Abstract
The skin, gills, and gut are the most extensively studied mucosal organs in fish. These mucosal structures provide the intimate interface between the internal and external milieus and serve as the indispensable first line of defense. They have highly diverse physiological functions. Their role in defense can be highlighted in three shared similarities: their microanatomical structures that serve as the physical barrier and hold the immune cells and the effector molecules; the mucus layer, also a physical barrier, contains an array of potent bioactive molecules; and the resident microbiota. Mucosal surfaces are responsive and plastic to the different changes in the aquatic environment. The direct interaction of the mucosa with the environment offers some important information on both the physiological status of the host and the conditions of the aquatic environment. Increasing attention has been directed to these features in the last year, particularly on how to improve the overall health of the fish through manipulation of mucosal functions and on how the changes in the mucosa, in response to varying environmental factors, can be harnessed to improve husbandry. In this short review, we highlight the current knowledge on how mucosal surfaces respond to various environmental factors relevant to aquaculture and how they may be exploited in fostering sustainable fish farming practices, especially in controlled aquaculture environments.
1. Introduction
Mucosal surfaces (i.e., skin, gills, gut, and olfactory organ) provide fish the crucial first line of defense against the threats present in the immediate environment [1,2]. Besides their role in defense, mucosal structures have other physiological functions—for example, skin in osmotic balance and sensory reception, gills in osmotic, ionic, and acid-base regulation as well as excretion of nitrogenous wastes, and the gut in catabolism and nutrient uptake [3,4,5]. The mucosal structures in fish have provided immense knowledge on the evolution of barrier functionality in vertebrates, particularly in cases where the organism and its environment are in constant interactions. The mucosal interface allow these highly organized structures to respond remarkably to external manipulations and perturbations. In recent years, extensive studies have been directed at understanding the role of mucosa to the overall health and welfare of fish and at securing optimum conditions in controlled aquaculture environments [6,7]. The current knowledge on the fundamental structures of the mucosa and the associated defense factors and mechanisms highlight both the complexity and peculiarity of mucosal surfaces in fish.
The immune system of teleost fish consists of primary lymphoid organs (i.e., thymus and head kidney) and the secondary lymphoid organs, comprising the spleen, the kidney, and mucosal-associated lymphoid tissues (MALT) present in peripheral immune tissues. Mucosal structures play a crucial role in immunity and MALT can be sub-categorized further into four main lymphoid tissues: skin-associated lymphoid tissue (SALT), gill-associated lymphoid tissue (GIALT), gut-associated lymphoid tissue (GALT), and nasal-associated lymphoid tissue (NALT) [2]. The first three MALTs are the most intensively characterized in fish and the majority of the current knowledge on teleost mucosal immunity is based on these tissues. Nasal-associated lymphoid tissue is a recently discovered and characterized MALT in fish and our current understanding is mainly on the fundamental aspects of its anatomy and physiology. Since there are limited information on how environmental changes in aquaculture affect their functions, they will not be discussed in this mini-review. For in depth discussion on mucosal immunity in teleost fish, kindly refer to the following highly relevant articles: Rombout et al., (2010), Sunyer (2013), Lazado and Caipang (2014), and Salinas (2015) [1,2,8,9].
An emblematic feature of mucosal surfaces is the presence of a mucus layer. This slimy polymer secreted by mucous and goblet cells contains inhibitory activity against pathogens, with potent molecules present in the matrix including mucins, lysozymes, complement proteins, lectins, antimicrobial peptides, and immunoglobulins, among many others [10,11]. Mucosal surfaces are also unique microenvironments for non-pathogenic and co-habitant microorganisms and they likewise provide another layer of defense by antagonizing pathogens through the production of bacteriocins, H2O2, and antimicrobial peptides and other inhibitory compounds. Disruption of microbial homeostasis may lead to increased susceptibility to infectious agents and eventually facilitates the development of disease [12]. There is mounting evidence showing that a balanced mucosal microbiota has a significant impact on barrier functionality and in totality, the health of the organism.
The general mechanism of immune response in the mucosal surfaces involves the concerted action of physical barriers, i.e., mucus, scales, and epithelium, which orchestrate the first line of defense by trapping and by direct elimination of pathogens (Figure 1). Once the pathogenic organism manages to infiltrate the physical barriers, pattern recognition receptors (PRR) of immune cells detect pathogenic agents through their pathogen-associated molecular patterns, triggering the innate immune system. Antigen uptake can have different pathways: (a) start of the inflammatory process brought about by the release of cytokine mediators and attractants corresponding to the type of cell and (b) presentation of the antigen that activates the action of antigen-specific lymphocytes that contain receptors which recognize specific molecules, characteristic of each pathogen. This elicits secondary responses, including processes involved in adaptive immunity [6].
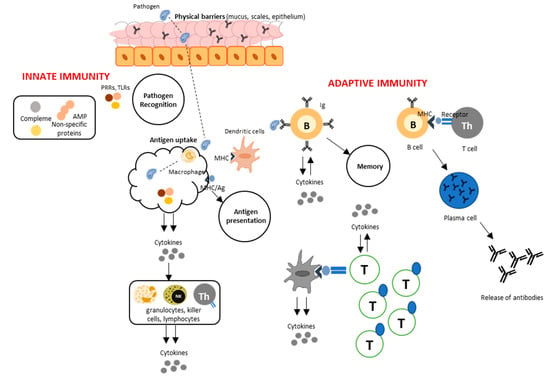
Figure 1.
