1. Introduction
Graysby (
Cephalophilis cruentata Lacepède 1802) (Family Serranidae) is a small member of the grouper family in the tropical western Atlantic, infrequently attaining lengths greater than 400 mm (mm) total length (TL). The species is a protogynous hermaphrodite, changing from female to male during its lifetime. Their distribution ranges from Brazil northward to North Carolina and Bermuda [
1] and is abundant throughout the Caribbean, being the most commonly observed serranid in coral reef habitats off La Parguera, Puerto Rico [
2]. Adults typically inhabit subtropical and tropical rocky ledge and coral reef areas, but adult graysby have been collected in Jamaican seagrass beds of 2–4 m depth [
3]. They were commonly observed at depths up to 170 m in Jamaica and 145 m in Belize [
4].
Graysby are currently included in the South Atlantic Fishery Management Council’s Snapper-Grouper Fishery Management Plan [
5]. The stock is regulated by inclusion in an aggregate grouper bag limit of three fish per person per day in the recreational fishery, and by inclusion in a spawning season closure of the shallow water grouper complex from January 1 through April 30 of each year [the complex includes gag
Mycteroperca microlepis (Goode and Beane 1879); black grouper
Mycteroperca bonaci (Poey 1860); red grouper
Epinephelus morio (Valenciennes 1828); scamp
Mycteroperca phenax (Jordan and Swain 1884); rock hind
Epinephelus adscensionis (Osbeck 1765); red hind
E. guttatus (Linnaeus 1758); coney
Cephalophilis fulva (Linnaeus 1758); yellowfin grouper
Mycteroperca venenosa (Linnaeus 1758); and yellowmouth grouper
M. interstitialis (Poey 1860)]. The stock is also included in the annual catch limits (ACL), or quotas, for the shallow water grouper complex. The ACLs are currently set at 25,193 kg for the commercial sector and 22,066 kg for the recreational sector. There are currently no size limits on graysby in either fishery sector.
Graysby are of limited economic importance to the southeastern United States (SEUS, North Carolina to Florida Keys, including the Dry Tortugas) reef fish fishery. Estimated annual landings of graysby from headboats (vessels carrying at least seven anglers engaged in recreational fishing) sampled by the Southeast Region Headboat Survey (SRHS) averaged 2982 fish (1503 kg) during the period 1981–2017 [
6] Estimated annual landings by anglers fishing from private recreational boats and charter boats averaged 11,772 fish (4118 kg) during the period 1981–2017 (unpub. data, available at
http://www.st.nmfs.noaa.gov/recreational-fisheries/data-and-documentation/queries/index). Combined landings from all recreational sectors show no consistent increasing or decreasing trends with the majority of fish coming from Florida-Georgia waters (
Figure 1). Total commercial landings of graysby in the SEUS during the period 1991–2015 were 34,986 kg, with 96% of these coming from FL-GA waters (unpub. data, available at:
https://www.st.nmfs.noaa.gov/st1/commercial/landings/annual_landings.html).
We studied graysby in the SEUS because of the increasing need for stock assessments of data-limited species. Our analyses relied on archived sagittal otoliths collected by long-term, systematic dockside sampling programs. The only previous study of graysby life history in SEUS waters was limited in scope (headboat-caught fish only) and sample size (
n = 118) [
7]. Thus, resource managers have had a paucity of biological information available to use in setting ACLs, the mechanism by which all fish species are now managed under the Magnuson-Stevens Fishery Management and Conservation Act. Our primary goal is to provide updated and comprehensive information on age-growth parameters and natural mortality rates for graysby from the SEUS, filling an important gap for this data-limited species.
2. Results
2.1. Age Determination and Timing of Opaque Zone Formation
A total of 1318 sagittae from graysby were sectioned. Opaque zones were counted on 1308 (99%) of graysby sections; ten sections were determined to be illegible and excluded from analyses. The majority of samples came from the North Carolina commercial fishery (
n = 534; 43%) and the Florida Keys recreational fishery (
n = 294; 22%), respectively. Samples were evenly distributed between fishery sectors, with 51% of graysby sampled from commercial fisheries and 49% from the recreational sector (
Table 1). All fish were measured to the nearest mm TL and, if available, whole weight (g).
Opaque zones on graysby otoliths were moderately easy to interpret (
Figure 2), with a within-reader index of average percent error (IAPE) of 3.18% (
n = 648, or 50% of sections), satisfying the acceptable value for IAPE (5% for species of moderate longevity and reading complexity) [
8]. Direct agreement between readings was 61%, and this agreement increased to 91% within ± one year. A final age was determined for all samples, and none were excluded from further analyses. When counting the opaque zones, the entire section was taken into consideration, but the most consistent counts were obtained from the dorsal portion and along the sulcal groove. Some otolith sections exhibited multiple banding, identifiable by discontinuous or incomplete orbits around the core of the section. Multiple banding was noted in earlier studies [
1,
7].
