Growth, Chemical Composition, Histology and Antioxidant Genes of Atlantic Salmon (Salmo salar) Fed Whole or Pre-Processed Nannochloropsis oceanica and Tetraselmis sp.
Abstract
:1. Introduction
2. Results
2.1. Volatile Organic Compounds
2.2. Growth
2.3. Whole Body Proximate Composition
2.4. Histology
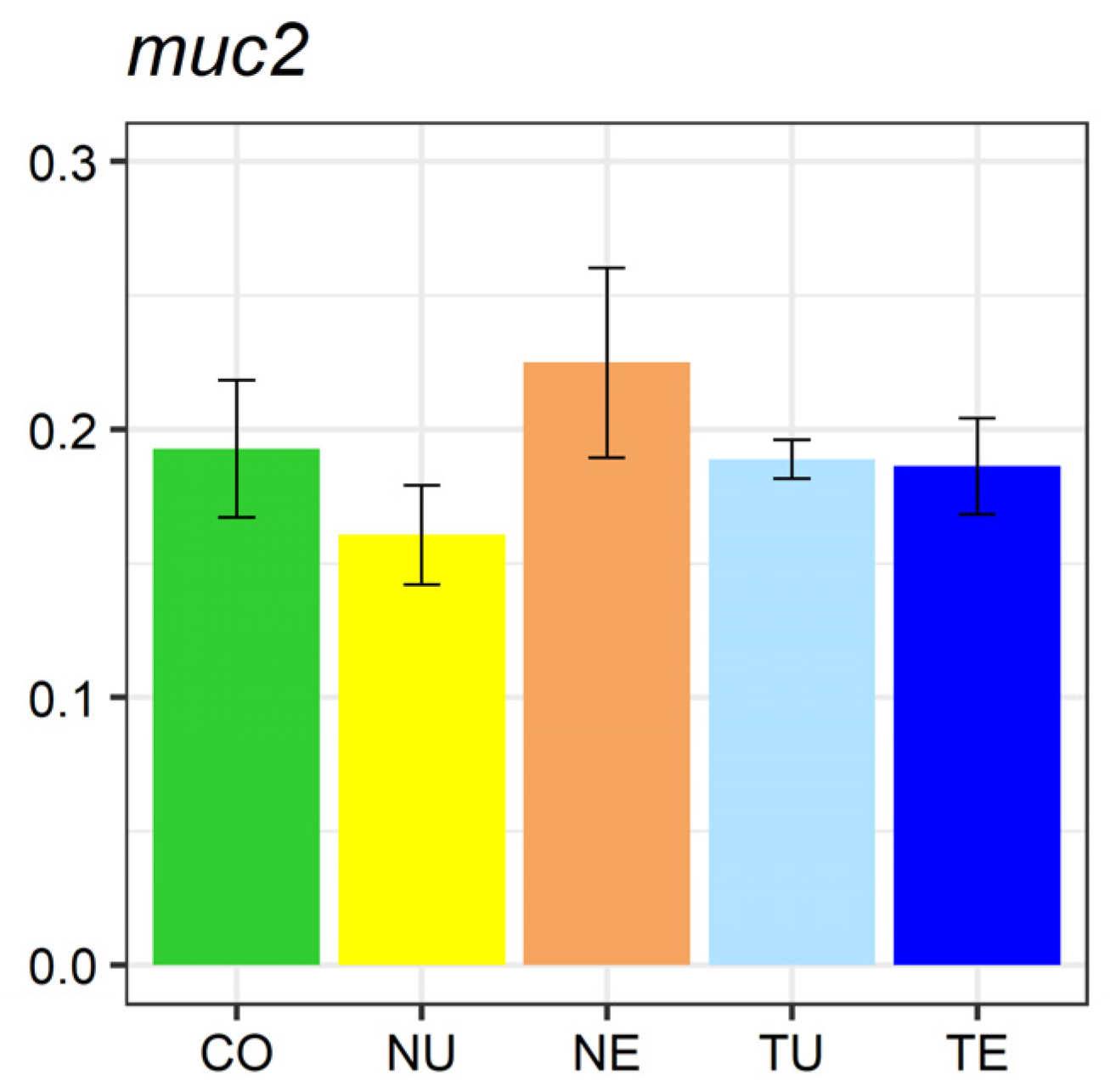
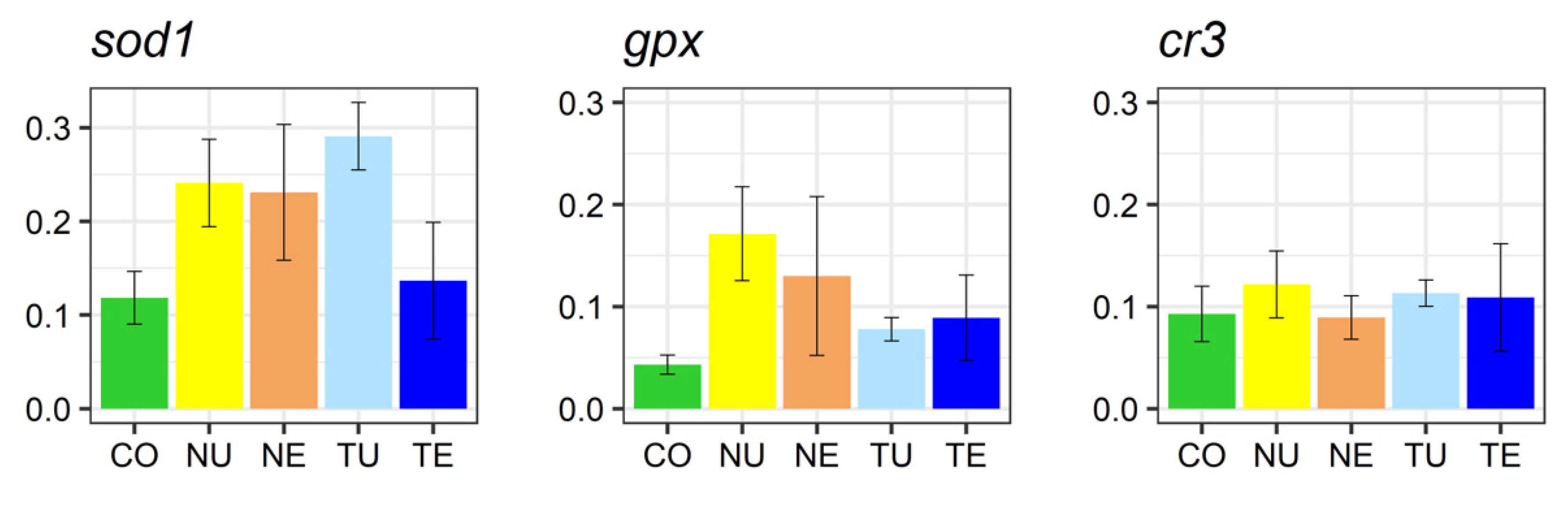
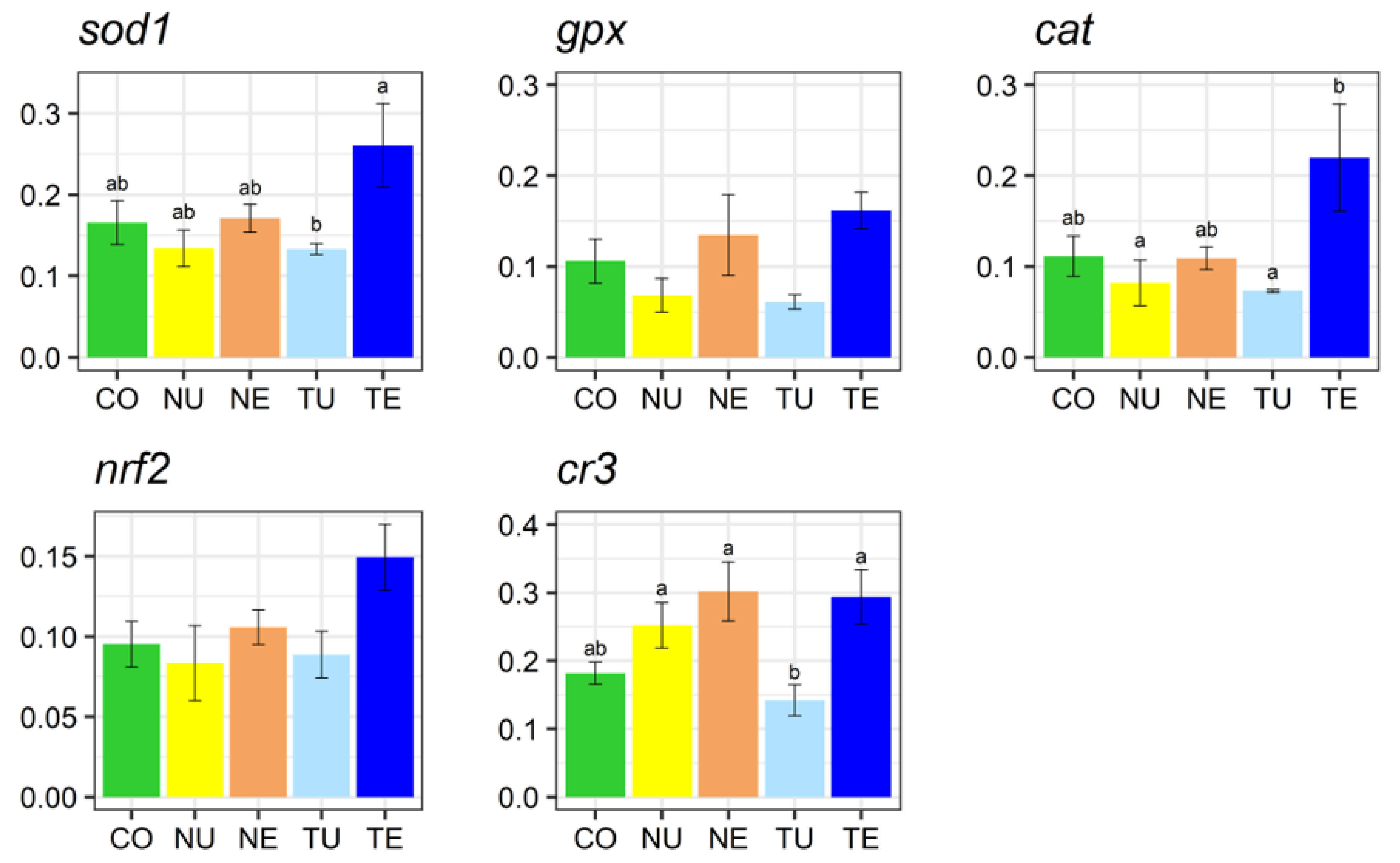
2.5. Gene Expression Analysis
3. Discussion
3.1. Growth and Chemical Composition
3.2. Intestinal Health
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Chowdhury, M.K.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in aquafeeds for a sustainable aquaculture industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- Gong, Y.; Guterres, H.A.D.S.; Huntley, M.; Sørensen, M.; Kiron, V. Digestibility of the defatted microalgae Nannochloropsis sp. and Desmodesmus sp. when fed to Atlantic salmon, Salmo salar. Aquac. Nutr. 2018, 24, 56–64. [Google Scholar] [CrossRef]
- Kiron, V.; Phromkunthong, W.; Huntley, M.; Archibald, I.; Scheemaker, G.d. Marine microalgae from biorefinery as a potential feed protein source for Atlantic salmon, common carp and whiteleg shrimp. Aquac. Nutr. 2012, 18, 521–531. [Google Scholar] [CrossRef]
- Kiron, V.; Sørensen, M.; Huntley, M.; Vasanth, G.K.; Gong, Y.; Dahle, D.; Palihawadana, A.M. Defatted biomass of the microalga, Desmodesmus sp., can replace fishmeal in the feeds for Atlantic salmon. Front. Mar. Sci. 2016, 3, 67. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, M.; Gong, Y.; Bjarnason, F.; Vasanth, G.K.; Dahle, D.; Huntley, M.; Kiron, V. Nannochloropsis oceanica-derived defatted meal as an alternative to fishmeal in Atlantic salmon feeds. PLoS ONE 2017, 12, e0179907. [Google Scholar] [CrossRef]
- Sørensen, M.; Berge, G.M.; Reitan, K.I.; Ruyter, B. Microalga Phaeodactylum tricornutum in feed for Atlantic salmon (Salmo salar)—Effect on nutrient digestibility, growth and utilization of feed. Aquaculture 2016, 460, 116–123. [Google Scholar] [CrossRef]
- Kousoulaki, K.; Østbye, T.-K.K.; Krasnov, A.; Torgersen, J.S.; Mørkøre, T.; Sweetman, J. Metabolism, health and fillet nutritional quality in Atlantic salmon (Salmo salar) fed diets containing n-3-rich microalgae. J. Nutr. Sci. 2015, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibbetts, S.M.; Mann, J.; Dumas, A. Apparent digestibility of nutrients, energy, essential amino acids and fatty acids