Immune response at the fish mucosa. When pathogens succeed to infiltrate the physical barriers, the innate immune system is triggered by the action of pathogen recognition receptors (PRRs). Antigen uptake results in the release of cytokines that activates different types of cells involved in inflammatory processes, and antigen presentation facilitated by lymphocytes containing receptors, which leads to subsequent responses as well as memory. This diagram is the simplified version from Beck and Peatman (2015) with Elsevier License number 4477021492352 [6].
2. Variability of Environmental Parameters in Aquaculture
Adaptations to varying environmetal pressures are often advantageous, since they can be crucial to the survival of an organism [13,14]. Phenotypic plasticity, the phenomenon of producing different phenotypes in response to change in environmental conditions, is a ubiquitous aspect of the organism’s evolutionary adaptation [15,16]. Fish are highly vulnerable to environmental changes because of the constant and intimate contact with the aquatic environment. In the wild, fish encounter fluctuating environmental factors throughout their lifetime. Some of these environmental variations are short-lived, while others may last for a considerable period, which may leave both phenotypic and physiological imprints in the organism. Many organisms exhibit an impressive repertoire of adaptive mechanisms to thrive in various environmental conditions, hence facilitating survival.
Fish domestication relies heavily on the control of environmental parameters. Successful and sustainable husbandry is fostering a rearing environment for fish to grow and survive under optimum controlled conditions. Environmental control in aquaculture often involves the manipulations of physico-chemical factors that support the physiological functions in fish. The changes in the environmental conditions in the production system may also be driven by the excretion products, both from fish and the microbial populations present in the rearing environment or the lack of equilibrium among the physico-chemical factors that support fish in the system. Some of the key environmental parameters that are critical in supporting the physiological processes in fish, especially in farming conditions, include dissolved oxygen, temperature, salinity, and photoperiod, among many others.
Moreover, these changes have pervasive impacts on the overall welfare status of fish. These environmental parameters can influence the survival and resistance of fish against diseases, stressful conditions, and more. The mucosal immune system, in particular, has inherent importance in the ability of fish to respond and adapt to these changes because of their direct connection with the environment. In a number of studies, it has been documented that mucosal surfaces respond exceptionally to these environmental changes—for example, phenotypic alterations (e.g., increase in mucus cell size and numbers), molecular responses (e.g., transcriptional and proteomic changes), and diversification (e.g., microbiota profile).
This short review discusses how different environmental factors relevant to aquaculture influence the basic physiological functions of the mucosal barriers and how the adaptive changes contribute to the plasticity of mucosal functionality. To the best of our knowledge, no published paper has synthesized the responses of fish to environmental changes, particularly within the context of mucosal barriers. Previous papers discussed several aspects of this theme separately. Studies that are enumerated in this short review highlight both the current knowledge and the gaps about this timely and highly relevant topic in aquaculture.
3. Dissolved Oxygen
Dissolved oxygen (DO) is the main limiting factor in an aquaculture system [17]. Oxygen is important in respiration and thus controls all cellular functions. Low DO (hypoxia) is known to alter the structure of the gills to accommodate more oxygen. Hypoxia-induced morphological adaptations in the gills were highly documented in cold-water carp species, which are known for their tolerance in low DO. Crucian carp (Carassius carassius) exposed to 0.75 mg L−1 DO display protruding gill lamellae accompanied by an increased interlamellar cell mass that resulted to a 7-fold surface area increase in at least 24 h of exposure [18]. A similar gill remodeling strategy was also observed in goldfish (Carassius auratus) [19]. In Qinghai scaleless carp (Gymnocypris przewalskii), increase in gill surface area occurred 8 h post-exposure to 0.3 mg L−1 DO and a shift into the small “shallow-basin” type of mitochondria rich-cells (MRC) from wavy-convex-type was observed 12 h post-exposure [20]. These changes induce gas transfer during hypoxia and were found to be reversible after exposure to normoxic conditions.
The sensitivity of the gut to hypoxic conditions has been observed in Atlantic salmon (Salmo salar). Exposure to low DO levels (50% saturation) caused chronic inflammation in the gut but the effect varied depending on the temperature. At 8 °C, mucosal neutrophil infiltration increased, together with a down-regulation in the expression of inhibitor of nuclear factor kB (ikB) gene, while at 16 °C, expression of the pro-inflammatory cytokine interleukin 1β (il-1 β) decreased, while expression of interleukin 10 (il-10) increased. Differential expression of immune factors was more striking at 16 °C, which shows that temperature intensifies the effects of hypoxia in this species [21].
