Abstract
Changes in different immune activities in the skin mucus of gilthead seabream (Sparus aurata L.) and European sea bass (Dicentrarchus labrax L.) specimens exposed to a constant light–dark photoperiod (12 h L:12 h D) were studied. Samples were collected at 08:00 (light on), 14:00, 20:00 (light off), 02:00, and again at 08:00 to determine immunoglobulin M (IgM) levels, several enzymes related to the immune system, and bactericidal activity. IgM levels were higher during the day in seabream and reached a minimum value at 20:00, but it was hardly affected in sea bass. No significant variations were recorded in the levels of protease and antiprotease. Peroxidase reached its maximum level in seabream at 02:00, the same time that it reached its minimum level in sea bass. Lysozyme showed little variation in seabream, but it was significantly lower at 14:00 than during the rest of the cycle in sea bass. Finally, different interspecific variations on bactericidal activity against Vibrio harveyi were recorded. The findings demonstrate that the immune parameters present in skin mucus of these important fish species are affected by the light–dark cycle and that there are substantial interspecies differences.
1. Introduction
Many environmental factors influence the life cycle of fish [1]. Most organisms are equipped with a biological system that allows them to maintain a circadian rhythm (daily cycle) to adapt to daily environmental changes in 24 h cycles generated by the Earth’s rotation [2]. Circadian rhythms are endogenously generated in vertebrates (including teleosts) and play a central role in the maintenance of homeostasis and growth. Hormone secretion, metabolism, and sleep are also influenced by circadian rhythms [3,4,5,6]. Furthermore, light and temperature can modulate these rhythms. Therefore, the behavior and physiology of fish are strongly influenced by light (both natural or seasonal and manipulated or artificial) conditions [2]. The teleost pineal is photoreceptive and considered to be essential for the generation, synchronization, and maintenance of biological rhythms, primarily via melatonin release. In fish, internal (circadian clock) and external (light) signals control melatonin production in the fish pineal [7]. More specifically, melatonin synthesis and secretion is suppressed by light and enhanced by darkness [8,9]. Peak levels in the dark are associated with age as well as various illnesses. Melatonin plays a role in regulating the sleep–wake cycle, pubertal development, and seasonal adaptation in humans [10]. Besides this, the role of the light–dark cycle or melatonin in the regulation of the immune system has been extensively described in mammals [8,11], and it is known that, in vertebrates (including fish), strong interactions exist between nervous and endocrine systems and also between these systems and the immune system [12]. Melatonin is the main hormone involved in the transduction of light information and the photoperiodic control of numerous significant physiological activities in fish.
Aquaculture production has continued to increase, and it is one of the fastest-growing animal-food-producing sectors [13]. However, the success of modern aquaculture is based on technological innovations as well as a good knowledge of the biology of the farmed fish, which is necessary to reach high production in controlled environmental conditions [12]. Although artificial photoperiods have been used to improve fish growth and manipulate reproduction [14], there is still very little information about the possibilities of altering the photoperiod to improve fish immune status. In this sense, it has been demonstrated that some components of the fish immune system also exhibit daily variations [8,12], but, to the best of our knowledge, the available information on this topic is very limited because such information mostly focus on seasonal variations (reviewed by [2]). In the above studies, daily rhythmicity was demonstrated in several components of fish humoral (such as complement, lysozyme, and peroxidase activities and globulin level) and cellular (leucocyte phagocytosis and production of reactive oxygen species) immunity [12,15,16]. Our group evaluated the effects of photoperiod on some seric immune parameters of gilthead seabream (S. aurata L.) and European sea bass (D. labrax) specimens [8], which are the most widely farmed fish species in the world [14]. Because it is known that the mucus layer constitutes the first barrier of defense of fish as it protects the skin against external aggressions, including those from opportunistic or obligated pathogens present in aquatic environments [17,18], the present study aimed to analyze the levels of different enzymes related to the immune system as well as the bactericidal activity present in skin mucus of these two marine fish species (gilthead seabream and European sea bass) in a light–dark (LD) cycle of 24 h. The knowledge gained on how daily rhythms regulate the main fish immune activities will help in attempts to improve fish welfare and health.
2. Results
2.1. Total IgM Levels
Immunoglobulin M (IgM) levels in skin mucus of gilthead seabream showed the highest values at 08:00 (when lights were turned on), after which they decreased as the light cycle progressed, reaching minimum values at 20:00 (when the lights were switched off); the differences between 20:00 and 08:00 were statistically significant. From 20:00 till 08:00, the IgM values increased (Figure 1A). By contrast, no significant variations were observed in the IgM levels in European seabass sampled at the different experimental times (Figure 1B). Furthermore, a significant daily rhythm was found for IgM in gilthead sea bream but not in European sea bass (Figure 1A).
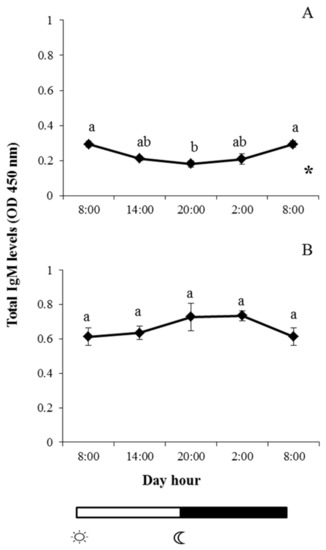
Figure 1.
