Oxygen Consumption and Ammonia Excretion of Marphysa sanguinea (Polychaeta: Eunicidae) in Relation to Body Mass and Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Worm Selection and Temporary Culture
2.2. Measurement of Oxygen Consumption and Ammonia Excretion Rates
2.3. Classic Q10 and Ratio of O:N
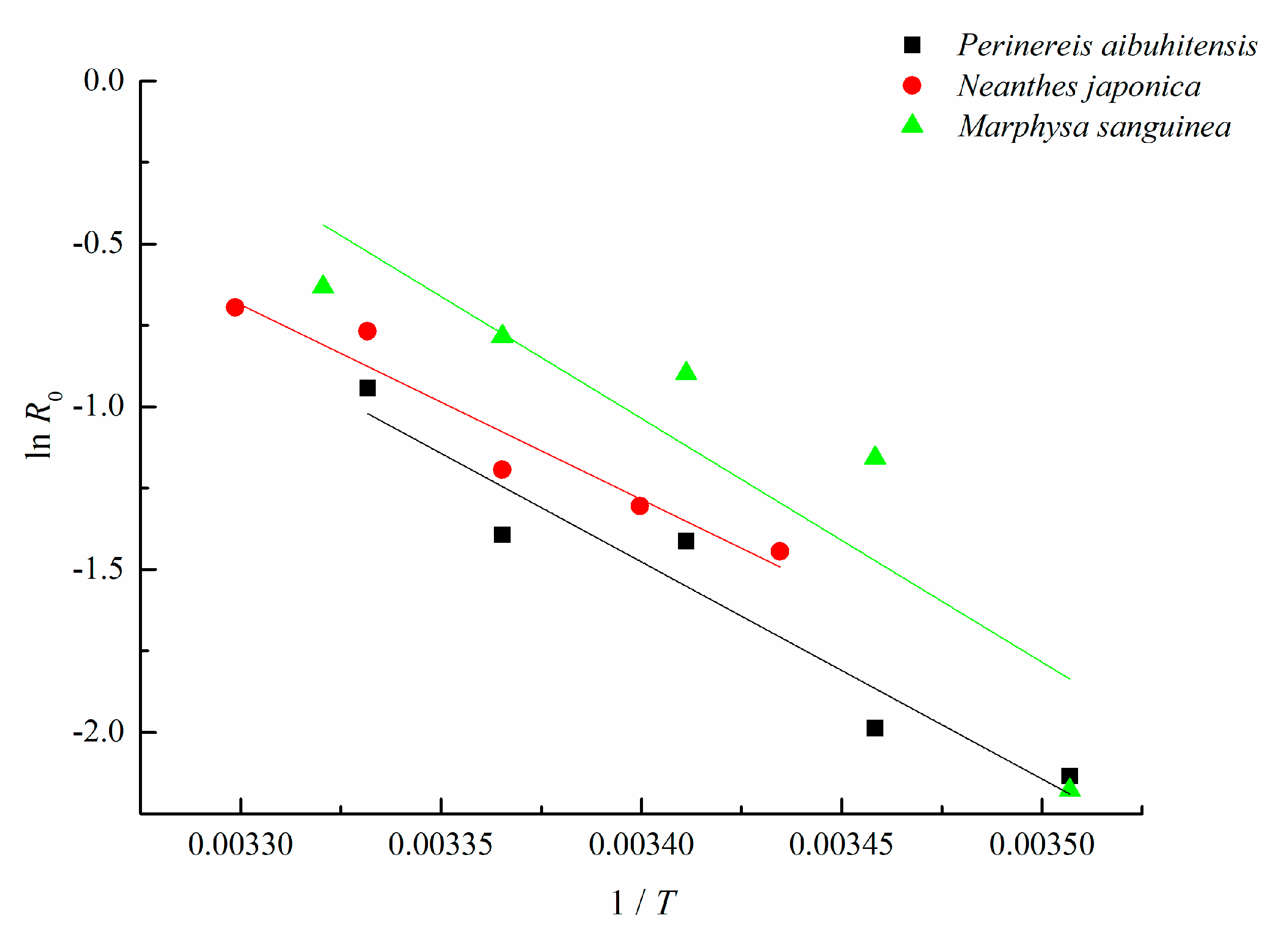
2.4. Activation Energy, UTD Value and Q10 of UTD Calculation
2.5. Statistical Analysis
3. Results
3.1. Oxygen Consumption and Ammonia Excretion of M. sanguinea with Different Body Sizes at Different Temperatures
3.2. Classical Q10 and O:N Ratio of M. sanguinea Metabolism
3.3. Activation Energy, UTD Value, and Q10 of UTD of M. sanguinea at Different Temperatures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jang, M.; Shim, W.J.; Han, G.M.; Song, Y.K.; Hong, S.H. Formation of microplastics by polychaetes (Marphysa sanguinea) inhabiting expanded polystyrene marine debris. Mar. Pollut. Bull. 2018, 131, 365–369. [Google Scholar] [CrossRef]
- Nel, H.; Froneman, P. Presence of microplastics in the tube structure of the reef-building polychaete Gunnarea gaimardi (Quatrefages 1848). Afr. J. Mar. Sci. 2018, 40, 87–89. [Google Scholar] [CrossRef]
- Mdaini, Z.; Tremblay, R.; Pharand, P.; Gagné, J.-P. Spatio-temporal variability of biomarker responses and lipid composition of Marphysa sanguinea, Montagu (1813) in the anthropic impacted lagoon of Tunis. Mar. Pollut. Bull. 2019, 144, 275–286. [Google Scholar] [CrossRef]
- Fries, E.; Dekiff, J.H.; Willmeyer, J.; Nuelle, M.-T.; Ebert, M.; Remy, D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci. Process. Impacts 2013, 15, 1949–1956. [Google Scholar] [CrossRef] [Green Version]