of juvenile Atlantic salmon (Salmo salar L.) diets containing whole-cell or cell-ruptured Chlorella vulgaris meals at five dietary inclusion levels. Aquaculture 2017, 481, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Dineshbabu, G.; Goswami, G.; Kumar, R.; Sinha, A.; Das, D. Microalgae–nutritious, sustainable aqua-and animal feed source. J. Funct. Foods 2019, 62, 103545. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Pelaseyed, T.; Svensson, F.; Johansson, M.E.V. Study of mucin turnover in the small intestine by in vivo labeling. Sci. Rep. 2018, 8, 5760. [Google Scholar] [CrossRef] [PubMed]
- Kraimi, N.; Dawkins, M.; Gebhardt-Henrich, S.G.; Velge, P.; Rychlik, I.; Volf, J.; Creach, P.; Smith, A.; Colles, F.; Leterrier, C. Influence of the microbiota-gut-brain axis on behavior and welfare in farm animals: A review. Physiol. Behav. 2019, 210, 112658. [Google Scholar] [CrossRef] [PubMed]
- Bjørgen, H.; Li, Y.; Kortner, T.M.; Krogdahl, Å.; Koppang, E.O. Anatomy, immunology, digestive physiology and microbiota of the salmonid intestine: Knowns and unknowns under the impact of an expanding industrialized production. Fish. Shellfish Immunol. 2020, 107, 172–186. [Google Scholar] [CrossRef] [PubMed]
- McGuckin, M.A.; Lindén, S.K.; Sutton, P.; Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef]
- Dang, M.; Pittman, K.; Sonne, C.; Hansson, S.; Bach, L.; Søndergaard, J.; Stride, M.; Nowak, B. Histological mucous cell quantification and mucosal mapping reveal different aspects of mucous cell responses in gills and skin of shorthorn sculpins (Myoxocephalus scorpius). Fish. Shellfish Immunol. 2020, 100, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, M.J. Encyclopedia of Immunobiology; Academic Press: Cambridge, MA, USA, 2016; Available online: https://www.sciencedirect.com/referencework/9780080921525/encyclopedia-of-immunobiology (accessed on 22 February 2021).
- Thornton, D.J.; Sheehan, J.K. From mucins to mucus. Proc. Am. Thorac. Soc. 2004, 1, 54–61. [Google Scholar] [CrossRef]
- Linden, S.K.; Sutton, P.; Karlsson, N.G.; Korolik, V.; McGuckin, M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008, 1, 183–197. [Google Scholar] [CrossRef] [Green Version]
- Lang, T.; Klasson, S.; Larsson, E.; Johansson, M.E.V.; Hansson, G.C.; Samuelsson, T. Searching the evolutionary origin of epithelial mucus protein components—Mucins and FCGBP. Mol. Biol. Evol. 2016, 33, 1921–1936. [Google Scholar] [CrossRef]
