1. Introduction
Over the past several decades, there has been growing concern over the potential health risks to humans and wildlife due to exposure to endocrine-disrupting chemicals (EDCs) in the environment [
1,
2]. Effluents of wastewater treatment plants (WWTPs) contain a variety of chemicals such as surfactants, pesticides, industrial materials, the human estrogens estrone (E1), 17β-estradiol (E2), and estriol (E3), and the pharmaceutical estrogen ethinylestradiol (EE2), also known as the birth control hormone, which possess estrogenic activity [
3]. To date, there are more than 50 chemicals from industrial, municipal, and agricultural sources that are known to interfere with endocrine systems of wildlife [
4,
5,
6].
These EDCs in the environment can have profound effects on both the physiology and behavior of wildlife and humans [
1,
7]. The most commonly studied negative effects of EDCs are on sex determination, onset of sexual maturation, secondary sex characteristics, and sperm and egg production [
8]. Endocrine disruption in rainbow trout (
Oncorhynchus mykiss) was documented when researchers detected vitellogenin (VTG) in males exposed to WWTP effluent [
9]. Vitellogenin is an egg yolk precursor protein normally only found in sexually mature females and is nondetectable in immature fish and mature male fish. Its production, however, is greatly stimulated by estrogenic compounds in water [
9,
10,
11]. In addition to protein expression, changes in reproductive behavior can be caused by exposure to EDCs. Declines in male reproductive behavior and fitness were caused by exposure to EE2 in sand gobies [
7] and zebra fish [
12]. Martinović et al. [
13] showed that male fathead minnows (
Pimephales promelas) exposed to the effluent of a wastewater treatment plant displayed decreased agonistic behavior as well as less defined secondary sex characteristics, resulting in reduced reproductive fitness.
In the 1990s and early 2000s, most studies exploring the effects of EDCs focused exclusively on either physiological impacts (i.e., protein expression) or behavior (i.e., aggression, courtship, and/or nest building), and relatively few looked into these effects simultaneously in the same model system [
14,
15]. In more recent years, it has become commonplace to investigate both physiological and behavioral changes induced by endocrine disrupting chemicals. A study using the brackish medaka,
Oryzias melastigma, found that both physiology and behavior were altered after fish were exposed to wastewater effluent brough into the laboratory [
16]. Studies in fathead minnows,
Pimephales promelas, have correlated changes in gene expression patterns, anatomy, behavior, and reproductive outputs in male and female fish exposed to varying levels of EDCs [
13,
17]. Studying changes in both physiology and behavior simultaneously provides a more comprehensive understanding of how EDCs can affect organisms and what the environmental relevance of these effects are. These studies, however, also have had limitations. Some focus on exposure to a specific compound or compounds [
17]. Others expose animals directly to wastewater effluent, rather than the natural environment that the effluent is released into, often subjecting animals to higher concentrations than are truly found in the aquatic environment [
13,
16].
Unlike previous studies that have investigated the effect of EDCs in WWTP effluent by bringing water samples into the lab, we exposed fish directly to the water in a receiving stream of a wastewater treatment plant. We placed juvenile and adult male fathead minnows in stream water above and below a WWTP effluent site. We assayed the levels of estrogenicity expressed as parts per trillion of 17β-estradiol equivalence at both locations weekly, measured VTG production in juvenile fish that should not be producing the protein and conducted behavioral experiments on adult males to assay reproductive behaviors. We hypothesized that downstream of the WWTP effluent, levels of estrogenicity would be higher, juveniles would have higher levels of VTG, and males would have altered reproductive behaviors when compared to individuals placed upstream.