The effects of high DO levels (hyperoxia) have been studied in some species. In Crucian carp and goldfish, high temperature and high DO (300% saturation) resulted in gill remodelling (i.e., increase in interlamellar cell mass) [22]. The exposure of Beluga sturgeon (Huso huso) to 115% saturation for 8 weeks resulted in changes in the gill structure, such as impairment of the secondary lamellae [23]. At the molecular level, hyperoxia of up to 200% saturation caused a decrease of expression of cyclooxygenase-2 (cox2), a gene important for pro-inflammatory pathways, in the gills of Senegalese sole (Solea senegalensis) after 24 h [24].
Suboptimal DO levels put the fish in a challenging condition that would result in the perturbations of mucosal barrier functions. These have been shown in the histological and molecular changes in the mucosal surfaces in response to variable DO levels. Disturbances in the functions of mucosal surfaces brought about by the highly demanding conditions at low DO may increase their permeability to pathogens. Thus, it may likely result in the increase of susceptibility to infection. Many fish pathogens are opportunistic; they cause infection when the environmental conditions are sub-optimal and host barriers are compromised.
4. Water pH
Next to DO, water pH is one of the secondary limiting factors in an aquaculture system [17]. Water pH is influenced by carbon dioxide (CO2) through the bicarbonate-carbonate balance, as well as alkalinity, which serves as a buffer system that prevents sudden changes in pH [25]. Non-CO2 change in water pH is driven by organic acids, inorganic substances, and anthropogenic factors (e.g., mining, industrial effluents) [26].
Changes in water pH as a result of pollution are a recurring problem, especially in freshwater aquaculture which is a major industry in areas near water basins. Responses of freshwater species to water pH beyond the normal physiological range have been studied, with considerable changes observed in mucosal organs. In brond snout (Chondrostoma regium), acute exposure (i.e., 24 h) to high pH (9.8–9.9) caused gill lamella hyperplasia and fusion of secondary lamella, while exposure to low pH (4.4) resulted in club-shaped cells and hypertrophy of the epithelium [27]. In zebrafish (Danio rerio) gills, pH 4.0 increased the density of H+-ATPase-rich cells, involved in acid secretion and Na+ secretion. [28]. These morphological changes allow the fish to adapt their respiratory structures to the physiological demands of variable water pH. Tight junctions, which limit the passage of molecules in spaces between epithelial cells [29], are also affected by water pH. In rainbow trout (Oncorhynchus mykiss), length of tight junctions between pavement epithelial cells and chloride cells decreased by 25% at pH 4.0 [30]. Claudins, a large class of tight junction proteins, are also regulated in acidic environments, as shown by the increase in expression of claudin-b (cldnb), linked to the tight junction between lamellar epithelium cells [31] in zebrafish gills after exposure to pH 3.8–4.0 [32]. The antioxidant defense at the mucosa is also responsive to changes in water pH. Zebrafish exposed to the same pH showed differential regulation of gene coding for superoxide dismutase (sod), catalase (cat), and glutathione peroxidase (gpx) in the gills. This reveals that the antioxidant system is not only important in combating oxidative damage in the presence of high oxygen radicals, but may also have key roles in the responses of the gills in pH variability [33].
In tambaqui (Colossoma macropomum), a freshwater fish found in the Amazon river, skin microbiota is affected by lower water pH (for example, pH 4.0) [34]. It was further shown that unlike the skin microbiota, the diversity and structure of the gut microbiota of tambaqui was more robust under lower pH, indicating that the microbial community in the gut is quite resistant to suboptimal pH levels. These alterations in mucosal features not only have implications on their functionality but can also serve as determinants of the extent of pollution in freshwater environments.
5. Carbon Dioxide
Carbon dioxide (CO2), which exists in the water as bicarbonate, carbonate and carbonic acid forms, is an environmental factor in an aquaculture system that has a striking impact on the mucosal functionality in fish [25]. CO2 can both directly impact the physiological functions of fish and indirectly affect the host by changing the water pH [17]. Carbonate concentration is important in maintaining the buffer capacity of the rearing water against pH fluctuations and can affect the toxicity of some components, such as ammonia and hydrogen sulfide.
The gills, being involved in acid-base regulation, are especially susceptible to high CO2 levels (hypercarbia). Mitochondria-rich cells (MRC) that are involved in acid-base regulation [35] have been studied to determine the effects of hypercarbia in the gills. The results varied across species. For instance, in channel catfish (Ictalurus punctatus) exposed to 8% CO2 and in olive flounder (Paralichthys olivaceus) exposed to 3–5% CO2, the apical opening surface area of MRCs increased to accommodate the rise in CO2 concentrations and ease the conversion to HCO3− and CO32− [36,37]. In contrast, a decrease in the MRC apical opening surface area was observed in brown bullhead (Ictalurus nebulosus) exposed to 2% CO2, in white sturgeon (Acipenser transmontanus) exposed to 1.53% CO2, and slightly in rainbow trout exposed to 1% CO2 [38,39,40]. It is apparent that changes in MRC are more likely to occur at higher concentrations of CO2, and interspecies differences are quite remarkable in the observed changes. A good acid-base regulation would result in healthy gills, which allows a fully functional mucosal barrier.