Photoperiod effect on total immunoglobulin M (IgM) levels found in skin mucus of gilthead seabream (A) and European sea bass (B), sampled at 6 h intervals throughout the day. Symbols  and
and  indicate lights on and off, respectively. Results are expressed as mean ± standard error of the mean (SEM) (n = 6). Different letters denote variations in the IgM levels at the sampling times (ANOVA), and the asterisk indicates a significant rhythmic pattern over 24 h (Ritme©). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
indicate lights on and off, respectively. Results are expressed as mean ± standard error of the mean (SEM) (n = 6). Different letters denote variations in the IgM levels at the sampling times (ANOVA), and the asterisk indicates a significant rhythmic pattern over 24 h (Ritme©). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
 and
and  indicate lights on and off, respectively. Results are expressed as mean ± standard error of the mean (SEM) (n = 6). Different letters denote variations in the IgM levels at the sampling times (ANOVA), and the asterisk indicates a significant rhythmic pattern over 24 h (Ritme©). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
indicate lights on and off, respectively. Results are expressed as mean ± standard error of the mean (SEM) (n = 6). Different letters denote variations in the IgM levels at the sampling times (ANOVA), and the asterisk indicates a significant rhythmic pattern over 24 h (Ritme©). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
2.2. Enzyme Activities in Skin Mucus
Protease, antiprotease, peroxidase, and lysozyme were studied in skin mucus of both fish species during the light–dark cycle. In the case of protease and antiprotease activities, a similar pattern was observed in both fish species, and no significant changes were observed at any of the studied times; furthermore, there were no significant daily rhythms in these activities (Figure 2 and Figure 3).
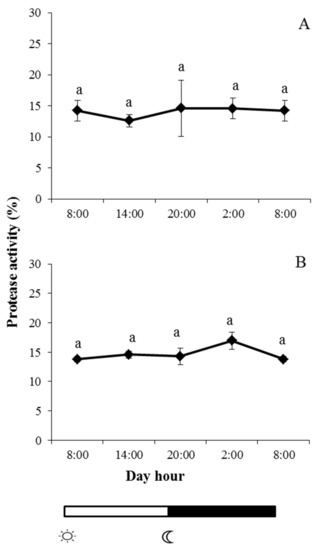
Figure 2.
Photoperiod effect on protease activity in skin mucus of gilthead seabream (A) and European sea bass (B), sampled at 6 h intervals throughout the day. Symbols  and
and  indicate lights on and off, respectively. Results are expressed as mean ± SEM (n = 6). Different letters denote variations in the protease activity at the sampling times (ANOVA). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
indicate lights on and off, respectively. Results are expressed as mean ± SEM (n = 6). Different letters denote variations in the protease activity at the sampling times (ANOVA). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
 and
and  indicate lights on and off, respectively. Results are expressed as mean ± SEM (n = 6). Different letters denote variations in the protease activity at the sampling times (ANOVA). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
indicate lights on and off, respectively. Results are expressed as mean ± SEM (n = 6). Different letters denote variations in the protease activity at the sampling times (ANOVA). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
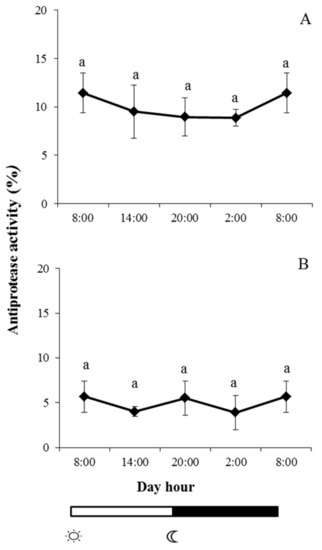
Figure 3.
Photoperiod effect on antiprotease activity in skin mucus of gilthead seabream (A) and European sea bass (B), sampled at 6 h intervals throughout the day. Symbols  and
and  indicate lights on and off, respectively. Results are expressed as mean ± SEM (n = 6). Different letters denote variations in the antiprotease activity at the sampling times (ANOVA). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
indicate lights on and off, respectively. Results are expressed as mean ± SEM (n = 6). Different letters denote variations in the antiprotease activity at the sampling times (ANOVA). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
 and
and  indicate lights on and off, respectively. Results are expressed as mean ± SEM (n = 6). Different letters denote variations in the antiprotease activity at the sampling times (ANOVA). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
indicate lights on and off, respectively. Results are expressed as mean ± SEM (n = 6). Different letters denote variations in the antiprotease activity at the sampling times (ANOVA). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
By contrast, peroxidase reached its maximum level in gilthead seabream at 02:00, the same time that it reached its minimum level in European sea bass (Figure 4). Peroxidase reached its minimum level in gilthead seabream at 08:00 (when lights were turned on), the same time that it reached its minimum level in European sea bass. A significant daily rhythm was obtained for gilthead sea bream peroxidase activity but not for European sea bass (Figure 4A).
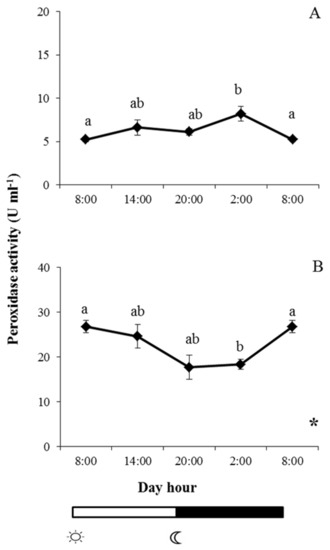
Figure 4.
Photoperiod effect on peroxidase activity in skin mucus of gilthead seabream (A) and European sea bass (B), sampled at 6 h intervals throughout the day. Symbols  and
and  indicate lights on and off, respectively. Results are expressed as mean ± SEM (n = 6). Different letters denote variations in the peroxidase activity at the sampling times (ANOVA), and the asterisk indicates a significant rhythmic pattern over 24 h (Ritme©). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
indicate lights on and off, respectively. Results are expressed as mean ± SEM (n = 6). Different letters denote variations in the peroxidase activity at the sampling times (ANOVA), and the asterisk indicates a significant rhythmic pattern over 24 h (Ritme©). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
 and
and  indicate lights on and off, respectively. Results are expressed as mean ± SEM (n = 6). Different letters denote variations in the peroxidase activity at the sampling times (ANOVA), and the asterisk indicates a significant rhythmic pattern over 24 h (Ritme©). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
indicate lights on and off, respectively. Results are expressed as mean ± SEM (n = 6). Different letters denote variations in the peroxidase activity at the sampling times (ANOVA), and the asterisk indicates a significant rhythmic pattern over 24 h (Ritme©). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
For its part, lysozyme activity in gilthead seabream skin mucus increased slightly throughout the light period but not to a statistically significant extent (Figure 5A). However, lysozyme activity was lower at 02:00 than at 08:00 (when lights were switched on), but the values recovered to reach similar levels to those registered in samples collected at 08:00 (Figure 5B). By contrast, significant daily rhythmicity was found in this activity for both fish species (Figure 5).