3. Discussion
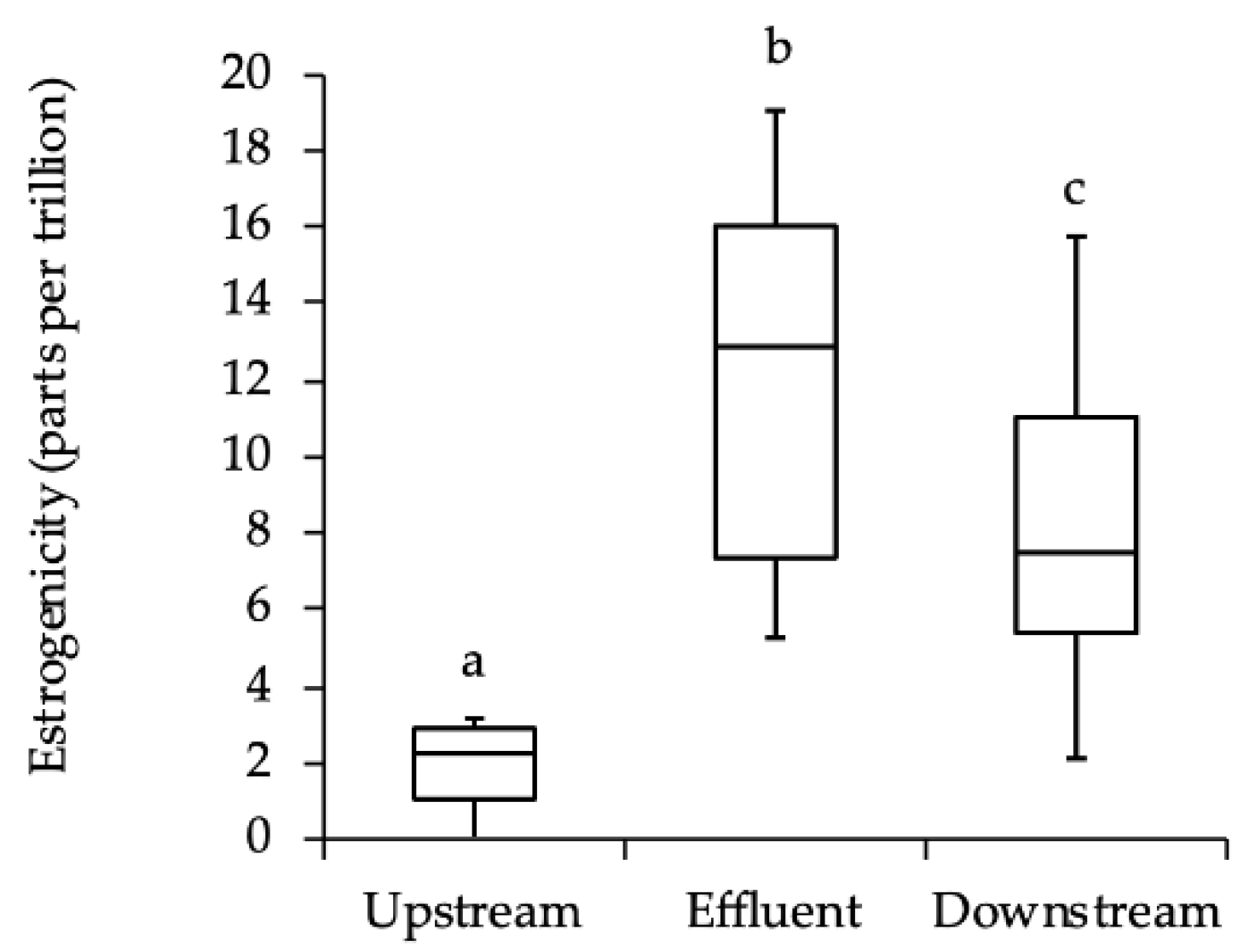
We hypothesized that the estrogenicity of stream water downstream of a WWTP effluent would be greater than that of water upstream, and furthermore, that fish exposed to this increase in estrogenicity would demonstrate physiological (VTG production) and behavioral effects. We indeed found differences in estrogenicity that correlated with changes in some behaviors, but we found no evidence of differences in VTG production. The estrogen yeast assay results demonstrate that the estrogenic activity of the effluent is significantly higher than the upstream water. Not surprisingly, the release of the SSTP effluent causes an increase in the estrogenicity of the downstream water, with levels of estrogenicity approximately 175% higher than the upstream environment. This difference suggests that any differences in reproductive behavior between fish above and below the effluent site may be due to exposure to different levels of estrogenicity in the water.
