Spatial and Temporal Variability of Parasite Communities: Implications for Fish Stock Identification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Declarations
2.2. Fish Sample
2.3. Bibliographic Search
2.4. Univariate Analysis
2.5. Multivariate Analysis
3. Results
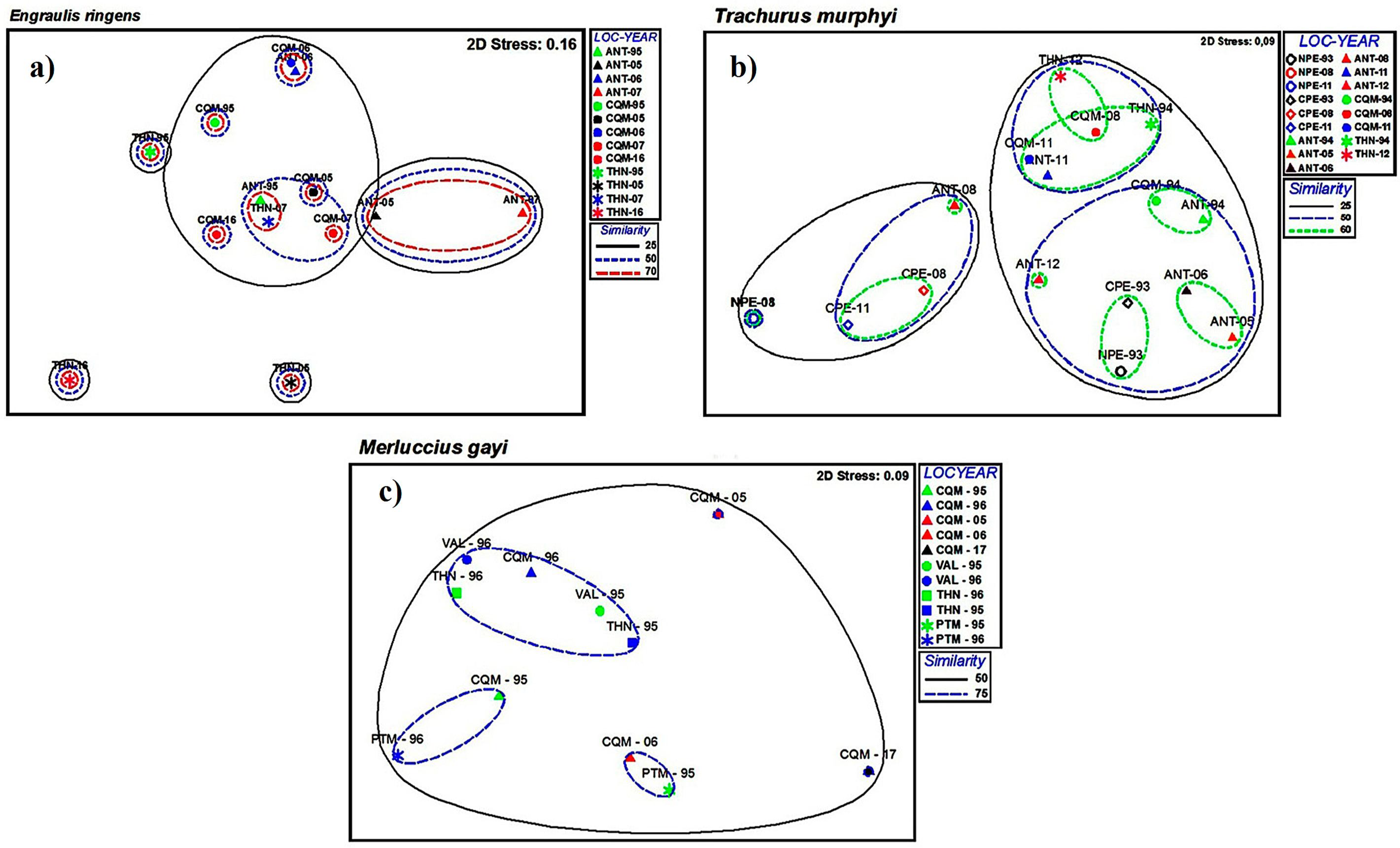
3.1. Spatial Analysis
3.2. Temporal Analysis
4. Discussion
4.1. Spatial Variability
4.2. Temporal Variability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cadrin, S.X. Defining spatial structure for fishery stock assessment. Fish. Res. 2020, 221, 105397. [Google Scholar] [CrossRef]
- Cadrin, S.X.; Friedland, K.D.; Waldman, J.R. Stock identification methods: An Overview. In Stock Identification Methods: Applications in Fishery Science, 1st ed.; Cadrin, S.X., Friedland, K.D., Waldman, J.R., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; pp. 2–6. [Google Scholar]
- Sparre, P.; Venema, C.S. Introduction to Tropical Fish Stock Assessment. Part I: Manual; FAO: Rome, Italy, 1998; p. 407. [Google Scholar]
- Timi, J.T.; MacKenzie, K. Parasites in fisheries and mariculture. Parasitology 2015, 142, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrington, W.C.; Bearse, H.M.; Firth, F.E. Observations on the life history, occurrence and distribution of the redfish parasite Sphyrion lumpi; United States Department of the Interior, Bureau of Fisheries: Washington, DC, USA, 1939; Volume 5, pp. 1–18. [Google Scholar]
- Dogiel, V.A.; Bychovsky, B.E. Parasites of the fishes of the Caspian Sea. Tr. Kompleks. Izucheniyu Kaspiikogo Morya 1939, 7, 1–150. [Google Scholar]
- Canel, D.; Levya, E.; Soares, I.A.; Braicovich, P.E.; Haimovicic, M.; Luque, J.L.; Timi, J.T. Stocks and migrations of the demersal fish Umbrina canosai (Sciaenidae) endemic from the subtropical and temperate Southwestern Atlantic revealed by its parasites. Fish. Res. 2019, 214, 10–18. [Google Scholar] [CrossRef]
- Hermida, M.; Cavaleiro, B.; Gouveia, L.; Saraiva, A. Seasonality of skipjack tuna parasites in the Eastern Atlantic provide an insight into its migratory patterns. Fish. Res. 2019, 216, 167–173. [Google Scholar] [CrossRef]
- Boudaya, L.; Feki, M.; Mosbahi, N.; Neifar, L. Stock discrimination of Chelidonichthys obscurus Triglidae in the central Mediterranean Sea using morphometric analysis and parasite markers. J. Helminthol. 2020, 94, E74. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, K. Parasites as biological tags in population studies of marine organisms: An update. Parasitology 2002, 124, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Braicovich, P.E.; Ieno, E.N.; Sáez, M.; Despos, J.; Timi, J.T. Assessing the role of host traits as drivers of the abundance of long-lived parasites in fish-stock assessment studies. J. Fish Biol. 2016, 89, 2419–2433. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, K.; Abaunza, P. Parasites as Biological Tags. In Stock Identification Methods: Applications in Fishery Science, 2nd ed.; Cadrin, S.X., Kerr, L.A., Mariani, S., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2014; pp. 185–203. [Google Scholar] [CrossRef]
