Relative Mass of Brain- and Intestinal Tissue in Juvenile Brown Trout: No Long-Term Effects of Compensatory Growth; with Additional Notes on Emerging Sex-Differences
Abstract
1. Introduction
2. Results
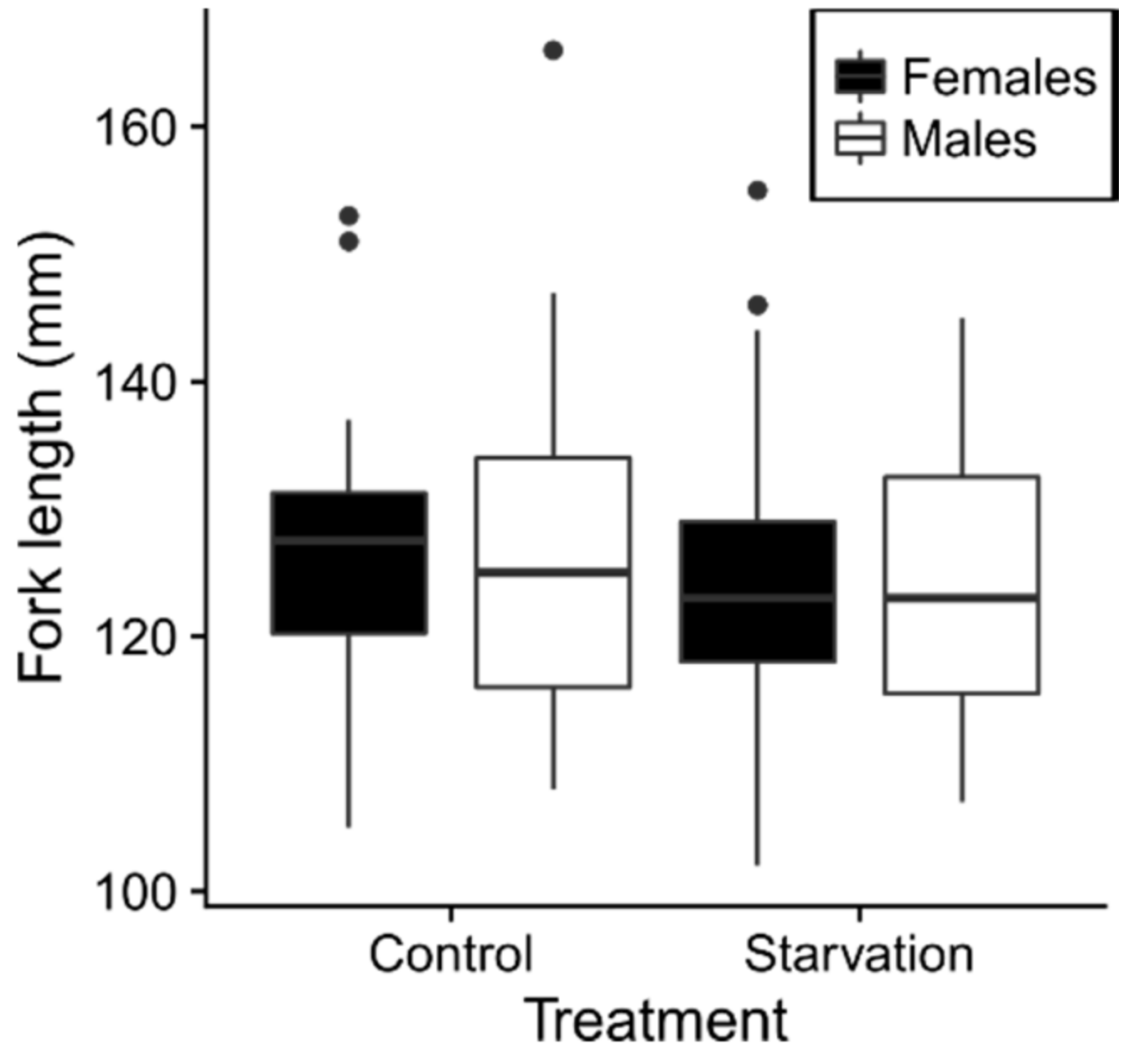
2.1. Body Size at Sampling
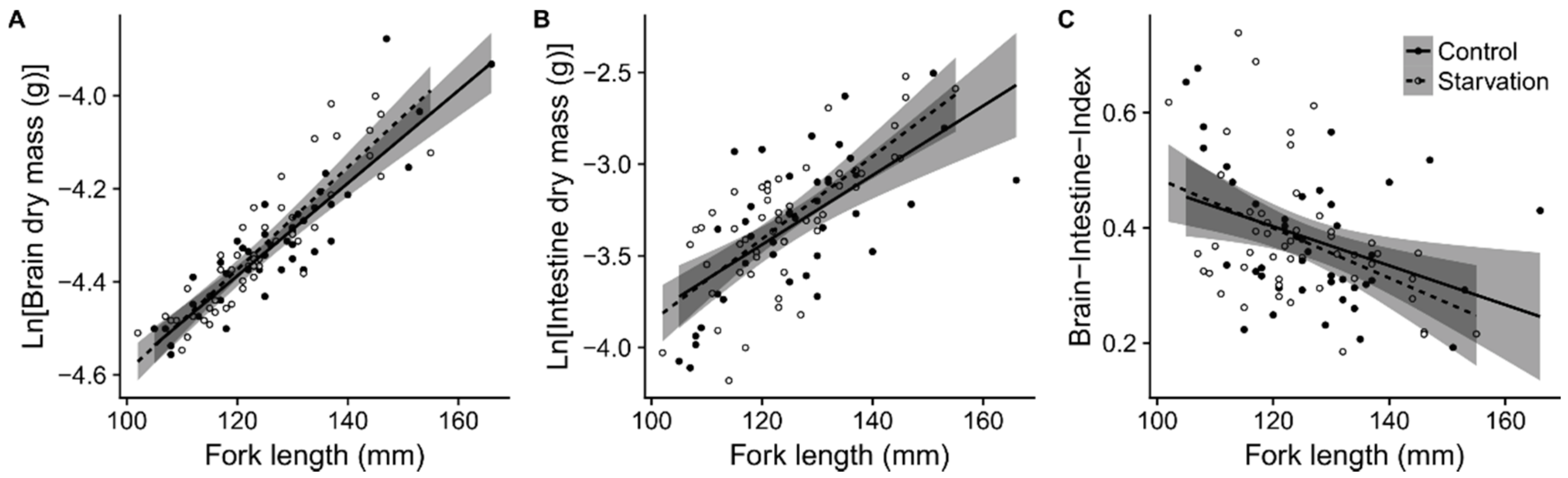
2.2. Effects of Compensatory Growth on Brain and Intestine Mass