- Olive, P.W. Polychaeta as a world resource: A review of patterns of exploitation as sea angling baits and the potential for aquaculture based production. Mémoires Muséum National D’histoire Naturelle 1994, 162, 603–610. [Google Scholar]
- Garcês, J.; Pereira, J. Effect of salinity on survival and growth of Marphysa sanguinea Montagu (1813) juveniles. Aquac. Int. 2011, 19, 523–530. [Google Scholar] [CrossRef]
- Pombo, A.; Baptista, T.; Granada, L.; Ferreira, S.M.; Gonçalves, S.C.; Anjos, C.; Sá, E.; Chainho, P.; da Fonseca, L.C.; e Costa, P.F. Insight into aquaculture’s potential of marine annelid worms and ecological concerns: A review. Rev. Aquac. 2020, 12, 107–121. [Google Scholar] [CrossRef]
- Park, Y.R.; Park, C.-I.; Soh, Y. Antioxidant and Anti-Inflammatory Effects of NCW Peptide from Clam Worm (Marphysa sanguinea). J. Microbiol. Biotechnol. 2020, 30, 1387–1394. [Google Scholar] [CrossRef]
- Yeon, S.J.; Shim, K.H.; Hong, J.S.; Shin, H.S. Identification of a new serine protease from polychaeta, Marphysa sanguinea, for its thrombolytic and anticoagulant activity. Korean J. Chem. Eng. 2017, 34, 781–786. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Qiu, J.-W. A new species in the Marphysa sanguinea complex (Annelida, Eunicidae) from Hong Kong. Zool. Stud. 2018, 57, e48. [Google Scholar]
- Molina-Acevedo, I.C.; Carrera-Parra, L.F. Reinstatement of three species of the Marphysa sanguinea complex (Polychaeta: Eunicidae) from the Grand Caribbean Region. Zootaxa 2015, 3925, 37–55. [Google Scholar] [CrossRef]
- Lavesque, N.; Daffe, G.; Bonifácio, P.; Hutchings, P. A new species of the Marphysa sanguinea complex from French waters (Bay of Biscay, NE Atlantic) (Annelida, Eunicidae). ZooKeys 2017, 761, 1. [Google Scholar] [CrossRef]
- Prevedelli, D.; N’Siala, G.M.; Ansaloni, I.; Simonini, R. Life cycle of Marphysa sanguinea (Polychaeta: Eunicidae) in the Venice lagoon (Italy). Mar. Ecol. 2007, 28, 384–393. [Google Scholar] [CrossRef]