The interaction of CO2 with other environmental variables, such as temperature and heavy metals, was also assessed in several studies. Hypercarbia at 10 °C resulted in an increase in gill tissue mass of Atlantic cod (Gadus morhua), but not at 18 °C [41]. The increase in size can be attributed to the response of fish to rapidly eliminate high CO2 concentrations [42]. Co-occurring acidification (CO2 + Hg) decreased the accumulation of Hg in Argyrosomus regius and decreased the activity of oxidative stress markers i.e. catalase, superoxide dismutase (SOD), and glutathione S-transferase activities in the gills [43]. These findings may give insights into the adaptive mechanisms of the gills to pollution in relation to projected ocean acidification, and consequently, on the future of marine cage culture.
Given the low water change that results in high CO2 accumulation, hypercarbia is a foreseen problem in recirculating aquaculture system (RAS). In a recent study, Mota et al. (2019) observed thinner epidermis in Atlantic salmon exposed to a CO2 concentration greater than 19 ppm, which renders the host more susceptible to pathogen infiltration [44]. This information shall give rise to more studies on the effect of high CO2 concentrations on the mucosal barriers of other aquaculture species reared in RAS.
6. Temperature
Temperature is a widely studied environmental cue in fish, not only in reproduction and animal behavior but also in immune response and the progression of infectious diseases [45,46]. Temperature can also intensify the effects of other physicochemical parameters (e.g., DO levels) in the rearing water [20].
Eurythermal species are a suitable model for determining the phenotypic changes in response to temperature variations within the physiological range. In Crucian carp and goldfish with temperature tolerances of 2–22 °C and 0–41 °C, respectively, hypertrophy of interlamellar cells was observed after a 30-day exposure to 7.5 °C [47]. In fathead minnow (Pimephales promela) with temperature tolerance of 0–33 °C acclimated to 5 °C, no changes occurred in interlamellar cells, although mucus cells in the gills were observed to be fewer but larger after acclimation [48].
Cellular responses varied when fish were exposed to the upper or lower temperature limits of their physiological tolerance. Lysozyme activity, hepcidin, and immunoglobulin M (IgM) increased in the skin of turbot (Scophthalmus maximus), with a temperature tolerance of 16–20 °C after exposure to 27 °C [49]. Genes coding for proteins that block viral replication in the early phase, macrophage activation, mucus secretion, and pro-inflammatory responses were upregulated while the genes coding for antigen presentation and immunoglobulins were downregulated in fathead minnow acclimated to 5 °C [48]. In channel catfish, the adaptive immune response to T-dependent antigens in the gills is inhibited by low temperature [50].
The antioxidant systems of mucosal surfaces are also responsive to thermal variability. The SOD levels in the skin mucus of turbot increased significantly when temperature was elevated from 16 °C to 20 °C. While it is within the normal range for turbot, it is apparent that in the upper temperature limits, the markers for oxidative stress were remarkably affected. The same tendency of increased SOD levels at a temperature higher than the normal rearing temperature (21–25 °C) was also documented in the gills of Asian stinging catfish (Heteropneustes fossilis) reared at 32 °C [51]. Reduced gill glutathione peroxidase (GPX) activity was observed in Antarctic fishes, Notothenia coriiceps, and N. rossi after exposure to 4 °C for 1 day (long-term) in contrast to 2 °C for 6 days (short-term) [52]. The temperature at 20 °C can also activate a protective antioxidant defense response in gills of discus fish (Symphysodon aequifasciatus), as evidenced by the increase in SOD and GPX activities, compared to a temperature of 28 °C [53].
Thermal plasticity of mucosal surfaces has a key role in the robustness of fish under variable temperatures. Thermal manipulations at early life stages have been observed to influence robustness at a later stage of fish, however, little is known on its role in mucosal immunomodulation.
7. Salinity
Salinity is important in the osmoregulatory functions of fish. The interesting overlap between osmoregulation and immunity purported some studies to determine the effect of changes in the salinity on the immune responses of fish. Acclimation to different salinity levels in euryhaline species, such as Atlantic salmon, an anadromous species, increases susceptibility to diseases during transitional stages. In fact, mortalities of up to 16% were observed after seawater transfer of post-smolts [54]. However, Karlsen et al. (2018) saw a progressive improvement in the protective functions in the skin of Atlantic salmon post-smolts, such as the increased mucus cell number and thickness observed 4 months after seawater transfer [55]. Furthermore, assessment of the mucus lysozyme activity of salmonids reared in either freshwater or seawater shows that Atlantic salmon had the least activity compared with coho salmon (Oncorhynchus kisutch) and rainbow trout when reared in seawater, while in freshwater, Atlantic salmon and coho salmon had the highest activity [56]. The variability in the results gives insights into the diversity of salmonid cutaneous defense system in response to various salinity conditions.