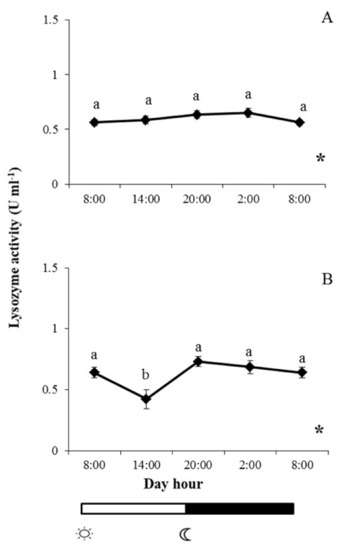
Figure 5.
Photoperiod effect on lysozyme activity in skin mucus of gilthead seabream (A) and European sea bass (B), sampled at 6 h intervals throughout the day. Symbols  and
and  indicate lights on and off, respectively. Results are expressed as mean ± SEM (n = 6). Different letters denote variations in the lysozyme activity at the sampling times (ANOVA), and the asterisk indicates a significant rhythmic pattern over 24 h (Ritme©). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
indicate lights on and off, respectively. Results are expressed as mean ± SEM (n = 6). Different letters denote variations in the lysozyme activity at the sampling times (ANOVA), and the asterisk indicates a significant rhythmic pattern over 24 h (Ritme©). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
 and
and  indicate lights on and off, respectively. Results are expressed as mean ± SEM (n = 6). Different letters denote variations in the lysozyme activity at the sampling times (ANOVA), and the asterisk indicates a significant rhythmic pattern over 24 h (Ritme©). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
indicate lights on and off, respectively. Results are expressed as mean ± SEM (n = 6). Different letters denote variations in the lysozyme activity at the sampling times (ANOVA), and the asterisk indicates a significant rhythmic pattern over 24 h (Ritme©). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
2.3. Bactericidal Activity
Bactericidal activity against V. harveyi was differently affected by the photoperiod in gilthead seabream and European sea bass (Figure 4). In the case of gilthead seabream, the activity increased throughout the day to show significant increases at 02:00. From this time, the activity decreased to reach minimum values at 08:00, with no significant daily rhythm (Figure 6A). By contrast, in European sea bass, bactericidal activity was significantly lower from 14:00 to 20:00 compared with the values recorded for skin mucus samples collected at 08:00, with a significant daily rhythm also being evident (Figure 6B).
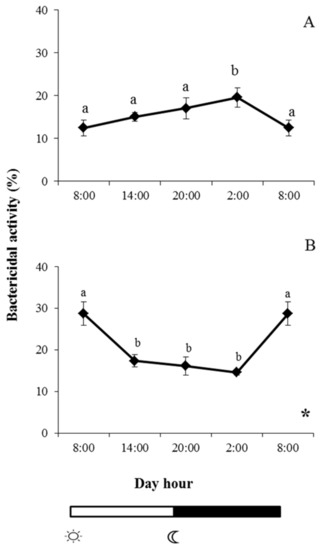
Figure 6.
Photoperiod effect on bactericidal activity against V. harveyi in skin mucus of gilthead seabream (A) and European sea bass (B), sampled at 6 h intervals throughout the day. Symbols  and
and  indicate lights on and off, respectively. Different letters denote variations in the bactericidal activity at the sampling times (ANOVA), and the asterisk indicates a significant rhythmic pattern over 24 h (Ritme©). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
indicate lights on and off, respectively. Different letters denote variations in the bactericidal activity at the sampling times (ANOVA), and the asterisk indicates a significant rhythmic pattern over 24 h (Ritme©). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
 and
and  indicate lights on and off, respectively. Different letters denote variations in the bactericidal activity at the sampling times (ANOVA), and the asterisk indicates a significant rhythmic pattern over 24 h (Ritme©). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
indicate lights on and off, respectively. Different letters denote variations in the bactericidal activity at the sampling times (ANOVA), and the asterisk indicates a significant rhythmic pattern over 24 h (Ritme©). The levels of significance were set at p < 0.05. Letters shared in common among experimental times indicate no significant differences.
3. Discussion
All living organisms are under the influence of different physical external rhythms, which have a great impact on their physiology [2]. Changes in day length (photoperiod) are the most potent signals of the time of year and allow seasonal changes in temperature and food availability to be anticipated, which is critical for reproductive success. In mammals, there are many studies about how photoperiod (environmental factor) influences neuronal function and melatonin secretion (internal factor) and also about how the hormone melatonin can act directly and indirectly to govern seasonal changes in the immunity of a variety of vertebrate taxa [19], including fish [1,8,12,20,21].
Apart from seasonal rhythms, there are also daily rhythms. The main regulator (a clock and a calendar) for daily (and seasonal) rhythms is also the periodicity of the external light–dark cycle. Light may also regulate immunity through hormonal modifications involving the neuroendocrine system [8,22,23]. Again, the external periodicity reflects the periodicity of melatonin secretion from the pineal gland (it is inhibited by light and induced during darkness). Melatonin levels peak during the night (both in diurnal and nocturnal species) [24]. The goal of the present study was to examine the effects of the 24 h light–dark cycle on the skin mucus levels of some important enzymes related to immunity. Two important marine-farmed fish were selected, namely, gilthead seabream and European sea bass. The transduction of seasonal information from the environment (i.e., photoperiod and water temperature) and daily rhythms have previously been studied in both fish species, and the results clearly suggest that the pineal gland is the major source of plasma melatonin [25,26].