- Elbarhoumi, M.; Scaps, P.; Djediat, C.; Zghal, F. Ultrastructural study of oogenesis in Marphysa sanguinea (Annelida: Polychaeta: Eunicida) from the Lagoon of Tunis. Sci. Mar. 2014, 78, 99–113. [Google Scholar]
- Yang, D.; Chen, F.; Zhou, Y.; Xiu, Z. Diel variation in metabolism and ammonia excretion of Marphysa sanguinea (Polychaeta: Eunicidae). Chin. J. Oceanol. Limnol. 2016, 34, 1209–1217. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, Y.; Chen, A.; Cao, X.; Liu, Y. The artificial breeding in Marphysa sanguinea (MONTAGU) in a laboratory. Fish. Sci. 2011, 30, 572–574. [Google Scholar]
- Kim, K.H.; Kim, B.K.; Kim, S.K.; Phoo, W.W.; Maran, B.V.; Kim, C.-H. Appropriate feeding for early juvenile stages of eunicid polychaete Marphysa sanguinea. Fish. Aquat. Sci. 2017, 20, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gillooly, J.F.; Brown, J.H.; West, G.B.; Savage, V.M.; Charnov, E.L. Effects of size and temperature on metabolic rate. Science 2001, 293, 2248–2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Shumway, S.E. The effects of body size, oxygen tension and mode of life on the oxygen uptake rates of polychaetes. Comp. Biochem. Physiol. Part A Physiol. 1979, 64, 273–278. [Google Scholar] [CrossRef]
- Cammen, L.M. Ingestion rate: An empirical model for aquatic deposit feeders and detritivores. Oecologia 1979, 44, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.M.; Eriksen, N.T.; Iversen, J.L.; Riisgård, H.U. Feeding, growth and respiration in the polychaetes Nereis diversicolor (facultative filter-feeder) and N. virens (omnivorous)-a comparative study. Mar. Ecol. Prog. Ser. 1995, 125, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Xian, W.; Sun, S. Metabolism of polychaete Neanthes japonica Izuka: Relations to temperature, salinity and body weight. Chin. J. Oceanol. Limnol. 2009, 27, 356–364. [Google Scholar] [CrossRef]
