Viability of Anisakis spp. Larvae After Direct Exposure to Different Processing Media and Non-Thermal Processing in Anchovy Fillets
Abstract
:1. Introduction
2. Results and Discussion
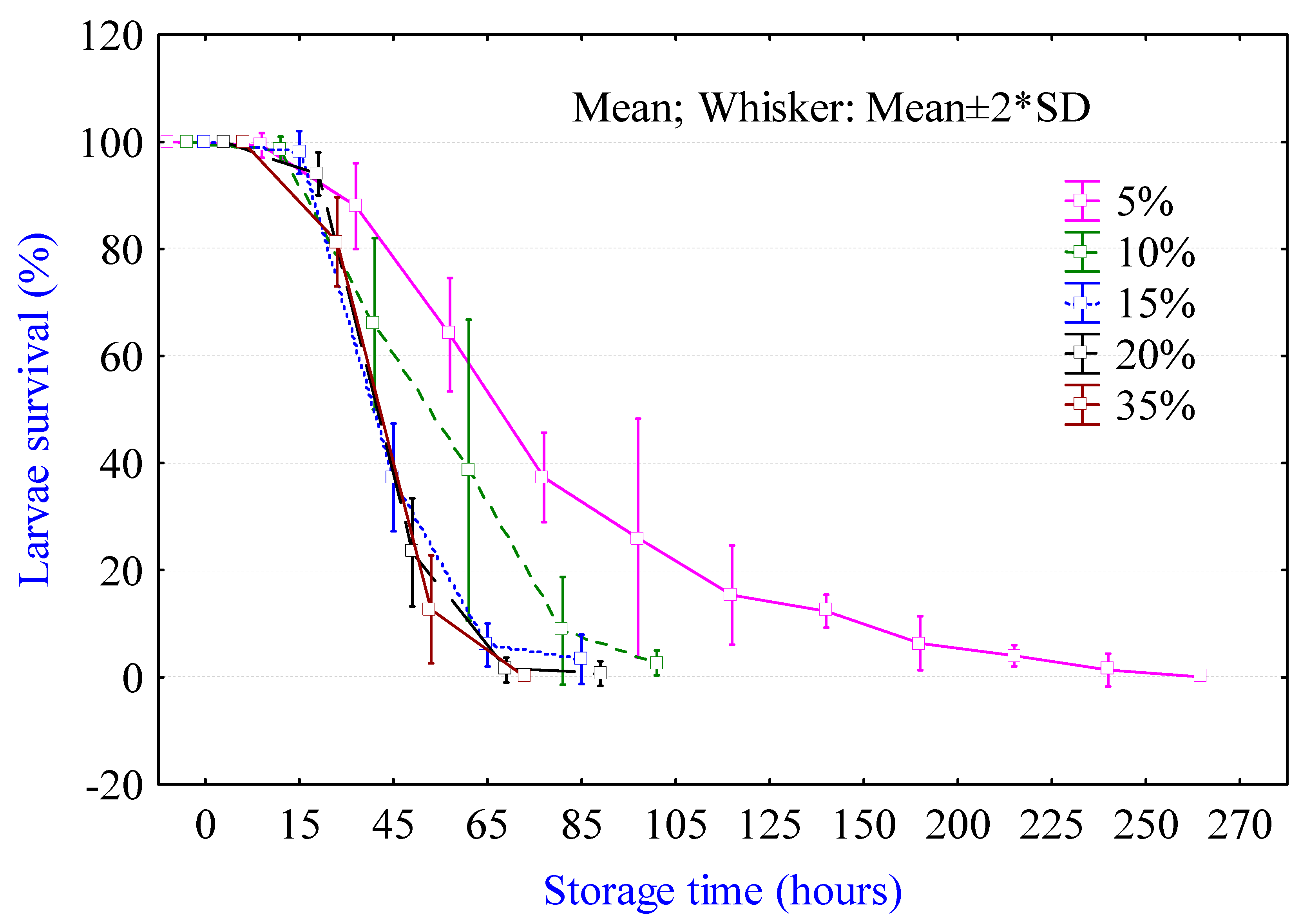
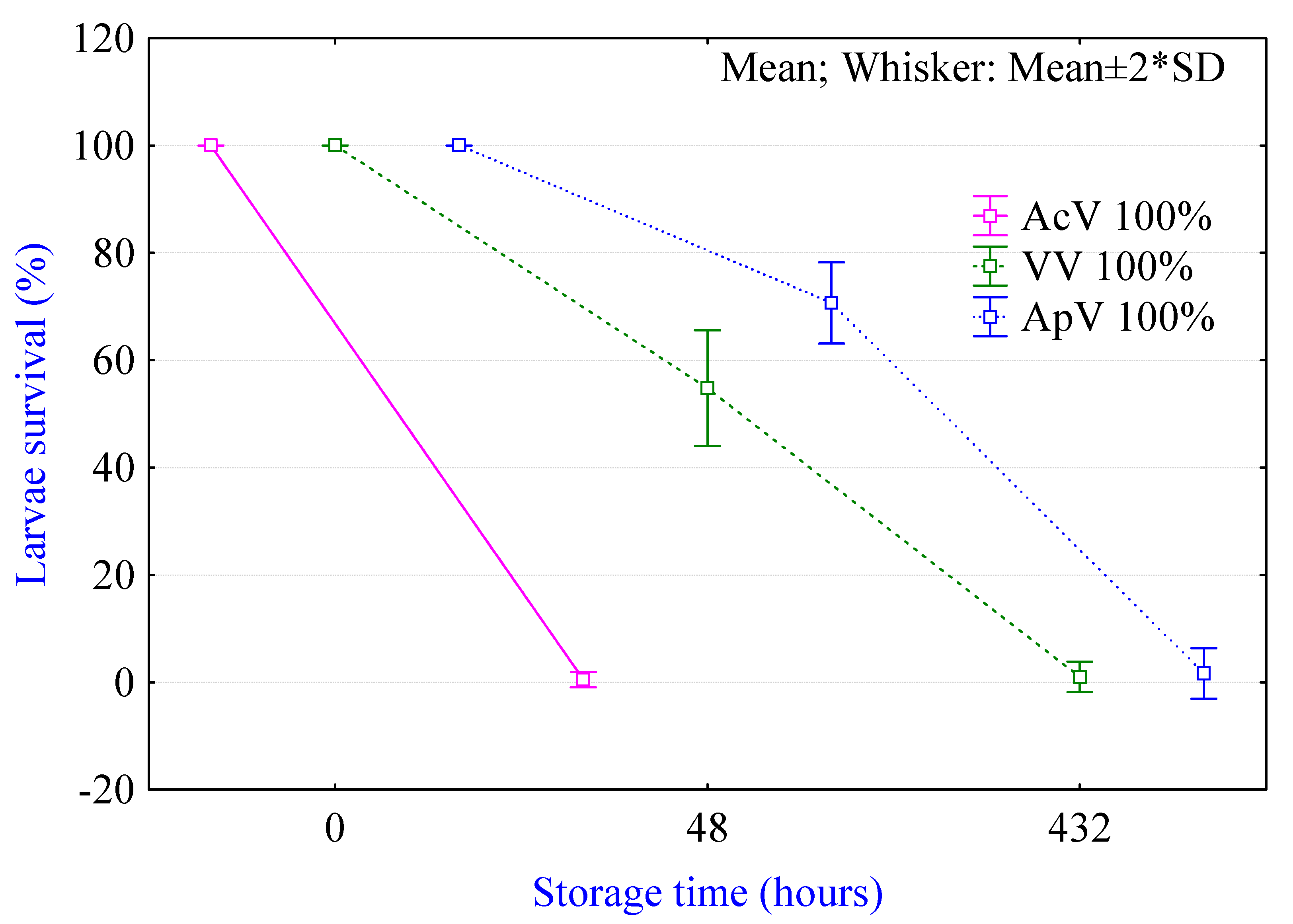
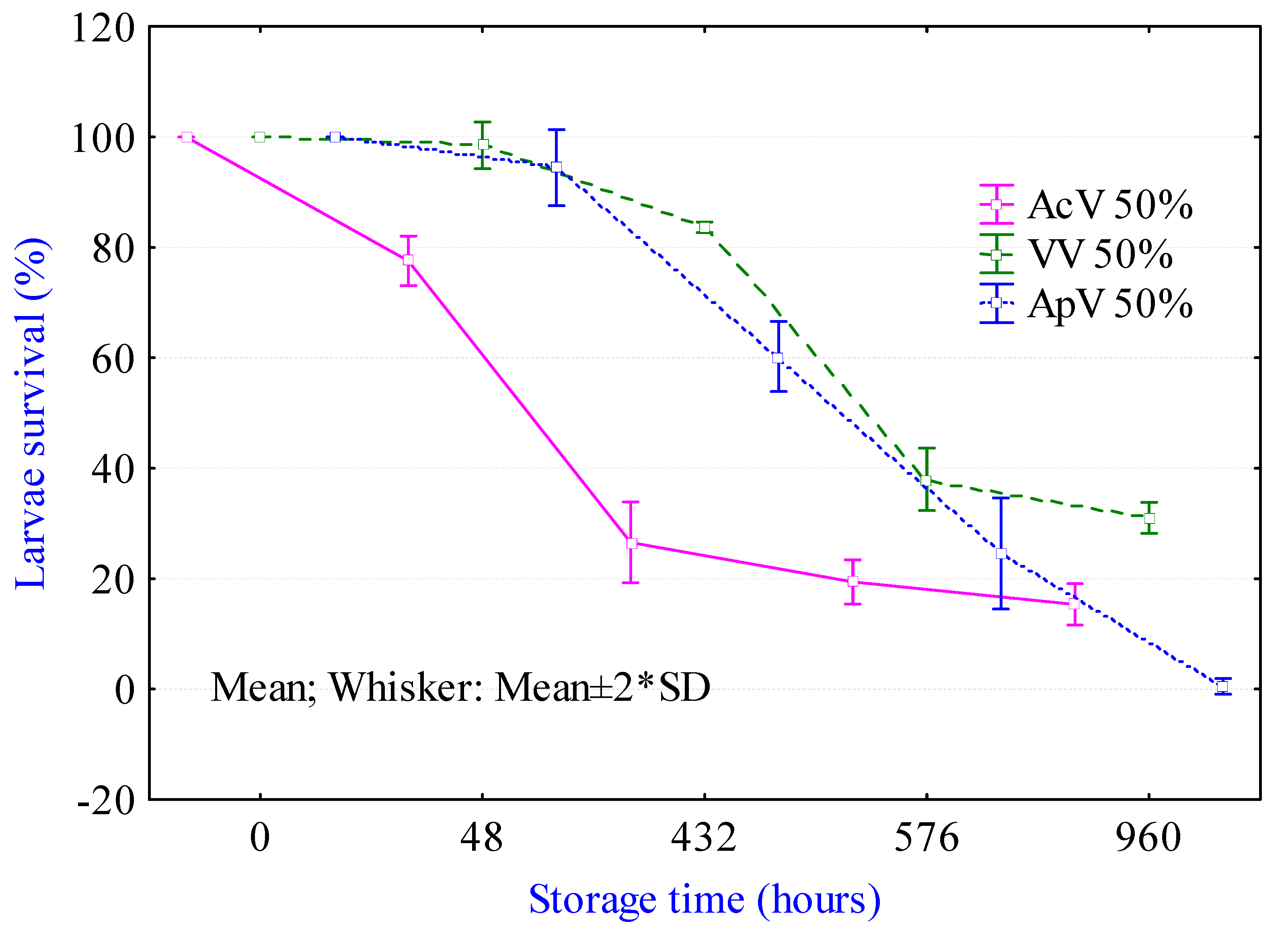
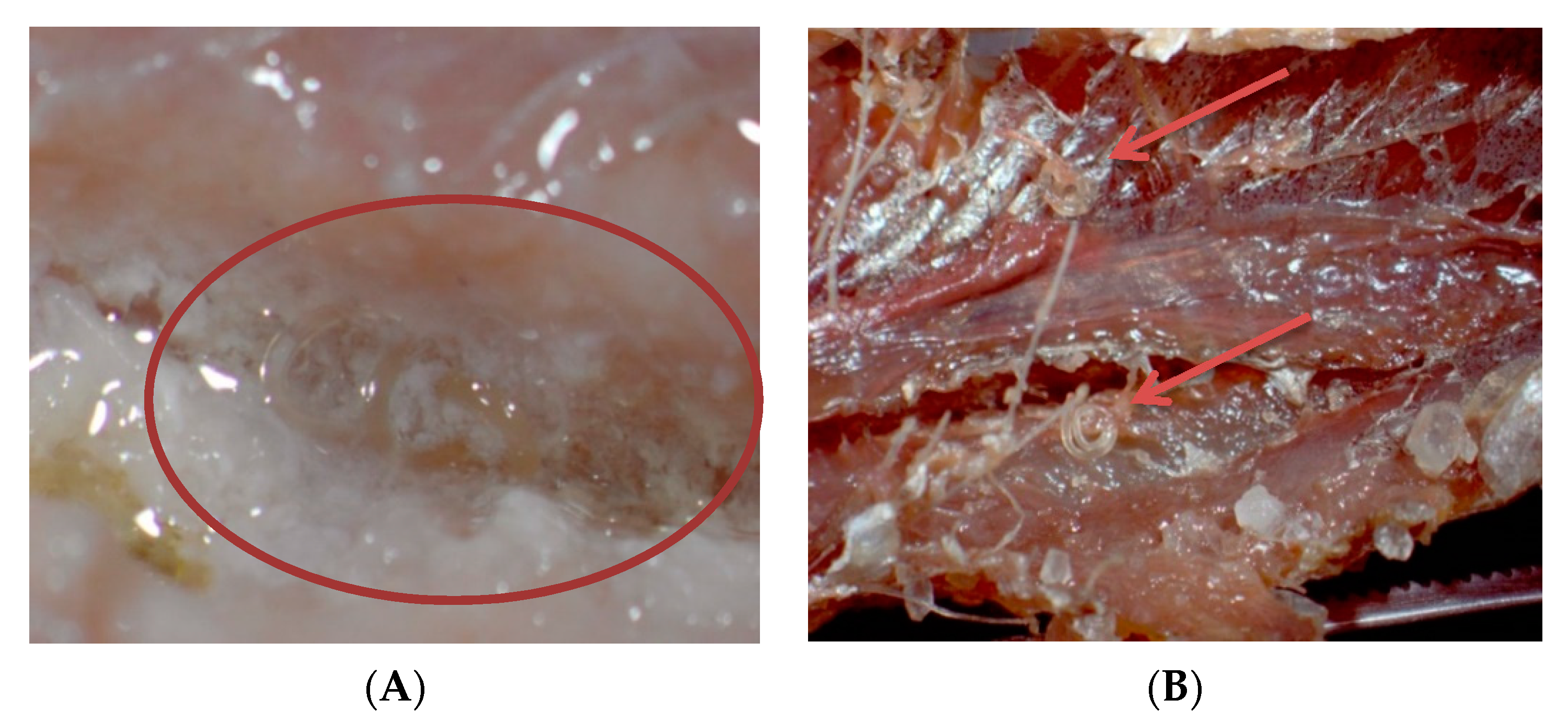
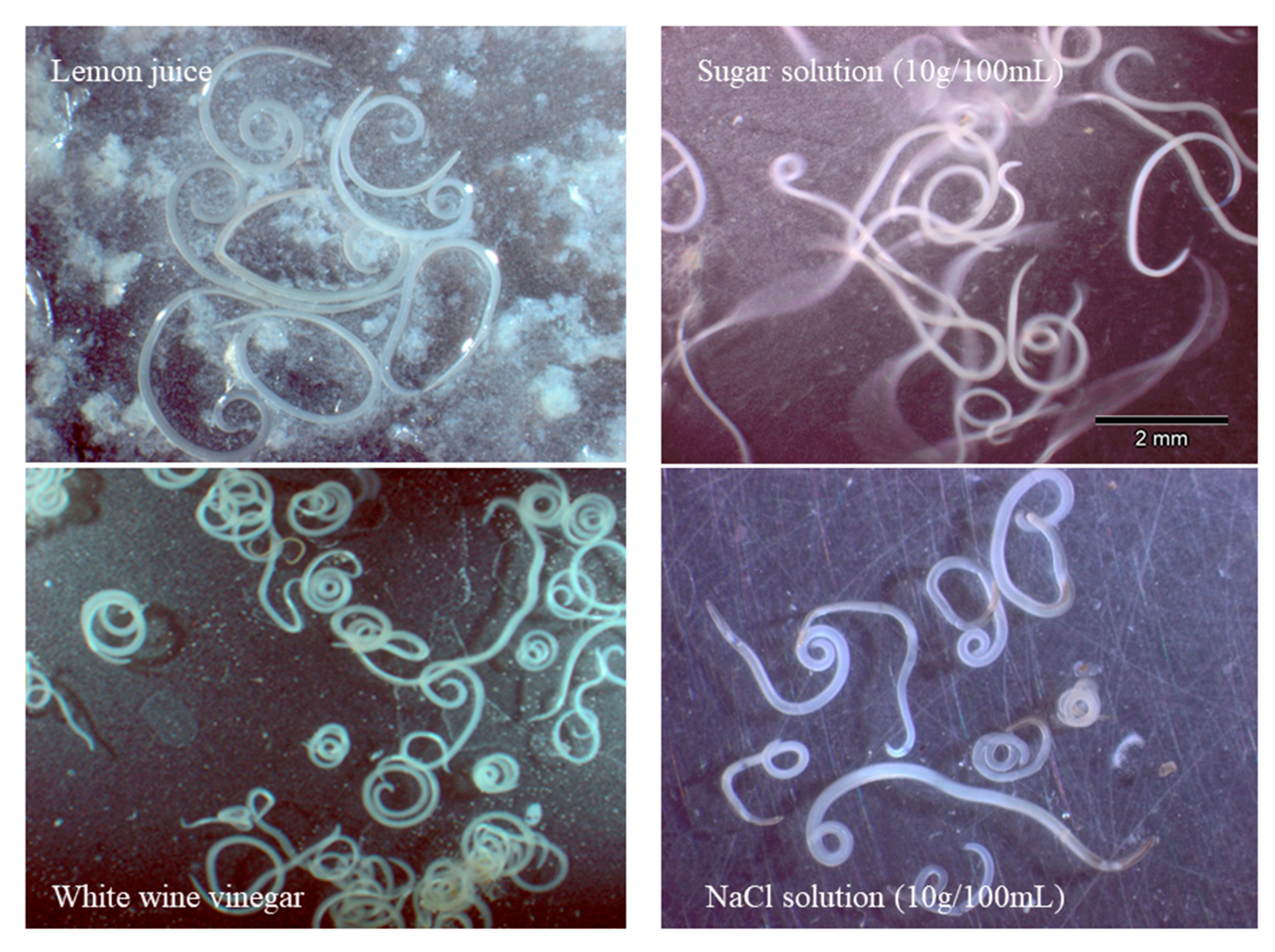
2.1. Viability of Larvae in Different Processing Media
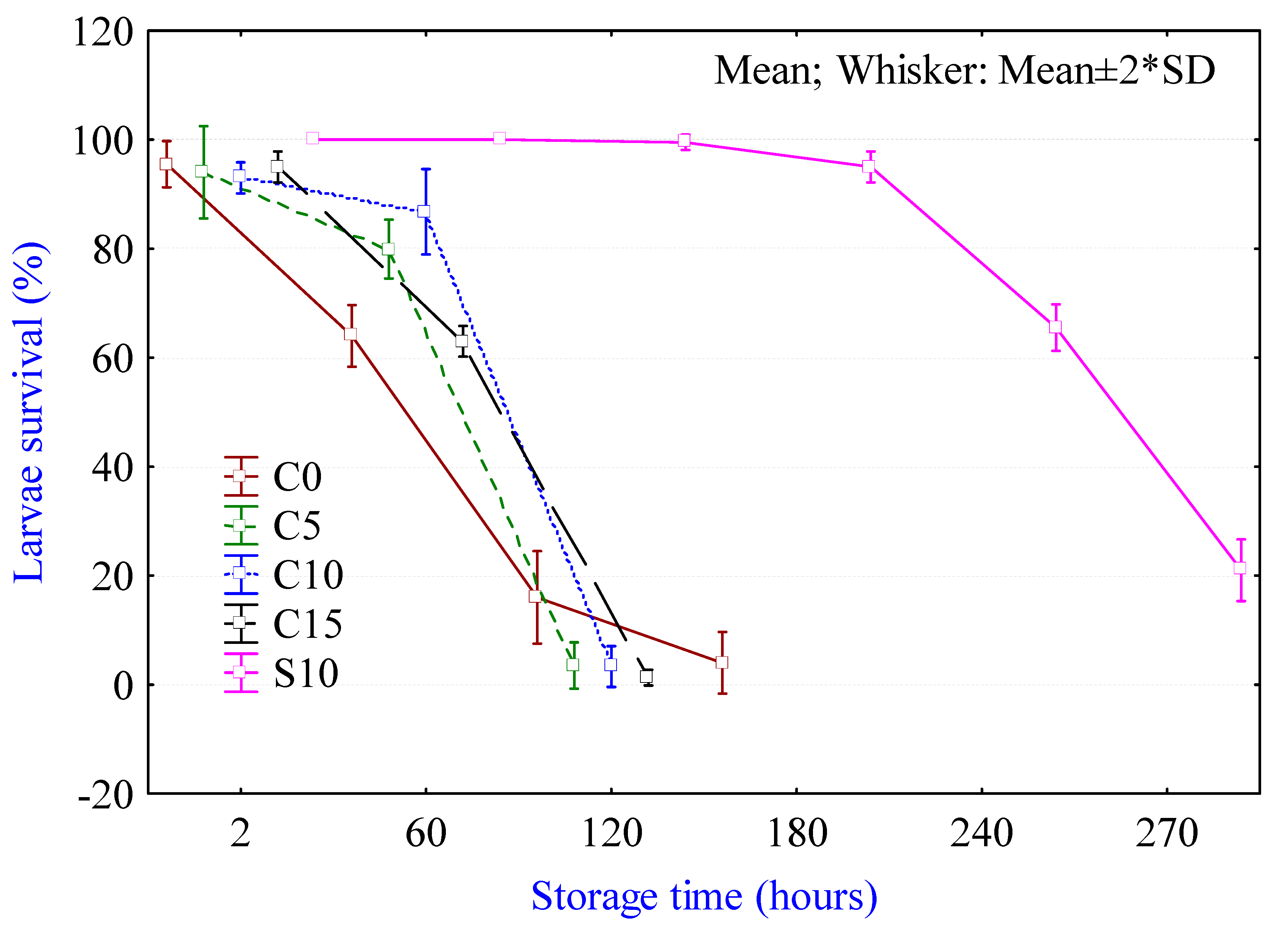
2.2. Viability of Larvae During Non-Thermal Processing in Fish Fillets
3. Materials and Methods
3.1. Viability Test in Processing Media
3.2. Viability Test during Non-Thermal Processing
3.3. pH and NaCl Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EFSA Panel on Biological Hazards (BIOHAZ). Scientifc Opinion on risk assessment of parasites in fshery products. EFSA J. 2010, 8, 1543. [Google Scholar] [CrossRef]
- van Thiel, P.; van Houten, H. The localization of the herringworm Anisakis marina in-and outside the human gastro-intestinal wall (with a description of the characteristics of its larval and juvenile stages). Trop. Geogr. Med. 1967, 19, 56–62. [Google Scholar] [PubMed]
