Molecular Characterization of Hsp47 in Grass Carp (Ctenopharyngodon idella) and Its Correlation with Type I Collagen in Response to Fish Aerobic Exercise
Abstract
1. Introduction
2. Results
2.1. Molecular Cloning of Hsp47
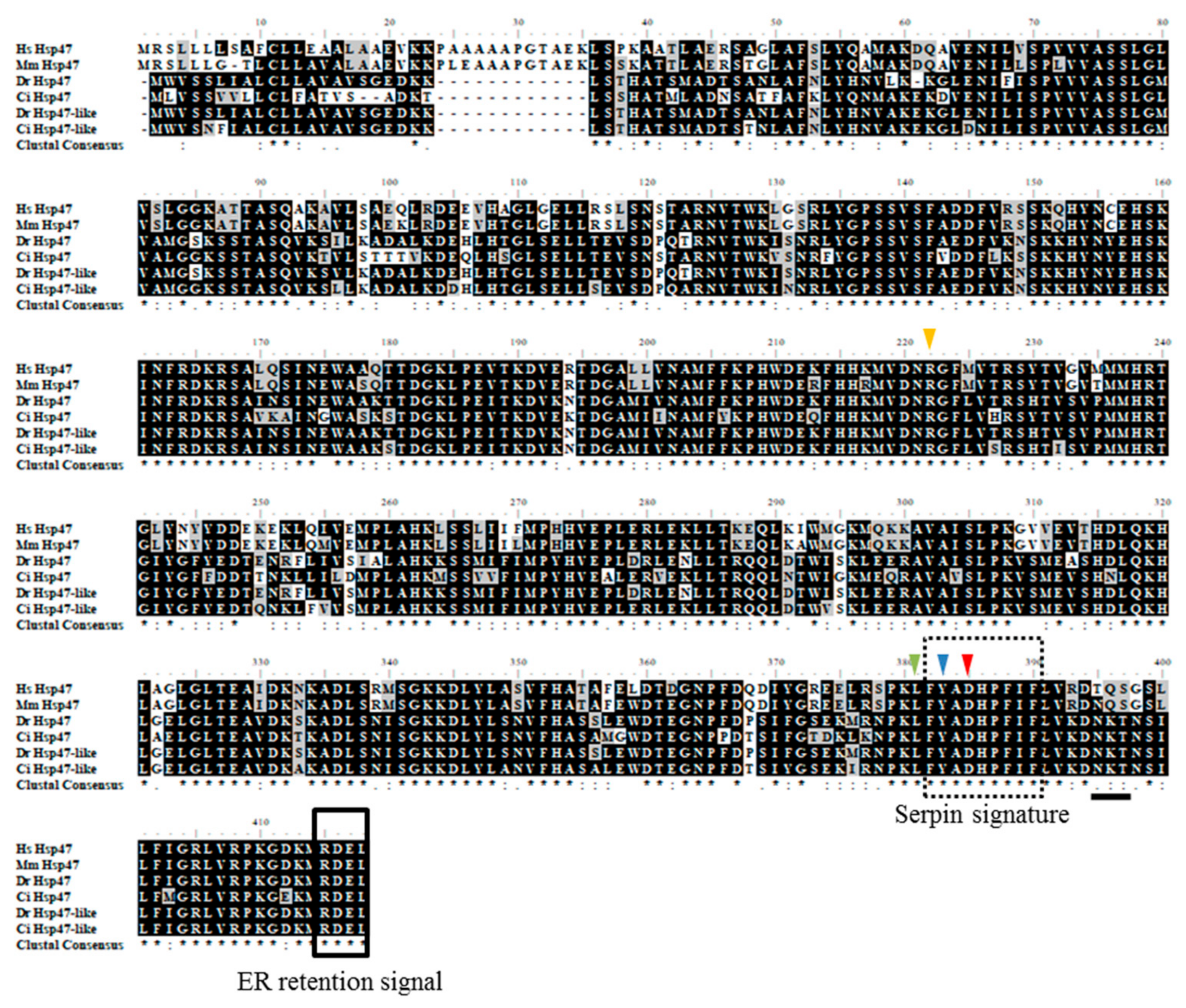
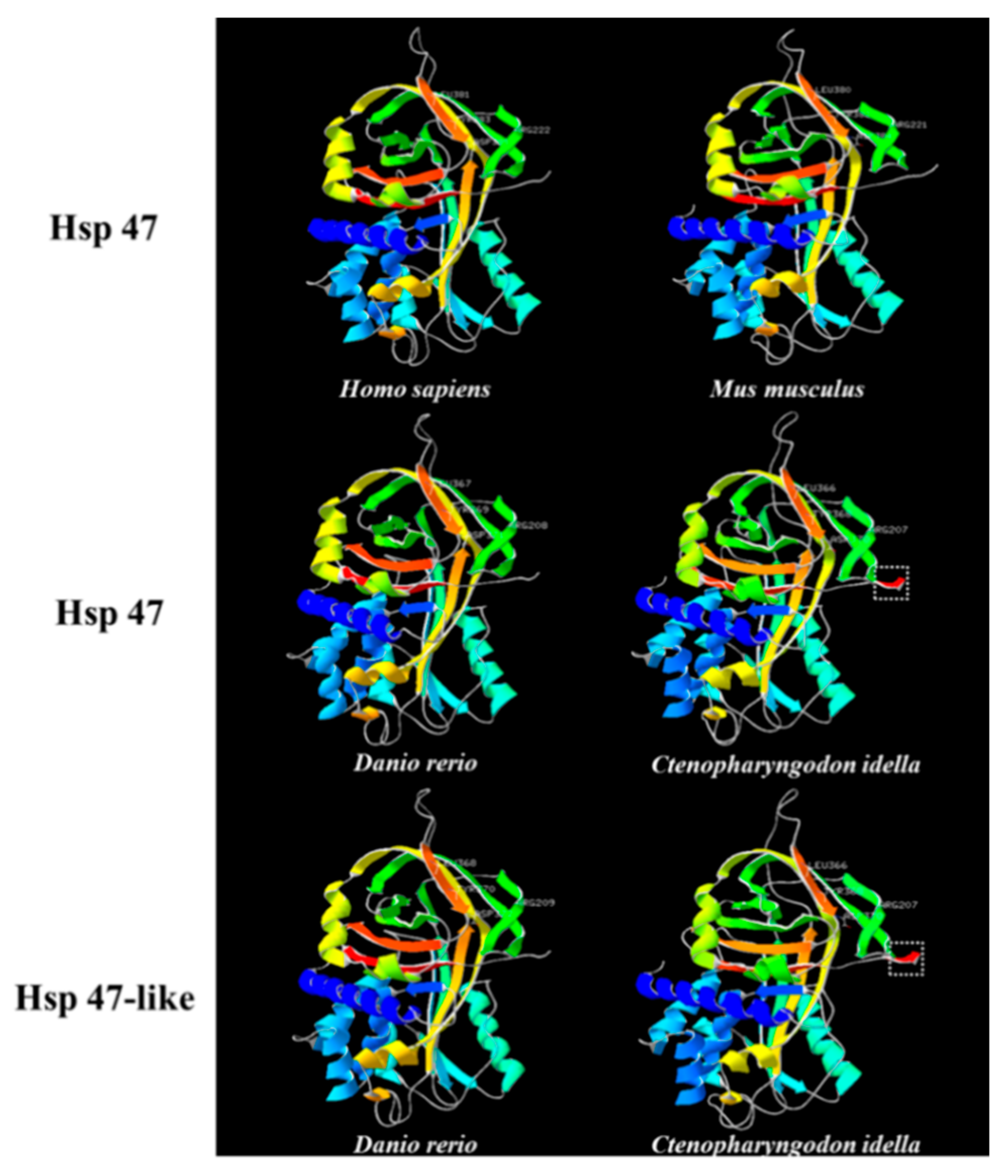
2.2. Structure Analysis and Characterization of Hsp47
2.3. Phylogenetic Analysis
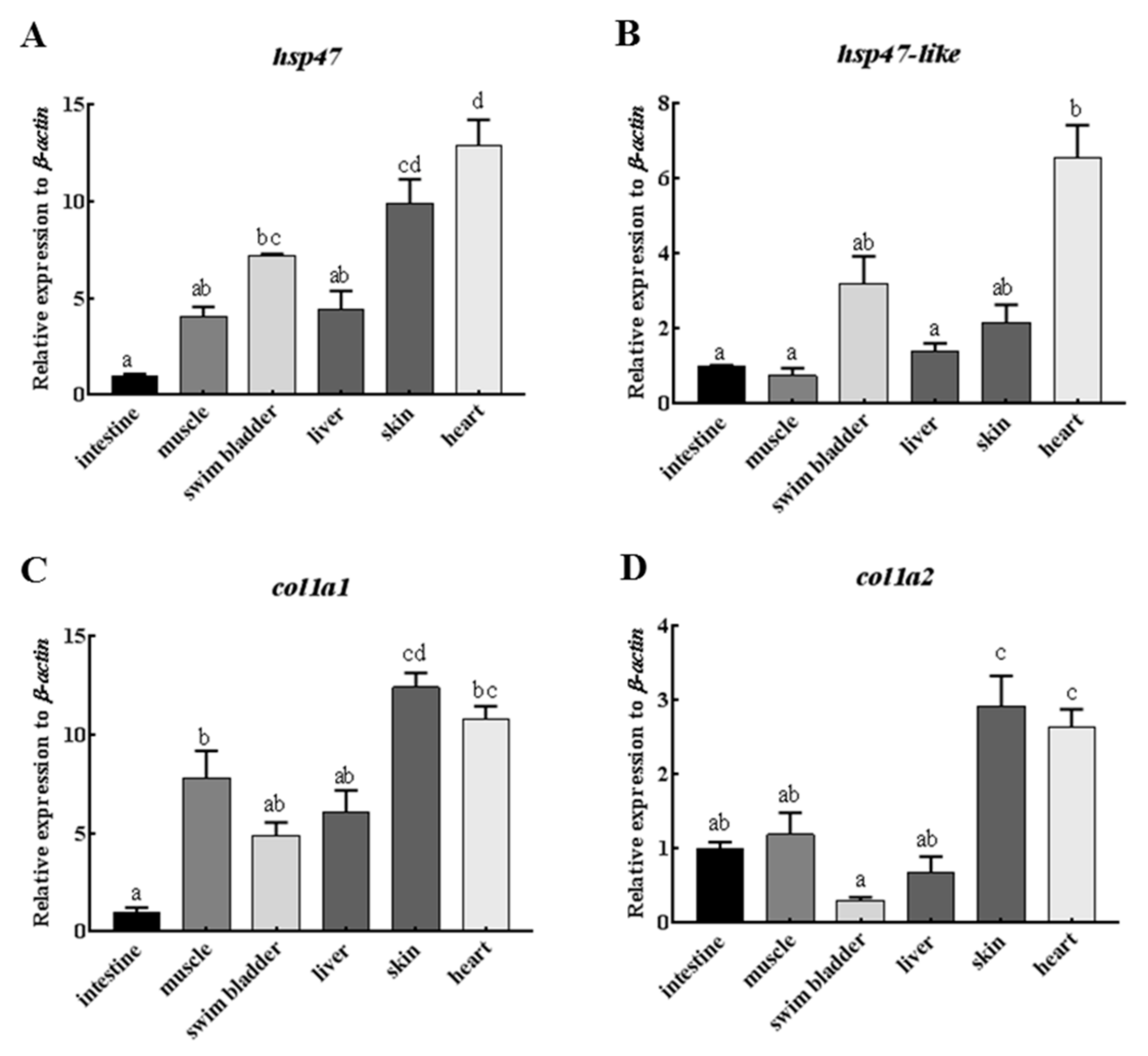
2.4. Comparative Expression of Hsp47, Hsp47-Like and Type I Collagen Genes in Various Organs
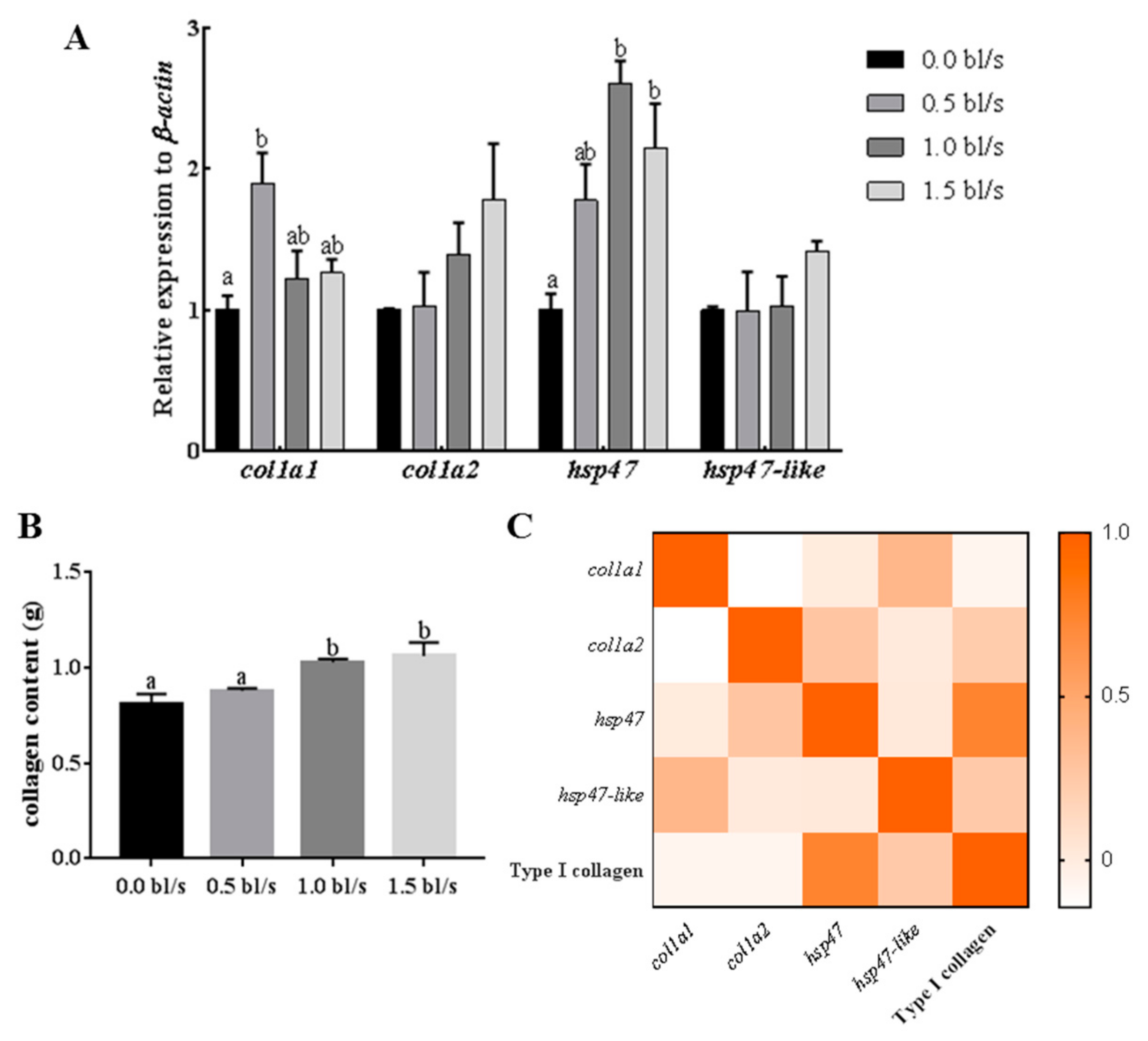
2.5. Effect of Aerobic Exercise on Expression of Muscle Hsp47, Hsp47-Like and Type I Collagen
3. Discussion
4. Materials and Methods
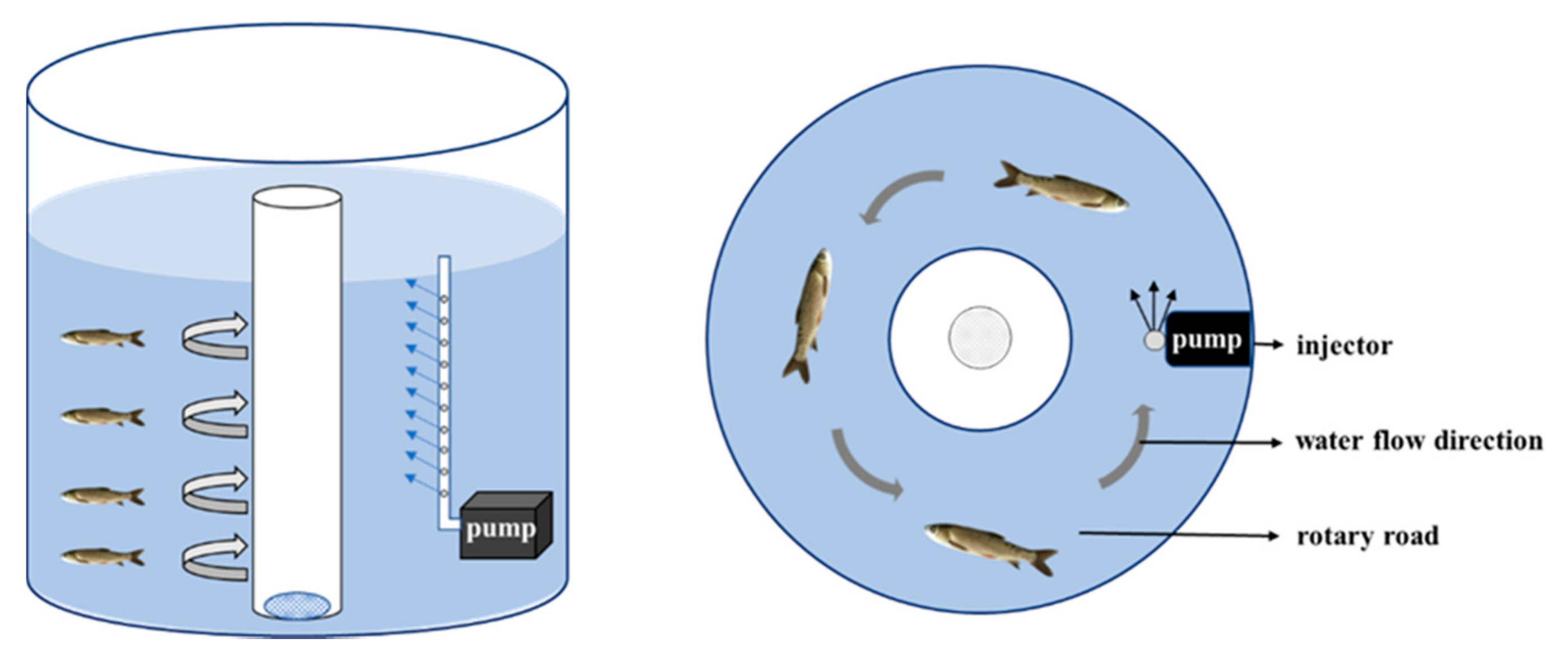
4.1. Experimental Fish and Sampling
4.2. Cloning of Hsp47 in Grass Carp
4.3. Characterization and Phylogenetic Analysis of Hsp47 in Grass Carp
4.4. Gene Expression and Collagen Content Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Nakai, A.; Satoh, M.; Hirayoshi, K.; Nagata, K. Involvement of the stress protein HSP47 in procollagen processing in the endoplasmic reticulum. J. Cell Biol. 1992, 117, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Nagata, K. Roles of the endoplasmic reticulum–resident, collagen-specific molecular chaperone Hsp47 in vertebrate cells and human disease. J. Biol. Chem. 2019, 294, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Hirayoshi, K.; Kudo, H.; Takechi, H.; Nakai, A.; Iwamatsu, A.; Yamada, K.M.; Nagata, K. HSP47: A tissue-specific, transformation-sensitive, collagen-binding heat shock protein of chicken embryo fibroblasts. Mol. Cell Biol. 1991, 11, 4036–4044. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Nagata, K. Biology of Hsp47 (serpin H1), a collagen specific molecular chaperone. Semin. Cell Dev. Biol. 2017, 62, 142–151. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Characterization of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 85, 81–89. [Google Scholar] [CrossRef]
- Zhang, X.; Adachi, S.; Ura, K.; Takagi, Y. Properties of collagen extracted from Amur sturgeon Acipenser schrenckii and assessment of collagen fibrils in vitro. Int. J. Biol. Macromol. 2019, 137, 809–820. [Google Scholar] [CrossRef]
- Zhu, S.; Yuan, Q.; Yang, M.; You, J.; Yin, T.; Gu, Z.; Hu, Y.; Xiong, S. A quantitative comparable study on multi-hierarchy conformation of acid and pepsin-solubilized collagens from the skin of grass carp (Ctenopharyngodon idella). Mater. Sci. Eng. C 2019, 96, 446–457. [Google Scholar] [CrossRef]
- Jain, N.; Brickenden, A.; Lorimer, I.; Ball, E.H.; Sanwal, B.D. Interaction of procollagen I and other collagens with colligin. Biochem. J. 1994, 304, 61–68. [Google Scholar] [CrossRef]
- Koide, T.; Takahara, Y.; Asada, S.; Nagata, K. Xaa-Arg-Gly triplets in the collagen triple helix are dominant binding sites for the molecular chaperone HSP47. J. Biol. Chem. 2002, 277, 6178–6182. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, J.; Zhang, X.; He, X.; Li, L.; Tang, R.; Chi, W.; Li, D. Diet affects muscle quality and growth traits of grass carp (Ctenopharyngodon idellus): A comparison between grass and artificial feed. Front. Physiol. 