- Wang, L.; Chen, A.; Zhao, X.; Wang, L.; Zhang, J.; Zhou, Y. Effect of temperature and body weight on respiration and excretion in Perinereis aibuhitensis Grube. J. Dalian Fish. Univ. 2004, 19, 176–181. (In Chinese) [Google Scholar]
- Galasso, H.L.; Richard, M.; Lefebvre, S.; Aliaume, C.; Callier, M.D. Body size and temperature effects on standard metabolic rate for determining metabolic scope for activity of the polychaete Hediste (Nereis) diversicolor. PeerJ 2018, 6, e5675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Cubber, L.; Lefebvre, S.; Lancelot, T.; Denis, L.; Gaudron, S.M. Annelid polychaetes experience metabolic acceleration as other Lophotrochozoans: Inferences on the life cycle of Arenicola marina with a Dynamic Energy Budget model. Ecol. Model. 2019, 411, 108773. [Google Scholar] [CrossRef]
- Montgomery, H.; Thom, N.; Cockburn, A. Determination of dissolved oxygen by the Winkler method and the solubility of oxygen in pure water and sea water. J. Appl. Chem. 1964, 14, 280–296. [Google Scholar] [CrossRef]
- Friedman, A.H.; Morgulis, S. The oxidation of amino acids with sodium hypobromite. J. Am. Chem. Soc. 1936, 58, 909–913. [Google Scholar] [CrossRef]
- Snow, N.; Williams, P.L. A simple method to determine the O: N ratio of small marine animals. J. Mar. Biol. Assoc. U. K. 1971, 51, 105–109. [Google Scholar] [CrossRef]
- Conover, R.; Mayzaud, P. Respiration and nitrogen excretion of neritic zooplankton in relation to potential food supply. In Proceedings of the 10th European Symposium on Marine Biology, Ostend, Belgium, 17–23 September 1975. [Google Scholar]