The fish immune system comprises numerous distinct and interdependent components that are necessary for organisms to defend themselves against invading pathogens [18]. In this sense, several environmental factors may influence the immune response, although photoperiod and temperature are the most important [1,12]. Thus, the management and control of the physical conditions, photoperiod in this case, in fisheries may be considered of great importance for fish welfare and health. In fact, photoperiod manipulation has been successfully used to accelerate growth, development, and survival of young stages in several fish species [27,28] and also to reduce unwanted sexual maturation [29,30]. However, there are no data about how photoperiod could be altered or modified in order to contribute to make the fish immune status more robust.
Regarding fish immunity, several studies have demonstrated the relationship between photoperiod and the immune system [8,9,12,15,31]. For example, a study conducted by our group (also on gilthead seabream and European sea bass) reported how the photoperiod affected some seric immune parameters (natural hemolytic complement, lysozyme and peroxidase activities) [8]. In the present work, we have studied how the photoperiod affects the levels of other immune parameters (IgM level, protease, antiprotease, peroxidase, lysozyme, and bactericidal activities) that are present in the mucus of skin, an organ of great importance because it is in direct contact with the environment. All these activities were chosen due to their involvement in the biostatic and biocidal activity in the skin mucus of several fish species [32,33,34]. The results indicated that the activities followed different patterns in response to the light–dark cycle. Furthermore, notable interspecific variations were observed between gilthead seabream and European sea bass samples. However, not all the studied activities showed rhythmicity; for example, IgM and peroxidase showed rhythmicity in the skin mucus of gilthead seabream, and bactericidal activity showed rhythmicity in European sea bass. Only lysozyme showed rhythmicity in both fish species. Such differences may be due to the different expression of clock genes, which generate daily rhythmic behavioral and physiological responses in vertebrates [35,36]. New studies on the expression of such genes in the skin of teleosts could help to understand the complex relationships between photoperiod and mucosal immunity.
Immunoglobulins, or antibodies, play a vital role in immune responses, and IgM is considered as the most ancient antibody molecule, which shares similar functions in all gnathostomes [37]. This Ig has a key role in both innate and adaptive immunity in fish, and its effector functions include complement activation (which both lyses and opsonizes microorganisms) [38]. Furthermore, IgM also mediates the agglutination of pathogens for phagocytosis and their removal [39]. Interestingly, the present results demonstrated that total IgM levels in skin mucus were lower during the night in gilthead seabream, with a significant decrease beginning when lights were switched off (08:00), while in European sea bass, IgM levels were lower during the day but without significant differences. As previously confirmed, specific antibodies can be generated in the mucosa of fish, including the skin [40], although new studies are needed to correlate the results obtained for daily changes of IgM in skin mucus with the IgM levels recorded in other mucosal surfaces (intestine and gills) and also with the systemic IgM levels present in serum, where it is the most prevalent Ig [37]. It should also be interesting to determine the levels of IgT/Z, which is considered the most important Ig of mucosal surfaces; however, to the best of our knowledge, there are no available antibodies for this fish species.
Intriguingly, in the present work, no significant variations were recorded in the levels of protease or antiprotease in the mucus from either of the studied fish species, results that agree with those previously obtained in Nile tilapia (Oreochromis niloticus) subjected to a LD cycle [31]. However, higher protease and antiprotease activities were recorded during the light period in Trachinotus falcatus [15]. Proteases are essential for the activation of innate and adaptive immune system, and they exert a protective role against pathogens [41]. On the other hand, antiproteases may help to combat the proteases produced by many microorganisms, which are crucial proteins for contributing to their growth and development. For these important reasons, these enzymes play critical roles in microbial infection and disease manifestation. Future studies aimed at understanding why the levels of these enzymes seem to be independent of the light–dark cycle in some teleost fish species will be welcome.
Peroxidase activity in skin mucus reached its maximum in gilthead seabream at 02:00, the time when it reached minimum levels in European sea bass. Curiously, when this enzyme was studied in serum from both species maintained in the same 12 h light: 12 h dark cycle, the peroxidase activity of seabream was significantly higher at 08:00 than during the rest of the cycle, while it showed little variation in sea bass [8]. These results indicate that the rhythms of this enzyme in the two fish species differ in serum and skin mucus. Taking into account that peroxidases are important microbicidal agents because they are able of efficiently eliminate H2O2 and maintain the redox balance of the immune system, the importance of this observation for mucosal immunity and skin defense is evident. The scarce results available on the influence of photoperiod on serum peroxidase levels suggest that peroxidase secretion depends on the fish species [8], as the results obtained in the present study suggest.
Lysozymes are ubiquitous antibacterial enzymes that are widely distributed within animals. Lysozymes display hydrolytic activity to specifically cleave the β-1,4-glycosidic bonds between the N-acetylglucosamine and N-acetylmuramic acid of peptidoglycan (an essential and single cross-linked bacterial cell wall heteropolymer that provides structural strength and protects the osmotically sensitive protoplast). Thus, the disruption of the peptidoglycan present in the bacterial cell walls results in cell lysis and destruction of the microorganisms [42]. Lysozyme activity in fish serum also follows a clear daily rhythm, although, once again, different interspecific patterns have been observed in gilthead seabream and European sea bass [8]. Similarly, a different pattern was observed in the levels of lysozyme in the skin mucus of these two fish species. Although lysozyme activity increased in both species in the dark, significantly lower levels were found at 14:00 in European sea bass. The present results are contrary to those obtained in O. niloticus, in which the lysozyme activity was highest in the light [8].