2018, 9, 283. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Tang, R.; He, X.; Li, L.; Takagi, Y.; Li, D. Improvement of muscle quality of grass carp (Ctenopharyngodon idellus) with a bio-floating bed in culture ponds. Front. Physiol. 2019, 10, 683. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, X.; Li, T.; Yang, H.; Zhang, H.; Regenstein, J.; Zhou, P. Extraction and characterization of acid- and pepsin-soluble collagens from the scales, skins and swim-bladders of grass carp (Ctenopharyngodon idella). Food Biosci. 2015, 9, 68–74. [Google Scholar] [CrossRef]
- Hu, J.H.; Li, T.C.; Liu, X.Y.; Liu, D.S. Seasonal variation of acid-soluble collagens extracted from the swim bladders and skins of bighead carp (Hypophthalmichthys nobilis) and grass carp (Ctenopharyngodon idella). Food Biosci. 2016, 15, 27–33. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Toriumi, S.; Ura, K.; Takagi, Y. Feasibility of collagens obtained from bester sturgeon Huso huso × Acipenser ruthenus for industrial use. Aquaculture 2020, 529, 735641. [Google Scholar] [CrossRef]
- Duan, R.; Zhang, J.J.; Du, X.Q.; Yao, X.C.; Konno, K. Properties of collagen from skin, scale and bone of carp (Cyprinus carpio). Food Chem. 2009, 112, 702–706. [Google Scholar] [CrossRef]