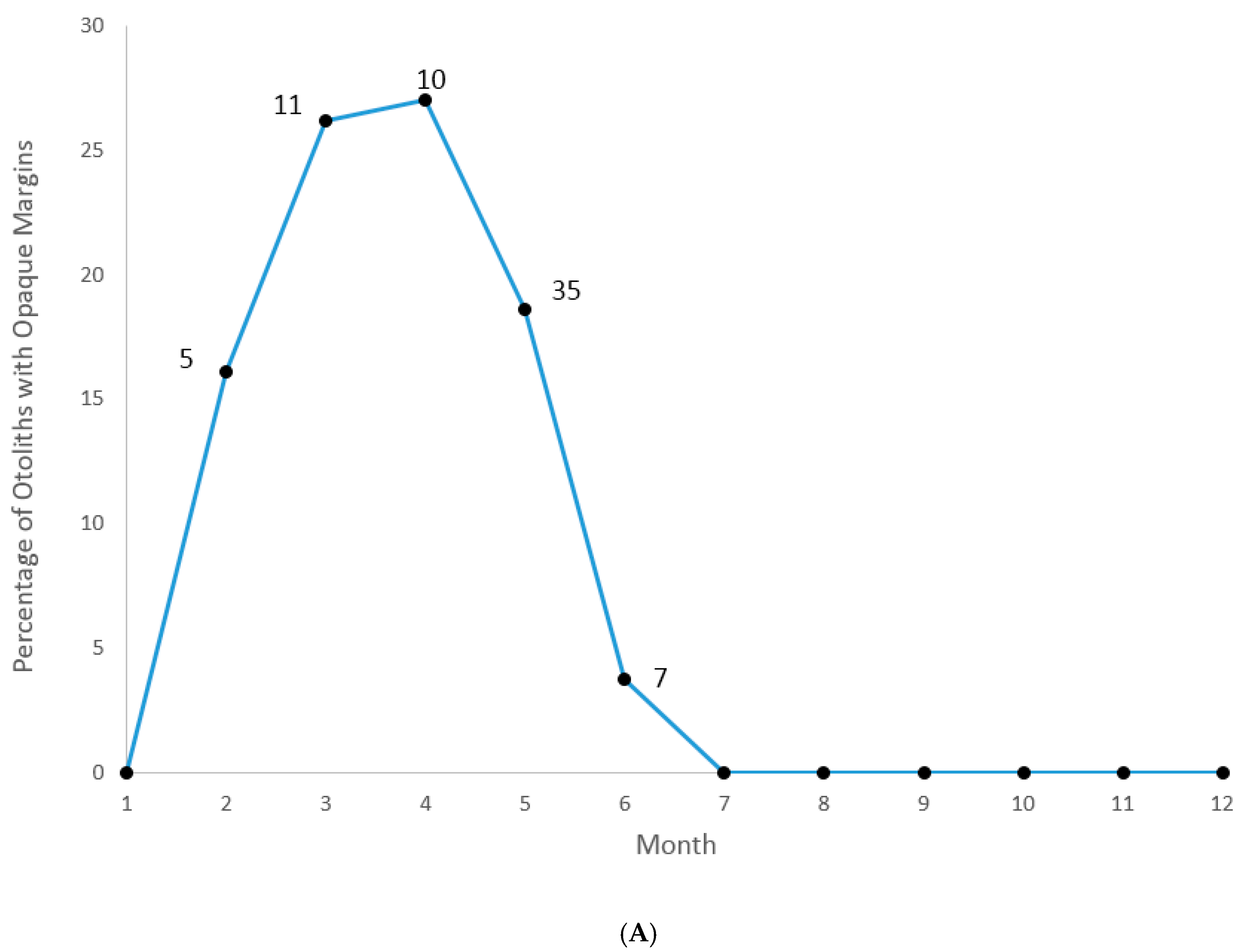
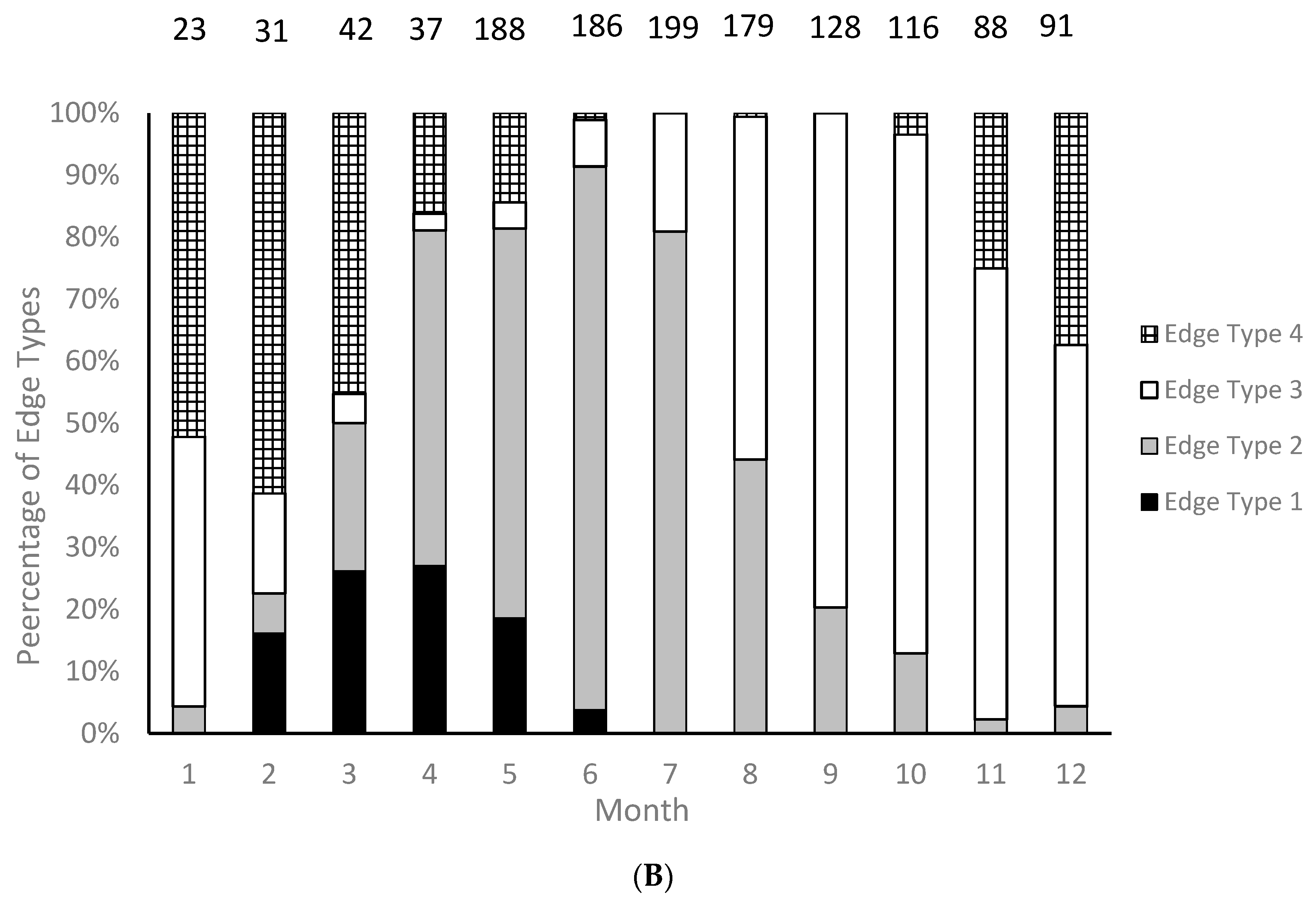
We were able to assign an edge type to all aged samples for our analysis of opaque zone formation timing. Graysby otoliths exhibited opaque zones on the margin February–June, with a peak in April (
Figure 3A). A shift to narrow translucent edge was noted in July, followed by a predominance of moderate to wide translucent edges from October through January and the widest translucent edges in January and February, immediately prior to opaque zone formation beginning in February (
Figure 3B). Graysby otoliths were without an opaque zone on the edge from July through January. We concluded that opaque zones on graysby otoliths were annuli.