Numerous studies have demonstrated the high antimicrobial activity present in fish skin mucus [43], a biochemical barrier that contains many different enzymes, such as those studied in the present work, which may be responsible for the antimicrobial activity in both individual enzymes and as a whole [44]. The present results indicated that mucus bactericidal activity against V. harveyi was also significantly affected by the light–dark cycle in gilthead sea bream and European sea bass. Our results agree with those obtained after a bacterial endotoxin challenge in O. niloticus [31] and with those obtained in Oncorhynchus mykiss focused on the dynamics and interplay of serum-mediated bacterial-killing activity against Flavobacterium psychrophilum and Yersinia ruckeri and on several immune defense factors during the daily light–dark cycle [45]. The above studies suggest that the serum responsiveness of humoral factors to a biological insult depend on the time of day. A deeper knowledge of the daily rhythm of bactericidal activity (not only in fish skin mucus but also in serum) would help us to anticipate the normal response of farmed fish in order to improve their welfare, to increase immune system robustness, or to prevent and/or treat possible diseases. Similarly, knowing the periodic changes that occur in fish immunocompetence could throw light on seasonal changes in disease incidence and severity in nature and provide a useful framework for understanding brain–immune interactions. Furthermore, the study of the influence of other light–dark patterns in the immune status of fish could be considered of great interest.
Immune cells have membrane receptors and nuclear orphan receptors to detect melatonin levels [46]. Comparative studies have demonstrated that the effects exerted by melatonin on immune parameters differ and depend on several factors (apart from melatonin level), including species, sex, age of animal, its immune system maturation, etc. and also on the parameters examined, experimental procedure, etc. In order to respond to the signal sent by melatonin, the immune cells become activated and secrete lymphoid organ-derived hormones as well as some cytokines. These lymphoid messages are understood by the pineal gland, completing the bidirectional regulatory loop between both systems [46]. However, much more data are needed to know the different steps that may be involved in these extremely complex networks of communication between neuroendocrine and the immune system in fish.
In conclusion, the present findings demonstrate that different enzymes related to the immune system and bactericidal activity of skin mucus varies substantially during the daily light–dark cycle in gilthead seabream and European sea bass. A better knowledge of the interactions between fish melatonin and the immune system will facilitate the development of novel practical suggestions (such as photoperiod manipulation) for enhancing the immune system and welfare of fish in hatcheries and on-growing farms. The complex interactions of the environment, host, and pathogens in farmed fish present numerous points that can be manipulated for research purposes and to improve production.
4. Materials and Methods
4.1. Animals
Thirty specimens of the hermaphroditic protandrous seawater teleost gilthead seabream (S. aurata) and 30 specimens of the gonocoric seawater teleost sea bass (D. labrax) obtained from a local farm were kept in recirculating seawater aquaria (400 L) with a flow rate of 900 L/h at 22 ± 2 °C and 25% salinity in the Marine Fish Facilities at the University of Murcia. Commercial diet (Skretting, Spain) was administrated at rate of 2% body weight/day. All the experimental protocols were approved by the Ethical Committee of the University of Murcia, following the guidelines of the European Union for animal handling (2010/63/UE) (permit number CEEA 357/2017).
An individual light source consisting of a blue bulb (Grolux; Silvania) was located at the top of each tank. The light–dark cycle (artificial photoperiod 12 h light: 12 h dark) was programmed by an electronic timer and set to switch on at 08:00 and off at 20:00. Specimens were reared under a 12 h L: 12 h D daily cycles for a month.
4.2. Experimental Design and Sampling
Skin mucus samples were collected at 08:00, 14:00, 20:00, 02:00, and again at 08:00 (six fish per time and per fish species). Sample collection during the dark phase was conducted with a red light. Prior to sampling, fish were anesthetized (20 mg/L of clove oil, Guinama®). Skin mucus was gently collected with a cell scraper (Sigma-Aldrich, Saint Louis, MO, USA) from the whole skin surface, avoiding blood, urine, and feces during collection [34]. Mucus samples were vigorously shaken and centrifuged (1400× g, 10 min, 4 °C), with the supernatant collected and kept frozen at −20 °C until use [17]. Protein concentration in each sample was determined by the Bradford method [47].
4.3. Total Immunoglobulin M Levels
Total IgM levels were analyzed for gilthead seabream using the enzyme-linked immunosorbent assay (ELISA) [48]. Thus, 10 µg/well of skin mucus proteins were placed in flat-bottomed 96-well plates in triplicate, and the proteins were coated by overnight incubation at 4 °C with 100 µL of carbonate–bicarbonate buffer (35 mM NaHCO3 and 15 mM Na2CO3, pH 9.6). After three rinses with PBS-T (phosphate buffer saline (PBS) and 0.05% Tween 20), plates were blocked for 2 h at room temperature with 200 µL/well blocking buffer with 3% bovine serum albumin (BSA, Sigma-Aldrich) in PBS-T and rinsed three times with PBS-T. The plates were then incubated for 1 h with 100 µL/well of mouse anti-gilthead seabream or anti-sea bass IgM monoclonal antibody (1:100 in blocking buffer; Aquatic Diagnostics Ltd., Scotland, UK), washed, and incubated for 1 h with the secondary antibody anti-mouse IgG-horseradish peroxidase (HRP) (1:1000 in blocking buffer; Sigma-Aldrich). After exhaustive rinsing with PBS-T, the samples were developed using 100 µL of a 0.42 mM3,3′,5,5′-tetramethylbenzidine hydrochloride (TMB, Sigma-Aldrich) prepared daily in distilled water containing 0.01% hydrogen peroxide (H2O2). The reaction was allowed to proceed for 1–10 min and stopped by the addition of 50 µL of 2 M sulfuric acid (H2SO4). The plates were read at 450 nm in a plate reader (BMG Labtech, Ortenberg, Germany). Negative controls consisted of samples without skin mucus or without primary antibody, whose optical density (OD) values were subtracted for each sample value.
4.4. Protease Activity
Protease activity was quantified using the azocasein hydrolysis assay [49]. Briefly, 100 µL of skin mucus were incubated with 100 mM ammonium bicarbonate buffer containing 100 µL of 0.7% azocasein (Sigma-Aldrich) for 24 h at 30 °C. The reaction was stopped by adding 250 µL of 4.6% trichloroacetic acid (TCA), and the mixture was centrifuged (6000× g, 5 min). The supernatants were transferred to a 96-well plate in triplicate containing 100 µL/well of 0.5 N sodium hydroxide (NaOH), and the OD was read at 450 nm using a plate reader (BMG Labtech). Skin mucus were replaced by trypsin solution (5 mg/mL, Sigma-Aldrich) as positive control (100% of protease activity) or by buffer as negative control (0% activity).
