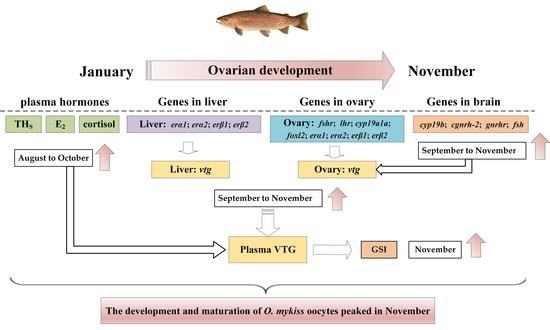
Seasonal Changes in Plasma Hormones, Sex-Related Genes Transcription in Brain, Liver and Ovary during Gonadal Development in Female Rainbow Trout (Oncorhynchus mykiss)
Abstract
1. Introduction
2. Materials and Methods
2.1. Fishes and Sample Collection
2.2. Determination of HSI and GSI
2.3. Plasma Hormones and Vitellogenin (VTG) Analyses
2.4. RNA Extraction and cDNA Synthesis
2.5. Gene Transcription
2.6. Statistical Analysis
3. Results
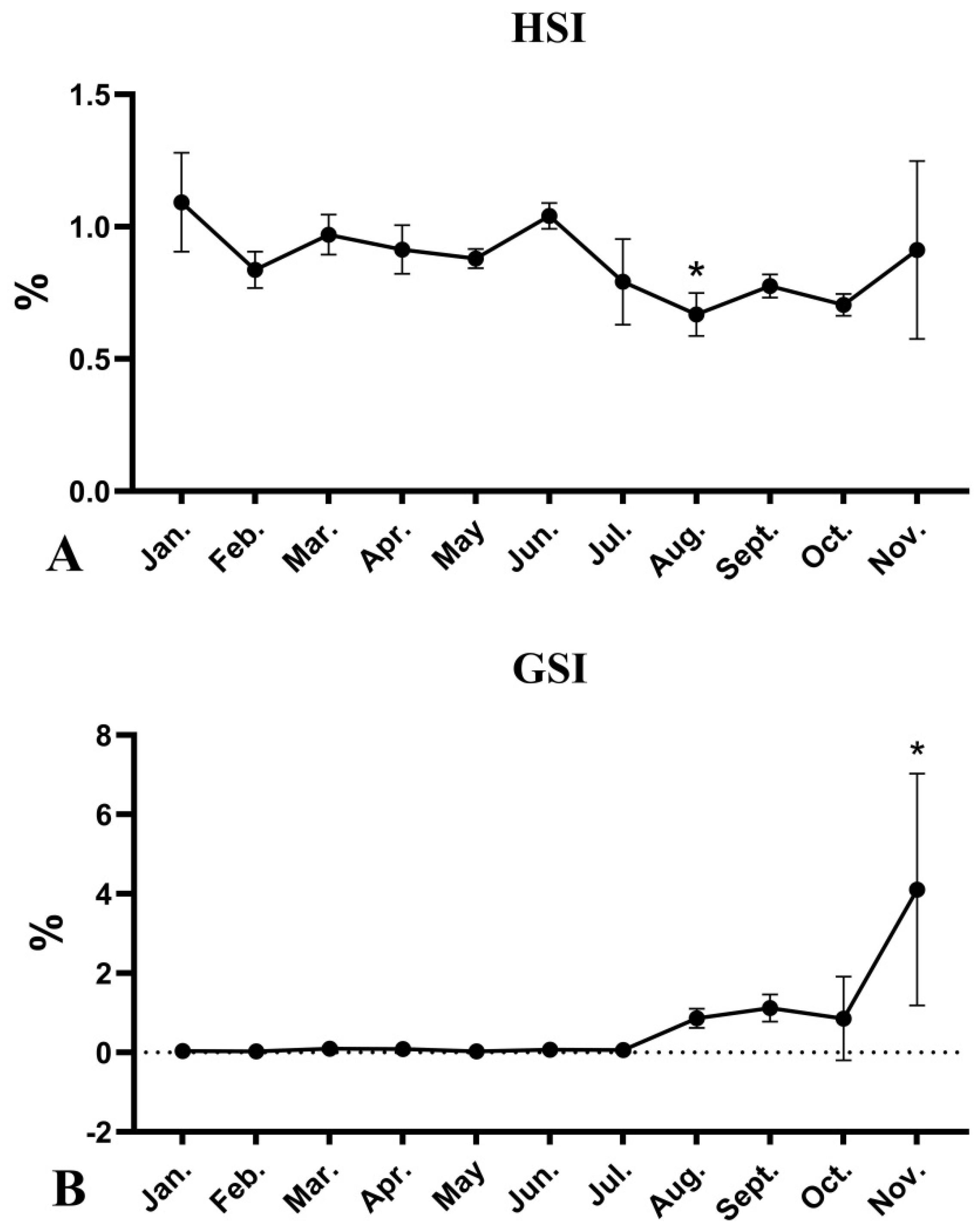
3.1. Seasonal Changes in HSI and GSI
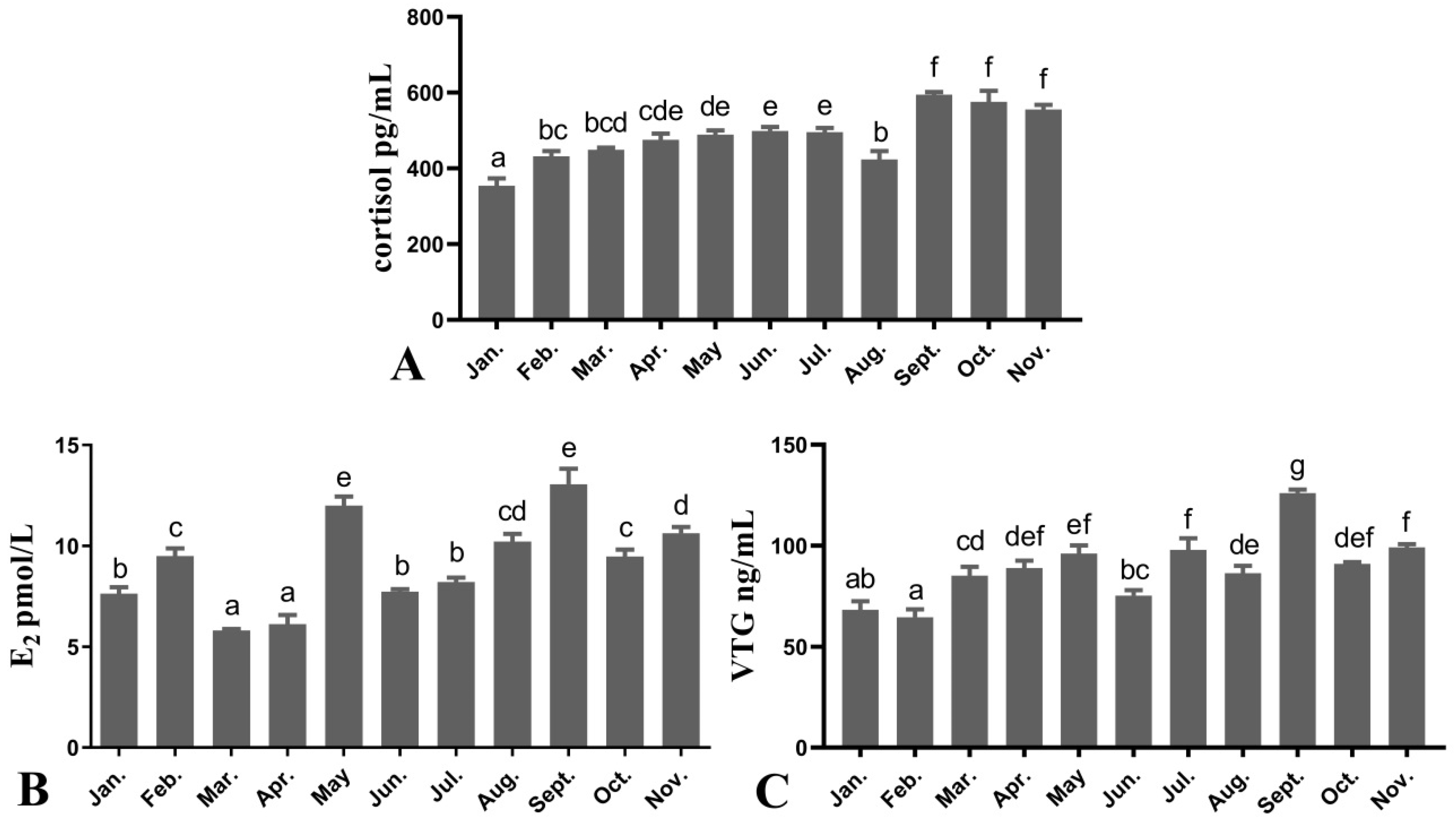
3.2. Plasma Cortisol, E2 and VTG Levels of Female O. mykiss during Oocyte Development
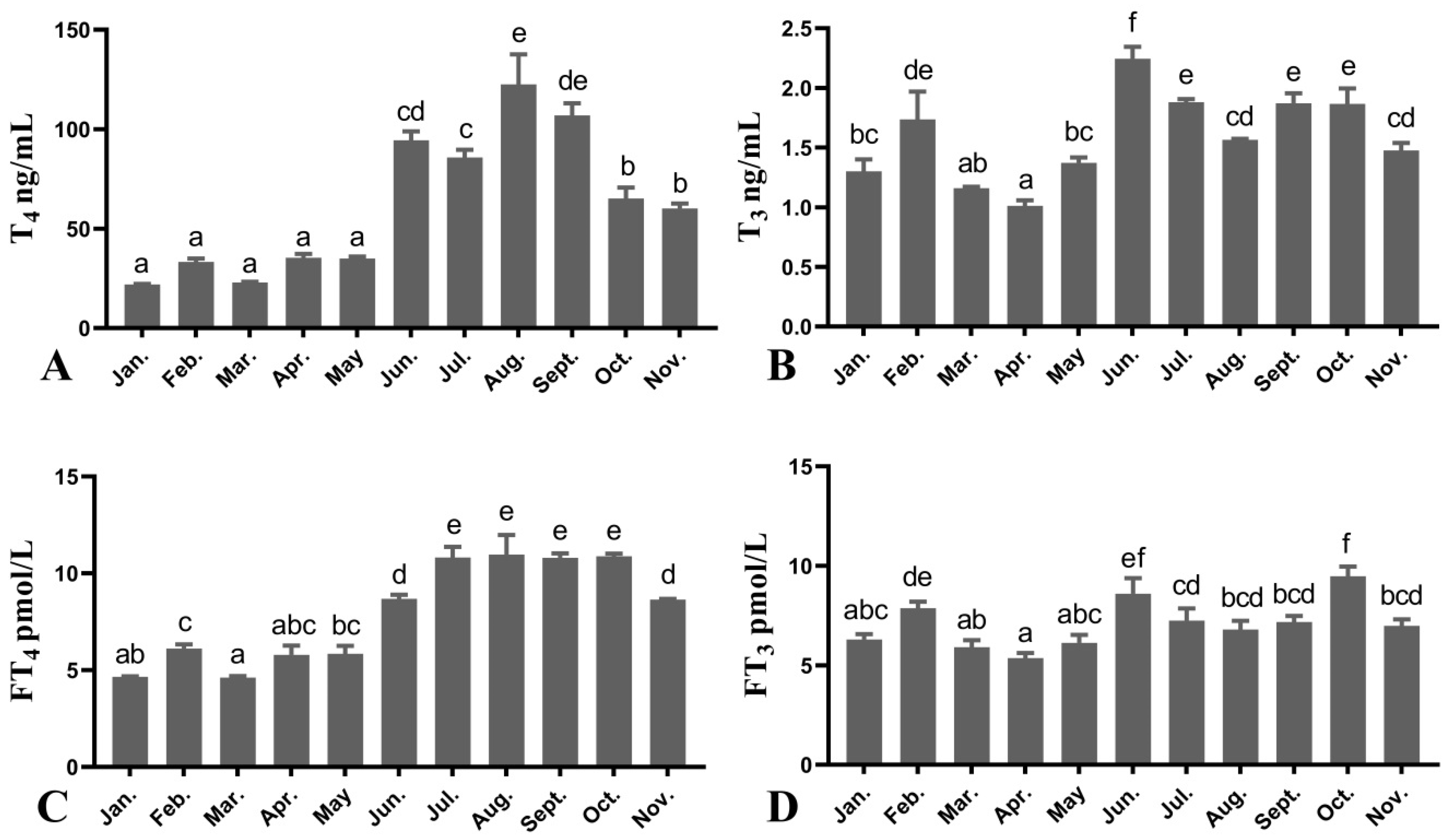
3.3. Plasma THS Levels of Female O. mykiss during Oocyte Development
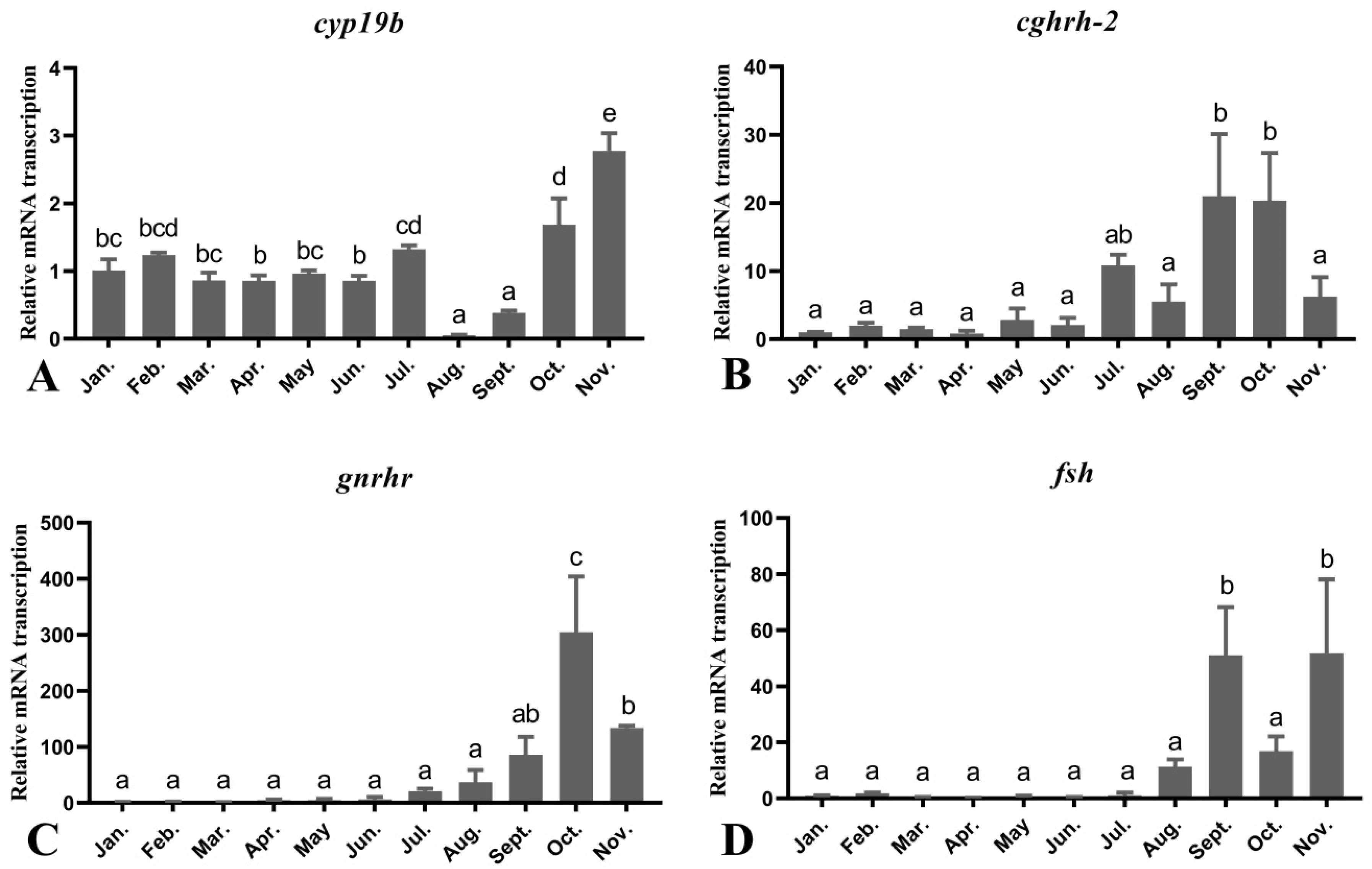
3.4. Seasonal Changes in the Levels of Female O. mykiss Brain Genes during Oocyte Development
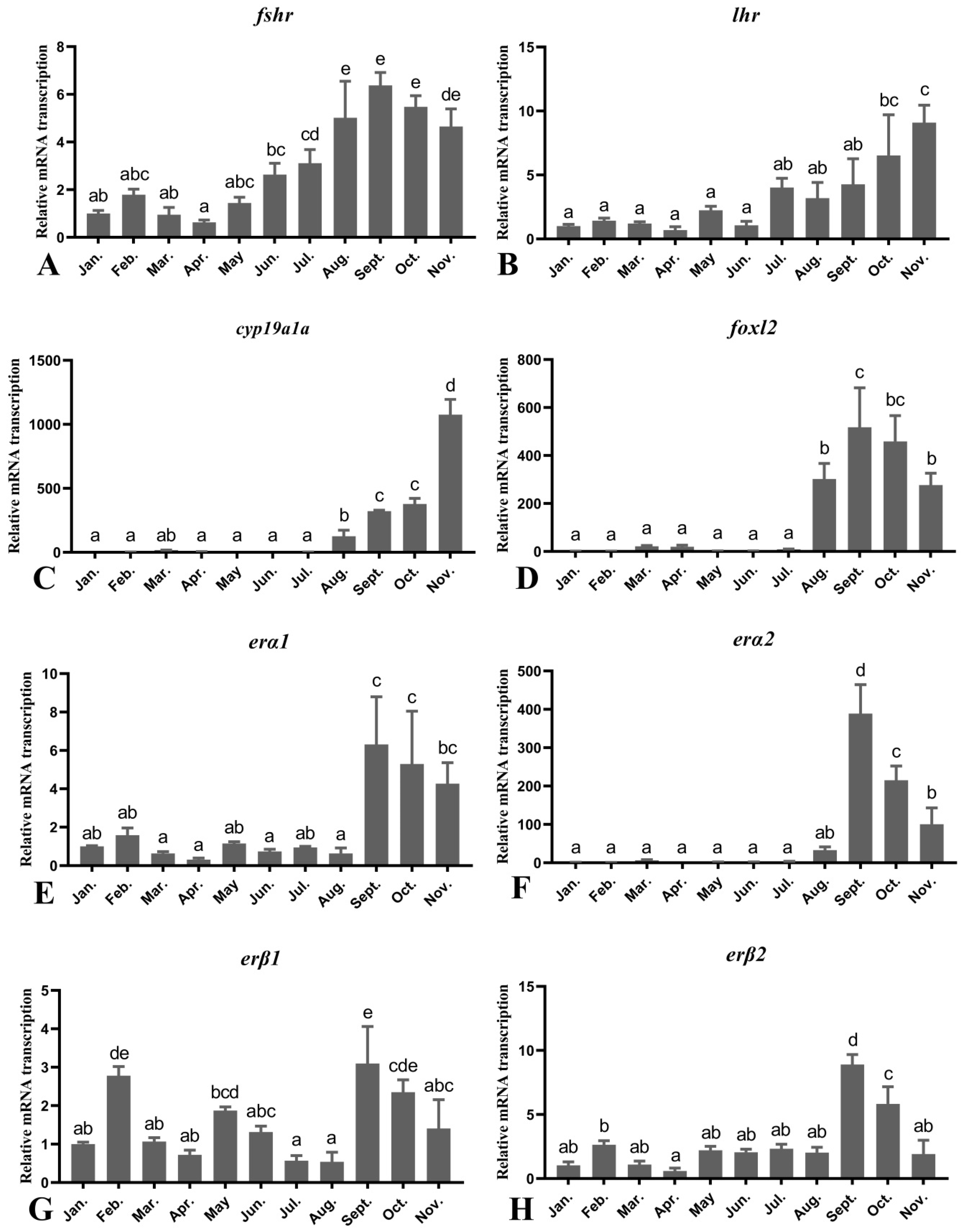
3.5. Seasonal Changes in the Levels of Female O. mykiss Gonadal Genes during Oocyte Development
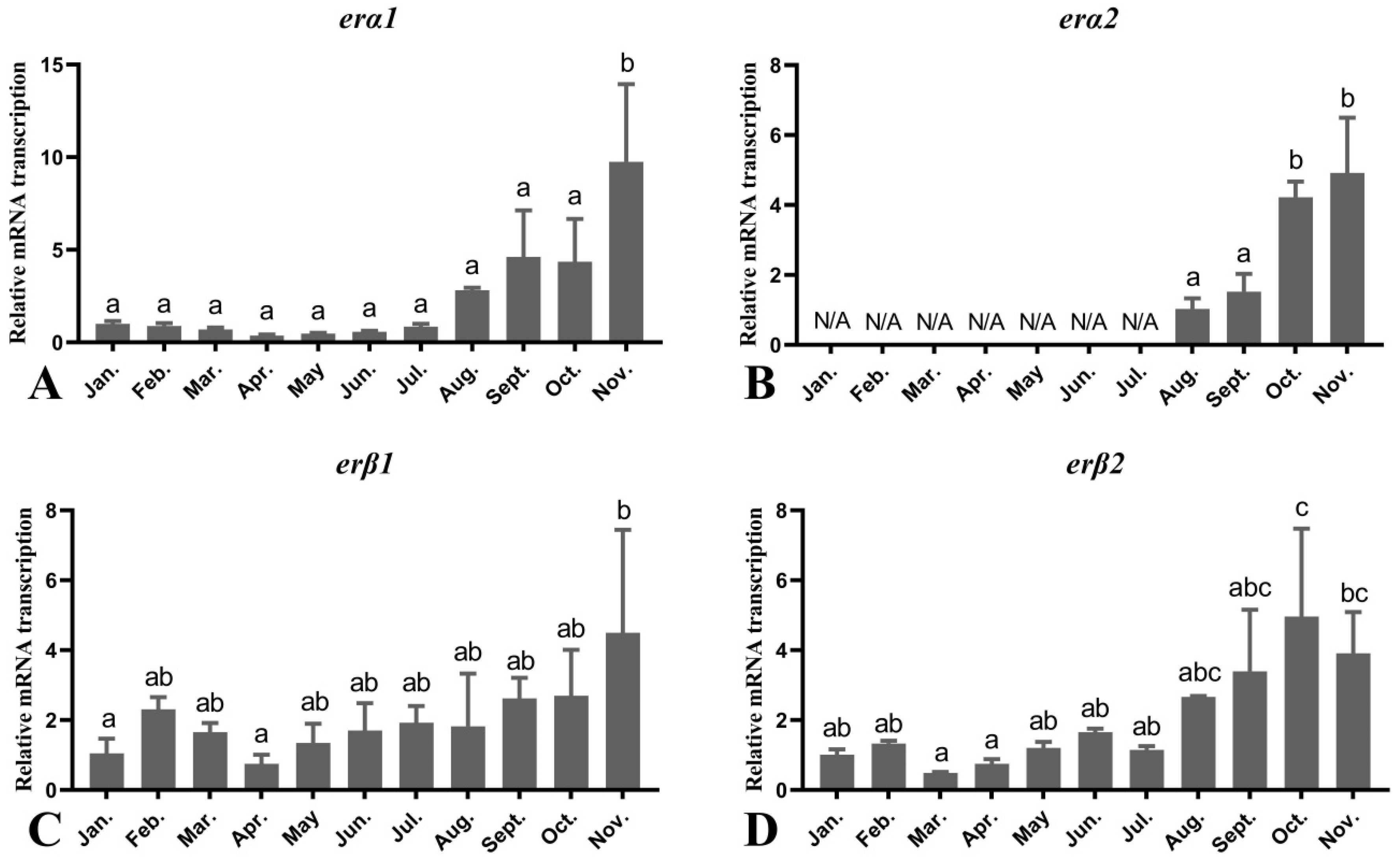
3.6. Seasonal Changes in the Levels of Female O. mykiss Liver Genes during Oocyte Development
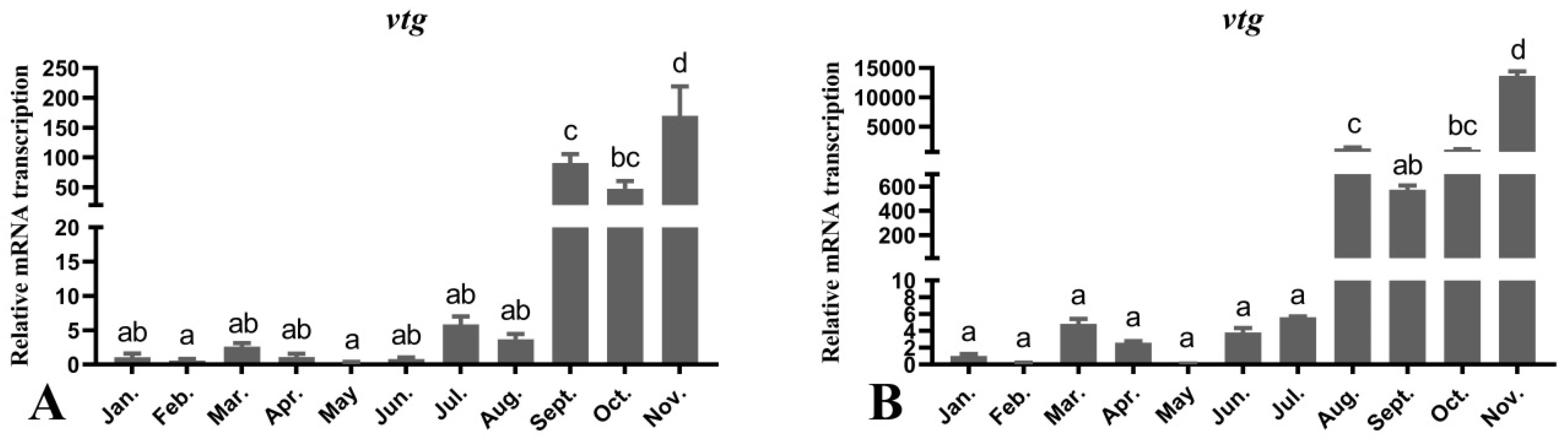
3.7. Seasonal Changes in the Levels of Female O. mykiss vtg Gene during Oocyte Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rocha, M.J. Fish Reproduction; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Karimi, S.; Kochanian, P.; Salati, A.P.; Gooraninejad, S. Plasma sex steroids and gonadosomatic index variations during ovarian development of female wild yellowfin seabream (Acanthopagrus latus). Ichthyol. Res. 2013, 61, 68–75. [Google Scholar] [CrossRef]
- Tolussi, C.E.; Gomes, A.D.; Ribeiro, C.D.S.; Caneppele, D.; Moreira, R.G.; Honji, R.M. Mobilization of energetic substrates in the endangered catfish Steindachneridion parahybae (Siluriformes: Pimelodidae): Changes in annual reproductive cycle in captivity. Neotrop. Ichthyol. 2018, 16. [Google Scholar] [CrossRef]
- Jan, M.; Ahmed, I. Assessment of fecundity, gonadosomatic index and hepatosomatic index of snow trout, Schizothorax plagiostomus in river Lidder, from Kashmir Himalaya, India. Int. J. Fish. Aquat. Stud. Delhi 2016, 4, 370–375. [Google Scholar]
- Jan, M.; Jan, N. Studies on the fecundity (F), gonadosomatic index (GSI) and hepatosomatic index (HSI) of Salmo trutta fario (Brown trout) at Kokernag trout fish farm, Anantnag, Jammu and Kashmir. Int. J. Fish. Aquat. Stud. Delhi 2017, 5, 170–173. [Google Scholar]