Mucosal barriers are influenced by salinity at the molecular level. For instance, freshwater acclimation reduced the expression of genes coding for tight junction proteins claudin-3 (cldn3) and claudin-4 (cldn4) in the gills of southern flounder (Paralichthys lethostigma) [57]. In green-spotted pufferfish (Tetraodon nigroviridis), claudin-10d (cldn10d) and cldn10e increased in the gills of fish acclimated to seawater versus freshwater, while claudin-6 (cldn6) decreased in the gills of fish acclimated to seawater [58]. Seawater acclimation of Atlantic salmon resulted in differential regulation of claudins (i.e., increase in the expression of claudin-10e (cldn10e) and decrease in the expression of claudin-27a and -30 (cldn27a, cldn30) in the gills) [59]. Moreover, an increase in the expression of genes encoding antigen presentation, complement, immunoglobulins, acute phase proteins, and lymphocytes, to mention a few, was observed in the skin Atlantic salmon 4 months after seawater transfer [55]. On the other hand, acclimating in seawater significantly reduced the expression of immune-related genes, i.e., C-reactive protein (crp), toll-like receptor 2 (tlr2), and interleukin-1 receptor type 2 (il1-1r2), in the gills of Japanese eel (Anguilla japonica), a catadromous species [60].
Acclimation to different salinities also impacts the microbiota at the mucosa. Transfer of Atlantic salmon from freshwater to seawater resulted in the destabilization of skin microbiota. The abundance of Proteobacteria, for instance, increased from 45% in freshwater to 89% in seawater. This drastic change reveals that microbiota of mucosal surfaces undergoes restructuring as a form of adaptation to salinity changes [61].
8. Photoperiod
Photoperiod, or the light-dark cycle, is an environmental cue that controls many biological activities in fish, including their defense mechanisms. Photoperiod manipulation is a practice to regulate sexual maturation and spawning. Though the use of artificial photoperiod has beneficial effects on regulating the reproduction cycles of farmed fish, it has been observed to cause morphological changes, such as skin lesions and ulcerative-type necrosis in rainbow trout, accompanied by mortalities of up to 36% in LD 14:10 and 25% in LD 24:0 compared to the control setup (7%; LD10:14) [62]. Flavobacterium psychrophilum was identified as the causative agent of the mortalities, though the isolation of Aeromonas, Pseudomonas, and Saprolegnia in the diseased fish implies an increased susceptibility of fish to opportunistic diseases.
The current understanding proposes that partitioning of the immune system characterizes the circadian impact to immune functions into a state of anticipation and enhanced immune activity, and a state of repair and regeneration [63]. Understanding the circadian rhythm of mucosal defenses in fish can be exploited to design photoperiod strategies that do not hamper normal barrier functions, and at the same time, can be used to deliver time-dependent strategies that aim at modulating fish immunity. The impact of daily rhythm to key defense factors in the mucus of permit (Trachinotus falcatus) reared under a 12L:12D cycle has been described by Lazado et al. (2015) [64]. Daily rhythmicity of defense enzymes in the mucus was observed in alkaline phosphatase (ALP) and GPX, with the latter having a dark-biased activity. The significant daily rhythmic pattern and similarity in acrophase of ALP in both serum and mucus suggests that this defense enzyme may be one of the vital components in the circadian-dependent immune defenses in permit.
9. Conclusions
The studies enumerated in this short review highlighted the remarkable influence of different environmental factors to fish mucosal barrier functionality. The changes in these factors not only impact the mucosal structural phenotypes and molecular response but also influence the mucus and microbiota that line the mucosal surfaces. It is quite apparent, however, that our current understanding of how environmental parameters in aquaculture systems impact mucosal barrier functions is quite limited and fragmentary. Future studies should be directed not only on how mucosal surfaces respond to environmental changes but also their mechanisms on adaption and the long-term impacts of the variability of environmental parameters. A thorough understanding of this interaction is particularly important in modern fish husbandry, in which production technologies are addressing strict control of aquaculture environments to foster heightened biosecurity and more sustainable production systems.
Author Contributions
Conceptualization, N.A.R.C.; writing—original draft preparation, N.A.R.C., C.C.L.; writing—review and editing, C.C.L., N.A.R.C.; visualization, N.A.R.C.
Funding
This research received no external funding.