- Ma, Y.B.; Zhang, J.X.; Zhou, X.Q.; Jiang, W.D.; Wu, P.; Liu, Y.; Kuang, S.Y.; Tang, L.; Feng, L. Effect of tea polyphenols on flavour, healthcare components, physicochemical properties, and mechanisms of collagen synthesis in growing grass carp (Ctenopharyngodon idella) muscle. Aquaculture 2021, 534, 736237. [Google Scholar] [CrossRef]
- Liu, G.Y.; Wu, Y.J.; Qin, X.H.; Shi, X.T.; Wang, X.L. The effect of aerobic exercise training on growth performance, innate immune response and disease resistance in juvenile Schizothorax prenanti. Aquaculture 2018, 486, 18–25. [Google Scholar] [CrossRef]
- Kirk, M.A.; Caudill, C.C.; Tonina, D.; Syms, J.C. Effects of water velocity, turbulence and obstacle length on the swimming capabilities of adult Pacific lamprey. Fish. Manag. Ecol. 2016, 23, 356–366. [Google Scholar] [CrossRef]
- Gutierrez, P.T.; Almeida, F.L.; Carani, F.R.; Vechetti, I.J.; Padovanic, C.R.; Salomão, R.A.S. Rearing temperature induces changes in muscle growth and gene expression in juvenile pacu (Piaractus mesopotamicus). Comp. Biochem. Phys. B 2014, 169, 31–37. [Google Scholar] [CrossRef]
- Pearson, D.S.; Kulyk, W.M.; Kelly, G.M.; Krone, P.H. Cloning and characterization of a cDNA encoding the collagen-binding stress protein hsp47 in zebrafish. DNA Cell Biol. 1996, 15, 263–272. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Y.; Li, X.; Cao, H.; Xu, M.; Dai, J. The identification of heat shock protein genes in goldfish (Carassius auratus) and their expression in a complex environment in Gaobeidian Lake, Widmer Beijing, China. Comp. Biochem. Phys. C 2007, 145, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Widmer, C.; Gebauer, J.M.; Brunstein, E.; Rosenbaum, S.; Zaucke, F.; Drögemüller, C.; Leeb, T.; Baumann, U. Molecular basis for the action of the collagen-specific chaperone Hsp47/SERPINH1 and its structure-specific client recognition. Proc. Natl. Acad. Sci. USA 2012, 109, 13243–13247. [Google Scholar] [CrossRef] [PubMed]
- Ojima, N.; Yamashita, M.; Watabe, S. Comparative expression analysis of two paralogous Hsp70s in rainbow trout cells exposed to heat stress. Biochim. Biophys. Acta 2005, 168, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Azuma, N.; Hagihara, S.; Adachi, S.; Ura, K.; Takagi, Y. Characterization of type I and II procollagen α1chain in Amur sturgeon (Acipenser schrenckii) and comparison of their gene expression. Gene 2016, 579, 8–16. [Google Scholar] [CrossRef]
- Vélez, E.J.; Sheida, A.; Dorothy, V.; Cristina, S.; Eamail, L.; Albert, S.M.; Isabel, N.; Joaquim, G.; Encarnacion, C.; Silvia, M.L. Proteolytic systems’ expression during myogenesis and transcriptional regulation by amino acids in gilthead sea bream cultured muscle cells. PLoS ONE 2017, 12, e0187339. [Google Scholar] [CrossRef]
- Ibarz, A.; Felip, O.; Fernández-Borràs, J.; Martín-Pérez, M.; Blasco, J.; Torrella, J.R. Sustained swimming improves muscle growth and cellularity in gilthead sea bream. J. Comp. Physiol. B 2011, 181, 209–217. [Google Scholar] [CrossRef]
- Kiessling, A.; Pickova, J.; Eales, J.G.; Dosanjh, B.; Higgs, D. Age, ration level, and exercise affect the fatty acid profile of chinook salmon (Oncorhynchus tshawytscha) muscle differently. Aquaculture 2004, 243, 345–356. [Google Scholar] [CrossRef]