- Lang, T.; Hansson, G.C.; Samuelsson, T. Gel-forming mucins appeared early in metazoan evolution. Proc. Natl. Acad. Sci. USA 2007, 104, 16209–16214. [Google Scholar] [CrossRef] [Green Version]
- Lang, T.; Alexandersson, M.; Hansson, G.C.; Samuelsson, T. Bioinformatic identification of polymerizing and transmembrane mucins in the puffer fish Fugu rubripes. Glycobiology 2004, 14, 521–527. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Sánchez, J.; Estensoro, I.; Redondo, M.J.; Calduch-Giner, J.A.; Kaushik, S.; Sitjà-Bobadilla, A. Mucins as diagnostic and prognostic biomarkers in a fish-parasite model: Transcriptional and functional analysis. PLoS ONE 2013, 8, e65457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Marel, M.; Adamek, M.; Gonzalez, S.F.; Frost, P.; Rombout, J.H.W.M.; Wiegertjes, G.F.; Savelkoul, H.F.J.; Steinhagen, D. Molecular cloning and expression of two β-defensin and two mucin genes in common carp (Cyprinus carpio L.) and their up-regulation after β-glucan feeding. Fish. Shellfish Immunol. 2012, 32, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Sveen, L.R.; Grammes, F.T.; Ytteborg, E.; Takle, H.; Jørgensen, S.M. Genome-wide analysis of Atlantic salmon (Salmo salar) mucin genes and their role as biomarkers. PLoS ONE 2017, 12, e0189103. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.R.; Mular, M.; Miller, I.; Farmer, C.; Trenerry, C. The vitamin content of microalgae used in aquaculture. J. Appl. Phycol. 1999, 11, 247–255. [Google Scholar] [CrossRef]
- Pataroa, G.; Carulloa, D.; Ferraria, G. PEF-assisted supercritical CO2 extraction of pigments from microalgae Nannochloropsis oceanica in a continuous flow system. Chem. Eng. 2019, 74. [Google Scholar] [CrossRef]
- Kokkali, M.; Martí-Quijal, F.J.; Taroncher, M.; Ruiz, M.-J.; Kousoulaki, K.; Barba, F.J. Improved extraction efficiency of antioxidant bioactive compounds from Tetraselmis chuii and Phaedoactylum tricornutum using pulsed electric fields. Molecules 2020, 25, 3921. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, S.M. The potential for ‘Next-Generation’, microalgae-based feed ingredients for salmonid aquaculture in context of the blue revolution. In Microalgal Biotechnol; IntechOpen: London, UK, 2018; Available online: https://www.intechopen.com/books/microalgal-biotechnology/the-potential-for-next-generation-microalgae-based-feed-ingredients-for-salmonid-aquaculture-in-cont (accessed on 3 February 2021).