- Estay, F.; Colihueque, N.; Araneda, C. Comparison of Oogenesis and Sex Steroid Profiles between Twice and Once Annually Spawning of Rainbow Trout Females (Oncorhynchus mykiss). Sci. World J. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Teitsma, C.; Desdoits-Lethimonier, C.; Tujague, M.; Anglade, I.; Saligaut, D.; Bailhache, T.; Pakdel, F.; Kah, O.; Ducouret, B. Identification of potential sites of cortisol actions on the reproductive axis in rainbow trout. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1998, 119, 243–249. [Google Scholar] [CrossRef]
- Cyr, D.G.; Eales, J.G. Influence of thyroidal status on ovarian function in rainbow trout, Salmo gairdneri. J. Exp. Zoöl. 1988, 248, 81–87. [Google Scholar] [CrossRef]
- Di Fiore, M.M.; Assisi, L.; Botte, V. Aromatase and testosterone receptor in the liver of the female green frog, Rana esculenta. Life Sci. 1998, 62, 1949–1958. [Google Scholar] [CrossRef]
- Burrone, L.; Santillo, A.; Pinelli, C.; Baccari, G.C.; Di Fiore, M.M. Induced synthesis of P450 Aromatase and 17β-estradiol by D-aspartate in frog brain. J. Exp. Biol. 2012, 215, 3559–3565. [Google Scholar] [CrossRef]
- Paolucci, M.; Di Fiore, M.M. Sex Steroid Binding Proteins in the Plasma of the Green Frog, Rana esculenta: Changes during the Reproductive Cycle and Dependence on Pituitary Gland and Gonads. Gen. Comp. Endocrinol. 1994, 96, 401–411. [Google Scholar] [CrossRef]
- Morris, J.A.; Jordan, C.L.; Breedlove, S.M. Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 2004, 7, 1034–1039. [Google Scholar] [CrossRef]
- Vizziano-Cantonnet, D.; Anglade, I.; Pellegrini, E.; Gueguen, M.-M.; Fostier, A.; Guiguen, Y.; Kah, O. Sexual dimorphism in the brain aromatase expression and activity, and in the central expression of other steroidogenic enzymes during the period of sex differentiation in monosex rainbow trout populations. Gen. Comp. Endocrinol. 2011, 170, 346–355. [Google Scholar] [CrossRef]
- Dickey, J.T.; Swanson, P. Effects of Salmon Gonadotropin-Releasing Hormone on Follicle Stimulating Hormone Secretion and Subunit Gene Expression in Coho Salmon (Oncorhynchus kisutch). Gen. Comp. Endocrinol. 2000, 118, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Von Schalburg, K.R.; Warby, C.M.; Sherwood, N.M. Evidence for Gonadotropin-Releasing Hormone Peptides in the Ovary and Testis of Rainbow Trout1. Biol. Reprod. 1999, 60, 1338–1344. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Okuzawa, K.; Amano, M.; Kobayashi, M.; Aida, K.; Hanyu, I.; Hasegawa, Y.; Miyamoto, K. Differences in salmon GnRH and chicken GnRH-II contents in discrete brain areas of male and female rainbow trout according to age and stage of maturity. Gen. Comp. Endocrinol. 1990, 80, 116–126. [Google Scholar] [CrossRef]