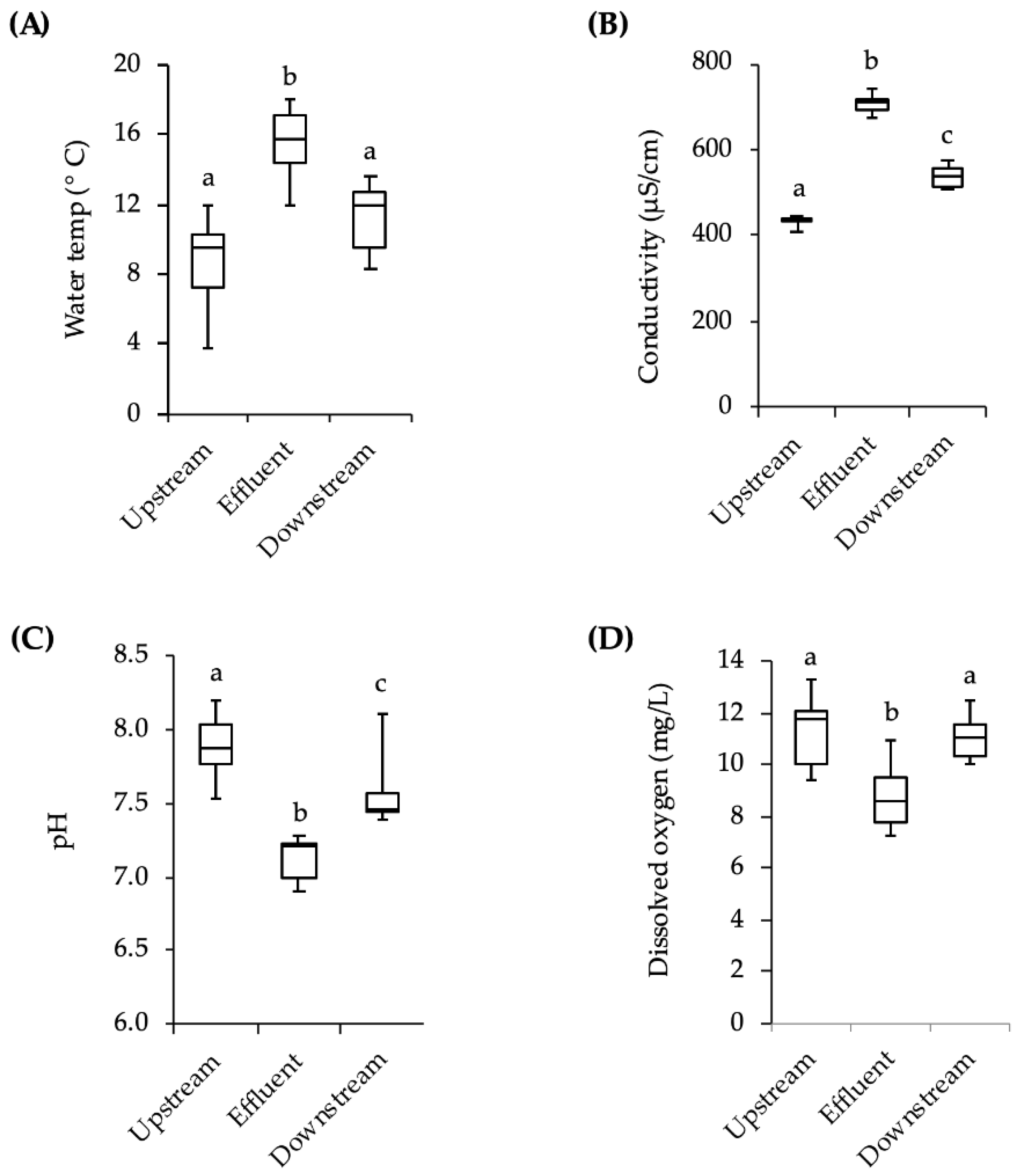
However, other variables must also be considered. The temperature and conductivity downstream of the effluent site were slightly higher than the values upstream of the effluent site and the pH downstream was slightly lower. There was also more debris covering the downstream cages at the end of the three-week exposures. There could have been other differences in upstream and downstream water conditions such as the speed of the current, the levels of nutrients, and the depth of the water. Additionally, apart from the chemicals with estrogenic activity, there are hundreds of pharmaceutical and personal care compounds as well as cleaning agents, fire retardants, and anti-stick chemicals in domestic wastewater that the fish may have been exposed to, but which were not monitored in this study [
18,
19,
20]. Little is known about the biological effects of most of these chemicals on fish either singly or in combination and they may have also affected the fish. There is no way to explicitly determine that one or more of these factors did not play a role in changing the behavior of males exposed to the downstream site, but the fact that estrogenicity was significantly different at least supports the hypothesis that it played a role in any noticeable effects.
Unexpectedly, the vitellogenin results of full body homogenates were inconclusive, and did not form any specific pattern. In general, without exposure to estrogenic compounds, male juvenile fathead minnows have much lower levels of vitellogenin than do juvenile females—approximately 30 ng/mL of plasma in males compared to 4000 ng/mL for females [
21]. This level increases dramatically (over 100×) in juveniles in the lab after exposure to 17β-estradiol [
21]. In our study, only three fish had vitellogenin concentrations greater than 1000 ng/g of fish. While these fish were in the downstream treatment group, the majority of fish in both treatments had levels of vitellogenin expected in fish unexposed to estrogenicity. This finding could in part be due to our low sample size, as well as the many challenges and limitations involved with measuring vitellogenin using enzyme immunoassays [
22]. Additionally, the complexity of the estrogen receptor complex with its multiple subtypes in teleost fish makes drawing a direct line between estrogen-like compounds in the environment and vitellogenin production problematic [
23]. However, at the time of the completion of this study, vitellogenin assays were one of the most common methods for detecting levels of concern of environmental estrogens, and they continue to be widely used today, despite legitimate concerns about the accuracy and usefulness of the process [
22].