- Lee, S.Y.; Cho, J.M.; Chang, Y.K.; Oh, Y.-K. Cell disruption and lipid extraction for microalgal biorefineries: A review. Bioresour. Technol. 2017, 244, 1317–1328. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Z.; Zhang, D.; Wang, Z.; Cong, W. Lipid extraction from Nannochloropsis oceanica biomass after extrusion pretreatment with twin-screw extruder: Optimization of processing parameters and comparison of lipid quality. Bioprocess. Biosyst. Eng. 2020, 43, 655–662. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, H.; Chen, S.; Wen, S.; Wu, X.; Zhang, D.; Yuan, Q.; Cong, W. Microalgal cell disruption via extrusion for the production of intracellular valuables. Energy 2018, 142, 339–345. [Google Scholar] [CrossRef]
- Teuling, E.; Schrama, J.W.; Gruppen, H.; Wierenga, P.A. Effect of cell wall characteristics on algae nutrient digestibility in Nile tilapia (Oreochromis niloticus) and African catfish (Clarus gariepinus). Aquaculture 2017, 479, 490–500. [Google Scholar] [CrossRef]
- Alhattab, M.; Kermanshahi-Pour, A.; Brooks, M.S.-L. Microalgae disruption techniques for product recovery: Influence of cell wall composition. J. Appl. Phycol. 2019, 31, 61–88. [Google Scholar] [CrossRef]
- Sarker, P.K.; Kapuscinski, A.R.; McKuin, B.; Fitzgerald, D.S.; Nash, H.M.; Greenwood, C. Microalgae-blend tilapia feed eliminates fishmeal and fish oil, improves growth, and is cost viable. Sci. Rep. 2020, 10, 19328. [Google Scholar] [CrossRef]
- Güroy, D.; Güroy, B.; Merrifield, D.L.; Ergün, S.; Tekinay, A.A.; Yiğit, M. Effect of dietary Ulva and Spirulina on weight loss and body composition of rainbow trout, Oncorhynchus mykiss (Walbaum), during a starvation period. J. Anim. Physiol. Anim. Nutr. 2011, 95, 320–327. [Google Scholar] [CrossRef]
- Barden, L.; Decker, E.A. Lipid oxidation in low-moisture food: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2467–2482. [Google Scholar] [CrossRef]
- Barriuso, B.; Astiasarán, I.; Ansorena, D. A review of analytical methods measuring lipid oxidation status in foods: A challenging task. Eur. Food Res. Technol. 2013, 236, 1–15. [Google Scholar] [CrossRef]
- Chen, Y.J.; Liu, Y.J.; Yang, H.J.; Yuan, Y.; Liu, F.J.; Tian, L.X.; Liang, G.Y.; Yuan, R.M. Effect of dietary oxidized fish oil on growth performance, body composition, antioxidant defence mechanism and liver histology of juvenile largemouth bass Micropterus salmoides. Aquac. Nutr. 2012, 18, 321–331. [Google Scholar] [CrossRef]
- Jasour, M.S.; Wagner, L.; Sundekilde, U.K.; Larsen, B.K.; Greco, I.; Orlien, V.; Olsen, K.; Rasmussen, H.T.; Hjermitslev, N.H.; Hammershøj, M.; et al. A comprehensive approach to assess feathermeal as an alternative protein source in aquafeed. J. Agric. Food Chem. 2017, 65, 10673–10684. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, R.S.; Blanchard, J.L.; Halpern, B.S.; Metian, M.; Froehlich, H.E. Global adoption of novel aquaculture feeds could substantially reduce forage fish demand by 2030. Nat. Food 2020, 1, 301–308. [Google Scholar] [CrossRef]
- Belghit, I.; Liland, N.S.; Waagbø, R.; Biancarosa, I.; Pelusio, N.; Li, Y.; Krogdahl, Å.; Lock, E.-J. Potential of insect-based diets for Atlantic salmon (Salmo salar). Aquaculture 2018, 491, 72–81. [Google Scholar] [CrossRef]
- Fisher, H.J.; Collins, S.A.; Hanson, C.; Mason, B.; Colombo, S.M.; Anderson, D.M. Black soldier fly larvae meal as a protein source in low fish meal diets for Atlantic salmon (Salmo salar). Aquaculture 2020, 521, 734978. [Google Scholar] [CrossRef]
- Belghit, I.; Waagbø, R.; Lock, E.-J.; Liland, N.S. Insect-based diets high in lauric acid reduce liver lipids in freshwater Atlantic salmon. Aquac. Nutr. 2019, 25, 343–357. [Google Scholar] [CrossRef] [Green Version]
- Kousoulaki, K.; Mørkøre, T.; Nengas, I.; Berge, R.K.; Sweetman, J. Microalgae and organic minerals enhance lipid retention efficiency and fillet quality in Atlantic salmon (Salmo salar L.). Aquaculture 2016, 451, 47–57. [Google Scholar] [CrossRef]
- Gong, Y.; Bandara, T.; Huntley, M.; Johnson, Z.I.; Dias, J.; Dahle, D.; Sørensen, M.; Kiron, V. Microalgae Scenedesmus sp. as a potential ingredient in low fishmeal diets for Atlantic salmon (Salmo salar L.). Aquaculture 2019, 501, 455–464. [Google Scholar] [CrossRef]
- Gong, Y.; Sørensen, S.L.; Dahle, D.; Nadanasabesan, N.; Dias, J.; Valente, L.M.P.; Sørensen, M.; Kiron, V. Approaches to improve utilization of Nannochloropsis oceanica in plant-based feeds for Atlantic salmon. Aquaculture 2020, 522, 735122. [Google Scholar] [CrossRef]
- Einen, O.; Roem, A.J. Dietary protein/energy ratios for Atlantic salmon in relation to fish size: Growth, feed utilization and slaughter quality. Aquac. Nutr. 1997, 3, 115–126. [Google Scholar] [CrossRef]
- Shearer, K.D. Factors affecting the proximate composition of cultured fishes with emphasis on salmonids. Aquaculture 1994, 119, 63–88. [Google Scholar] [CrossRef]
- Dallaire, V.; Lessard, P.; Vandenberg, G.; de la Noüe, J. Effect of algal incorporation on growth, survival and carcass composition of rainbow trout (Oncorhynchus mykiss) fry. Bioresour. Technol. 2007, 98, 1433–1439. [Google Scholar] [CrossRef]
- Mustafa, G.; Wakamatsu, S.; Takeda, T.-a.; Umino, T.; Nakagawa, H. Effects of algae meal as feed additive on growth, feed efficiency, and body composition in red sea bream. Fish. Sci. 1995, 61, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, M.C. Effect of processing on high-protein feedstuffs: A review. Biol.Wastes 1989, 29, 123–138. [Google Scholar] [CrossRef]
- Pickford, J.R. Effects of processing on the stability of heat labile nutrients in animal feeds. In Recent Advances in Animal Nutrition; Garnsworthy, P.C., Haresign, W., Cole, D.J.A., Eds.; Redwood Press: Melksham, UK, 1992; Available online: https://agris.fao.org/agris-search/search.do?recordID=US201301481830 (accessed on 22 January 2021).
- Bender, A.E. Food Processing and Nutrition; Academic Press Inc. (London) Ltd.: London, UK, 1978; p. 243. Available online: http://saspjournals.com/wp-content/uploads/2015/08/SJAVS-24A304-311.pdf (accessed on 20 February 2021).