- Chang, X.; Kobayashi, T.; Senthilkumaran, B.; Kobayashi-Kajura, H.; Sudhakumari, C.C.; Nagahama, Y. Two types of aromatase with different encoding genes, tissue distribution and developmental expression in Nile tilapia (Oreochromis niloticus). Gen. Comp. Endocrinol. 2005, 141, 101–115. [Google Scholar] [CrossRef]
- Cyr, D.; Bromage, N.; Duston, J.; Eales, J. Seasonal patterns in serum levels of thyroid hormones and sex steroids in relation to photoperiod-induced changes in spawning time in rainbow trout, Salmo gairdneri. Gen. Comp. Endocrinol. 1988, 69, 217–225. [Google Scholar] [CrossRef]
- Maclatchy, D.L.; Eales, J. Short-term treatment with testosterone increases plasma 3,5,3′-triiodo-l-thyronine and hepatic l-thyroxine 5′-monodeiodinase levels in arctic charr, Salvelinus alpinus. Gen. Comp. Endocrinol. 1988, 71, 10–16. [Google Scholar] [CrossRef]
- Blanton, M.L.; Specker, J.L. The Hypothalamic-Pituitary-Thyroid (HPT) Axis in Fish and Its Role in Fish Development and Reproduction. Crit. Rev. Toxicol. 2007, 37, 97–115. [Google Scholar] [CrossRef]
- Leatherland, J.F. Reflections on the thyroidology of fishes: From molecules to humankind. Guelph Ichthyol. Rev. 1994, 2, 1–67. [Google Scholar]
- Power, D.M.; Llewellyn, L.; Faustino, M.; Nowell, M.A.; Björnsson, B.T.; Einarsdóttir, I.E.; Canario, A.V.; Sweeney, G.E. Thyroid hormones in growth and development of fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 447–459. [Google Scholar] [CrossRef]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Mu, W.J.; Wen, H.S.; Li, J.F.; He, F. Cloning and expression analysis of Foxl2 during the reproductive cycle in Korean rockfish, Sebastes schlegeli. Fish Physiol. Biochem. 2013, 39, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-D.; Zhang, G.-R.; Wei, K.-J.; Ji, W.; Gardner, J.P.A.; Yang, R.-B.; Chen, K.-C. Molecular identification and expression of the Foxl2 gene during gonadal sex differentiation in northern snakehead Channa argus. Fish Physiol. Biochem. 2015, 41, 1419–1433. [Google Scholar] [CrossRef]