- Zhu, Z.M.; Song, B.L.; Lin, X.T.; Xu, Z.N. Effect of sustained training on glycolysis and fatty acids oxidation in swimming muscles and liver in juvenile tinfoil barb Barbonymus schwanenfeldii (bleeker, 1854). Fish. Physiol. Biochem. 2016, 42, 1–11. [Google Scholar] [CrossRef]
- Lemoine, C.; Craig, P.M.; Dhekney, K.; Kim, J.J.; Mcclelland, G.B. Temporal and spatial patterns of gene expression in skeletal muscles in response to swim training in adult zebrafish (Danio rerio). J. Comp. Physiol. B 2010, 180, 151–160. [Google Scholar] [CrossRef]
- Palstra, A.P.; Rovira, M.; Rizo-Roca, D.; Torrella, J.; Spaink, H.P.; Planas, J.V. Swimming-induced exercise promotes hypertrophy and vascularization of fast skeletal muscle fibres and activation of myogenic and angiogenic transcriptional programs in adult zebrafish. BMC Genom. 2014, 15, 1136. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Han, Z.; Zeng, X.A. Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: A review. Comp. Rev. Food Sci. 2014, 13, 52–61. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Z.; Qi, T.; Xi, R.; Liang, X.; Li, L.; Tang, R.; Li, D. Slight increases in salinity improve muscle quality of grass carp (Ctenopharyngodon idellus). Fishes 2021, 6, 7. [Google Scholar] [CrossRef]
- Murakami, S.; Toda, Y.; Seki, T.; Munetomo, E.; Kondo, Y.; Sakurai, T.; Furukawa, Y.; Matsuyama, M.; Nagate, T.; Hosokawa, N.; et al. Heat shock protein (HSP) 47 and collagen are upregulated during neointimal formation in the balloon-injured rat carotid artery. Atherosclerosis 2001, 157, 361–368. [Google Scholar] [CrossRef]