- Audicana, M.T.; Kennedy, M.W. Anisakis simplex: From obscure infectious worm to inducer of immune hypersensitivity. Clin. Microbiol. Rev. 2008, 21, 360–379. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Pierce, G.J.; Pascual, S.; González-Munõz, M.; Mattiucci, S.; Mladineo, I.; Cipriani, P.; Bušelić, I.; Strachan, N.J.C. Assessing the risk of an emerging zoonosis of worldwide concern: Anisakiasis. Sci. Rep. 2017, 7, 43699. [Google Scholar] [CrossRef] [PubMed]
- Guardone, L.; Armani, A.; Nucera, D.; Costanzo, F.; Mattiucci, S.; Bruschi, F. Human anisakiasis in Italy: A retrospective epidemiological study over two decades. Parasite 2018, 25, 41. [Google Scholar] [CrossRef] [PubMed]
- Mattiucci, S.; Cipriani, P.; Levsen, A.; Paoletti, M.; Nascetti, G. Molecular Epidemiology of Anisakis and Anisakiasis: An Ecological and Evolutionary Road Map. Adv. Parasitol. 2018, 99, 93–263. [Google Scholar] [PubMed]
- Cavallero, S.; Martini, A.; Migliara, G.; De Vito, C.; Iavicoli, S.; D’Amelio, S. Anisakiasis in Italy: Analysis of hospital discharge records in the years 2005–2015. PLoS ONE 2018, 13, e0208772. [Google Scholar] [CrossRef]