- Herndon, M.K.; Nilson, J.H. Maximal Expression of Foxl2 in Pituitary Gonadotropes Requires Ovarian Hormones. PLoS ONE 2015, 10, e0126527. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, D.S.; Kobayashi, T.; Zhou, L.Y.; Nagahama, Y. Molecular cloning and gene expression of Foxl2 in the Nile tilapia, Oreochromis niloticus. Biochem. Biophys. Res. Commun. 2004, 320, 83–89. [Google Scholar] [CrossRef]
- Nagler, J.J.; Cavileer, T.; Sullivan, J.; Cyr, D.G.; Rexroad, C., III. The complete nuclear estrogen receptor family in the rainbow trout: Discovery of the novel ERα2 and both ERβ isoforms. Gene 2007, 392, 164–173. [Google Scholar] [CrossRef][Green Version]
- Guiguen, Y.; Fostier, A.; Piferrer, F.; Chang, C.-F. Ovarian aromatase and estrogens: A pivotal role for gonadal sex differentiation and sex change in fish. Gen. Comp. Endocrinol. 2010, 165, 352–366. [Google Scholar] [CrossRef]
- Lubzens, E.; Young, G.; Bobe, J.; Cerdà, J. Oogenesis in teleosts: How fish eggs are formed. Gen. Comp. Endocrinol. 2010, 165, 367–389. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Delalande, C.; Goupil, A.-S.; Lareyre, J.-J.; Le Gac, F. Differential expression patterns of three aromatase genes and of four estrogen receptors genes in the testes of trout (Oncorhynchus mykiss). Mol. Reprod. Dev. 2015, 82, 694–708. [Google Scholar] [CrossRef]
- Hou, Z.-S.; Wen, H.-S.; Li, J.-F.; He, F.; Li, Y.; Tao, Y.-X. Hypothalamus-pituitary-gonad axis of rainbow trout (Oncorhynchus mykiss) during early ovarian development and under dense rearing condition. Gen. Comp. Endocrinol. 2016, 236, 131–138. [Google Scholar] [CrossRef]
- Vizziano, D.; Randuineau, G.; Baron, D.; Cauty, C.; Guiguen, Y. Characterization of early molecular sex differentiation in rainbow trout, Oncorhynchus mykiss. Dev. Dyn. 2007, 236, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- van Bohemen, C.; Lambert, J.; Peute, J. Annual changes in plasma and liver in relation to vitellogenesis in the female rainbow trout, Salmo gairdneri. Gen. Comp. Endocrinol. 1981, 44, 94–107. [Google Scholar] [CrossRef]
- Zin, T.; Than, A.A.; Naing, T.T. Fecundity (F), gonadosomatic index (GSI), hepatosomatic index (HSI), condition factor (K) and length-weight relationship (LWR) in Channa orientalis Bloch & Schneider, 1801. Univ. Res. J. 2011, 4, 47–62. [Google Scholar]
- Soranganba, N.; Singh, I. Role of some steroidogenic hormones in fish reproduction. Chem. Sci. Rev. Lett. 2019, 8, 64–69. [Google Scholar]
- Lamba, V.J.; Goswami, S.V.; Sundararaj, B.I. Circannual and circadian variations in plasma levels of steroids (cortisol, estradiol-17β estrone, and testosterone) correlated with the annual gonadal cycle in the catfish, Heteropneustes fossilis (Bloch). Gen. Comp. Endocrinol. 1983, 50, 205–225. [Google Scholar] [CrossRef]