Based on the above-reported timing of opaque zone formation, calendar ages were assigned as follows: for fish caught January through June and having an edge type of 3 or 4, the annuli count was increased by one; for fish caught in that same time period with an edge type of 1 or 2, calendar age was equivalent to annuli count; for fish caught from July to December, the calendar age was equivalent also to the annuli count.
2.2. Growth
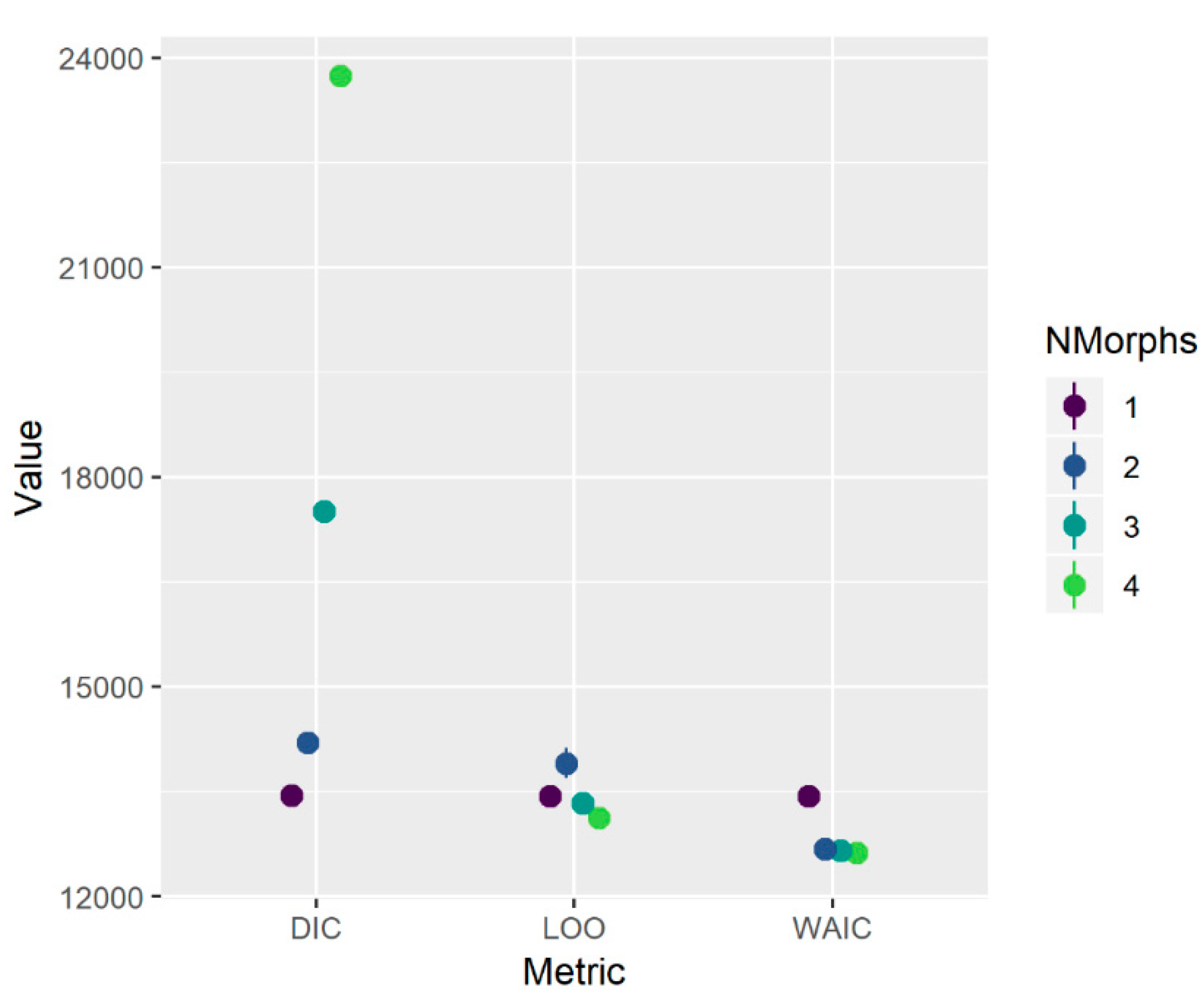
The initial growth morph analysis with identity treated as unknown provided support for either one or two distinct growth trajectories (
Figure A1). Although this initial fitting procedure made no assumptions a priori about the underlying source of each morph, subsequent inspection of the two-morph model showed that the smaller morph comprised fish primarily from the Florida Keys (FLK; southern region) while the larger morph comprised fish from an area encompassing North Carolina through the southeast coast of Florida (NC-EFL; northern region). This observation was supported by statistical analysis of a