4.5. Antiprotease Activity
Total antiprotease activity was determined by the capacity of skin mucus to inhibit trypsin activity [50]. Briefly, 10 µL of skin mucus samples were incubated (10 min, 22 °C) with the same volume of standard trypsin solution (5 mg/mL). After adding 100 µL of 100 mM ammonium bicarbonate buffer and 125 µL of 0.7% azocasein, samples were incubated (2 h, 30 °C) and, following the addition of 250 µL, 4.6% TCA, a new incubation (30 min, 30 °C) was done. The mixture was then centrifuged (13,000× g, 5 min), with the supernatants transferred to a 96-well plate in triplicate containing 100 µL/well 0.5N NaOH, and the OD was read at 450 nm using a plate reader. Skin mucus were replaced by buffer as positive control (100% protease and 0% antiprotease activity) and by trypsin as negative control (0% protease and 100% antiprotease activity). The percentage of inhibition of trypsin activity by each sample was calculated.
4.6. Peroxidase Activity
The peroxidase activity in skin mucus samples was measured [51]. For this, 10 µL of skin mucus was diluted with 40 µL of Hank’s buffer (HBSS) without Ca2+ or Mg2+ in flat-bottomed 96-well plates. As substrates, 100 µL of 10 mM TMB solution (Sigma-Aldrich) and 0.015% H2O2 were added. The color-change reaction was stopped after 2 min by adding 50 µL of 2 M H2SO4, and the OD was read at 450 nm in a plate reader. Standard samples without skin mucus were used as blanks. One unit was defined as the amount producing an absorbance change of 1, and the activity was expressed as U/mL.
4.7. Lysozyme Activity
Lysozyme activity was measured according to the turbidimetric method described by [52] with some modifications. Briefly, 20 μL of skin mucus were placed in flat-bottomed 96-well plates. To each well, 180 µL of freeze-dried Micrococcus lysodeikticus (0.2 mg/mL, Sigma-Aldrich) in 40 mM sodium phosphate (pH 6.2) was added as lysozyme substrate. As blanks of each sample, 20 μL of skin mucus were added to 180 μL of sodium phosphate buffer. The absorbance at 450 nm was measured after 20 min at 35 °C in a microplate reader (BMG Labtech). The amounts of lysozyme present in skin mucus were obtained from a standard curve made with hen egg white lysozyme (HEWL, Sigma) through serial dilutions in the above buffer. Skin mucus lysozyme values were expressed as U/mL equivalent of HEWL activity.
4.8. Bactericidal Activity
An opportunist marine pathogenic bacterium (V. harveyi) was used to determine the bactericidal activity present in the mucus samples. Bacteria were grown in agar plates at 25 °C in tryptic soy both medium (TSB, Sigma). Then, fresh single colonies of 1–2 mm were diluted in 5 mL of appropriate liquid culture medium and cultured for 16 h at 25 °C at 200–250 rpm until exponential growing, at which point bacteria were resuspended in TSB and adjusted to 1 × 106 cfu/mL. The mucus bactericidal activity was then determined following the method of Graham et al. [53] with some modifications. Briefly, 20 μL of skin mucus was added to triplicate wells of a U-shaped 96-well plate. Hank’s balanced salt solution was added to some wells instead of sample and served as positive control. To each well, 20 μL of V. harveyi was added, and the plates were incubated for 5 h at 25 °C. To each well, 25 μL of 3-(4,5 dimethyl-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, 1 mg/mL, Sigma) were added, and the plates were incubated for 10 min to allow the formation of formazan. Plates were then centrifuged (2000× g, 10 min), and the precipitate was dissolved in 200 μL of dimethyl sulfoxide (DMSO, Sigma). The absorbance of the dissolved formazan was measured at 560 nm in a microplate reader (BMG Labtech). Bacterial viability was expressed as percentage, calculated from the difference between the surviving bacteria and the number of bacteria from positive controls (100%).
4.9. Statistical Analysis
The results are expressed as mean ± standard error of the mean (SEM). All data were analyzed by one-way ANOVA and Tukey’s post-hoc test to determine differences among groups. Normality of the data was previously assessed using a Shapiro–Wilk test, and homogeneity of variance was also verified using the Levene test. All the statistical analyses were conducted using Statistical Package for Social Science (SPSS for Windows; v23.0, Chicago, IL, USA). The daily rhythms for each activity were analyzed using the Ritme© software package (Antoni Diez-Noguera, University of Barcelona, Barcelona, Spain). Differences for both analyses were considered statistically significant when p < 0.05.
Author Contributions
Conceptualization, M.Á.E.; methodology, D.C.-F. and A.C.; software, D.C.-F.; validation, A.C.; formal analysis, M.Á.E.; investigation, D.C.-F. and A.C.; resources, M.Á.E.; data curation, A.C.; writing—original draft preparation, D.C.-F.; writing—review and editing, A.C. and M.Á.E.; supervision, M.Á.E.; funding acquisition, M.Á.E. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Spanish Ministry of Economy and Competitiveness (MINECO; Grant No. AGL2014-51839-C5-1-R) co-funded by the European Regional Development Funds (ERDF/FEDER) and Fundación Seneca de la Región de Murcia (Grupo de Excelencia Grant No. 19883/GERM/15).