- Wingfield, J.; Grimm, A. Seasonal changes in plasma cortisol, testosterone and oestradiol-17β in the plaice, Pleuronectes platessa L. Gen. Comp. Endocrinol. 1977, 31, 1–11. [Google Scholar] [CrossRef]
- Pickering, A.D.; Christie, P. Changes in the concentrations of plasma cortisol and thyroxine during sexual maturation of the hatchery-reared brown trout, Salmo trutta L. Gen. Comp. Endocrinol. 1981, 44, 487–496. [Google Scholar] [CrossRef]
- Kusakabe, M.; Nakamura, I.; Young, G. 11β-Hydroxysteroid Dehydrogenase Complementary Deoxyribonucleic Acid in Rainbow Trout: Cloning, Sites of Expression, and Seasonal Changes in Gonads. Endocrinology 2003, 144, 2534–2545. [Google Scholar] [CrossRef]
- Shankar, D.S.; Kulkarni, R.S. Tissue cholesterol and serum cortisol level during different reproductive phases of the female freshwater fish Notopterus notopterus (Pallas). J. Environ. Biol. 2007, 28, 137–139. [Google Scholar] [PubMed]
- Hou, Y.Y.; Han, X.D.; Suzuki, Y. Annual changes in plasma levels of cortisol and sex steroid hormones in male rainbow trout, Oncorhynchus mykiss. Chin. J. Oceanol. Limnol. 2001, 19, 217–221. [Google Scholar]
- Abdollahpour, H.; Falahatkar, B.; Efatpanah, I.; Meknatkhah, B.; Van Der Kraak, G. Hormonal and physiological changes in Sterlet sturgeon Acipenser ruthenus treated with thyroxine. Aquaculture 2019, 507, 293–300. [Google Scholar] [CrossRef]
- Gorbman, A. Thyroid function and its control in fishes—ScienceDirect. Fish Physiol. 1969, 2, 241–274. [Google Scholar]
- Grau, E.G. Environmental Influences on Thyroid Function in Teleost Fish. Am. Zoöl. 1988, 28, 329–335. [Google Scholar] [CrossRef]
- Leatherland, J.F. Endocrine Factors Affecting Thyroid Economy of Teleost Fish. Am. Zoöl. 1988, 28, 319–328. [Google Scholar] [CrossRef]
- Peter, M.S. The role of thyroid hormones in stress response of fish. Gen. Comp. Endocrinol. 2011, 172, 198–210. [Google Scholar] [CrossRef]
- Kang, D.-Y.; Chang, Y.J. Effects of maternal injection of 3,5,3′-triiodo-l-thyronine (T3) on growth of newborn offspring of rockfish, Sebastes schlegeli. Aquaculture 2004, 234, 641–655. [Google Scholar] [CrossRef]
- Cyr, D.G.; Idler, D.R.; Audet, C.; McLeese, J.M.; Ealesd, J.G. Effects of Long-Term Temperature Acclimation on Thyroid Hormone Deiodinase Function, Plasma Thyroid Hormone Levels, Growth, and Reproductive Status of Male Atlantic Cod, Gadus morhua. Gen. Comp. Endocrinol. 1998, 109, 24–36. [Google Scholar] [CrossRef]