- Pascual, S.; Abollo, E.; González, A.F. Biobanking and genetic markers for parasites in fish stock studies. Fish. Res. 2016, 173, 214–220. [Google Scholar] [CrossRef]
- Chavez, R.A.; Valdivia, I.M.; Oliva, M.E. Local variability in metazoan parasites of the pelagic fish species, Engraulis ringens: Implications for fish stock assessment using parasites as biological tags. J. Helminthol. 2007, 81, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Braicovich, P.E.; Timi, J.T. Seasonal stability in parasite assemblages of the Brazilian flathead, Percophis brasiliensis (Perciformes: Percophidae): Predictable tools for stock identification. Folia Parasitol. 2010, 57, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George-Nascimento, M.; Moscoso, D. Variación local y geográfica de las infracomunidades de parásitos de la anchoveta Engraulis ringens en Chile. Rev. Biol. Mar. Oceanogr. 2013, 48, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Levy, E.; Canel, D.; Rossin, M.A.; Hernández-Orts, J.S.; González-Castro, M.; Timi, J.T. Parasites as indicators of fish population structure at two different geographical scales in contrasting coastal environments of the south-western Atlantic. Estuar. Coast. Shelf Sci. 2019, 229, 106400. [Google Scholar] [CrossRef]
- Charters, R.A.; Lester, R.J.G.; Buckworth, R.C.; Newman, S.J.; Ovendend, J.R.; Broderick, D.; Kravchuke, O.; Ballaghf, A.; Welchfg, D.J. The stock structure of grey mackerel Scomberomorus semifasciatus in Australia as inferred from its parasite fauna. Fish. Res. 2010, 101, 94–99. [Google Scholar] [CrossRef]
- Lester, R.J.G.; MacKenzie, K. The use and abuse of parasites as stock markers for fish. Fish. Res. 2009, 97, 1–2. [Google Scholar] [CrossRef]
- Connell, J.H.; Sousa, W.P. On the evidence needed to judge ecological stability or persistence. Am. Nat. 1983, 121, 789–824. [Google Scholar] [CrossRef]
- Poulin, R.; Kamiya, T. Parasites as biological tags of fish stocks: A meta-analysis of their discriminatory power. Parasitology 2015, 142, 145–155. [Google Scholar] [CrossRef]
- Bennett, S.N.; Adamson, M.L.; Margolis, L. Long-term changes in parasites of sockeye salmon (Oncorhynchus nerka) smolts. Can. J. Fish. Aquat. Sci. 1998, 55, 977–986. [Google Scholar] [CrossRef]
- Grutter, A.S. Spatial and temporal variations of the ectoparasites of seven reef fish species from Lizard Island and Heron Island, Australia. Mar. Ecol. Prog. Ser. 1994, 115, 21–30. [Google Scholar] [CrossRef]
- Ferrer-Castelló, E.; Raga, J.A.; Aznar, F.J. Parasites as fish population tags and pseudoreplication problems: The case of striped red mullet Mullus surmuletus in the Spanish Mediterranean. J. Helminthol. 2007, 81, 169–178. [Google Scholar] [CrossRef]