- Deng, D.-F.; Hemre, G.-I.; Storebakken, T.; Shiau, S.-Y.; Hung, S.S.O. Utilization of diets with hydrolyzed potato starch, or glucose by juvenile white sturgeon (Acipenser transmontanus), as affected by Maillard reaction during feed processing. Aquaculture 2005, 248, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Opstvedt, J.; Miller, R.; Hardy, R.W.; Spinelli, J. Heat-induced changes in sulfhydryl groups and disulfide bonds in fish protein and their effect on protein and amino acid digestibility in rainbow trout (Salmo gairdneri). J. Agric. Food Chem. 1984, 32, 929–935. [Google Scholar] [CrossRef]
- Andorsdòttir, G. Protein Quality, Methionine Supplementation and Fat Levels in Starter Diets for Salmon Fry. Ph.D. Thesis, University of Oslo, Oslo, Norway, 1985. [Google Scholar]
- Ljøkjel, K.; Sørensen, M.; Storebakken, T.; Skrede, A. Digestibility of protein, amino acids and starch in mink (Mustela vison) fed diets processed by different extrusion conditions. Can. J. Anim. Sci. 2004, 84, 673–680. [Google Scholar] [CrossRef] [Green Version]
- Ljøkjel, K.; Harstad, O.M.; Skrede, A. Effect of heat treatment of soybean meal and fish meal on amino acid digestibility in mink and dairy cows. Anim. Feed Sci. Technol. 2000, 84, 83–95. [Google Scholar] [CrossRef]
- Hurrell, R.F. Reactions of food proteins during processing and storage and their nutritional consequences. In Developments in Food Proteins—3; Hudson, B.J.F., Ed.; Elsevier: London, UK, 1984; pp. 213–244. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201302567346 (accessed on 15 January 2021).
- Sissener, N.H. Are we what we eat? Changes to the feed fatty acid composition of farmed salmon and its effects through the food chain. J. Exp. Biol. 2018, 221, jeb161521. [Google Scholar] [CrossRef] [Green Version]
- Sprague, M.; Dick, J.R.; Tocher, D.R. Impact of sustainable feeds on omega-3 long-chain fatty acid levels in farmed Atlantic salmon, 2006–2015. Sci. Rep. 2016, 6, 21892. [Google Scholar] [CrossRef] [Green Version]
- Teimouri, M.; Yeganeh, S.; Amirkolaie, A.K. The effects of Spirulina platensis meal on proximate composition, fatty acid profile and lipid peroxidation of rainbow trout (Oncorhynchus mykiss) muscle. Aquac. Nutr. 2016, 22, 559–566. [Google Scholar] [CrossRef]
- Mizambwa, H.E. Effects of Replacing Fish Oil with Microalgae Biomass (Schizochytrium spp.) as a Source of n-3 LC-PUFA to Atlantic salmon (Salmo salar) on Growth Performance, Fillet Quality and Fatty Acid Composition. Master’s Thesis, Norwegian University of Life Sciences, Ås, Norway, 2017. [Google Scholar]
- Walker, A.B.; Berlinsky, D.L. Effects of partial replacement of fish meal protein by microalgae on growth, feed intake, and body composition of Atlantic cod. N. Am. J. Aquac. 2011, 73, 76–83. [Google Scholar] [CrossRef]
- Baeverfjord, G.; Krogdahl, A. Development and regression of soybean meal induced enteritis in Atlantic salmon, Salmo salar L., distal intestine: A comparison with the intestines of fasted fish. J. Fish. Dis. 1996, 19, 375–387. [Google Scholar] [CrossRef]
- Sørensen, S.L.; Park, Y.; Gong, Y.; Vasanth, G.K.; Dahle, D.; Korsnes, K.; Phuong, T.H.; Kiron, V.; Øyen, S.; Pittman, K.; et al. Nutrient digestibility, growth, mucosal barrier status, and activity of leucocytes from head kidney of Atlantic salmon fed marine- or plant-derived protein and lipid sources. Front. Immunol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Bou, M.; Berge, G.M.; Baeverfjord, G.; Sigholt, T.; Østbye, T.-K.; Romarheim, O.H.; Hatlen, B.; Leeuwis, R.; Venegas, C.; Ruyter, B. Requirements of n-3 very long-chain PUFA in Atlantic salmon (Salmo salar L.): Effects of different dietary levels of EPA and DHA on fish performance and tissue composition and integrity. Br. J. Nutr. 2017, 117, 30–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benoit, B.; Bruno, J.; Kayal, F.; Estienne, M.; Debard, C.; Ducroc, R.; Plaisancié, P. Saturated and unsaturated fatty acids differently modulate colonic goblet cells in vitro and in rat pups. J. Nutr. 2015, 145, 1754–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messina, M.; Bulfon, C.; Beraldo, P.; Tibaldi, E.; Cardinaletti, G. Intestinal morpho-physiology and innate immune status of European sea bass (Dicentrarchus labrax) in response to diets including a blend of two marine microalgae, Tisochrysis lutea and Tetraselmis suecica. Aquaculture 2019, 500, 660–669. [Google Scholar] [CrossRef]
- Cerezuela, R.; Fumanal, M.; Tapia-Paniagua, S.T.; Meseguer, J.; Moriñigo, M.Á.; Esteban, M.Á. Histological alterations and microbial ecology of the intestine in gilthead seabream (Sparus aurata L.) fed dietary probiotics and microalgae. Cell Tissue Res. 2012, 350, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Kousoulaki, K.; Berge, G.M.; Mørkøre, T.; Krasnov, A.; Baeverfjord, G.; Ytrestøyl, T.; Carlehög, M.; Sweetman, J.; Ruyter, B. Microalgal Schizochytrium limacinum biomass improves growth and filet quality when used long-term as a replacement for fish oil, in modern salmon diets. Front. Mar. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Maradonna, F.; Nozzi, V.; Santangeli, S.; Traversi, I.; Gallo, P.; Fattore, E.; Mita, D.G.; Mandich, A.; Carnevali, O. Xenobiotic-contaminated diets affect hepatic lipid metabolism: Implications for liver steatosis in Sparus aurata juveniles. Aquat. Toxicol. 2015, 167, 257–264. [Google Scholar] [CrossRef]