- Ikeda, T. Nutritional ecology of marine zooplankton. Mem. Fac. Fish. Hokkaido Univ. 1974, 22, 1–97. [Google Scholar]
- Kleiber, M. Body size and metabolic rate. Physiol. Rev. 1947, 27, 511–541. [Google Scholar] [CrossRef]
- Enquist, B.J.; Brown, J.H.; West, G.B. Allometric scaling of plant energetics and population density. Nature 1998, 395, 163–165. [Google Scholar] [CrossRef]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for the origin of allometric scaling laws in biology. Science 1997, 276, 122–126. [Google Scholar] [CrossRef]
- White, C.R.; Seymour, R.S. Mammalian basal metabolic rate is proportional to body mass2/3. Proc. Natl. Acad. Sci. USA 2003, 100, 4046–4049. [Google Scholar] [CrossRef] [Green Version]
- McKechnie, A.E.; Wolf, B.O. The allometry of avian basal metabolic rate: Good predictions need good data. Physiol. Biochem. Zool. 2004, 77, 502–521. [Google Scholar] [CrossRef] [Green Version]
- White, C.R.; Phillips, N.F.; Seymour, R.S. The scaling and temperature dependence of vertebrate metabolism. Biol. Lett. 2006, 2, 125–127. [Google Scholar] [CrossRef] [Green Version]
- Clarke, A.; Fraser, K. Why does metabolism scale with temperature? Funct. Ecol. 2004, 18, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Pörtner, H.O.; Knust, R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 2007, 315, 95–97. [Google Scholar] [CrossRef] [Green Version]
- Lasserre, P. Metabolic activities of benthic microfauna and meiofauna. In The Benthic Boundary Layer; Springer: Berlin/Heidelberg, Germany, 1976; pp. 95–142. [Google Scholar]
- Ott, J.; Schiemer, F. Respiration and anaerobiosis of free living nematodes from marine and limnic sediments. Neth. J. Sea Res. 1973, 7, 233–243. [Google Scholar] [CrossRef]