- Kawasaki, K.; Ushioda, R.; Ito, S.; Ikeda, K.; Masago, Y.; Nagata, K. Deletion of the collagen-specific molecular chaperone hsp47 causes endoplasmic reticulum stress-mediated apoptosis of hepatic stellate cells. J. Biol. Chem. 2015, 290, 3639–3646. [Google Scholar] [CrossRef]
- Miyamura, T.; Sakamoto, N.; Kakugawa, T.; Taniguchi, H.; Akiyama, Y.; Okuno, D.; Moriyama, S.; Hara, A.; Kido, T.; Ishimoto, H.; et al. Small molecule inhibitor of HSP47 prevents pro-fibrotic mechanisms of fibroblasts in vitro. Biochem. Bioph. Res. Co. 2020, 530, 561–565. [Google Scholar] [CrossRef]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics 2006, 22, 195–201. [Google Scholar] [CrossRef]

| Primers | Primer Sequence (5′–3′) |
|---|---|
| hsp47 01-F1 | TGAGCTGGGTCTGACTGAAG |
| hsp47 01-R1 | CAAAAGGATGGTCGGCGTAG |
| hsp47 02-F1 | GCCCAGTTCTGTTAGCTTCG |
| hsp47 02-R1 | ACCACGATTGTCCACCATCT |
| hsp47 03-F1 | AGATGGTGGACAATCGTGGT |
| hsp47 03-R1 | CAAAAGGATGGTCGGCGTAG |
| hsp47 5′race-outer | CCATCTTGTGATGGAACTGCTCATCC |
| hsp47 5′race-inner | GCGCTTGTCACGGAAGTTTATTTTGG |
| hsp47 3′race-outer | CAGGGAAGAAAGACCTCTACCTGTCCAATG |
| hsp47 3′race-inner | GAAATCCACCCGATACGAGCATCTTTGG |
| hsp47-like F1 | TCTCTACCACAATGTCGCCA |
| hsp47-like R1 | CGCTCACCTCGCTCAGAA |
| hsp47-qF | TGAGCTGGGTCTGACCGAAG |
| hsp47-qR | TGAAGGGATGGTCAGCGTAG |
| hsp47-like-qF | TCTCTACCACAATGTCGCCA |