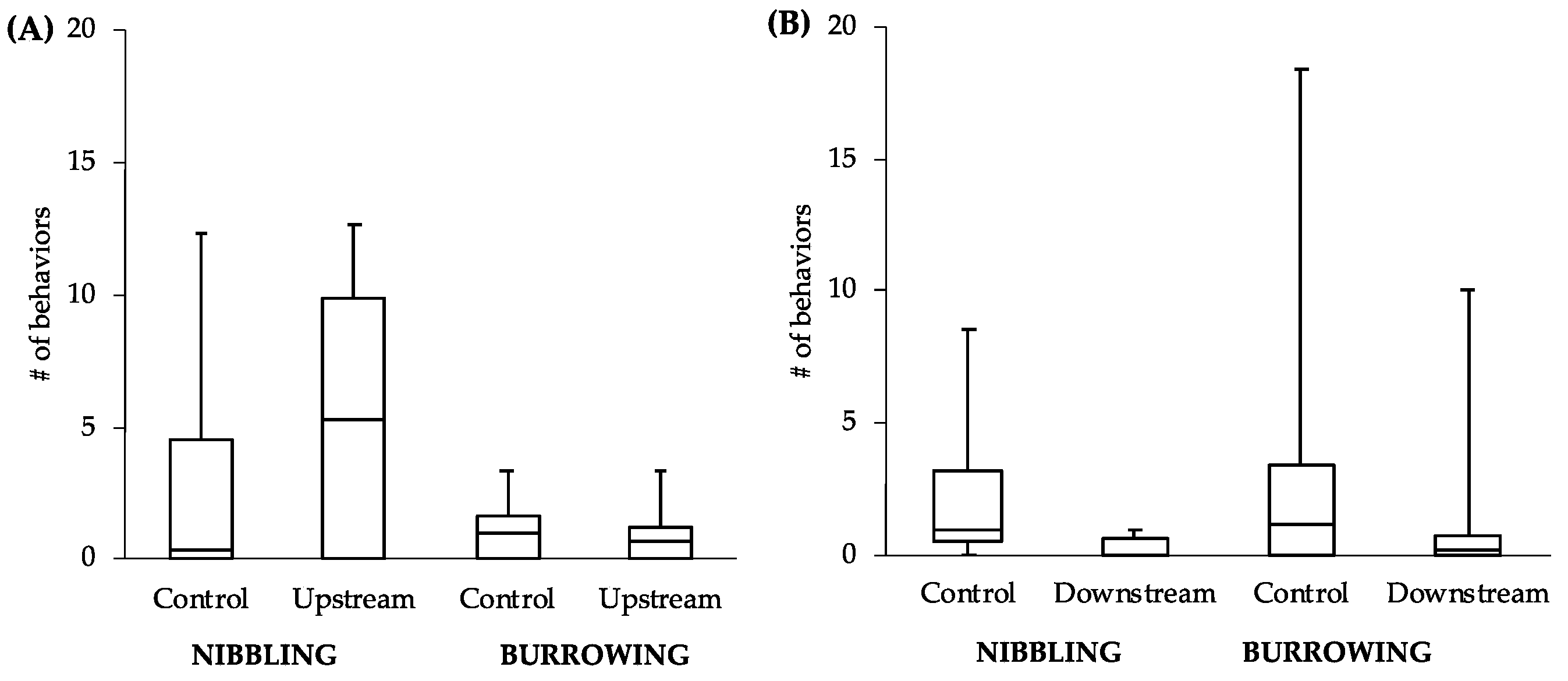
It is worth noting that while we did not detect significant differences between upstream and downstream fish in vitellogenin, we did find slight behavioral differences. If we had not completed a behavioral assay and focused exclusively on vitellogenin, our conclusion would be that the difference in levels of estrogenicity upstream and downstream of the WWTP effluent was not enough to produce this commonly studied effect. While unpredicted, our results are not entirely unique as Soffker and Tyler [
15] cite multiple studies where behavioral effects were observed but physiological effects were not found. Our assay revealed some slight but significant differences in behavior. In relation to controls, two of the reproductive behaviors of downstream males, (male-directed butting/biting and nest nibbling) were found to occur significantly less often than those same behaviors in the upstream males. Similar results were detected for fathead minnows in other studies [
3,
17,
24] and in three-spined sticklebacks (
Gasterosteus aculeatus) [
25] and zebrafish (
Danio rerio) [
12,
26], but, unlike our study, these studies used higher concentrations of 17β-estradiol equivalent than is normally found in the aquatic environment [
15]. Male-directed butting/biting is an aggressive behavior commonly used in territorial disputes that is likely to impact a male’s ability to secure a territory. On the other hand, nest-nibbling is a behavior used by males in preparing a nest site for egg deposition from females. Males showing lower levels of this behavior are likely not preparing for reproduction. A decrease in either of these types of behavior should have direct fitness consequences.