, area-by-morph contingency table (
, df = 1,
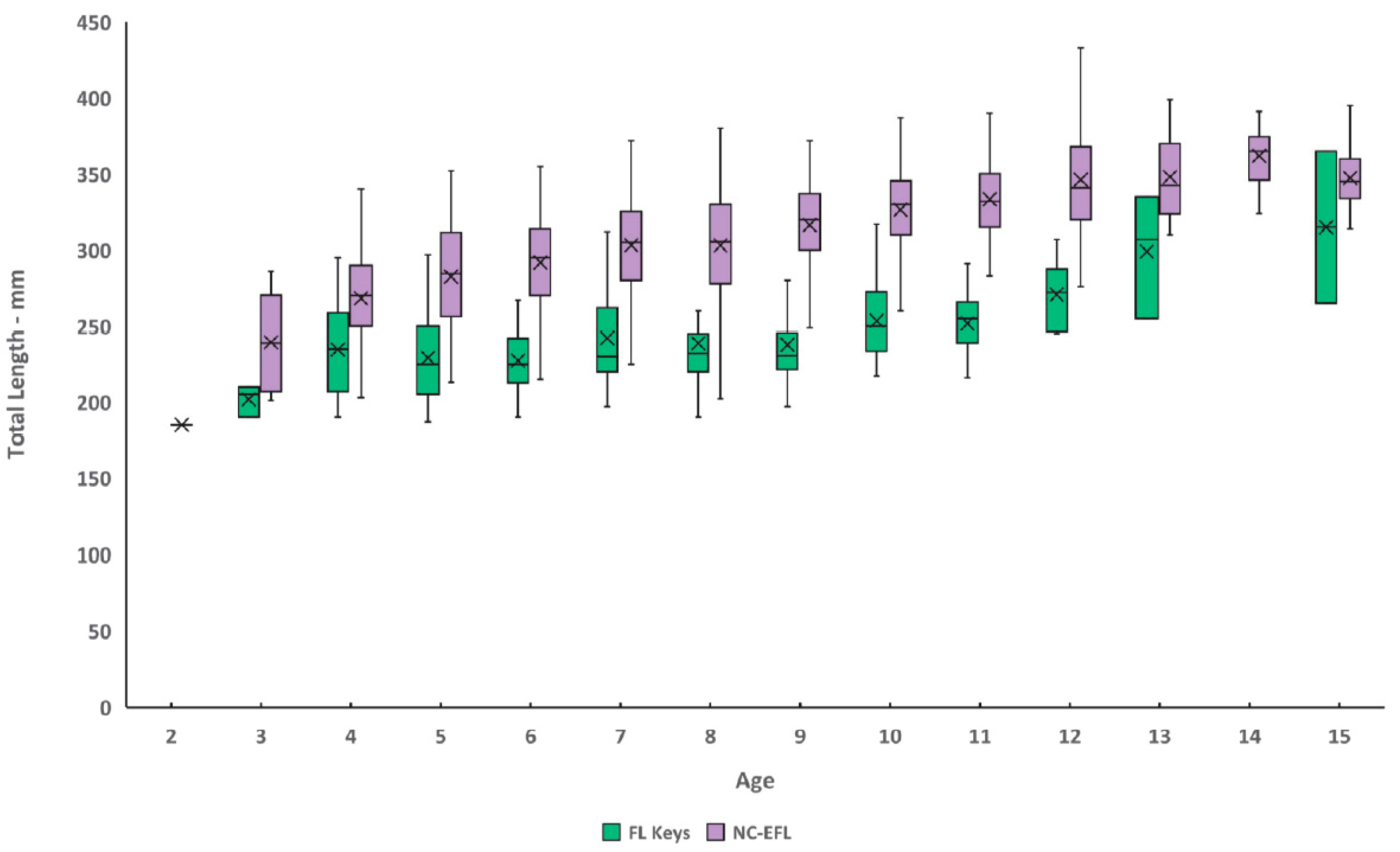
P < 0.001). Size of graysby from the FLK ranged 187–365 mm TL and ages 3–15 (
Table 2,
Figure 4), while sizes from NC-EFL ranged 185–453 mm TL and ages 2–21 (
Table 3,
Figure 4). Mean length-at-age was significantly different (paired
t-tests,
p < 0.05) between regions for 9 out of 9 ages for which samples were adequate for analysis (
Table 4). When the growth morph analysis was repeated using one or two morphs with identity treated as known (FLK or NC-EFL), information criteria provided clear support for the model with two distinct growth trajectories (
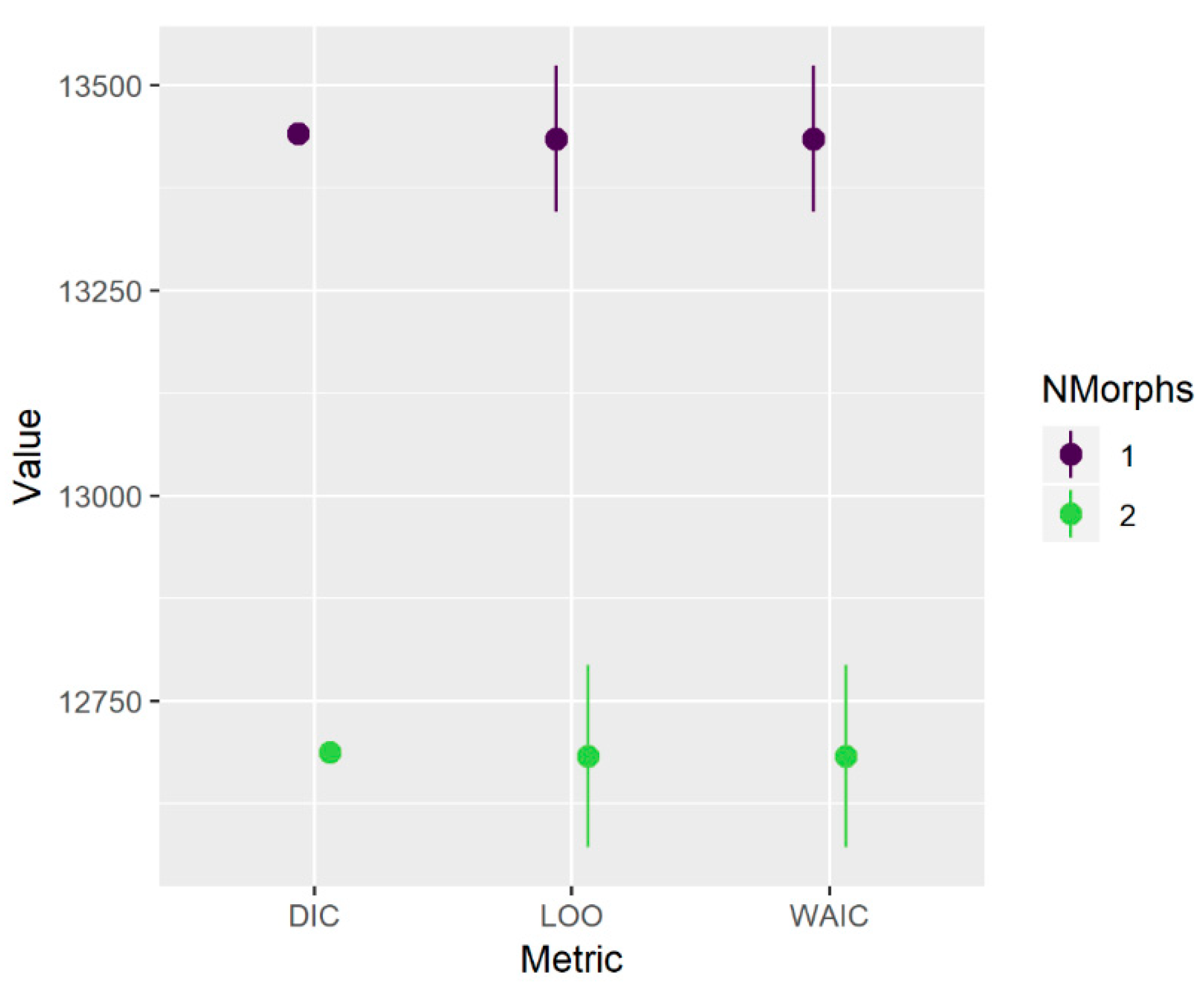
Figure A2).
After sub-setting the data by area, n = 294 individuals were from the southern (FLK) region and n = 1014 individuals were from the northern (NC-EFL) region. For the FLK region, posterior median parameter estimates (95% credible intervals) of the von Bertalanffy growth model were , , and . For the NC-EFL region, the estimates were , , and .