- Marcon, L.; Bazzoli, N.; Honor Mounteer, A.; Anjos Benjamin, L.D. Histological and histometric evaluation of the liver in Astyanax bimaculatus (Teleostei: Characidae), exposed to different concentrations of an organochlorine insecticide. Anat. Rec. 2015, 298, 1754–1764. [Google Scholar] [CrossRef] [Green Version]
- Vizcaíno, A.J.; Fumanal, M.; Sáez, M.I.; Martínez, T.F.; Moriñigo, M.A.; Fernández-Díaz, C.; Anguis, V.; Balebona, M.C.; Alarcón, F.J. Evaluation of Ulva ohnoi as functional dietary ingredient in juvenile Senegalese sole (Solea senegalensis): Effects on the structure and functionality of the intestinal mucosa. Algal Res. 2019, 42, 101608. [Google Scholar] [CrossRef]
- Skalli, A.; Firmino, J.P.; Andree, K.B.; Salomón, R.; Estévez, A.; Puig, P.; Sabater-Martínez, M.; Hechavarria, T.; Gisbert, E. The inclusion of the microalga Scenedesmus sp. in diets for rainbow trout, Onchorhynchus mykiss, juveniles. Animals 2020, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Jasour, M.S.; Wagner, L.; Sundekilde, U.K.; Larsen, B.K.; Rasmussen, H.T.; Hjermitslev, N.H.; Hammershøj, M.; Dalsgaard, A.J.T.; Dalsgaard, T.K. Fishmeal with different levels of biogenic amines in aquafeed: Comparison of feed protein quality, fish growth performance, and metabolism. Aquaculture 2018, 488, 80–89. [Google Scholar] [CrossRef]
- Caballero, M.J.; Obach, A.; Rosenlund, G.; Montero, D.; Gisvold, M.; Izquierdo, M.S. Impact of different dietary lipid sources on growth, lipid digestibility, tissue fatty acid composition and histology of rainbow trout, Oncorhynchus mykiss. Aquaculture 2002, 214, 253–271. [Google Scholar] [CrossRef]
- Tao, Y.-F.; Qiang, J.; Bao, J.-W.; Chen, D.-J.; Yin, G.-J.; Xu, P.; Zhu, H.-J. Changes in physiological parameters, lipid metabolism, and expression of microRNAs in Genetically Improved Farmed Tilapia (Oreochromis niloticus) with fatty liver induced by a high-fat diet. Front. Physiol. 2018, 9, 1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliakbarpour, H.R.; Chamani, M.; Rahimi, G.; Sadeghi, A.A.; Qujeq, D. The Bacillus subtilis and lactic acid bacteria probiotics influences intestinal mucin gene expression, histomorphology and growth performance in broilers. Asian-Australas. J. Anim. Sci. 2012, 25, 1285–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayed, A.E.H.; Authman, M.M.N. The protective role of Spirulina platensis to alleviate the sodium dodecyl sulfate toxic effects in the catfish Clarias gariepinus (Burchell, 1822). Ecotoxicol. Environ. Saf. 2018, 163, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.; Risha, E. Modulatory role of dietary Chlorella vulgaris powder against arsenic-induced immunotoxicity and oxidative stress in Nile tilapia (Oreochromis niloticus). Fish. Shellfish Immunol. 2014, 41, 654–662. [Google Scholar] [CrossRef]
- Perlemuter, G.; Davit-Spraul, A.; Cosson, C.; Conti, M.; Bigorgne, A.; Paradis, V.; Corre, M.-P.; Prat, L.; Kuoch, V.; Basdevant, A.; et al. Increase in liver antioxidant enzyme activities in non-alcoholic fatty liver disease. Liver Int. 2005, 25, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Tsuru, H.; Osaka, M.; Hiraoka, Y.; Yoshida, M. HFD-induced hepatic lipid accumulation and inflammation are decreased in Factor D deficient mouse. Sci. Rep. 2020, 10, 17593. [Google Scholar] [CrossRef]
- Vorup-Jensen, T.; Jensen, R.K. Structural immunology of complement receptors 3 and 4. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Storebakken, T.; Sørensen, M.; Bjerkeng, B.; Harris, J.; Monahan, P.; Hiu, S. Stability of astaxanthin from red yeast, Xanthophyllomyces dendrorhous, during feed processing: Effects of enzymatic cell wall disruption and extrusion temperature. Aquaculture 2004, 231, 489–500. [Google Scholar] [CrossRef]
- Martínez-Yusta, A.; Goicoechea, E.; Guillén, M.D. A review of thermo-oxidative degradation of food lipids studied by 1H NMR spectroscopy: Influence of degradative conditions and food lipid nature. Compr. Rev. Food Sci. Food Saf. 2014, 13, 838–859. [Google Scholar] [CrossRef]
- Shahidi, F. Headspace volatile aldehydes as indicators of lipid oxidation in foods. In Headspace Analysis of Foods and Flavors: Theory and Practice; Rouseff, R.L., Cadwallader, K.R., Eds.; Springer: Boston, MA, USA, 2001; pp. 113–123. [Google Scholar] [CrossRef]
- Riaz, M.N.; Rokey, G.J. (Eds.) Impact of particle size and other ingredients on extruded foods and feeds. In Extrusion Problems Solved; Woodhead Publishing: Cambridge, UK, 2012; pp. 55–63. [Google Scholar] [CrossRef]
- Paraman, I.; Wagner, M.E.; Rizvi, S.S.H. Micronutrient and protein-fortified whole grain puffed rice made by supercritical fluid extrusion. J. Agric. Food Chem. 2012, 60, 11188–11194. [Google Scholar] [CrossRef] [PubMed]
- Standard Norge. Atlantic Salmon Measurement of Colour and Fat. NS9401/9402. 1994, p. 5. Available online: https://www.standard.no/no/nettbutikk/produktkatalogen/produktpresentasjon/?ProductID=135397 (accessed on 3 February 2021).