Acknowledgments
The writing of this short review is made possible through SFI CtrlAQUA—Centre for Closed Containment Aquaculture funded by the Norwegian Research Council (ref. 237856).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lazado, C.C.; Caipang, C.M.A. Mucosal immunity and probiotics in fish. Fish Shellfish Immunol. 2014, 39, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Salinas, I. The Mucosal Immune System of Teleost Fish. Biology 2015, 4, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.W. The absorption and excretion of water and salts by marine teleosts. Am. J. Physiol. Legacy Content 1930, 93, 480–505. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Grossel, M.; Farrell, A.; Brauner, C. Fish Physiology: The Multifunctional Gut of Fish, 1st ed.; Academic Press: Cambridge, MA, USA, 2010; Volume 30. [Google Scholar]
- Beck, B.H.; Peatman, E. Mucosal Health in Aquaculture, 1st ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Lazado, C.C.; Caipang, C.M.A.; Estante, E.G. Prospects of host-associated microorganisms in fish and penaeids as probiotics with immunomodulatory functions. Fish Shellfish Immunol. 2015, 45, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Rombout, J.H.W.M.; Yang, G.; Kiron, V. Adaptive immune responses at mucosal surfaces of teleost fish. Fish Shellfish Immunol. 2014, 40, 634–643. [Google Scholar] [CrossRef]
- Sunyer, J.O. Fishing for mammalian paradigms in the teleost immune system. Nature Immunol. 2013, 14, 320. [Google Scholar] [CrossRef] [PubMed]
- Nigam, A.K.; Kumari, U.; Mittal, A.K. Comparative analysis of innate immune parameters of the skin mucous secretions from certain freshwater teleosts, inhabiting different ecological niches. Fish Physiol. Biochem. 2012, 38, 1245–1256. [Google Scholar] [CrossRef]
- Ellis, A.E. Innate host defense mechanisms of fish against viruses and bacteria. Dev. Comp. Immunol. 2001, 25, 827–839. [Google Scholar] [CrossRef]
- Boutin, S.; Bernatchez, L.; Audet, C.; Derôme, N. Network analysis highlights complex interactions between pathogen, host and commensal microbiota. PLoS ONE 2013, 8, e84772. [Google Scholar] [CrossRef]
- Dudley, S.A.; Schmitt, J. Testing the Adaptive Plasticity Hypothesis: Density-Dependent Selection on Manipulated Stem Length in Impatiens capensis. Am. Nat. 1996, 147, 445–465. [Google Scholar] [CrossRef]
- Meyers, L.A.; Bull, J.J. Fighting change with change: Adaptive variation in an uncertain world. Trends Ecol. Evol. 2002, 17, 551–557. [Google Scholar] [CrossRef]
- Travis, J. Evaluating the adaptive role of morphological plasticity. In Ecological Morphology: Integrative Organismal Biology; University of Chicago Press: Chicago, IL, USA, 1994; pp. 99–122. [Google Scholar]
- West-Eberhard, M.J. Developmental Plasticity and Evolution; Oxford University Press: Cary, NC, USA, 2003; ISBN 978-0-19-512235-0. [Google Scholar]
- Fivelstad, S. Long-term carbon dioxide experiments with salmonids. Aqua. Eng. 2013, 53, 40–48. [Google Scholar] [CrossRef]
- Sollid, J.; De Angelis, P.; Gundersen, K.; Nilsson, G.E. Hypoxia induces adaptive and reversible gross morphological changes in crucian carp gills. J. Exp. Biol. 2003, 206, 3667–3673. [Google Scholar] [CrossRef] [PubMed]
- Sollid, J.; Nilsson, G.E. Plasticity of respiratory structures—Adaptive remodeling of fish gills induced by ambient oxygen and temperature. Respir. Physiol. Neurobiol. 2006, 154, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Matey, V.; Richards, J.G.; Wang, Y.; Wood, C.M.; Rogers, J.; Davies, R.; Murray, B.W.; Chen, X.-Q.; Du, J.; Brauner, C.J. The effect of hypoxia on gill morphology and ionoregulatory status in the Lake Qinghai scaleless carp, Gymnocypris przewalskii. J. Exp. Biol. 2008, 211, 1063–1074. [Google Scholar] [CrossRef]
- Niklasson, L.; Sundh, H.; Fridell, F.; Taranger, G.L.; Sundell, K. Disturbance of the intestinal mucosal immune system of farmed Atlantic salmon (Salmo salar), in response to long-term hypoxic conditions. Fish Shellfish Immunol. 2011, 31, 1072–1080. [Google Scholar] [CrossRef]
- Tzaneva, V.; Bailey, S.; Perry, S.F. The interactive effects of hypoxemia, hyperoxia, and temperature on the gill morphology of goldfish (Carassius auratus). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1344–R1351. [Google Scholar] [CrossRef] [PubMed]
- Bagherzadeh Lakani, F.; Sattari, M.; Falahatkar, B. Effect of different oxygen levels on growth performance, stress response and oxygen consumption in two weight groups of great sturgeon Huso huso. Iran. J. Fish. Sci. 2013, 12, 533–549. [Google Scholar]
- Machado, M.; Malheiro, D.; Couto, A.; Wilson, J.M.; Guerreiro, M.; Azeredo, R.; Svendsen, J.C.; Afonso, A.; Serradeiro, R.; Costas, B. Acute hyperoxia induces systemic responses with no major changes in peripheral tissues in the Senegalese sole (Solea senegalensis Kaup, 1858). Fish Shellfish Immunol. 2018, 74, 260–267. [Google Scholar] [CrossRef]
- Boyd, C.E.; Tucker, C.S. Pond Aquaculture Water Quality Management; Springer: New York, NY, USA, 1998. [Google Scholar]