Acknowledgments
The authors gratefully acknowledge the assistance of A.I. Salvá. D.C.F. is grateful to the MINECO for a F.P.I. fellowship (Grant No. BES-2015-074726).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Valero, Y.; García-Alcázar, A.; Esteban, M.Á.; Cuesta, A.; Chaves-Pozo, E. Seasonal variations of the humoral immune parameters of European sea bass (Dicentrarchus labrax L.). Fish Shellfish Immunol. 2014, 39, 185–187. [Google Scholar] [CrossRef]
- Bowden, T.J.; Thompson, K.D.; Morgan, A.L.; Gratacap, R.M.; Nikoskelainen, S. Seasonal variation and the immune response: A fish perspective. Fish Shellfish Immunol. 2007, 22, 695–706. [Google Scholar] [CrossRef]
- Kalra, S.P.; Dube, M.G.; Pu, S.; Xu, B.; Horvath, T.L.; Kalra, P.S. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr. Rev. 1999, 20, 68–100. [Google Scholar] [CrossRef] [PubMed]
- Boeuf, G.; Le Bail, P.Y. Does light have an influence on fish growth? Aquaculture 1999, 177, 129–152. [Google Scholar] [CrossRef]
- Mogi, M.; Yokoi, H.; Suzuki, T. Analyses of the cellular clock gene expression in peripheral tissue, caudal fin, in the Japanese flounder, Paralichthys olivaceus. Gen. Comp. Endocrinol. 2017, 248, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ramsey, K.M.; Marcheva, B.; Bass, J. Circadian rhythms, sleep, and metabolism. J. Clin. Invest. 2011, 121, 2133–2141. [Google Scholar] [CrossRef]
- McStay, E.; Migaud, H.; Vera, L.M.; Sánchez-Vázquez, F.J.; Davie, A. Comparative study of pineal clock gene and AANAT2 expression in relation to melatonin synthesis in Atlantic salmon (Salmo salar) and European seabass (Dicentrarchus labrax). Comp. Biochem. Physiol. B 2014, 169, 77–89. [Google Scholar] [CrossRef]
- Esteban, M.Á.; Cuesta, A.; Rodríguez, A.; Meseguer, J. Effect of photoperiod on the fish innate immune system: A link between fish pineal gland and the immune system. J. Pineal Res. 2006, 41, 261–266. [Google Scholar] [CrossRef]
- Liebmann, P.M.; Wölfler, A.; Felsner, P.; Hofer, D.; Schauenstein, K. Melatonin and the immune system. Int. Arch. Allergy Immunol. 1997, 112, 203–211. [Google Scholar] [CrossRef]
- Emet, M.; Ozcan, H.; Ozel, L.; Yayla, M.; Halici, Z.; Hacimuftuoglu, A. A review of melatonin, its receptors and drugs. Eurasian J. Med. 2016, 48, 135. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Guerrero, J.M.; Lardone, P.J.; Reiter, R.J. A review of the multiple actions of melatonin on the immune system. Endocr. Res. 2005, 27, 189–200. [Google Scholar] [CrossRef]
- Esteban, M.Á.; Cuesta, A.; Chaves-Pozo, E.; Meseguer, J. Influence of melatonin on the immune system of fish: A review. Int. J. Mol. Sci. 2013, 14, 7979–7999. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018. [Google Scholar]
- Maitra, S.K.; Hasan, K.N. The Role of melatonin as a hormone and an antioxidant in the control of fish reproduction. Front. Endocrinol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cabillon, N.A.R.; Lazado, C.C. Mucosal barrier functions of fish under changing environmental conditions. Fishes 2019, 4, 2. [Google Scholar] [CrossRef]
- Kaplan, J.E.; Chrenek, R.D.; Morash, J.G.; Ruksznis, C.M.; Hannum, L.G. Rhythmic patterns in phagocytosis and the production of reactive oxygen species by zebrafish leukocytes. Comp. Biochem. Physiol. A 2008, 151, 726–730. [Google Scholar] [CrossRef]
- Cordero, H.; Cuesta, A.; Meseguer, J.; Esteban, M.Á. Changes in the levels of humoral immune activities after storage of gilthead seabream (Sparus aurata) skin mucus. Fish shellfish immunol. 2016, 58, 500–507. [Google Scholar] [CrossRef]
- Esteban, M.Á. An Overview of the Immunological Defenses in Fish Skin. ISRN Immunol. 2012, 2012, 1–29. [Google Scholar] [CrossRef]
- Weil, R.J.; Borniger, Z.M.; Cisse, J.C.; Salloum, Y.M.; Nelson, B.A.A. Neuroendocrine control of photoperiodic changes in immune function. Front. Neuroendocr. 2015, 37, 108–118. [Google Scholar] [CrossRef]
- Nakanishi, T. Seasonal changes in the humoral immune response and the lymphoid tissues of the marine teleost, Sebastiscus marmoratus. Vet. Immunol. Immunopathol. 1986, 12, 213–221. [Google Scholar] [CrossRef]
- Bly, L.W.; Quiniou, J.E.; Clem, S.M. Environmental effects on fish immune mechanisms. Dev. Biol. Stand. 1997, 90, 33–43. [Google Scholar]
- Haldar, R.; Ahmad, C. Photoimmunomodulation and melatonin. J. Photochem. Photobiol. B Biol. 2010, 98, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.E. Light and immunomodulation. Ann. N. Y. Acad. Sci. 2000, 917, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Płytycz, S.; Seljelid, R. Rhythms of immunity. Arch. Immunol. Ther. Exp. 1997, 45, 157–162. [Google Scholar]
- Molina-Borja, M.; Falcón, J.; Ravault, J.P. Production of melatonin by the gilthead sea bream pineal: An in vivo and in vitro study. Fish Physiol. Biochem. 1996, 15, 413–419. [Google Scholar] [CrossRef]
- García-Allegue, R.; Madrid, J.A.; Sánchez-Vázquez, F.J. Melatonin rhythms in European sea bass plasma and eye: Influence of seasonal photoperiod and water temperature. J. Pineal Res. 2001, 31, 68–75. [Google Scholar] [CrossRef]
- Tandler, A.; Helps, S. The effects of photoperiod and water exchange rate on growth and survival of gilthead sea bream (Sparus aurata, Linnaeus; Sparidae) from hatching to metamorphosis in mass rearing systems. Aquaculture 1985, 48, 71–82. [Google Scholar] [CrossRef]