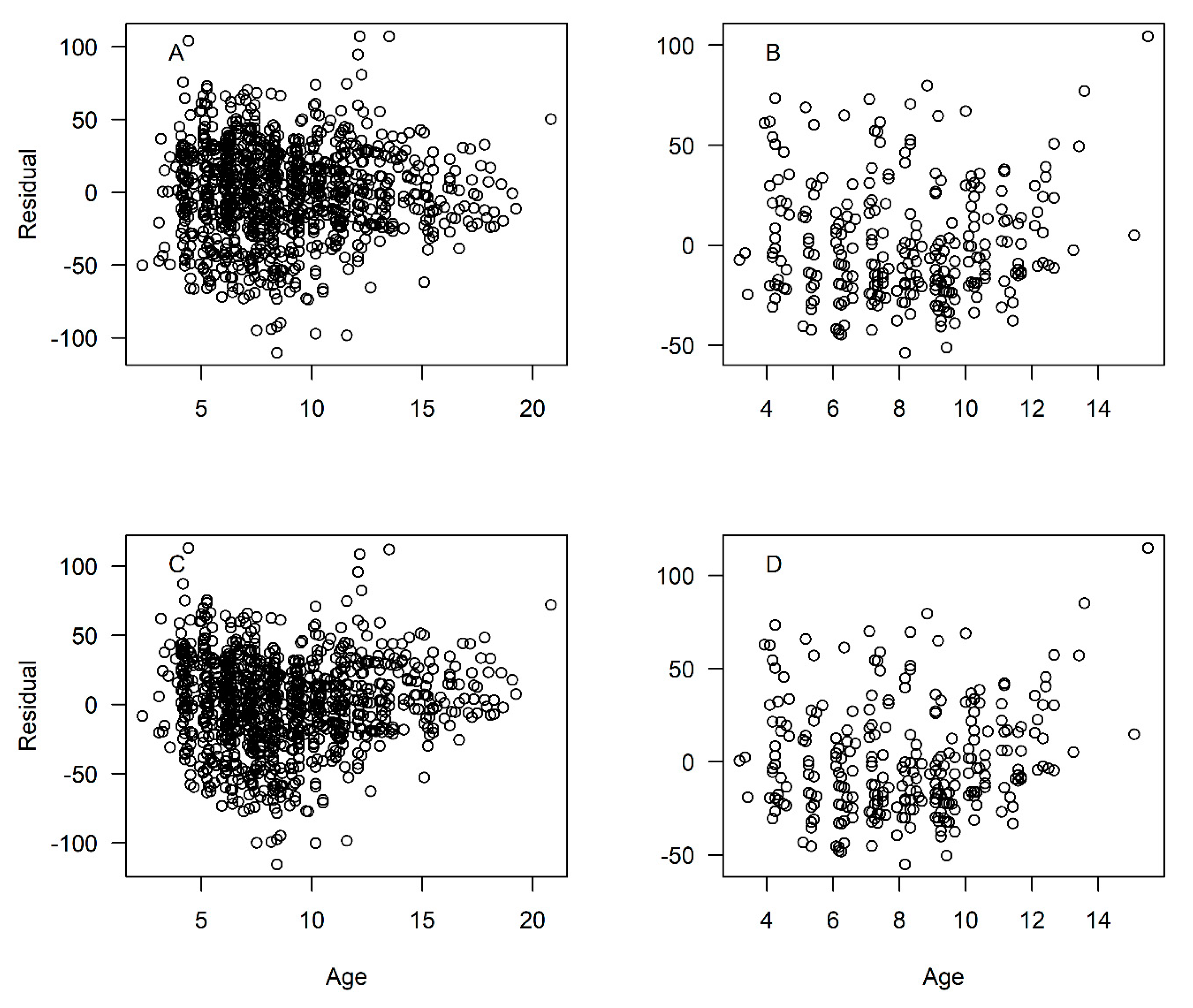
Because our data included few fish younger than age-3 (and no age-0 or age-1 fish), we do not believe the model was able to accurately capture initial growth for fish of ages 0–3, thus explaining the large negative estimates of
t0 (theoretical age at a length of zero). The lack of smaller fish is likely explained by gear selectivity, as our samples were all fishery-dependent. We re-estimated growth using a fixed value of
, which is consistent with spawning in the first quarter of the year as observed in graysby [
12] and other grouper species [
13]. The lower value of
has the effect of pulling the growth curve down to simulate smaller fish length at age for the youngest ages (
Figure 5; Residuals shown in
Figure A3). With
fixed, posterior median parameter estimates (95% credible intervals) were
and
for the FLK region, and
and
for the NC-EFL region.