- Wilkie, M.P.; Wood, C.M. The adaptations of fish to extremely alkaline environments. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1996, 113, 665–673. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mahboobi-Soofiani, N.; Farhadian, O.; Malekpouri, P. Metabolic and NH4 excretion rate of fresh water species, Chondrostoma regium in response to environmental stressors, different scenarios for temperature and pH. Sci. Total Environ. 2019, 648, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.J.; Horng, J.L.; Yan, J.J.; Hsiao, C.D.; Hwang, P.P. The transcription factor, glial cell missing 2, is involved in differentiation and functional regulation of H+-ATPase-rich cells in zebrafish (Danio rerio). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1192–R1201. [Google Scholar] [CrossRef] [PubMed]
- Günzel, D.; Fromm, M. Claudins and Other Tight Junction Proteins. In Comprehensive Physiology; American Cancer Society: Atlanta, GA, USA, 2012; pp. 1819–1852. ISBN 978-0-470-65071-4. [Google Scholar]
- Freda, J.; Sanchez, D.A.; Bergman, H.L. Shortening of Branchial Tight Junction Acid-Exposed Rainbow Trout (Oncorhynchus mykiss). Can. J. Fish. Aquat. Sci. 1991, 48, 2028–2033. [Google Scholar] [CrossRef]
- Kwong, R.W.M.; Perry, S.F. Cortisol regulates epithelial permeability and sodium losses in zebrafish exposed to acidic water. J. Endocrinol. 2013, 217, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Kumai, Y.; Bahubeshi, A.; Steele, S.; Perry, S.F. Strategies for maintaining Na+ balance in zebrafish (Danio rerio) during prolonged exposure to acidic water. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 160, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Tiedke, J.; Cubuk, C.; Burmester, T. Environmental acidification triggers oxidative stress and enhances globin expression in zebrafish gills. Biochem. Biophys. Res. Commun. 2013, 441, 624–629. [Google Scholar] [CrossRef]
- Sylvain, F.-É.; Cheaib, B.; Llewellyn, M.; Gabriel Correia, T.; Barros Fagundes, D.; Luis Val, A.; Derome, N. pH drop impacts differentially skin and gut microbiota of the Amazonian fish tambaqui (Colossoma macropomum). Sci. Rep. 2016, 6, 32032. [Google Scholar] [CrossRef]
- Sloman, K.A. Mitochondria-Rich Cell Subtypes in Fish Gill. J. Exp. Biol. 2003, 206, 7–8. [Google Scholar] [CrossRef]
- Cameron, J.N.; Iwama, G.K. Compensation of Progressive Hypercapnia in Channel Catfish and Blue Crabs. J. Exp. Biol. 1987, 133, 183–197. [Google Scholar]
- Hayashi, M.; Kikkawa, T.; Ishimatsu, A. Morphological changes in branchial mitochondria-rich cells of the teleost Paralichthys olivaceus as a potential indicator of CO2 impacts. Mar. Pollut. Bull. 2013, 73, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Goss, G.G.; Laurent, P.; Perry, S.F. Evidence for a morphological component in acid-base regulation during environmental hypercapnia in the brown bullhead (Ictalurus nebulosus). Cell Tissue Res. 1992, 268, 539–552. [Google Scholar] [CrossRef]
- Goss, G.G.; Perry, S.F. Physiological and morphological regulation of acid–base status during hypercapnia in rainbow trout (Oncorhynchus mykiss). Can. J. Zool. 1993, 71, 1673–1680. [Google Scholar] [CrossRef]
- Baker, D.W.; Matey, V.; Huynh, K.T.; Wilson, J.M.; Morgan, J.D.; Brauner, C.J. Complete intracellular pH protection during extracellular pH depression is associated with hypercarbia tolerance in white sturgeon, Acipenser transmontanus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1868–R1880. [Google Scholar] [CrossRef] [PubMed]
- Kreiss, C.M.; Michael, K.; Lucassen, M.; Jutfelt, F.; Motyka, R.; Dupont, S.; Pörtner, H.O. Ocean warming and acidification modulate energy budget and gill ion regulatory mechanisms in Atlantic cod (Gadus morhua). J. Comp. Physiol. B 2015, 185, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Kreiss, C.M.; Michael, K.; Bock, C.; Lucassen, M.; Pörtner, H.O. Impact of long-term moderate hypercapnia and elevated temperature on the energy budget of isolated gills of Atlantic cod (Gadus morhua). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 182, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, E.; Lopes, A.R.; Francisco, S.; Paula, J.R.; Pimentel, M.; Maulvault, A.L.; Repolho, T.; Grilo, T.F.; Pousão-Ferreira, P.; Marques, A.; et al. Ocean acidification dampens physiological stress response to warming and contamination in a commercially-important fish (Argyrosomus regius). Sci. Total Environ. 2018, 618, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Mota, V.C.; Nilsen, T.O.; Gerwins, J.; Gallo, M.; Ytteborg, E.; Baeverfjord, G.; Kolarevic, J.; Summerfelt, S.T.; Terjesen, B.F. The effects of carbon dioxide on growth performance, welfare, and health of Atlantic salmon post-smolt (Salmo salar) in recirculating aquaculture systems. Aquaculture 2019, 498, 578–586. [Google Scholar] [CrossRef]
- Bly, J.E.; Clem, L.W. Temperature and teleost immune functions. Fish Shellfish Immunol. 1992, 2, 159–171. [Google Scholar] [CrossRef]
