal Contaminants in Tissues of the Orinoco Sailfin Catfish Pterygoplichthy smultiradiatus, (Hancock, 1828). Sri Lanka Nat. Sci. 2014, 12, 1–4. [Google Scholar]
- Kong-Yap, C.; Jusoh, A.; Leong, W.J.; Karami, A.; Hock-Ong, G. Potential human health risk assessment of heavy metals via the consumption of tilapia Oreochromis mossambicus collected from contaminated and uncontaminated ponds. Environ. Monit Assess 2015, 187, 584. [Google Scholar] [CrossRef]
- Hossain, I.; Saha, B.; Begum, M.; Jahan-Punom, N.; Begum, K.; Shamsur-Rahman, M. Bioaccumulation of heavy metals in nile tilapia Oreochromis niloticus (Linnaeus 1758) fed with commercial fish fee. Bangladesh J. Sci. Res. 2016, 29, 89–99. [Google Scholar] [CrossRef]
- Rafati-Rahimzadeh, M.; Rafati-Rahimzadeh, M.; Kazemi, S.; Moghadamnia, A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135–145. [Google Scholar] [CrossRef]
- Souza-Arroyo, V.; Martínez-Flores, K.; Bucio-Ortiz, L.; Gómez-Quiroz, L.E.; Gutiérrez-Ruiz, M.C. Liver and Cadmium Toxicity. J. Drug Metab. Toxicol 2012, S5, 1. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 1881/2006 of the European Parliament and the Council of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Communities. 2006, L364, 5–24. [Google Scholar]
- Official Diary. NORMA Oficial Mexicana NOM-242-SSA1-2009, Productos y Servicios, Productos de la Pesca Frescos, Refrigerados, Congelados y Procesados, Especificaciones Sanitarias y Métodos de Prueba, Official Gazette of the Federation Mexico, 10 February 2011. 2009. Available online: http://dof.gob.mx/nota_detalle.php?codigo=5177531&fecha=10/02/2011 (accessed on 12 May 2021).
- USFDA. Fish and Fishery Products Hazards and Controls Guidance; Center for Food Safety and Applied Nutrition’s; US Food and Drug Administration: College Park, MD, USA, 2001. [Google Scholar]
- SNI. Standardisasi Nasional Indonesia. Indonesia National Standard. In Maximum Limits for Heavy Metal Impurities in Food; Badan Standardisasi Nasional: Jakarta, Indonesia, 2009. [Google Scholar]
- SNI. Standardisasi Nasional Indonesia. Indonesia National Standard. Chemical means of trials section 6: The heavy metal levels Lead (Pb) and Cadmium (Cd). In Fishery Product; Badan Standardisasi Nasional: Jakarta, Indonesia, 2011. [Google Scholar]
- World Health Organization (WHO). Health criteria other supporting information. In Guidelines for Drinking Water Quality, 2nd ed.; WHO: Geneva, Switzerland, 1996; pp. 31–388. [Google Scholar]
- WHO. Exposure to Cadmium: A Major Public Health Concern; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]

| Invasive fish species | |||
| Sampling site | N | Cd | Pb |
| S1 | 3 | 5.593 ± 1.245 | 4.670 ± 0.603 |
| S2 | 5 | 5.682 ± 0.880 | 5.290 ± 0.803 |
| S3 | 2 | 6.065 ± 0.403 | 5.340 ± 0.000 |
| S4 | 3 | 6.960 ± 0.533 | 6.080 ± 0.640 |
| S5 | 2 | 6.820 ± 0.098 | 5.510 ± 0.226 |
| S6 | 3 | 5.993 ± 0.340 | 5.480 ± 0.370 |
| S7 | 2 | 6.500 ± 0.070 | 6.160 ± 0.410 |
| LCOR | 5 | 6.328 ± 1.027 | 5.374 ± 0.725 |
| S8 | 3 | 7.210 ± 0.704 | 6.680 ± 0.302 |
| S9 | 3 | 5.403 ± 0.370 | 5.070 ± 0.480 |
| LCOL | 2 | 5.975 ± 1.053 | 5.590 ± 1.456 |
| S10 | 3 | 6.053 ± 1.317 | 5.243 ± 0.998 |
| LDUL2 | 3 | 6.693 ± 0.821 | 5.516 ± 0.858 |
| LCAR | 1 | 7.870 | 2.320 |
| S11 | 2 | 7.155 ± 0.261 | 6.355 ± 0.601 |
| LLAR | 1 | 7.660 | 7.760 |
| LCRU | 1 | 6.890 | 6.450 |
| TOTAL | 44 | 6.345 ± 0.917 | 5.545 ± 0.965 |
| Native fish species | |||
| Sampling site | N | Cd | Pb |
| S2 | 1 | 5.340 | 4.560 |
| LFRE | 1 | 3.450 | 4.340 |
| LDUL | 3 | 5.523 ± 0.356 | 4.896 ± 0.825 |
| LCOL | 1 | 4.900 | 4.560 |
| LCAR | 1 | 5.840 | 5.540 |
| Total | 7 | 5.157 ± 0.830 | 4.812 ± 0.614 |
| Invasive Species | Native Species | ||||||
|---|---|---|---|---|---|---|---|
| Species Code | N | Pb | Cd | Species Code | N | Pb | Cd |
| AS | 33 | 5.645 ± 1.047 | 6.547 ± 0.873 | TC | 2 | 5.080 ± 1.046 | 4.685 ± 1.746 |
| GC | 3 | 5.376 ± 0.063 | 6.283 ± 0.453 | BS | 2 | 4.885 ± 0.926 | 5.535 ± 0.431 |
| CC | 2 | 5.980 ± 0.905 | 6.630 ± 0.127 | MC | 3 | 4.586 ± 0.046 | 5.220 ± 0.280 |
| NT | 6 | 4.930 ± 0.464 | 5.173 ± 0.565 | ||||
| Total | 44 | 5.545 ± 0.965 | 6.345 ± 0.917 | Total | 7 | 4.812 ± 0.614 | 5.157 ± 0.830 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañeda-Chávez, M.d.R.; Lango-Reynoso, F.; Navarrete-Rodríguez, G.; Wakida-Kusunoki, A.T. Concentration of Metals in Native and Invasive Species of Fish in the Fluvial-Lagoon-Deltaic System of the Palizada River, Campeche. Fishes 2021, 6, 72. https://doi.org/10.3390/fishes6040072
Castañeda-Chávez MdR, Lango-Reynoso F, Navarrete-Rodríguez G, Wakida-Kusunoki AT. Concentration of Metals in Native and Invasive Species of Fish in the Fluvial-Lagoon-Deltaic System of the Palizada River, Campeche. Fishes. 2021; 6(4):72. https://doi.org/10.3390/fishes6040072
Chicago/Turabian StyleCastañeda-Chávez, María del Refugio, Fabiola Lango-Reynoso, Gabycarmen Navarrete-Rodríguez, and Armando Toyokazu Wakida-Kusunoki. 2021. "Concentration of Metals in Native and Invasive Species of Fish in the Fluvial-Lagoon-Deltaic System of the Palizada River, Campeche" Fishes 6, no. 4: 72. https://doi.org/10.3390/fishes6040072
APA StyleCastañeda-Chávez, M. d. R., Lango-Reynoso, F., Navarrete-Rodríguez, G., & Wakida-Kusunoki, A. T. (2021). Concentration of Metals in Native and Invasive Species of Fish in the Fluvial-Lagoon-Deltaic System of the Palizada River, Campeche. Fishes, 6(4), 72. https://doi.org/10.3390/fishes6040072