2.3. Body–Size Relationships
Given the differences in growth by region, we analyzed the
W–TL relationships by region as well. Statistical analyses revealed a multiplicative error term (variance increasing with size) in the residuals of the
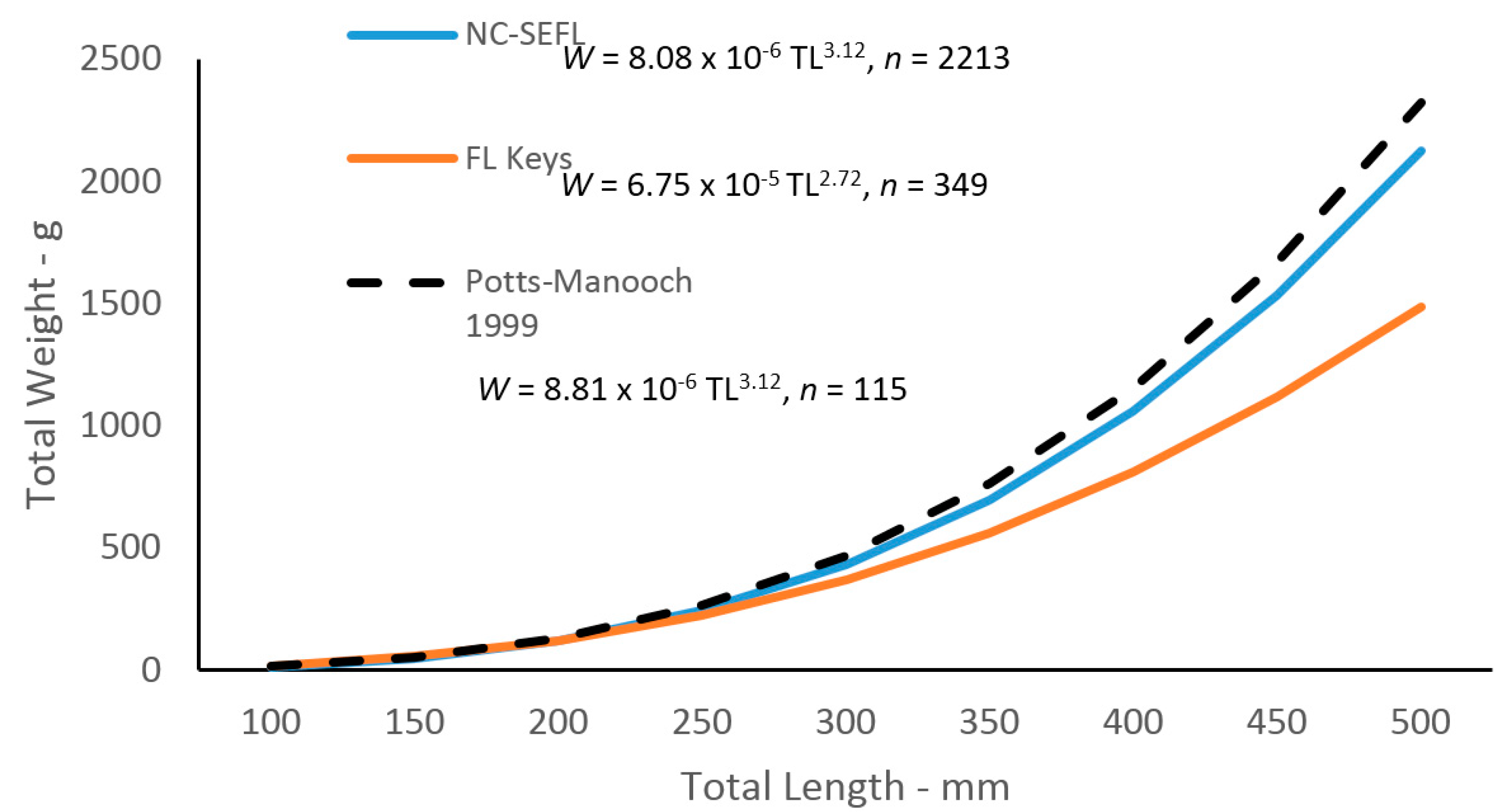
W–TL relationships for graysby, indicating a linearized ln-transform fit of the data was appropriate for both regions. The relationships are described by the following regressions:
and
These equations were transformed back to the form W =
a(L)
b after adjusting the intercepts for log-transformation bias with the addition of one-half of the mean square error (MSE) [
14], resulting in the following relationships (
Figure 6):
2.4. Natural Mortality
The age-invariant value [
11] of natural mortality (
M) was estimated to be 0.30 y
−1 for graysby, using the maximum age of 21 years from all samples. Age-specific estimates of
M [
10] are presented in
Table 2 and
Table 3. For these values, we used the constrained growth estimates (i.e., with
).