- Bowden, T.J.; Thompson, K.D.; Morgan, A.L.; Gratacap, R.M.L.; Nikoskelainen, S. Seasonal variation and the immune response: A fish perspective. Fish Shellfish Immunol. 2007, 22, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Sollid, J.; Weber, R.E.; Nilsson, G.E. Temperature alters the respiratory surface area of crucian carp Carassius carassius and goldfish Carassius auratus. J. Exp. Biol. 2005, 208, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Wentworth, S.A.; Thede, K.; Aravindabose, V.; Monroe, I.; Thompson, A.W.; Molyneaux, N.; Owen, C.L.; Burns, J.R.; Gonzalez-Vicente, A.; Garvin, J.L.; et al. Transcriptomic analysis of changes in gene expression of immune proteins of gill tissue in response to low environmental temperature in fathead minnows (Pimephales promelas). Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 25, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-H.; Ma, A.-J.; Wang, X.-A. The immune response of turbot, Scophthalmus maximus (L.), skin to high water temperature. J. Fish Dis. 2011, 34, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Clem, L.W.; Faulmann, E.; Miller, N.W.; Ellsaesser, C.; Lobb, C.J.; Cuchens, M.A. Temperature-mediated processes in teleost immunity: Differential effects of in vitro and in vivo temperatures on mitogenic responses of channel catfish lymphocytes. Dev. Comp. Immunol. 1984, 8, 313–322. [Google Scholar] [CrossRef]
- Parihar, M.S.; Javeri, T.; Hemnani, T.; Dubey, A.K.; Prakash, P. Responses of superoxide dismutase, glutathione peroxidase and reduced glutathione antioxidant defenses in gills of the freshwater catfish (Heteropneustes fossilis) to short-term elevated temperature. J. Therm. Biol. 1997, 22, 151–156. [Google Scholar] [CrossRef]
- Klein, R.D.; Borges, V.D.; Rosa, C.E.; Colares, E.P.; Robaldo, R.B.; Martinez, P.E.; Bianchini, A. Effects of increasing temperature on antioxidant defense system and oxidative stress parameters in the Antarctic fish Notothenia coriiceps and Notothenia rossii. J. Therm. Biol. 2017, 68, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Jin, S.R.; Chen, Z.Z.; Gao, J.-Z. Physiological responses to cold stress in the gills of discus fish (Symphysodon aequifasciatus) revealed by conventional biochemical assays and GC-TOF-MS metabolomics. Sci. Total Environ. 2018, 640–641, 1372–1381. [Google Scholar] [CrossRef]
- Bleie, H.; Skrudland, A. Tap av laksefisk i sjø. Rapport Fra Mattilsynet; Mattilsynet: Oslo, Norway, 2014.
- Karlsen, C.; Ytteborg, E.; Timmerhaus, G.; Høst, V.; Handeland, S.; Jørgensen, S.M.; Krasnov, A. Atlantic salmon skin barrier functions gradually enhance after seawater transfer. Sci. Rep. 2018, 8, 9510. [Google Scholar] [CrossRef]
- Fast, M.D.; Sims, D.E.; Burka, J.F.; Mustafa, A.; Ross, N.W. Skin morphology and humoral non-specific defence parameters of mucus and plasma in rainbow trout, coho and Atlantic salmon. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 132, 645–657. [Google Scholar] [CrossRef]
- Tipsmark, C.K.; Luckenbach, J.A.; Madsen, S.S.; Kiilerich, P.; Borski, R.J. Osmoregulation and expression of ion transport proteins and putative claudins in the gill of southern flounder (Paralichthys lethostigma). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 150, 265–273. [Google Scholar] [CrossRef]
- Bui, P.; Kelly, S.P. Claudin-6, -10d and -10e contribute to seawater acclimation in the euryhaline puffer fish Tetraodon nigroviridis. J. Exp. Biol. 2014, 217, 1758–1767. [Google Scholar] [CrossRef] [PubMed]
- Tipsmark, C.K.; Kiilerich, P.; Nilsen, T.O.; Ebbesson, L.O.E.; Stefansson, S.O.; Madsen, S.S. Branchial expression patterns of claudin isoforms in Atlantic salmon during seawater acclimation and smoltification. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1563–R1574. [Google Scholar] [CrossRef]
- Gu, J.; Dai, S.; Liu, H.; Cao, Q.; Yin, S.; Lai, K.P.; Tse, W.K.F.; Wong, C.K.C.; Shi, H. Identification of immune-related genes in gill cells of Japanese eels (Anguilla japonica) in adaptation to water salinity changes. Fish Shellfish Immunol. 2018, 73, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Lokesh, J.; Kiron, V. Transition from freshwater to seawater reshapes the skin-associated microbiota of Atlantic salmon. Sci. Rep. 2016, 6, 19707. [Google Scholar] [CrossRef]
- Valenzuela, A.; Campos, V.; Yañez, F.; Alveal, K.; Gutiérrez, P.; Rivas, M.; Contreras, N.; Klempau, A.; Fernandez, I.; Oyarzun, C. Application of artificial photoperiod in fish: A factor that increases susceptibility to infectious diseases? Fish Physiol. Biochem. 2012, 38, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.M.; Bellet, M.M.; Sassone-Corsi, P.; O’Neill, L.A.J. Circadian clock proteins and immunity. Immunity 2014, 40, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Lazado, C.C.; Lund, I.; Pedersen, P.B.; Nguyen, H.Q. Humoral and mucosal defense molecules rhythmically oscillate during a light–dark cycle in permit, Trachinotus falcatus. Fish Shellfish Immunol. 2015, 47, 902–912. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).