- Bouwknegt, M.; Devleesschauwer, B.; Graham, H.; Robertson, L.J.; van der Giessen, J.W.; the Euro-FBP workshop participants. Prioritisation of food-borne parasites in Europe, 2016. Eurosurveillance 2018, 23, 17-00161. [Google Scholar] [CrossRef]
- Broglia, A.; Kapel, C. Changing dietary habits in a changing world: Emerging drivers for the transmission of foodborne parasitic zoonoses. Vet. Parasitol. 2011, 182, 2–13. [Google Scholar] [CrossRef]
- Hochberg, N.S.; Hamer, D.H. Anisakidosis: Perils of the Deep. Clin. Infect. Dis. 2010, 51, 806–812. [Google Scholar] [CrossRef]
- Arizono, N.; Yamada, M.; Tegoshi, T.; Yoshikawa, M. Anisakis simplex sensu stricto and Anisakis pegreffii: Biological characteristics and pathogenetic potential in human anisakiasis. Foodborne Pathog. Dis. 2012, 9, 517–521. [Google Scholar] [CrossRef] [PubMed]
- del Carmen Romero, M.; Valero, A.; Navarro-Moll, M.C.; Martín-Sánchez, J. Experimental comparison of pathogenic potential of two sibling species Anisakis simplex s.s. and Anisakis pegreffii in Wistar rat. Trop. Med. Int. Health 2013, 18, 979–984. [Google Scholar] [CrossRef]