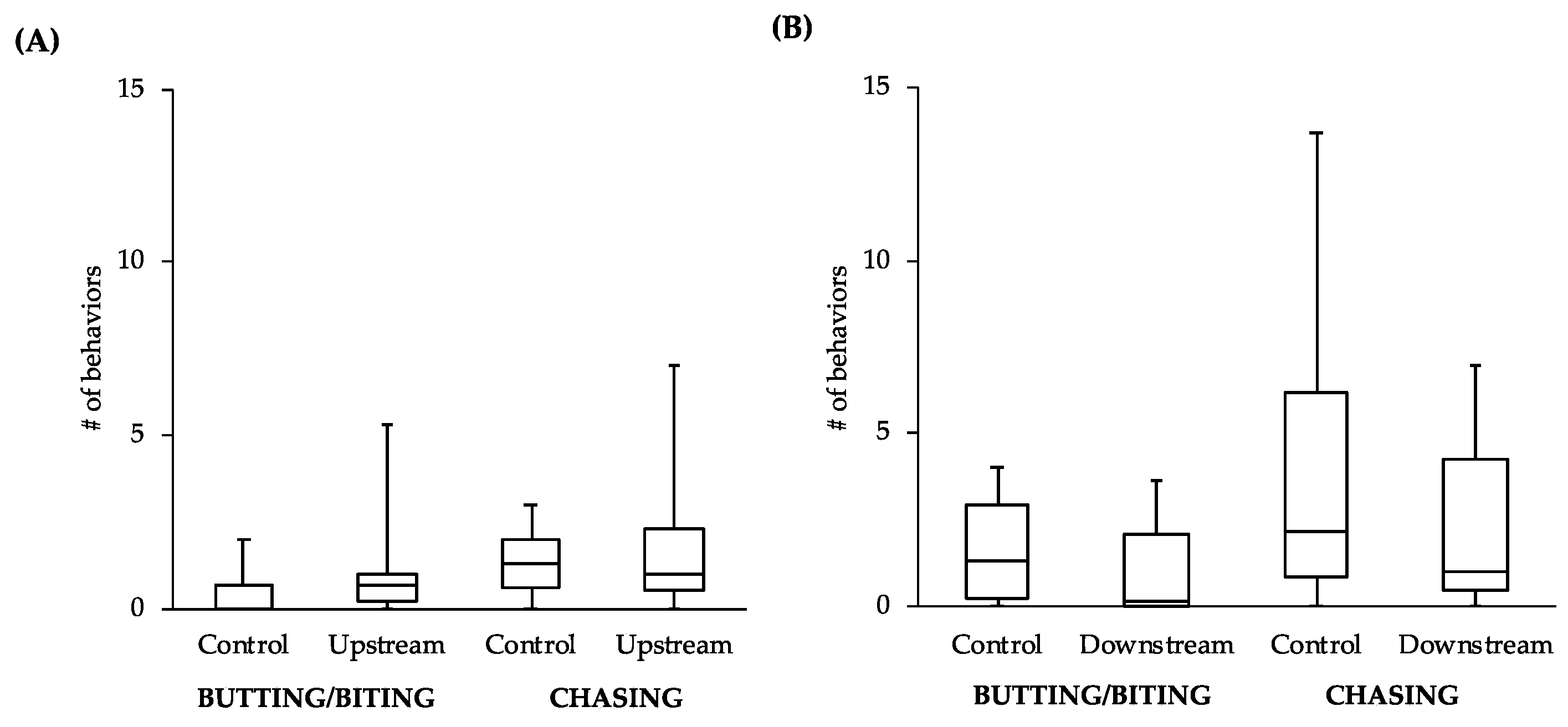
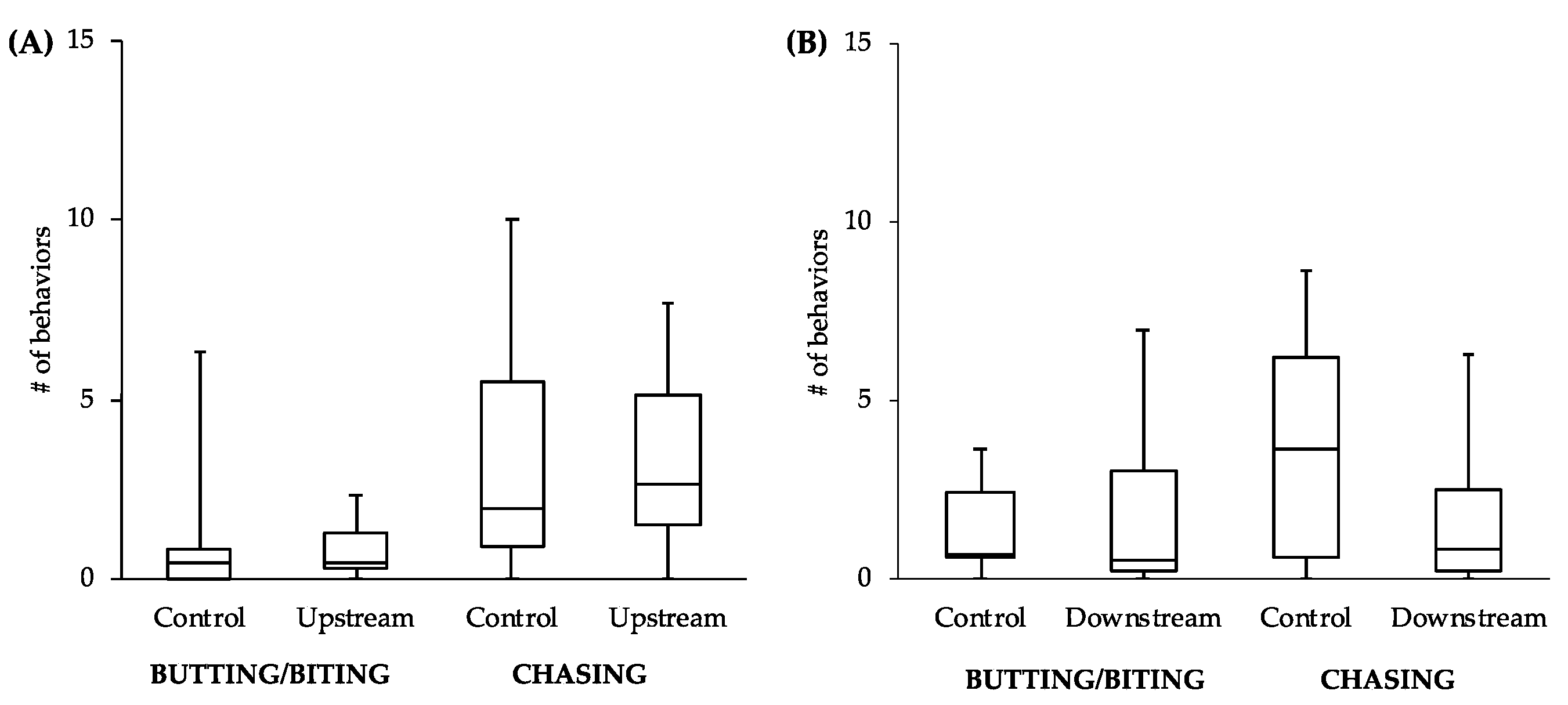
While the comparisons between other behaviors did not reveal statistically significant differences, the general patterns of the data follow what would be expected of estrogenic influence. For example, the male-to-male behavior of control fish in the tanks with the upstream males was similar to that level of behavior of the upstream males themselves (
Figure 4A). However, the level of male-to-male behavior of the control fish in the tanks with the downstream males (
Figure 4B) more closely resembled the levels of male-to-female behavior of the controls in tanks with both upstream and downstream males (
Figure 5). This intriguing pattern suggests that not only might the behavior of downstream males be influenced by exposure to EDCs, but also that the response of conspecifics toward these males could be affected. As social interactions between males are important for securing nest sites and attracting females, the response of others in the population to individuals that have been exposed to EDCs could have more dramatic effects on fitness than any change in behavior of the individuals themselves.
Although only two behaviors examined did reveal significant differences in the downstream males, we feel this study likely underestimates the influence of estrogen on reproductive behavior in this system due to the small sample size and relatively short exposure time. The fact that any significant differences were found in such a conservative study suggests that estrogenicity at this site may have a very powerful effect on reproductive behaviors. This finding, coupled with the lack of results in the vitellogenin assay, may very well mean that environmental estrogens are having a much greater effect on wildlife more generally than previously thought.