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metcalfe, L.D.; Schmitz, A.A.; Pelka, J.R. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal. Chem. 1966, 38, 514–515. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Diet Groups | CO | NU | NE | TU | TE | |
|---|---|---|---|---|---|---|
| VOCs | ||||||
| SUM LMW Amines | 790 | 694 | 722 | 595 | 657 | |
| Propanal | 5 | 6 | 2 | 7 | 12 | |
| Propanal, 2-methyl- | 27 | 49 | 96 | 33 | 45 | |
| 2-Propenal | 0 | 3 | 9 | 2 | 2 | |
| Butanal | 18 | 33 | 37 | 33 | 39 | |
| Butanal, 2-methyl- | 53 | 101 | 4 | 72 | 102 | |
| Butanal, 3-methyl- | 310 | 651 | 406 | 372 | 550 | |
| 1-Penten-3-one | 33 | 38 | 62 | 31 | 35 | |
| 2-Butenal | 7 | 33 | 77 | 13 | 16 | |
| Hexanal | 51 | 107 | 116 | 48 | 77 | |
| 2-Pentenal, (E)- | 16 | 32 | 98 | 16 | 34 | |
| 1-Penten-3-ol | 307 | 428 | 678 | 244 | 580 | |
| 2-Hexenal, (E)- | 43 | 80 | 72 | 36 | 82 | |
| 2-Penten-1-ol, (E)- | 135 | 57 | 189 | 5 | 170 | |
| Growth Indices | CO | NU | NE | TU | TE |
|---|---|---|---|---|---|
| IBW(g) | 154.4 ± 0.1 | 154.0 ± 0.1 | 153.9 ± 0.3 | 154.4 ± 0.2 | 154.3 ± 0.2 |
| FBW (g) | 307.8 ± 1.6 a | 288.9 ± 5.0 b | 292.9 ± 1.7 ab | 282.9 ± 3.4 b | 285.1 ± 3.6 b |
| WG (%) | 99.4 ± 2.0 a | 87.6 ± 6.3 b | 90.3 ± 3.0 ab | 83.3 ± 4.3 b | 84.8 ± 4.8 b |
| SGR (% day−1) | 1.15 ± 0.01 a | 1.05 ± 0.03 b | 1.07 ± 0.01 ab | 1.01 ± 0.02 b | 1.02 ± 0.02 b |
| Parameter | CO | NU | NE | TU | TE |
| Dry matter (g/kg) | 28.8 ± 0.09 a | 28.8 ± 0.19 ac | 29.6 ± 0.11 b | 29.3 ± 0.16 abc | 29.6 ± 0.28 bc |
| Dry matter (%) | |||||
| Protein | 62.6 ± 0.42 a | 61.1 ± 0.45 abc | 60.4 ± 0.29 bc | 61.4 ± 0.6 ac | 59.6 ± 0.43 b |
| Lipid | 32.5 ± 0.54 ab | 31.7 ± 0.53 a | 32.0 ± 0.3 a | 32.0 ± 0.57 a | 35.0 ± 0.54 b |
| Ash | 6.8 ± 0.09 ab | 7.0 ± 0.13 a | 7.0 ± 0.21 ab | 6.1 ± 0.2 bc | 5.6 ± 0.05 c |
| Energy | 25.6 ± 0.07 a | 25.6 ± 0.02 a | 25.6 ± 0.06 a | 26.1 ± 0.11 b | 26.3 ± 0.13 b |
| Diet Groups | CO | NU | NE | TU | TE | |
|---|---|---|---|---|---|---|
| Fatty Acids | ||||||
| C14:0 | 3.1 ± 0.04 ab | 3.1 ± 0.02 b | 3.3 ± 0.01 a | 3.4 ± 0.4 b | 3.1 ± 0.02 b | |
| C16:0 | 13.0 ± 0.18 a | 13.0 ± 0.13 a | 14.5 ± 0.26 b | 12.5 ± 0.67 a | 13.8 ± 0.23 ab | |
| C18:0 | 2.9 ± 0.05 a | 2.8 ± 0.05 ab | 2.9 ± 0.05 a | 2.7 ± 0.07 b | 2.9 ± 0.05 ab | |
| ∑SFA | 19.0 ± 0.24 a | 18.8 ± 0.15 a | 20.7 ± 0.31 b | 17.98 ± 0.94 a | 19.70 ± 0.27 ab | |
| C16:1 | 3.4 ± 0.01 ab | 4.0 ± 0.04 ac | 4.6 ± 0.05 c | 3.2 ± 0.03 b | 3.4 ± 0.07 ab | |
| C18:1n-9 | 21.4 ± 0.51 ab | 22.8 ± 0.2 b | 20.6 ± 0.39 a | 19.5 ± 1.67 a | 22.7 ± 0.22 b | |
| C18:1n-7 | 2.8 ± 0.01 ab | 2.9 ± 0.02 b | 2.7 ± 0.02 a | 2.7 ± 0.04 a | 2.7 ± 0.01 a | |
| C20:1n-9 | 3.8 ± 0.15 a | 4.3± 0.02 b | 3.9 ± 0.09 a | 4.0 ± 0.04 a | 4.1 ± 0.06 ab | |
| C22:1n-9 | 3.8 ± 0.02 a | 4.3 ± 0.03 b | 3.9 ± 0.04 abc | 3.8 ± 0.07 ac | 3.9 ± 0.01 bc | |
| ∑ MUFA | 35.1 ± 0.56 ab | 38.3 ± 0.23 c | 35.7 ± 0.38 ab | 29.8 ± 5.08 b | 36.9 ± 0.20 ac | |
| C18:2n-6 | 6.8 ± 0.19 a | 7.7 ± 0.15 b | 7.1 ± 0.15 a | 6.8 ± 0.33 a | 7.3 ± 0.12 ab | |
| C18:3n-3 | 2.3 ± 0.20 ab | 2.3 ± 0.08 ab | 2.1 ± 0.06 a | 2.8 ± 0.05 bc | 3.2 ± 0.17 c | |
| C18:4n-3 | 4.4 ± 0.28 a | 1.3 ± 0.01 b | 1.2 ± 0.03 b | 1.4 ± 0.02 ab | 4.1 ± 0.11 a | |
| C20:4n-3 | 1.1 ± 0.05 abc | 1.0 ± 0.00 ab | 1.0 ± 0.01 a | 1.3 ± 0.03 bc | 1.3 ± 0.01 c | |
| C20:5n-3 | 4.9 ± 0.62 a | 5.4 ± 0.25 ab | 6.5 ± 0.16 b | 4.9 ± 0.40 a | 5.2 ± 0.08 a | |
| C22:5n-3 | 1.5 ± 0.09 a | 1.1 ± 0.07 b | 1.2 ± 0.06 b | 2.2± 1.03 ab | 1.2 ± 0.09 ab | |
| C22:6n-3 | 21.4 ± 0.51 ab | 22.8 ± 0.22 b | 20.6 ± 0.39 a | 19.5 ± 1.67 a | 22.7 ± 0.22 b | |
| ∑ PUFA | 34.2 ± 4.77 | 34.1 ± 0.25 | 29.9 ± 3.95 | 34.4± 0.91 | 33.1 ± 3.97 | |
| ∑ n-6FA | 6.8 ± 0.19 a | 7.7 ± 0.15 b | 7.1 ± 0.15 a | 6.8 ± 0.33 a | 7.4 ± 0.12 ab | |
| ∑ n-3FA | 27.4 ± 4.59 a | 26.3 ± 0.18 b | 22.9 ± 4.03 ab | 27.6 ± 0.65 ab | 25.8 ± 3.87 ab | |
| ∑ EPA+DHA | 18.6 ± 4.30 a | 20.8 ± 0.11 ab | 17.6 ± 3.71 ab | 21.3 ± 0.50 a | 16.0 ± 3.55 b | |