- Jeon, C.H.; Kim, J.H. Pathogenic Potential of Two Sibling Species, Anisakis simplex (s. s.) and Anisakis pegreffii (Nematoda: Anisakidae): In Vitro and In Vivo Studies. Biomed. Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Šimat, V.; Bogdanović, T. Seasonal changes in proximate composition of anchovy (Engraulis encrasicolus, L.) from the central Adriatic. Acta Adriat. 2012, 53, 125–132. [Google Scholar]
- European Commission. Regulation of the European Parliament and of the Council (29 April 2004). Laying down specific hygiene rules for the hygiene of foodstuffs, 853/2004/EC. Off. J. Eur. Union 2004, L 139, 55–206. [Google Scholar]
- European Commission. Regulation of the European Parliament and of the Council (8 December 2011) amending Annex III to Regulation (EC) No 853/2004 of the European Parliament and of the Council as regards the treatment to kill viable parasites in fishery products for human cons. Off. J. Eur. Union 2011, L 327, 39–41. [Google Scholar]
- Mladineo, I.; Šimat, V.; Miletić, J.; Beck, R.; Poljak, V. Molecular identification and population dynamic of Anisakis pegreffii (Nematoda: Anisakidae Dujardin, 1845) isolated from the European anchovy (Engraulis encrasicolus L.) in the Adriatic Sea. Int. J. Food Microbiol. 