Cumulative survivorship to each age (2+) based on the age-specific
M [
10] was similar between the two regions. Survivorship of graysby from the southern region ranged from 41% at age-2 to 0.5% surviving to age-15 (
Table 2). Survivorship of graysby from the northern region ranged from 41% at age-2 to 0.5% at age-15 and 0.1% surviving to age-21 (
Table 3).
Cumulative survivorship using the age-specific M was quite different from that using the age-invariant method. As expected, use of the age-invariant estimate of M, which was considerably less than the age-specific M for younger ages, resulted in a greater proportion of the population surviving to each age. The survivorship estimates for graysby using the age-invariant method ranged from 74% at age-2 to 1.5% at age-15 and 0.2% at age-21.
3. Discussion
Otolith edge analysis demonstrated that graysby deposited one annulus per year from February–June, with peak annulus formation occurring in April. This agrees with a previous study that found a minimum marginal increment on the otolith edge in April [
7]. These results are also similar to timing of annulus formation for other smaller members of the family Serranidae in the SEUS, which tend to form annuli in the late spring-summer months: coney—
Cephalophilis fulva Linnaeus 1758, [
15]; rock hind—
Epinephelus adscensionis Osbeck 1765 [
16].
Graysby attained an average observed size of 202 mm TL for Florida Keys fish and 239 mm TL for northern region fish by age-3. Northern region fish grew faster than southern fish, attaining average observed lengths by age-10 of 327 mm and 254 mm, respectively. This compares with a previous study [
7] which reported an average observed size of 351 mm TL for graysby from the combined SEUS coast. While growth appears at first glance to be relatively slow (three years to achieve 200 mm), it should be noted that graysby attain about 75% of their L
∞ by age-4 for northern fish and by age-2 for southern fish. While we may think of this species as slow-growing, the values of
K, the von Bertalanffy growth coefficient, for each regional group indicate that they reach their theoretical maximum size at a moderate rate.
One limitation of this study is the lack of younger age classes, due to the fishery-dependent nature of our samples and the selectivity of fishing gear. This factor is likely the reason that we had a single fish younger than age-3. This lack of young fish common to studies dominated by fishery-dependent samples can lead to problems in estimating the initial trajectory of the growth curve for the youngest ages. This issue resulted in large negative values of
t0, which we took into account by re-estimating the growth parameters using a fixed value of
t0 = −0.75. This procedure had the effect of pulling down the initial trajectory of the growth curve (
Figure 5), simulating a more realistic size-at-age for the youngest fish. Caution should be taken, however, when interpreting these estimated lengths-at-age beyond the range of the data, as any extrapolation may be unreliable.
Growth may also vary inter-annually in response to internal factors (e.g., density dependence) or external factors (e.g., environmental variables such as temperature, size-selective fishing pressure). Although we did not have enough samples to analyze whether there are inter-annual patterns in growth, this type of analysis would be valuable. It could be done in the future with increased sampling intensity (landings estimates indicate >10,000 fish landed annually on average).
Body–size relationships were different between regions in this study. The regression for the northern region was almost identical to the regression estimated for the previous study [
7], and we speculate that this is due to the fact that 95% of their samples came from the northern region. We do not know exactly why fish from the southern region are smaller at a given age than northern fish. We hypothesize that environmental factors influence nutritional or energetic constraints. Such constraints underlie the trade-offs among growth, survival, and reproduction, and thus shape the life-history characteristics [
17].
Natural mortality of wild populations of fish is difficult to measure but is an important component of stock assessments. For marine fishes in general, M is likely to be age-specific, decreasing as fish grow larger [
10]. We have no reason to expect that graysby deviate from this pattern, but we also acknowledge that our age-specific estimates of M are uncertain, as they depend on growth curves that are themselves extrapolations at the youngest ages. By the age at which the fish are fully exploited in the fishery, age-7, the natural mortality rate at each age has generally leveled off and is close to the age-invariant estimate derived using maximum age [
11]. Thus, either estimator would likely be suitable for the purpose of stock assessment [
18].
When considering the cumulative estimate of survivorship to the oldest age, the age-invariant method [
11] estimates 0.2% survivorship for the combined region. Estimates derived using the age-specific method of calculating
M [
10] are 0.1% and 0.5% for the northern fish (age-21) and southern fish (age-15), respectively. These estimates are supported by the age frequencies from both groups of fishes that indicate that the chance of survivorship to the oldest age may be less than 1%. These observations give weight to the argument to use the age-specific estimate of
M at age.
4. Materials and Methods
4.1. Age Determination and Timing of Opaque Zone Formation
Graysby samples (n = 1318 fish) were collected dockside by NMFS and state agencies sampling landings from the recreational and commercial sectors along the SEUS coast during the period 2001–2016. All specimens were captured by either conventional vertical hook and line gear or divers with spears. Both fisheries sectors employed the same gear types, thus variable gear selectivity should not have influenced size of fish caught. All specimens were measured for total length (TL, mm). Additionally, fish landed by the recreational headboat fishery (n = 2465) were weighed (whole weight, W, grams), and these weights were used in a W-TL regression analysis. Fish landed by commercial fisheries were eviscerated at sea, thus whole weights were not available.