- Pavlidis, M.; Greenwood, L.; Mourot, B.; Kokkari, C.; Le Menn, F.; Divanach, P.; Scott, A. Seasonal Variations and Maturity Stages in Relation to Differences in Serum Levels of Gonadal Steroids, Vitellogenin, and Thyroid Hormones in the Common Dentex (Dentex dentex). Gen. Comp. Endocrinol. 2000, 118, 14–25. [Google Scholar] [CrossRef]
- Eales, J. Modes of action and physiological effects of thyroid hormones in fish. Fish Endocrinol. 2006, 2, 767–808. [Google Scholar]
- Supriya, A.; Raghuveer, K.; Swapna, I.; Rasheeda, M.K.; Kobayashi, T.; Nagahama, Y.; Gupta, A.D.; Majumdar, K.C.; Senthilkumaran, B. Thyroid hormone modulation of ovarian recrudescence of air-breathing catfish Clarias gariepinus. Fish Physiol. Biochem. 2005, 31, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, M.; Dessypris, A.; Christofidis, I. Seasonal fluctuations in plasma thyroid hormones, in two strains of rainbow trout (Oncorhynchus mykiss), during the first and second reproductive cycle: Relation with their photoperiodically altered spawning time. Aquaculture 1991, 99, 365–385. [Google Scholar] [CrossRef]
- Millar, R.P. GnRHs and GnRH receptors. Anim. Reprod. Sci. 2005, 88, 5–28. [Google Scholar] [CrossRef]
- Sherwood, N.M.; Lovejoy, D.A.; Coe, I.R. Origin of mammalian gonadotropin-releasing hormones. Endocr. Rev. 1993, 14, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Yaron, Z.; Levavi-Sivan, B. Endocrine regulation of fish reproduction. Encycl. Fish Physiol. Genome Environ. 2011, 2, 1500–1508. [Google Scholar]
- Nyuji, M.; Kazeto, Y.; Izumida, D.; Tani, K.; Suzuki, H.; Hamada, K.; Mekuchi, M.; Gen, K.; Soyano, K.; Okuzawa, K. Greater amberjack Fsh, Lh, and their receptors: Plasma and mRNA profiles during ovarian development. Gen. Comp. Endocrinol. 2016, 225, 224–234. [Google Scholar] [CrossRef]
- Pfaff, D.W.; Jorgenson, K.; Kow, L. Luteinizing Hormone-Releasing Hormone in Rat Brain: Gene Expression, Role as Neuromodulator, and Functional Effects. Ann. N. Y. Acad. Sci. 1987, 519, 323–333. [Google Scholar] [CrossRef]
- Tchoudakova, A.; Kishida, M.; Wood, E.; Callard, G.V. Promoter characteristics of two cyp19 genes differentially expressed in the brain and ovary of teleost fish. J. Steroid Biochem. Mol. Biol. 2001, 78, 427–439. [Google Scholar] [CrossRef]
- Chiang, E.F.L.; Yan, Y.L.; Tong, S.K.; Hsiao, P.H.; Guiguen, Y.; Postlethwait, J.; Chung, B.C. Characterization of duplicated zebrafish cyp19 genes. J. Exp. Zool. 2001, 290, 709–714. [Google Scholar] [CrossRef]