2012, 157, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, P.; Sbaraglia, G.L.; Palomba, M.; Giulietti, L.; Bellisario, B.; Bušelić, I.; Mladineo, I.; Cheleschi, R.; Nascetti, G.; Mattiucci, S. Anisakis pegreffii (Nematoda: Anisakidae) in European anchovy Engraulis encrasicolus from the Mediterranean Sea: Fishing ground as a predictor of parasite distribution. Fish. Res. 2018, 202, 59–68. [Google Scholar] [CrossRef]
- AAITO-IFIACI Anisakis Consortium. Anisakis hypersensitivity in Italy: Prevalence and clinical features: A multicenter study. Allergy 2011, 66, 1563–1569. [Google Scholar] [CrossRef]
- Fumarola, L.; Monno, R.; Ierardi, E.; Rizzo, G.; Giannelli, G.; Lalle, M.; Pozio, E. Anisakis pegreffi etiological agent of gastric infections in two Italian women. Foodborne Pathog. Dis. 2009, 6, 1157–1159. [Google Scholar] [CrossRef]
- Mladineo, I.; Poljak, V.; Martínez-Sernández, V.; Ubeira, F.M. Anti-Anisakis IgE seroprevalence in the healthy Croatian coastal population and associated risk factors. PLoS Negl. Trop. Dis. 2014, 8, e2673. [Google Scholar] [CrossRef]