Sagittal otoliths were removed during dockside sampling and stored dry. Otoliths were sectioned using a low-speed saw, two serial 0.5 mm sections were taken near the otolith core, mounted on microscope slides with thermal cement and covered with mounting medium before analysis [
19]. The sections were viewed under a dissecting microscope at 12.5× using transmitted light. Each sample was assigned an opaque zone count by an experienced reader with extensive experience interpreting otolith sections [
20,
21]. Opaque zones were counted regardless even if they were not yet completely formed (i.e., no translucent zone beginning to form between the opaque zone and the edge). Sections were read with no knowledge of location or date of capture or fish size. After the initial reading, the otolith sections were set aside for several months and a subset (
n = 648) was re-read by the same reader. We calculated an index of within-reader average percent error IAPE [
22]. Where readings for a specimen disagreed, the sections were viewed a third time and a final age determination was made.
Timing of opaque zone formation was assessed based on the distance between the outermost opaque zone and the edge of the otolith. Edge type descriptions are: 1 = opaque zone forming on edge of otolith section; 2 = narrow translucent zone of the edge, generally less than 30% of the width of the previous translucent zone; 3 = moderate translucent zone on the edge, generally 30%–60% of width of previous translucent zone; and 4 = wide translucent zone on the edge, generally greater than 60% of width of previous translucent edge [
9]. The edge types were plotted by month of capture to determine if the opaque zones were deposited primarily in one season or month. Based upon edge frequency analysis, all samples were assigned a calendar age, obtained by increasing the opaque zone count by one if the fish was caught before that year’s increment was formed and had an edge which was a moderate to wide translucent zone (type 3 or 4). Fish caught during the time of year of opaque zone formation with an edge type of 1 or 2, as well as fish caught after the time of opaque zone formation, were assigned a calendar age equivalent to the opaque zone count. Then, each fish was assigned a fractional age calculated from the calendar age, month of peak spawning and month of capture. Because we did not have a direct observation of the spawning season of graysby in our study area, we selected April as month of peak spawning based on observations on the species in the Caribbean [
12] and other groupers [
13]. Fractional ages were used for estimation of growth.
4.2. Growth
Growth was modeled for combined sexes due to the protogynous nature of graysby. We fitted the length-at-age (calendar age) data using the von Bertalanffy growth equation,
where
is TL at age
a,
is the theoretical maximum length,
k is the Brody growth coefficient, or the rate at which maximum size is attained, and
t0 is the theoretical age at size 0 [
23]. We tested for whether the data were best explained by a single or multiple growth curves, using a new growth-morph approach [
24]. The approach is described elsewhere in detail [
24], and so we describe it here only briefly. In essence, it treats the growth data as a mixture of one or more components (growth morphs) from which an individual’s identity may or may not be known a priori. We initially fitted the model allowing up to four morphs, with the identity (morph) of all individuals treated as unknown. We then considered three metrics in concert to indicate the optimal number of morphs: the deviance information criterion (DIC), leave-one-out cross validation (LOO), and the widely applicable information criterion (WAIC).
Based on results of the initial growth morph analysis, we re-fitted the model with identity treated as known, assigning identities based on geographic location (FLK or NC–EFL). We anticipated there would be few fish of the youngest age classes available to us, as our samples were primarily fishery-dependent, and hook-and-line gear or fishers generally selected for older or larger fish. Consequently, the model would be unable to depict initial growth of the youngest fish, leading to difficulty in accurately estimating size at the youngest ages. We therefore re-ran the growth model with t0 fixed to a value of −0.75.
We assigned a uniform prior distribution to the asymptotic length of the largest growth morph, . The asymptotic length of smaller morphs was computed as a proportion c of the largest morph’s , similarly following a uniform prior distribution, . We also assumed a uniform prior distribution for the growth coefficient, , and a truncated normal distribution for t0 with mean of 0.0 and precision (inverse variance) of 0.1. We used a truncated distribution to constrain values of t0 to be negative.
The mixture model was fit in a Bayesian framework using JAGS version 4.3.0 [
25], implemented in R version 3.4 [
26]. The fitting procedure utilized three independent Markov chains, each of length 500,000 iterations. Posterior distributions excluded the first 100,000 iterations as the burn-in period, and we thinned by keeping every tenth iteration, because of data storage limits [
27]. None of the posterior distributions were limited by the specified ranges of the prior distributions. We assessed convergence with visual inspection of trace, density, and autocorrelation plots, as well as with the Brooks–Gelman–Rubin statistic [
28].
We conducted paired t-tests to determine if there were statistical differences in length-at age between geographic areas. For this analysis we used calendar ages, and only those ages for which we had adequate sample sizes (n ≥ 10) from each area for comparison.
4.3. Body–Size Relationships
For weight–length relationships we regressed
W on TL (
n = 2465) using all fish sampled for lengths and whole weights from the recreational headboat fishery during the period 1975–2015. We examined both a non-linear fit,
W =
a TL
b, using nonlinear least squares estimation [
29] and a linearized fit of the log-transformed data, ln (
W) =
a +
b ln(TL). Residuals were examined for patterns to determine which regression provided the best fit to the data.
4.4. Natural Mortality
We estimated the instantaneous rate of natural mortality (
M) using two methods. The first method was based on maximum age of the whole sample, and resulted in an age-invariant estimate of
M:
where
tmax is the maximum age of the fish in the sample [
11]. The second method was based on growth parameters:
where
Ma is natural mortality rate at age
a,
L∞ and
k are the von Bertalanffy growth equation parameters and
La is fish length at age
a [
10]. For these calculations, we used the midpoint of each age (i.e., 1.5, 2.5, 3.5, etc.), because the midpoint better represents the mean annual size.
The latter method, which incorporates life-history information via the growth parameters, is based upon evidence suggesting that
M decreases as a power function of body size. This method generates age-specific rates of
M as is commonly used in Southeast Data Assessment and Review (SEDAR) stock assessments e.g., [
30].
Cumulative survival (
ϕ) to each age 2+ was calculated with both age-invariant and age-specific natural mortality rates:
where
is the natural mortality rate at age a, and
A is the age of interest (for age-invariant estimates,
is constant). These survival rates represent expectation in the absence of fishing.