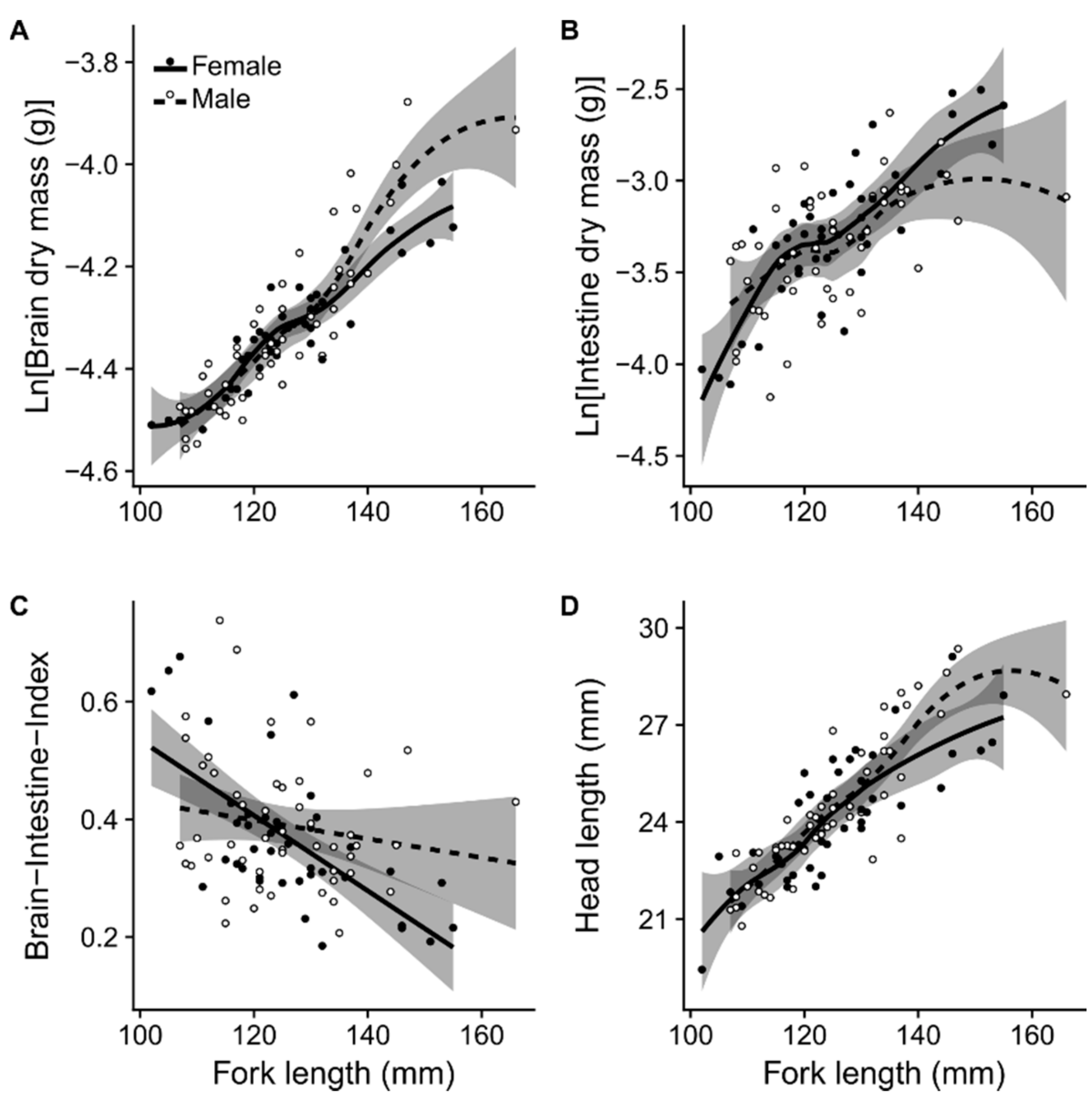
2.3. Effects of Sex on Relative Brain- and Intestine Mass and Head Length
2.4. Effects of Sex in Smolts
3. Discussion
3.1. No Long-Term Effects of Compensatory Growth on Brain or Gut Size
3.2. Emerging Sex Differences, Depending on Sex Bias Within Different Migration Strategies
3.3. Brain and Intestine Size: The Expensive-Tissue Hypothesis