- Kissil, G.W.; Lupatsch, I.; Elizur, A.; Zohar, Y. Long photoperiod delayed spawning and increased somatic growth in gilthead seabream (Sparus aurata). Aquaculture 2001, 200, 363–379. [Google Scholar] [CrossRef]
- Bromage, N.; Porter, M.; Randall, C. The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture 2001, 197, 63–98. [Google Scholar] [CrossRef]
- Chi, L.; Li, X.; Liu, Q.; Liu, Y. Photoperiod regulate gonad development via kisspeptin/kissr in hypothalamus and saccus vasculosus of Atlantic salmon (Salmo salar). PLoS ONE 2017, 12, e0169569. [Google Scholar] [CrossRef]
- Lazado, C.C.; Skov, P.V.; Pedersen, P.B. Innate immune defenses exhibit circadian rhythmicity and differential temporal sensitivity to a bacterial endotoxin in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2016, 55, 613–622. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Cuesta, A.; Abellán, E.; Meseguer, J.; Esteban, M.Á. Comparative analysis of the humoral immunity of skin mucus from several marine teleost fish. Fish Shellfish Immunol. 2014, 40, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Itami, I. Defense mechanism of Ayu skin mucus. J. Shimonoseki Univ. Fish. 1993, 42, 71. [Google Scholar]
- Palaksha, K.J.; Shin, G.W.; Kim, Y.R.; Jung, T.S. Evaluation of non-specific immune components from the skin mucus of olive flounder (Paralichthys olivaceus). Fish shellfish immunol. 2008, 24, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Vera, L.M.; Negrini, P.; Zagatti, C.; Frigato, E.; Sanchez-Vazquez, F.J.; Bertolucci, C. Light and feeding entrainment of the molecular circadian clock in a marine teleost (Sparus aurata). Chronobiol. Int. 2013, 30, 649–661. [Google Scholar] [CrossRef]
- Herrero, J.M.; Lepesant, M.J. Daily and seasonal expression of clock genes in the pituitary of the European sea bass (Dicentrarchus labrax). Gen. Comp. Endocrinol. 2014, 208, 30–38. [Google Scholar] [CrossRef]
- Flajnik, M.F. Comparative analyses of immunoglobulin genes: Surprises and portents. Nat. Rev. Immunol. 2002, 2, 688–698. [Google Scholar] [CrossRef]
- Boshra, J.O.; Gelman, H.; Sunyer, A.E. Structural and functional characterization of complement C4 and C1s-like molecules in teleost fish: Insights into the evolution of classical and alternative pathways. J. Immunol. 2004, 173, 349–359. [Google Scholar] [CrossRef]
- Ye, J.; Kaattari, I.M.; Ma, C.; Kaattari, S. The teleost humoral immune response. Fish Shellfish Immunol. 2013, 35, 1719–1728. [Google Scholar] [CrossRef]
- Cain, R.L.; Jones, K.D.; Raison, D.R. Characterisation of mucosal and systemic immune responses in rainbow trout (Oncorhynchus mykiss) using surface plasmon resonance. Fish Shellfish Immunol. 2000, 10, 651–666. [Google Scholar] [CrossRef]
- Subramanian, N.W.; MacKinnon, S.; Ross, S.L. A comparative study on innate immune parameters in the epidermal mucus of various fish species. Comp. Biochem. Physiol. B 2007, 148, 256–263. [Google Scholar] [CrossRef]
- Watts, M.; Munday, B.L.; Burke, C.M. Immune responses of teleost fish. Aust. Vet. J. 2001, 79, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Aranichi, F.; Mano, V.; Nakane, M.; Hirose, H. Effects of thermal stress on skin defence lysins of European eel, Anguilla anguilla L. J. Fish Dis. 1999, 22, 227–229. [Google Scholar] [CrossRef]
- Subramanian, S.; Ross, N.W.; MacKinnon, S.L. Comparison of antimicrobial activity in the epidermal mucus extracts of fish. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 150, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Lazado, C.C.; Gesto, M.; Madsen, L.; Jokumsen, A. Interplay between daily rhythmic serum-mediated bacterial killing activity and immune defence factors in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2018, 72, 418–425. [Google Scholar] [CrossRef]
- Skwarlo-Sonta, K. Melatonin in immunity: Comparative aspects. Neuro Endocrinol. Lett. 2002, 23, 61–66. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Cuesta, A.; Meseguer, J.; Esteban, M.A. Total serum immunoglobulin M levels are affected by immunomodulators in seabream (Sparus aurata L.). Vet. Immunol. Immunop. 2004, 101, 203–210. [Google Scholar] [CrossRef]
- Ross, N.W.; Firth, K.J.; Wang, A.; Burka, J.F.; Johnson, S.C. Changes in hydrolytic enzyme activities of naive Atlantic salmon Salmo salar skin mucus due to infection with the salmon louse Lepeophtheirus salmonis and cortisol implantation. Dis. Aquat. Org. 2000, 41, 43–51. [Google Scholar] [CrossRef]
- Hanif, A.; Bakopoulos, V.; Dimitriadis, G.J. Maternal transfer of humoral specific and non-specific immune parameters to sea bream (Sparus aurata) larvae. Fish Shellfish Immunol. 2004, 17, 411–435. [Google Scholar] [CrossRef]
- Quade, M.J.; Roth, J.A. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet. Immunol. Immunopathol. 1997, 58, 239–248. [Google Scholar] [CrossRef]
- Swain, P.; Dash, S.; Sahoo, P.K.; Routray, P.; Sahoo, S.K.; Gupta, S.D.; Sarangi, N. Non-specific immune parameters of brood Indian major carp Labeo rohita and their seasonal variations. Fish Shellfish Immunol. 2007, 22, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.G.; Jeffries, A.H. A novel assay to detect macrophage bactericidal activity in fish; factors influencing the killing of Aeromonas salmonicida. J. Fish Dis. 1988, 11, 389–396. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).