- Timi, J.T.; Lanfranchi, A.L.; Etchegoin, J.A. Seasonal stability and spatial variability of parasites in Brazilian sandperch Pinguipes brasilianus from the Northern Argentine Sea: Evidence for stock discrimination. J. Fish Biol. 2009, 74, 1206–1225. [Google Scholar] [CrossRef] [PubMed]
- Espínola-Novelo, J.F.; González, M.T.; Pacheco, A.S.; Luque, J.L.; Oliva, M.E. Testing for deterministic succession in metazoan parasite communities of marine fish. Ecol. Lett. 2020, 23, 631–641. [Google Scholar] [CrossRef]
- George-Nascimento, M.; Oliva, M.E. Fish population studies using parasites from the Southeastern Pacific Ocean: Considering host population changes and species body size as sources of variability of parasite communities. Parasitology 2015, 142, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, I.M.; Chávez, R.A.; Oliva, M.E. Metazoan parasites of Engraulis ringens as tools for stock discrimination along the Chilean coast. J. Fish Biol. 2007, 70, 1504–1511. [Google Scholar] [CrossRef]
- George-Nascimento, M.; Arancibia, H. Stocks ecológicos del jurel (Trachurus symmetricus murphyi Nichols) en tres zonas de pesca frente a Chile, detectados mediante comparación de su fauna parasitaria y morfometría. Rev. Chil. Hist. Nat. 1992, 65, 453–470. [Google Scholar]
- Aldana, M.; Oyarzún, J.; George-Nascimento, M. Isópodos parásitos como indicadores poblacionales del jurel Trachurus symmetricus murphyi (Nichols, 1920) (Pisces: Carangidae) frente a las costas de Chile. Biol. Pesq. 1995, 24, 23–32. [Google Scholar]
- Oliva, M.E. Metazoan parasites of the jack mackerel Trachurus murphyi (Teleostei, Carangidae) in a latitudinal gradient from South America (Chile and Peru). Parasite 1999, 6, 223–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George-Nascimento, M. Geographical variations in the jack mackerel Trachurus symmetricus murphyi populations in the southeastern Pacific Ocean as evidenced from the associated parasite communities. J. Parasitol. 2000, 86, 929–932. [Google Scholar] [CrossRef]
- George-Nascimento, M. Populations and assemblages of parasites in hake, Merluccius gayi, from the southeastern Pacific Ocean: Stock implications. J. Fish Biol. 1996, 48, 557–568. [Google Scholar] [CrossRef]
- Oliva, M.E.; Ballón, I. Metazoan parasites of the Chilean hake Merluccius gayi gayi as a tool for stock discrimination. Fish. Res. 2002, 56, 313–320. [Google Scholar] [CrossRef]
- Canales, M.T.; Leal, E. Parámetros de historia de vida de la anchoveta Engraulis ringens Jenyns, 1842, en la zona centro norte de Chile. Rev. Biol. Mar. Oceanogr. 2009, 44, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Cisterna, L.; Arancibia, H. Age of Jack mackerel Trachrus murphyi (Carangidae) using daily growth rings in sagittae otoliths. Gayana 2017, 81, 28–31. [Google Scholar] [CrossRef] [Green Version]
- Froese, R.; Pauly, D. World Wide Web Electronic Publication. Fishbase. 2021. Available online: www.fishbase.org (accessed on 4 August 2021).