- Baron, D.; Cocquet, J.; Xia, X.; Fellous, M.; Guiguen, Y.; Veitia, R.A. An evolutionary and functional analysis of FoxL2 in rainbow trout gonad differentiation. J. Mol. Endocrinol. 2004, 33, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-S.; Kobayashi, T.; Zhou, L.-Y.; Paul-Prasanth, B.; Ijiri, S.; Sakai, F.; Okubo, K.; Morohashi, K.-I.; Nagahama, Y. Foxl2 Up-Regulates Aromatase Gene Transcription in a Female-Specific Manner by Binding to the Promoter as Well as Interacting with Ad4 Binding Protein/Steroidogenic Factor 1. Mol. Endocrinol. 2007, 21, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Mu, X.; Gui, L.; Su, M.; Li, H.; Zhang, G.; Liu, Z.; Zhang, J. Characterization and gonadal expression of FOXL2 relative to Cyp19a genes in spotted scat Scatophagus argus. Gene 2015, 561, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, S.; Miyagawa, S.; Lange, A.; Ogino, Y.; Mizutani, T.; Tatarazako, N.; Katsu, Y.; Ihara, M.; Tanaka, H.; Ishibashi, H.; et al. Understanding the Molecular Basis for Differences in Responses of Fish Estrogen Receptor Subtypes to Environmental Estrogens. Environ. Sci. Technol. 2015, 49, 7439–7447. [Google Scholar] [CrossRef]
- Griffin, L.B.; January, K.E.; Ho, K.W.; Cotter, K.; Callard, G.V. Morpholino-Mediated Knockdown of ERα, ERβa, and ERβb mRNAs in Zebrafish (Danio rerio) Embryos Reveals Differential Regulation of Estrogen-Inducible Genes. Endocrinology 2013, 154, 4158–4169. [Google Scholar] [CrossRef]
- Nelson, E.; Habibi, H.R. Functional Significance of Nuclear Estrogen Receptor Subtypes in the Liver of Goldfish. Endocrinology 2010, 151, 1668–1676. [Google Scholar] [CrossRef]
| Month | Body Weight (g) | Liver Weight (g) | Ovary Weight (g) |
|---|---|---|---|
| Jan. | 82.37 ± 11.19 | 0.91 ± 0.24 | 0.03 ± 0.02 |
| Feb. | 154.93 ± 1.46 | 1.30 ± 0.12 | 0.04 ± 0.002 |
| Mar. | 181.40 ± 57.93 | 1.75 ± 0.57 | 0.18 ± 0.13 |
| Apr. | 308.87 ± 62.43 | 2.84 ± 0.72 | 0.28 ± 0.06 |
| May | 291.47 ± 62.76 | 2.58 ± 0.64 | 0.09 ± 0.04 |
| Jun. | 550.73 ± 149.84 | 5.77 ± 1.77 | 0.40 ± 0.13 |
| Jul. | 661.73 ± 162.31 | 5.17 ± 1.34 | 0.34 ± 0.24 |
| Aug. | 909.17 ± 49.99 | 6.08 ± 0.86 | 7.90 ± 2.54 |
| Sept. | 1047.23 ± 212.67 | 8.07 ± 1.26 | 11.76 ± 4.85 |
| Oct. | 991.93 ± 60.76 | 6.70 ± 0.81 | 8.91 ± 11.29 |
| Nov. | 1033.43 ± 286.97 | 9.98 ± 5.62 | 47.86 ± 37.95 |
| Gene | Primer Name | Sequence (5′–3′) | GenBank No. or Article Source |