- Šimat, V.; Miletić, J.; Bogdanović, T.; Poljak, V.; Mladineo, I. Role of biogenic amines in the post-mortem migration of Anisakis pegreffii (Nematoda: Anisakidae Dujardin, 1845) larvae into fish fillets. Int. J. Food Microbiol. 2015, 214, 179–186. [Google Scholar] [CrossRef]
- Rello, F.J.; Adroher, F.J.; Benítez, R.; Valero, A. The fishing area as a possible indicator of the infection by anisakids in anchovies (Engraulis encrasicolus) from southwestern Europe. Int. J. Food Microbiol. 2009, 129, 277–281. [Google Scholar] [CrossRef]
- Guardone, L.; Nucera, D.; Lodola, L.B.; Tinacci, L.; Acutis, P.L.; Guidi, A.; Armani, A. Anisakis spp. larvae in different kinds of ready to eat products made of anchovies (Engraulis encrasicolus) sold in Italian supermarkets. Int. J. Food Microbiol. 2018, 268, 10–18. [Google Scholar] [CrossRef]
- Karl, H.; Roepstorff, A.; Huss, H.H.; Bloemsma, B. Survival of Anisakis larvae in marinated herring fillets. Int. J. Food Sci. Technol. 1995, 29, 661–670. [Google Scholar] [CrossRef]
- Sánchez-Monsalvez, I.; de Armas-Serra, C.; Martínez, J.; Dorado, M.; Sánchez, A.; Rodríguez-Caabeiro, F. A new procedure for marinating fresh anchovies and ensuring the rapid destruction of Anisakis larvae. J. Food Prot. 2005, 68, 1066–1072. [Google Scholar] [CrossRef]