| ∑ n-6/n-3 | 0.28 ± 0.06 a | 0.29 ± 0.01 b | 0.36 ± 0.10 ab | 0.24 ± 0.01 a | 0.31 ± 0.06 ab | |
| Diet Groups | CO | NU | NE | TU | TE | |
|---|---|---|---|---|---|---|
| Ingredients (%) | ||||||
| Fishmeal LT70 1 | 52.0 | 36.4 | 36.4 | 36.4 | 36.4 | |
| Nannochloropsis 2 | 30.0 | |||||
| Nannochloropsis (pre-extruded) 2 | 30.0 | |||||
| Tetraselmis2 | 30.0 | |||||
| Tetraselmis (pre-extruded) 2 | 30.0 | |||||
| Wheat gluten 3 | 15.0 | 10.5 | 10.5 | 10.5 | 10.5 | |
| Wheat meal 4 | 8.480 | 5.936 | 5.936 | 5.936 | 5.936 | |
| Pea starch | 6.0 | 4.2 | 4.2 | 4.2 | 4.2 | |
| Fish oil 5 | 17.50 | 12.25 | 12.25 | 12.25 | 12.25 | |
| Vitamin Mineral Premix PV01 6 | 1.0 | 0.7 | 0.7 | 0.7 | 0.7 | |
| Proximate composition (g/100 g) | ||||||
| Dry matter (DM) | 92.6 | 92.5 | 91.9 | 93.4 | 90.4 | |
| Per 100 g DM | ||||||
| Protein | 52.4 | 47.0 | 47.0 | 44.8 | 45.3 | |
| Lipid | 20.9 | 18.4 | 18.8 | 16.0 | 17.5 | |
| Ash | 8.7 | 17.2 | 16.9 | 16.6 | 16.1 | |
| Carbohydrates | 18.0 | 17.4 | 17.3 | 22.6 | 21.1 | |
| Energy (MJ/1000 g) | 23.4 | 21.5 | 21.7 | 20.7 | 21.2 | |
| Gene Name | Primer Sequence | Amplicon Size (bp) | PCR Efficiency (%) | Accession # (UniProt/GenBank) |
|---|---|---|---|---|
| Target genes | ||||
| muc2(.1/2) | GAGTGGGCTCTCAGATCCAG-F | 99 | 96.8 | XM_014184683.1/XM_014170386.1 |
| GATGATGCGGACGGTAGTTT-R | ||||
| muc5ac1 | GACCTGCTCTGTGGAAGGAG-F | 120 | 96.7 | XM_014127075.1 |
| AGCACGGTGAATTCAGTTCC-R | ||||
| muc5ac2 (/4) | TTTTCTCAGTTGCCGCTTTT-F | 92 | 98.9-99.8 | XM_014182329.1 |
| AGTCGGAGCCCATAAGAGGT-R | ||||
| muc5b | ATTAAGAGCGATGTCTTCACAGC-F | 85 | 97.4-102.4 | XM_014175874.1/XM_014126057.1 |
| AAGCACATGAGTCTCTCACACAA-R | ||||
| cat | CCGACCGTCCGTAAATGCTA-F | 140 | 96.1 | BT045615.1 |
| GCTTTTCAGATAGGCTCTTCATGTAA-R | ||||
| gpx | GCAATCAGTTCGGACATCAGG-F | 131 | 91.1 | XM_014133872 |
| GTCCTTCCCATTCACATCCAC-R | ||||
| nrf2 | TCAACAGGACATCGACCTAAT-F | 83 | 81.9 | BT059007.1 |
| GGCAGTAGTCAAACACCTCT-R | ||||
| sod1 | CCACGTCCATGCCTTTGG-F | 141 | 94.1 | AY736282.1 |
| TCAGCTGCTGCAGTCACGTT-R | ||||
| cr3 (itgb2) | ATGACATGGACTACCCATCTGTT-F | 151 | 110.5 | BT058776.1 |
| TCTGACAATACTCCCACCTCA-R | ||||
| Reference genes | ||||
| ef1ab | TGCCCCTCCAGGATGTCTAC-F | 59 | 100.8 | BG933853 |
| CACGGCCCACAGGTACTG-R | ||||
| rpl13 | CGCTCCAAGCTCATCCTCTTCCC-F | 79 | 94.0 | BT048949.1 |
| CCATCTTGAGTTCCTCCTCAGTGC-R | ||||
| rps29 | GGGTCATCAGCAGCTCTATTGG-F | 167 | 91.1 | BT043522.1 |
| AGTCCAGCTTAACAAAGCCGATG-R | ||||
| ubi | AGCTGGCCCAGAAGTACAACTGTG-F | 162 | 91.1 | AB036060.1 |
| CCACAAAAAGCACCAAGCCAAC-R | ||||
| hprt1 | CCGCCTCAAGAGCTACTGTAAT-F | 255 | 82.5 | BT043501 |
| GTCTGGAACCTCAAACCCTATG-R | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sørensen, S.L.; Ghirmay, A.; Gong, Y.; Dahle, D.; Vasanth, G.; Sørensen, M.; Kiron, V. Growth, Chemical Composition, Histology and Antioxidant Genes of Atlantic Salmon (Salmo salar) Fed Whole or Pre-Processed Nannochloropsis oceanica and Tetraselmis sp. Fishes 2021, 6, 23. https://doi.org/10.3390/fishes6030023
Sørensen SL, Ghirmay A, Gong Y, Dahle D, Vasanth G, Sørensen M, Kiron V. Growth, Chemical Composition, Histology and Antioxidant Genes of Atlantic Salmon (Salmo salar) Fed Whole or Pre-Processed Nannochloropsis oceanica and Tetraselmis sp. Fishes. 2021; 6(3):23. https://doi.org/10.3390/fishes6030023
Chicago/Turabian StyleSørensen, Solveig Lysfjord, Ateshm Ghirmay, Yangyang Gong, Dalia Dahle, Ghana Vasanth, Mette Sørensen, and Viswanath Kiron. 2021. "Growth, Chemical Composition, Histology and Antioxidant Genes of Atlantic Salmon (Salmo salar) Fed Whole or Pre-Processed Nannochloropsis oceanica and Tetraselmis sp." Fishes 6, no. 3: 23. https://doi.org/10.3390/fishes6030023
APA StyleSørensen, S. L., Ghirmay, A., Gong, Y., Dahle, D., Vasanth, G., Sørensen, M., & Kiron, V. (2021). Growth, Chemical Composition, Histology and Antioxidant Genes of Atlantic Salmon (Salmo salar) Fed Whole or Pre-Processed Nannochloropsis oceanica and Tetraselmis sp. Fishes, 6(3), 23. https://doi.org/10.3390/fishes6030023