- Beis, I.; Manousis, A.; Barrett, J. Studies on the respiration of the polychaete Ophelia bicornis. Comp. Biochem. Physiol. Part A Physiol. 1980, 67, 303–305. [Google Scholar] [CrossRef]
- Clarke, A. Is there a universal temperature dependence of metabolism? Funct. Ecol. 2004, 18, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Brauer, V.S.; De Jonge, V.N.; Buma, A.G.; Weissing, F.J. Does universal temperature dependence apply to communities? An experimental test using natural marine plankton assemblages. Oikos 2009, 118, 1102–1108. [Google Scholar] [CrossRef]
- Gillooly, J.; Allen, A.; Savage, V.; Charnov, E.; West, G.; Brown, J. Response to Clarke and Fraser: Effects of temperature on metabolic rate. Funct. Ecol. 2006, 20, 400–404. [Google Scholar] [CrossRef]
- Dell, A.I.; Pawar, S.; Savage, V.M. Systematic variation in the temperature dependence of physiological and ecological traits. Proc. Natl. Acad. Sci. USA 2011, 108, 10591–10596. [Google Scholar] [CrossRef] [Green Version]
- Kooijman, B.; Kooijman, S. Dynamic Energy Budget Theory for Metabolic Organization; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- De Cubber, L.; Lefebvre, S.; Lancelot, T.; Duong, G.; Gaudron, S.M. Investigating down-shore migration effects on individual growth and reproduction of the ecosystem engineer Arenicola marina. J. Mar. Syst. 2020, 211, 103420. [Google Scholar] [CrossRef]
- Kraemer, B.M.; Chandra, S.; Dell, A.I.; Dix, M.; Kuusisto, E.; Livingstone, D.M.; Schladow, S.G.; Silow, E.; Sitoki, L.M.; Tamatamah, R. Global patterns in lake ecosystem responses to warming based on the temperature dependence of metabolism. Glob. Chang. Biol. 2017, 23, 1881–1890. [Google Scholar] [CrossRef]
- Clarke, A.; Johnston, N.M. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 1999, 68, 893–905. [Google Scholar] [CrossRef]