- Smaldone, G.; Marrone, R.; Palma, G.; Sarnelli, P.; Anastasio, A. Preliminary study on the inactivation of anisakid larvae in baccalà prepared according to traditional methods. Ital. J. Food Saf. 2017, 6, 6964. [Google Scholar] [CrossRef]
- Mladineo, I.; Bušelić, I.; Hrabar, J.; Vrbatović, A.; Radonić, I. Population parameters and mito-nuclear mosaicism of Anisakis spp. in the Adriatic Sea. Mol. Biochem. Parasitol. 2017, 212, 46–54. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]

| Carpaccio Marinade | White Wine Vinegar Marinade | Dry-Salted Fillets | |
|---|---|---|---|
| pH of the fillet | 3.94 ± 0.03 | 3.82 ± 0.24 | 5.78 ± 0.24 |
| Salt content in the fillet (%) | 3.96 ± 0.24 | 2.41 ± 0.24 | 16.37 ± 0.67 |
| Duration of maturation | 7 days at 4 °C | 7 days at 4–8 °C | 10 days at 16 °C |
| Recovered larvae after maturation (%) | 100 | 100 | 100 |
| Larvae found in the fillet pocket (%) | 81.0 ± 2.5 | 68.0 ± 2.4 | 97.0 ± 2.8 |
| Larvae found in the fillet outside the pocket (%) | 6.3 ± 2.1 | 2.7 ± 0.3 | 3.0 ± 0.1 |
| Larvae found in the marinating bath (%) | 13.2 ± 3.1 | 31.4 ± 4.8 | - |