4. Materials and Methods
4.1. Test Fish and Study Site
Adult fathead minnows (
n = 32) to be used in the behavioral assay (approximately five months old; mean standard length (SL) = 5.03 cm; mass = 3.20 g) were acquired from Aquatic Bio Systems in Fort Collins, Colorado. Males were identified as sexually mature by the presence of tubercles on their heads and spongy dorsal pads. Juvenile minnows of similar size (
n = 80; mean SL = 5.45 cm; mass = 2.15 g), but lacking tubercles and dorsal pads, were bought from Chris’s Bait and Tackle, in Mertztown, PA. Juvenile fish were used for the vitellogenin assay only. Field sites were located 10 m upstream and 10 m downstream of the wastewater treatment effluent of the Upper Saucon Township’s Wastewater Treatment Plant in Lehigh County, PA (40°33′ N, 75°23′ W, 113 m elevation). Based on data in [
27], the drainage area of the Saucon Creek watershed upstream of the Upper Saucon Township Wastewater Treatment plant is about 50 km
2; land use is approximately 30% agriculture, 30% suburban, 20% forested, and 10% a variety of other land uses. The average daily discharge by the Upper Saucon Township Wastewater Treatment plant is 53 L/s and the average flow of the Saucon Creek is 1700 L/s [
28]; personal communication by Karl E. Schreiter of Schreiter Engineering Associates]. The collection system is comprised of approximately 96 miles of underground pipe ranging from 8 inches to 30 inches in diameter and there are approximately 7000 customers [
28]. The wastewater treated at the plant is approximately 90% domestic waste. The plant uses aerobic activated sludge for the digestion of organic matter and the water passes through banks of UV lights for sanitation before discharge to the creek (personal communication; John Guinet, Assistant Director Water and Sewer Resources Upper Saucon Township). The study occurred between October and November 2013. All animal subjects were treated in accordance to guidelines set out by the National Research Council and the Guide for the Care and Use of Laboratory Animals.
4.2. Experimental Design
For exposure to stream water, fish were placed in wire cages made from 3.3 mm mesh galvanized wire hardware cloth (d × l = 22 cm × 56 cm). The upstream end of the cage was covered by an 11 L black plastic bucket, which provided protection from the stream current. To prevent movement and maintain the cages in the water column, each wire mesh cage had a cement block attached to it by a metal cable. On the day fish were to be put in the stream, 16 adult male minnows and approximately 40 juveniles were moved from laboratory holding tanks to 2-L flasks of stream water that had been previously collected and allowed to warm to room temperature. The flasks were then placed in a 4 °C refrigerator for approximately 2 h to allow the fish to acclimate by gradually lowering the temperature of the water. The flasks were transported to the field site in coolers and, upon arrival, placed in the stream for approximately 30 min for further temperature acclimation. Fish were placed into two cages positioned approximately 10 m downstream from the effluent site, and two cages positioned 10 m upstream (4 adults and 10 juveniles per cage). The first group of fish was placed in the stream on 28 October 2013. After 21 days the fish were retrieved and returned to the lab in 2-L flasks in a cooler for analysis. A second group of fish was placed in the stream in the same way on 11 November 2013 and retrieved after 21 days. Due to low survival rates in the cages (~50% of adults, ~20% of juveniles), individuals from both exposures were pooled into a single analysis for behavior and vitellogenin. While fish were being exposed to stream water, temperature, pH, conductivity, and dissolved oxygen levels were measured weekly with hand-held meters in the vicinity of the cages. The meters were calibrated each measurement day.
4.3. Estrogenicity Assay
For assaying levels of estrogenicity in the water, samples were obtained and filtered on site through a 0.2 µm sterile bacteriological filter and kept on ice during transport back to the lab. One upstream, one downstream, and one effluent sample of 100 mL each was collected approximately weekly from 28 October 2013 until 2 December 2013 for a total of 7 samples from each source. Estrogenic activity was measured using the YESne, a yeast estrogen assay developed by Colosi and Kney [
29] as a modification of the YES bioassay [
18]. In brief, 0.5 mL of stream water was added to 1.5 mL microfuge tubes. Either a spike of 1.56 × 10
−8 M 17β-estradiol in 10 µL of dimethyl sulfoxide (DMSO) or 10 µL of DMSO alone was added to each tube. Yeast (1 µL, a 1 to 100 dilution of stock; OD620 = 1.2) was added to each tube in 0.5 mL 2× medium. A set of standards with 17β-estradiol in concentrations ranging from 0 to 9.38 E-11 molar in 10 µL of DMSO was included with each sample run. All samples and standard curve concentrations were run in triplicate. The tubes were incubated at 30 °C for 24 h. After incubation, 100 µL of the original mixture was added to 900 µL of ortho-nitrophenyl-β-galactoside (ONPG) in Z-buffer (final concentration of ONPG was 0.36 mg/mL) in a new tube and incubated at 37 °C for 60 min. In the first incubation, the yeast produced the enzyme β-galactosidase in proportion to the estrogenicity in the water. In the second incubation, the β-galactosidase cleaved the ONPG producing the yellow ortho-nitrophenol. A 400 µL of stop buffer (1 M Na
2CO
3) was added to each tube, and 200 µL of the solution was transferred to a microtiter plate, which was read at 414 nm.