- Hansen, P.J.; Bjørnsen, P.K.; Hansen, B.W. Zooplankton grazing and growth: Scaling within the 2-2,-μm body size range. Limnol. Oceanogr. 1997, 42, 687–704. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Stephanopoulou, S.; Miliou, H.; Moraitou-Apostolopoulou, M.; Verriopoulos, G. Oxygen consumption and ammonia excretion of Octopus vulgaris (Cephalopoda) in relation to body mass and temperature. Mar. Biol. 2005, 146, 725–732. [Google Scholar] [CrossRef]
- Weber, S.B.; Blount, J.D.; Godley, B.J.; Witt, M.J.; Broderick, A.C. Rate of egg maturation in marine turtles exhibits ‘universal temperature dependence’. J. Anim. Ecol. 2011, 80, 1034–1041. [Google Scholar] [CrossRef] [Green Version]
- Katsanevakis, S.; Xanthopoulos, J.; Protopapas, N.; Verriopoulos, G. Oxygen consumption of the semi-terrestrial crab Pachygrapsus marmoratus in relation to body mass and temperature: An information theory approach. Mar. Biol. 2007, 151, 343–352. [Google Scholar] [CrossRef]
- Qian, P.-Y. Larval settlement of polychaetes. Reproductive strategies and developmental patterns in annelids. In Developments in Hydrobiology; Springer: Berlin/Heidelberg, Germany, 1999; pp. 239–253. [Google Scholar]
- Iles, A.C. Toward predicting community-level effects of climate: Relative temperature scaling of metabolic and ingestion rates. Ecology 2014, 95, 2657–2668. [Google Scholar] [CrossRef]
- Deutsch, C.; Ferrel, A.; Seibel, B.; Pörtner, H.-O.; Huey, R.B. Climate change tightens a metabolic constraint on marine habitats. Science 2015, 348, 1132–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
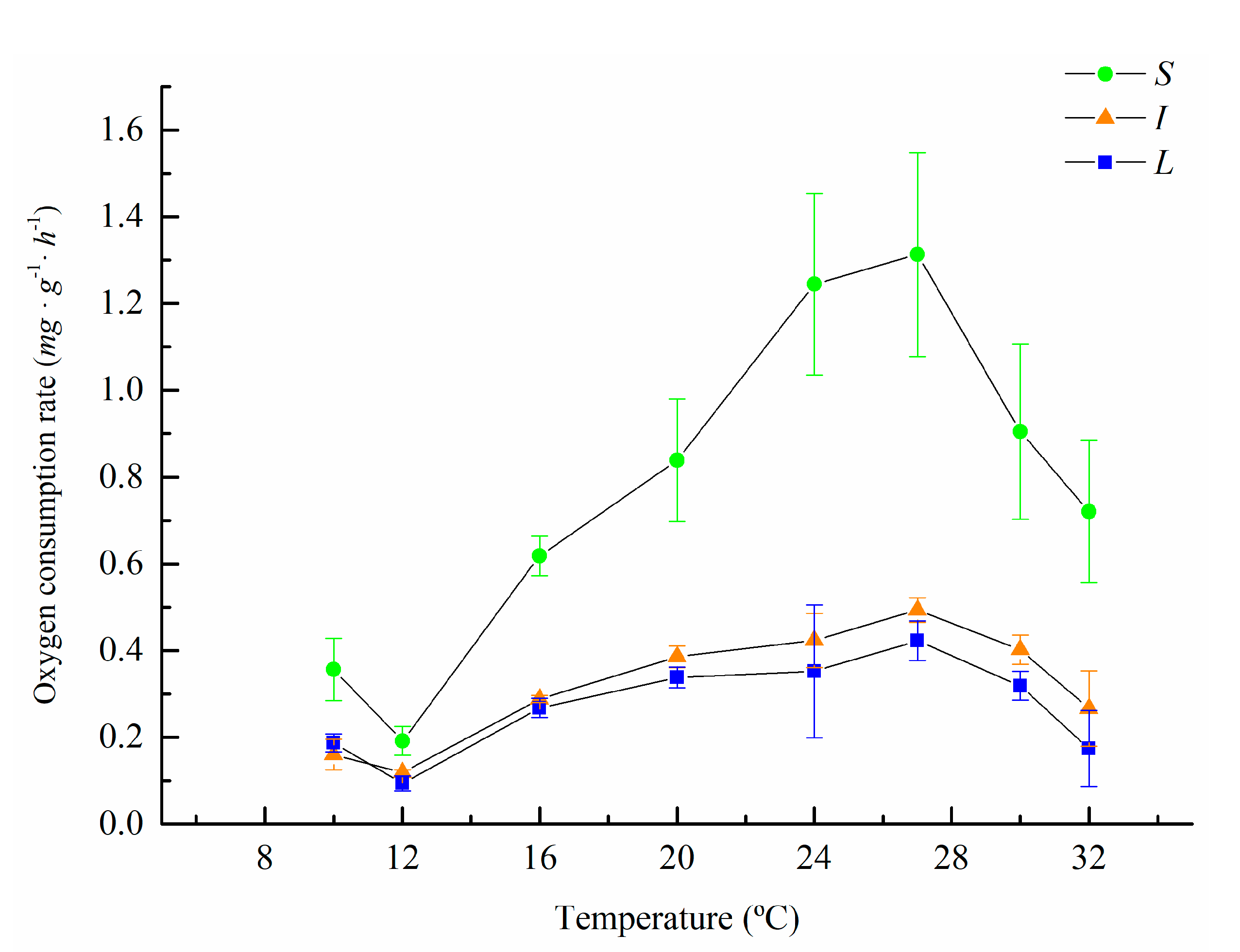
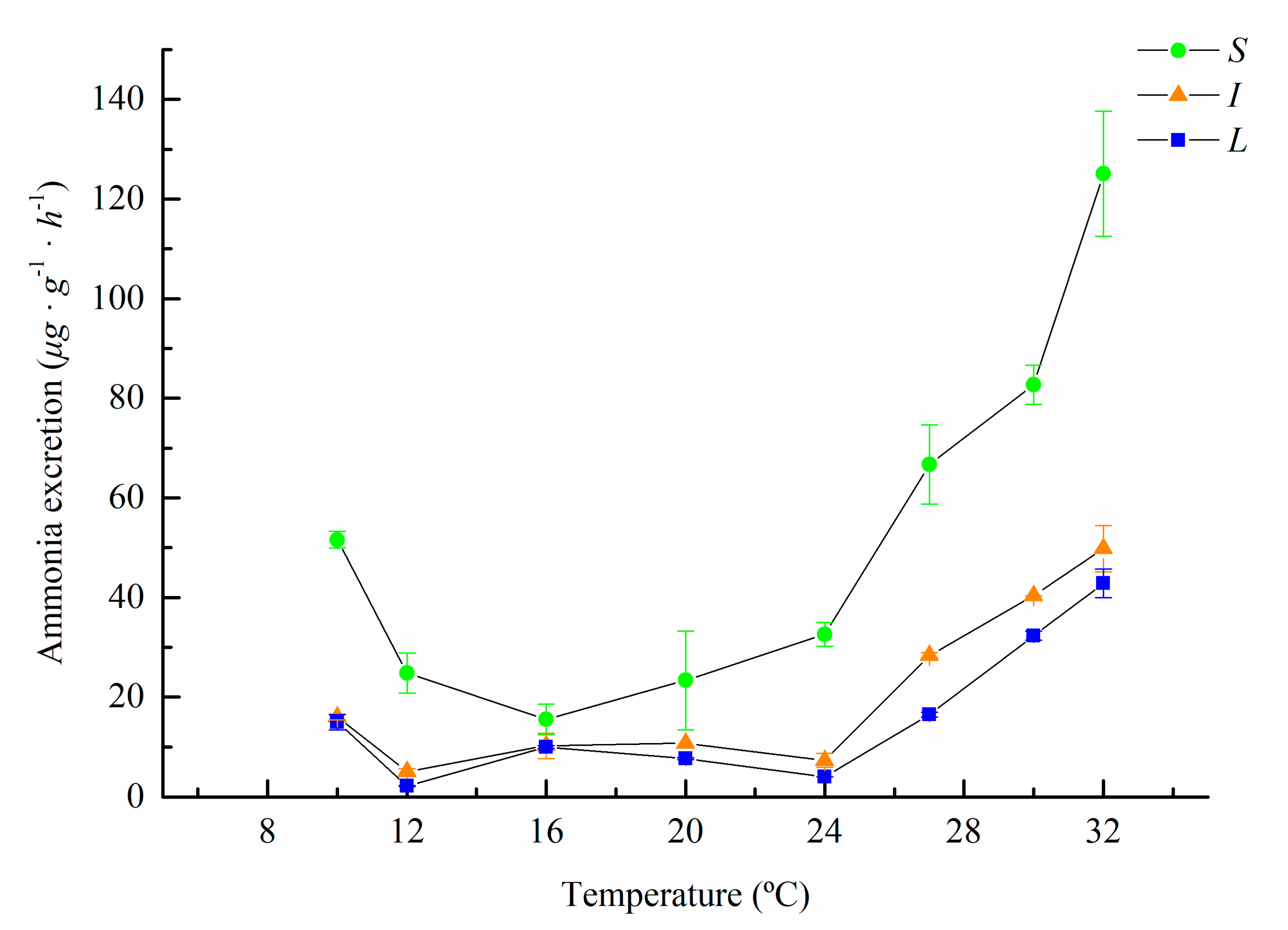
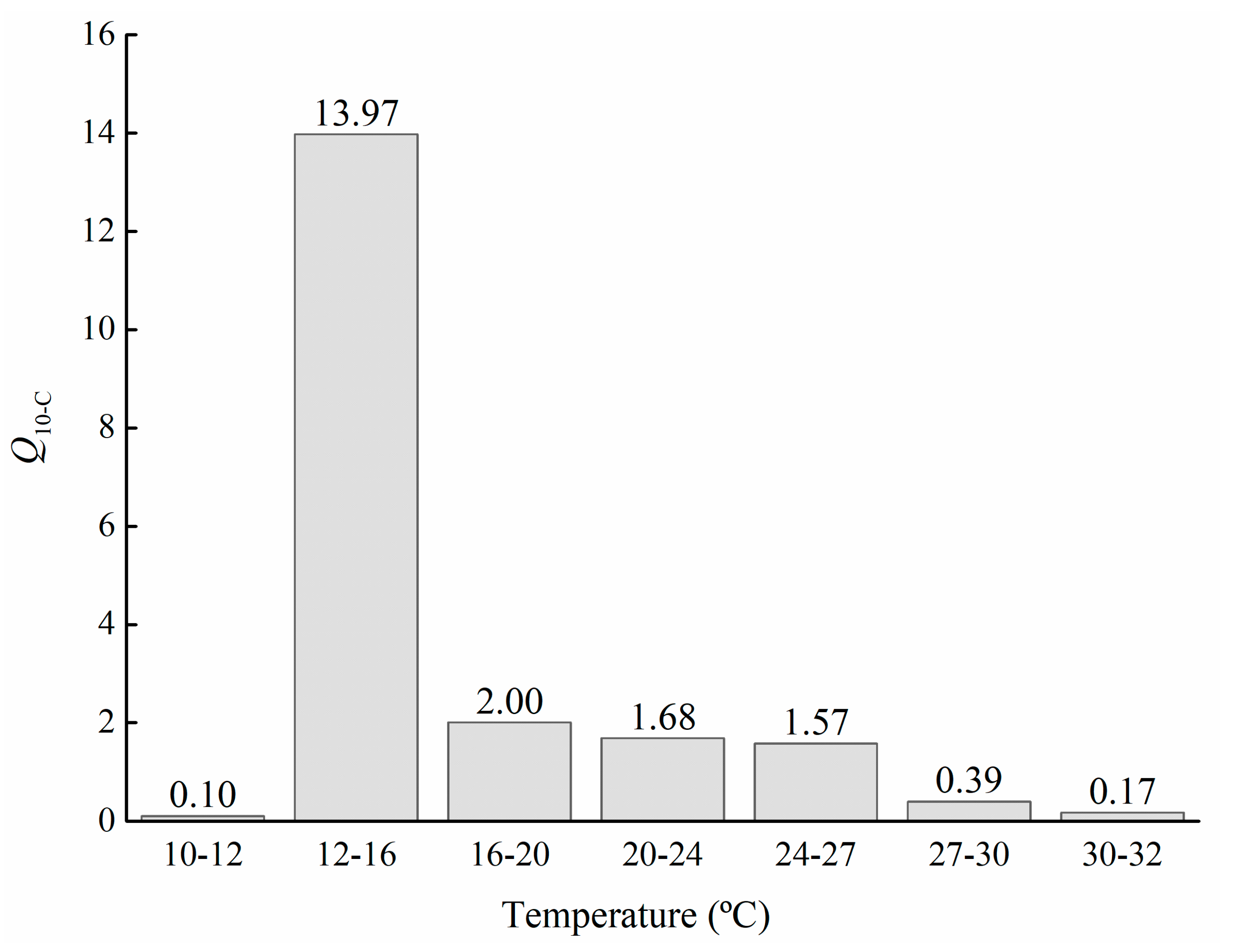
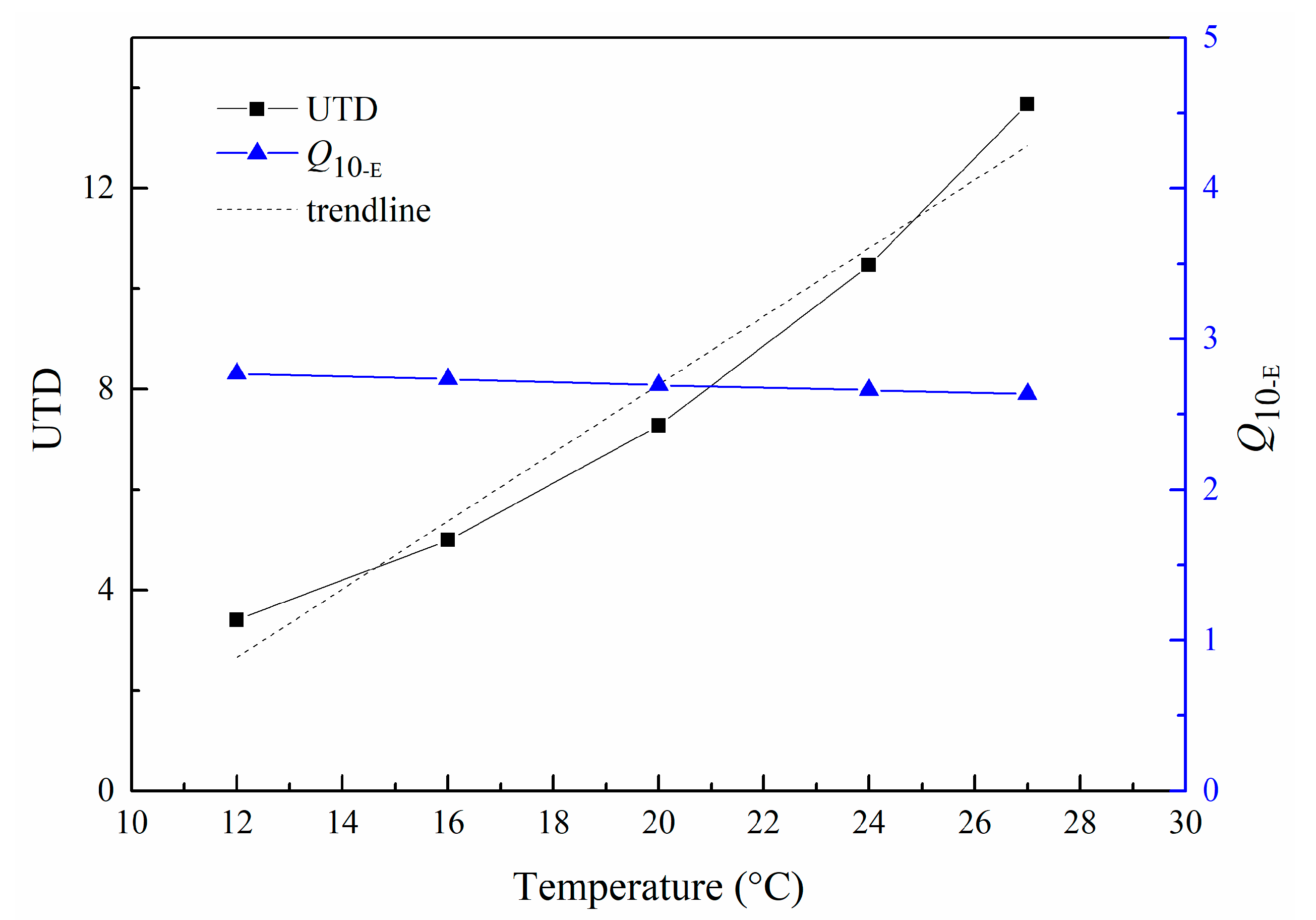
| Temperature (°C) | Body Weight Groups | ||
|---|---|---|---|
| S | I | L | |