| Percentage of live larvae after maturation | 39.1 ± 7.8 | 54.3 ± 4.3 | 0 |
| Percentage of live larvae after 30 days of storage | 0.3 ± 0.4 | 1.6 ± 8.1 | 0 * |
| Percentage of live larvae after 60 days of storage | 0 | 0 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimat, V.; Trumbić, Ž. Viability of Anisakis spp. Larvae After Direct Exposure to Different Processing Media and Non-Thermal Processing in Anchovy Fillets. Fishes 2019, 4, 19. https://doi.org/10.3390/fishes4010019
Šimat V, Trumbić Ž. Viability of Anisakis spp. Larvae After Direct Exposure to Different Processing Media and Non-Thermal Processing in Anchovy Fillets. Fishes. 2019; 4(1):19. https://doi.org/10.3390/fishes4010019
Chicago/Turabian StyleŠimat, Vida, and Željka Trumbić. 2019. "Viability of Anisakis spp. Larvae After Direct Exposure to Different Processing Media and Non-Thermal Processing in Anchovy Fillets" Fishes 4, no. 1: 19. https://doi.org/10.3390/fishes4010019
APA StyleŠimat, V., & Trumbić, Ž. (2019). Viability of Anisakis spp. Larvae After Direct Exposure to Different Processing Media and Non-Thermal Processing in Anchovy Fillets. Fishes, 4(1), 19. https://doi.org/10.3390/fishes4010019