4.4. Vitellogenin Assay
Juveniles were euthanized using tricaine methanesulfonate (MS222) at concentrations greater than 250 mg/L after retrieval from the exposure site. The fish were then weighed and stored at −20 °C. Following the procedure described in “Preparation of whole-body homogenates from small fish using a hand-held homogenizer” (Biosense Laboratories AS, Bergen, Norway, 2004.1), ice cold sample buffer (PBS, 1% BSA with 3–7 TIU Aprotinin/mg) and fish were placed into a homogenizer at a 1:2, weight to volume ratio. The samples were homogenized until the tissue was finely processed, and then placed into labeled tubes on ice. The samples were centrifuged at 4 °C, 20,000× g for 30 min. Then, keeping the tubes on ice, the supernatant was carefully collected to avoid any fat in the sample. The homogenate samples were stored at −80 °C.
Vitellogenin was assessed for all but the smallest 2 of the 14 juvenile fish that survived both sets of the three-week exposure in the creek with a vitellogenin assay kit (Biosense Laboratories, Bergen, Norway). The kit contained pre-coated microplates, plate sealers, dilution buffer, PBS/ Tween tablets, detecting antibody, TMB substrate, and fathead minnow VTG standard, used to measure VTG in the plasma. Dilution series for each full body homogenate sample was established using the provided buffer at 1:25, 1:2500, and 1:250,000 dilutions. The standard curve included 11 dilution steps with a final concentration of 0.05 ng VTG/mL. In duplicate, 100 μL of each VTG standard dilution and each sample dilution was added to the appropriate wells. The plate was sealed, incubated at room temperature for 90 min, and then washed 3× with 300 μL washing buffer per well. The detecting antibody was diluted 1:500 and 100 μL was added to all wells. The plate was sealed, incubated at room temperature for 30 min, then washed 5× with 300 μL of washing buffer per well before adding 100 μL of TMB substrate to each well. The plate was left to incubate in the dark at room temperature for 20 min. The reaction was stopped by adding 100 μL 0.3 M H2SO4 to each well and then the plate was read in a microplate reader at 450 nm.
4.5. Behavior Assay
After retrieval from the field site, adult male fathead minnows (n = 15) were assayed for reproductive behavior in experimental tanks in a controlled laboratory setting (temperature = 22 ± 2 °C; 12:12 light:dark). Prior to testing, each male had a small clip taken from the bottom tailfin for identification purposes and was then placed in separate 37 L experimental aquaria. An equal number of control males that were not exposed to stream water had the upper tail fin clipped before being added to each experimental aquarium. Finally, a sexually mature female was placed in each tank along with two potential nest sites (terra cotta flower pots). Approximately half of the experimental males came from cages placed upstream of the wastewater treatment plant outflow (n = 7) and the other males came from cages placed downstream (n = 8). After a 24-h acclimation period, each tank was video-recorded 10 min/day for 3 consecutive days. Observers were not present in the room during the recording of behavior.
Observers blind to treatment groups analyzed video recordings for 10 behaviors in 3 categories: (1) Male to male (butting/biting, chasing, and tail beating), (2) male to female (butting/biting, chasing, tail beating, leading, and quivering), and (3) male to nest (nibbling and burrowing/rubbing). An ethogram was modified from Martinović et al. [
13] after preliminary observations of minnows prior to the start of the study (
Table 2). The number of behaviors per observation was averaged across the three days for both control and experimental males in the aquarium.
4.6. Statistical Analysis
Environmental parameters of stream water (temperature, pH, conductivity, dissolved O2, and estrogenicity) were compared between effluent, upstream, and downstream sites at each sampling date using a one-way ANOVA with Fishers LSD post-hoc tests. Levels of VTG were compared between downstream and upstream fish using an independent samples t-test. For the behavior assay, the number of behaviors was not normally distributed, so a non-parametric Wilcoxon Signed Ranks test was used to compare the behavior between control and experimental males in both upstream and downstream conditions. Statistical analyses were conducted using SPSS 17.0 and alpha was set at p = 0.05.