| hsp47-like-qR | CGCTCACCTCGCTCAGAA |
| col1α1-qF | GCATGGGGCAAGACAGTCA |
| col1α1-qR | ACGCACACAAACAATCTCAAGT |
| col1α2-qF | ACATTGGTGGCGCAGATCA |
| col1α2-qR | TCTCCGATAGAGCCCAGCTT |
| β-actin-qF | GCCGTGACCTGACTGACT |
| β-actin-qR | CAAGACTCCATACCCAAGAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Wan, Y.; Shen, Z.; Liu, Y.; Li, D.; Li, L.; Tang, R.; Zhang, X. Molecular Characterization of Hsp47 in Grass Carp (Ctenopharyngodon idella) and Its Correlation with Type I Collagen in Response to Fish Aerobic Exercise. Fishes 2021, 6, 17. https://doi.org/10.3390/fishes6020017
Liang X, Wan Y, Shen Z, Liu Y, Li D, Li L, Tang R, Zhang X. Molecular Characterization of Hsp47 in Grass Carp (Ctenopharyngodon idella) and Its Correlation with Type I Collagen in Response to Fish Aerobic Exercise. Fishes. 2021; 6(2):17. https://doi.org/10.3390/fishes6020017
Chicago/Turabian StyleLiang, Xiao, Ying Wan, Zhiyuan Shen, Yanmei Liu, Dapeng Li, Li Li, Rong Tang, and Xi Zhang. 2021. "Molecular Characterization of Hsp47 in Grass Carp (Ctenopharyngodon idella) and Its Correlation with Type I Collagen in Response to Fish Aerobic Exercise" Fishes 6, no. 2: 17. https://doi.org/10.3390/fishes6020017
APA StyleLiang, X., Wan, Y., Shen, Z., Liu, Y., Li, D., Li, L., Tang, R., & Zhang, X. (2021). Molecular Characterization of Hsp47 in Grass Carp (Ctenopharyngodon idella) and Its Correlation with Type I Collagen in Response to Fish Aerobic Exercise. Fishes, 6(2), 17. https://doi.org/10.3390/fishes6020017