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Reiczigel, J.; Marozzi, M.; Fábián, I.; Rózsa, L. Biostatistics for Parasitologists—A Primer to Quantitative Parasitology. Trends Parasitol. 2019, 35, 277–281. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 17 January 2020).
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; Primer-E: Plymouth, UK, 2008. [Google Scholar]
- Anderson, M.J.; Willis, T.J. Canonical analysis of principal coordinates: A useful method of constrained ordination for ecology. Ecology 2003, 84, 511–525. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. Primer V6: User Manual/Tutorial; Primer-E: Plymouth, UK, 2006. [Google Scholar]
- Braicovich, P.E.; Timi, J.T. Parasites as biological tags for stock discrimination of the Brazilian flathead Percophis brasiliensis in the south-west Atlantic. J. Fish Biol. 2008, 73, 557–571. [Google Scholar] [CrossRef]
- Bottari, T.; Gaglio, G.; Mobilia, V.; Garofalos, G.; Laria, C.; Fiorentino, F. Discrimination of Red Mullet Populations (Teleostean, Mullidae) Off the Sicilian Coasts (Mediterranean Sea) on the Basis of Metazoan Parasites. Thalassas 2000, 6, 357–363. [Google Scholar] [CrossRef]
- Moore, B.R.; Stapley, J.; Allsop, Q.; Newman, S.J.; Ballagh, A.; Welch, D.J.; Lester, R.J.G. Stock structure of blue threadfin Eleutheronema tetradactylum across northern Australia, as indicated by parasites. J. Fish Biol. 2011, 78, 923–936. [Google Scholar] [CrossRef]
- Campbell, N.; Cross, M.A.; Chubb, J.C.; Cunningham, C.O.; Hatfield, E.M.C.; MacKenzie, K. Spatial and temporal variations in parasite prevalence and infracommunity structure in herring (Clupea harengus L.) caught to the west of the British Isles and in the North and Baltic Seas: Implications for fisheries science. J. Helminthol. 2007, 81, 137–146. [Google Scholar] [CrossRef]
- Moore, B.R.; Welch, D.J.; Newman, S.J.; Lester, R.J.G. Parasites as indicators of movement and population connectivity of a non-diadromous, tropical estuarine teleost: King threadfin Polydactylus macrochir. J. Fish Biol. 2012, 81, 230–252. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.F.; Reed, C.C.; Hendricks, M.; Winker, H.; van der Lingen, C.D. Stock discrimination of South African sardine (Sardinops sagax) using a digenean parasite biological tag. Fish. Res. 2015, 164, 120–129. [Google Scholar] [CrossRef]
- Moore, A.B.M. Metazoan parasites of the lesser-spotted dogfish Scyliorhinus canicula and their potential as stock discrimination tools. J. Mar. Biol. Assoc. UK 2001, 81, 1009–1013. [Google Scholar] [CrossRef] [Green Version]
- Cantatore, D.M.P.; Timi, J.T. Marine parasites as biological tags in South American Atlantic waters, current status and perspectives. Parasitology 2015, 142, 5–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espínola-Novelo, J.F.; Oliva, M.E. Spatial and Temporal Variability of Parasite Communities: Implications for Fish Stock Identification. Fishes 2021, 6, 71. https://doi.org/10.3390/fishes6040071
Espínola-Novelo JF, Oliva ME. Spatial and Temporal Variability of Parasite Communities: Implications for Fish Stock Identification. Fishes. 2021; 6(4):71. https://doi.org/10.3390/fishes6040071
Chicago/Turabian StyleEspínola-Novelo, Juan F., and Marcelo E. Oliva. 2021. "Spatial and Temporal Variability of Parasite Communities: Implications for Fish Stock Identification" Fishes 6, no. 4: 71. https://doi.org/10.3390/fishes6040071
APA StyleEspínola-Novelo, J. F., & Oliva, M. E. (2021). Spatial and Temporal Variability of Parasite Communities: Implications for Fish Stock Identification. Fishes, 6(4), 71. https://doi.org/10.3390/fishes6040071