| 10 | 6.04 | 8.75 | 10.94 |
| 12 | 6.75 | 20.46 | 37.01 |
| 16 | 34.85 | 24.68 | 23.47 |
| 20 | 31.43 | 31.49 | 38.48 |
| 24 | 35.43 | 50.55 | 56.44 |
| 27 | 17.22 | 15.20 | 22.40 |
| 30 | 9.57 | 8.71 | 8.63 |
| 32 | 5.04 | 4.68 | 3.55 |
| Temperature (°C) | Regression Equation | R2 |
|---|---|---|
| 12 | lnR = −2.1281 + 0.6876lnM | 0.9997 |
| 16 | lnR = −1.1052 + 0.6194lnM | 0.9705 |
| 20 | lnR = −0.8354 + 0.5908lnM | 0.9758 |
| 24 | lnR = −0.6968 + 0.4318lnM | 0.9183 |
| 27 | lnR = −0.5535 + 0.4890lnM | 0.9426 |
| Temperature (°C) | Regression Equation | R2 |
|---|---|---|
| 12 | lnU = 1.6246 − 0.0504lnM | 0.8956 |
| 16 | lnU = 2.4235 + 0.8016lnM | 0.9973 |
| 20 | lnU = 2.3882 + 0.5099lnM | 0.9893 |
| 24 | lnU = 2.0717 + 0.0834lnM | 0.5816 |
| 27 | lnU = 3.3072 + 0.3988lnM | 0.9968 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Kou, N.; Liu, X.; Yang, D. Oxygen Consumption and Ammonia Excretion of Marphysa sanguinea (Polychaeta: Eunicidae) in Relation to Body Mass and Temperature. Fishes 2021, 6, 52. https://doi.org/10.3390/fishes6040052
Wang C, Kou N, Liu X, Yang D. Oxygen Consumption and Ammonia Excretion of Marphysa sanguinea (Polychaeta: Eunicidae) in Relation to Body Mass and Temperature. Fishes. 2021; 6(4):52. https://doi.org/10.3390/fishes6040052
Chicago/Turabian StyleWang, Chengjian, Na Kou, Xiaowei Liu, and Dazuo Yang. 2021. "Oxygen Consumption and Ammonia Excretion of Marphysa sanguinea (Polychaeta: Eunicidae) in Relation to Body Mass and Temperature" Fishes 6, no. 4: 52. https://doi.org/10.3390/fishes6040052
APA StyleWang, C., Kou, N., Liu, X., & Yang, D. (2021). Oxygen Consumption and Ammonia Excretion of Marphysa sanguinea (Polychaeta: Eunicidae) in Relation to Body Mass and Temperature. Fishes, 6(4), 52. https://doi.org/10.3390/fishes6040052