|---|---|---|---|
| erα1 | erα1-F | CCCTGCTGGTGACAGAGAGAA | [28] |
| erα1-R | ATCCTCCACCACCATTGAGACT | ||
| erα2 | erα2-F | GTGGCACTGCTGGTGACAAC | [28] |
| erα2-R | ACCACCGAAGCTGCTGTTCT | ||
| erβ1 | erβ1-F | CCCAAGCGGGTCCTAGCT | [28] |
| erβ1-R | TCCTCATGTCCTTCTGGAGGAA | ||
| erβ2 | erβ2-F | CTGACCCCAGAACAGCTGATC | [28] |
| erβ2-R | TCGGCCAGGTTGGTAAGTG | ||
| vtg | vtg-F | GTGGACTGGATGAAGGGACA | AY049952.1 |
| vtg-R | AGAGCGGCTCAGGTTGGAAT | ||
| cyp19b | cyp19b-F | GAGGAAGGCACTGGAAGATGAC | [32] |
| cyp19b-R | GCTGGAAGAAACGACTGGGC | ||
| fsh | fsh-F | GCGAAACAACGGACCTGAACTAT | [33] |
| fsh-R | GGACCACTCCTTGAAGTTACACA | ||
| cgnrh-II | cgnrh-II-F | CTGTGAGGCAGGAGAATG | [33] |
| cgnrh-II-R | ACGGTTGATAGGTTGTCTAA | ||
| gnrhr | gnrhr-F | GTCTTTTCCAACCCAGGATGTC | AJ272116.1 |
| gnrhr-R | GGAAACTGGGACATGTTTGAGAG | ||
| fshr | fshr-F | TCAGTCACCTGACGATCTGCAA | [33] |
| fshr-R | TCCTGCAGGTCCAGCAGAAACG | ||
| lhr | lhr-F | CTTCTCAACCTCAATGAAATCTTC | [33] |
| lhr-R | GGATATACTCAGATAACGCAGCTT | ||
| cyp19a1a | cyp19a1a-F | CTCTCCTCTCATACCTCAGGTT | [34] |
| cyp19a1a-R | AGAGGAACTGCTGAGTATGAAT | ||
| foxl2 | foxl2-F | TGTGCTGGATTTGTTTTTTGTT | [34] |
| foxl2-R | GTGTCGTGGACCATCAGGGCCA | ||
| ef1α | ef1α-F | AGGCCATCTGATCTACAAGTGC | AF498320.1 |
| ef1α-R | GGTGATACCACGCTCCCTCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Bi, B.; Kong, L.; Rong, H.; Su, Y.; Hu, Q. Seasonal Changes in Plasma Hormones, Sex-Related Genes Transcription in Brain, Liver and Ovary during Gonadal Development in Female Rainbow Trout (Oncorhynchus mykiss). Fishes 2021, 6, 62. https://doi.org/10.3390/fishes6040062
Chen H, Bi B, Kong L, Rong H, Su Y, Hu Q. Seasonal Changes in Plasma Hormones, Sex-Related Genes Transcription in Brain, Liver and Ovary during Gonadal Development in Female Rainbow Trout (Oncorhynchus mykiss). Fishes. 2021; 6(4):62. https://doi.org/10.3390/fishes6040062
Chicago/Turabian StyleChen, Huiqin, Baoliang Bi, Lingfu Kong, Hua Rong, Yanhua Su, and Qing Hu. 2021. "Seasonal Changes in Plasma Hormones, Sex-Related Genes Transcription in Brain, Liver and Ovary during Gonadal Development in Female Rainbow Trout (Oncorhynchus mykiss)" Fishes 6, no. 4: 62. https://doi.org/10.3390/fishes6040062
APA StyleChen, H., Bi, B., Kong, L., Rong, H., Su, Y., & Hu, Q. (2021). Seasonal Changes in Plasma Hormones, Sex-Related Genes Transcription in Brain, Liver and Ovary during Gonadal Development in Female Rainbow Trout (Oncorhynchus mykiss). Fishes, 6(4), 62. https://doi.org/10.3390/fishes6040062