4. Materials and Methods
4.1. Compensatory Growth: Effects on Relative Brain and Intestine Mass
4.1.1. Growth Manipulation
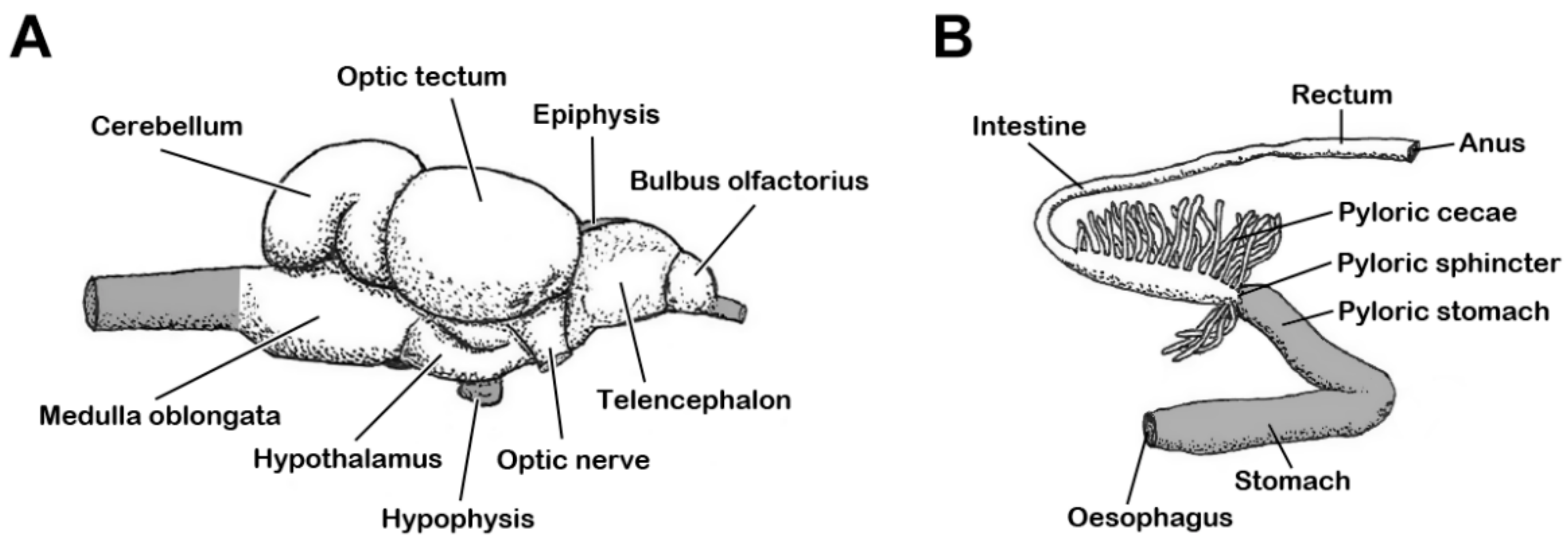
4.1.2. Tissue Sampling and Measurements
4.1.3. Data Notes and Comments
4.1.4. Statistical Analyses
4.2. Investigating Sex Effects in Smoltified Individuals
4.3. General Notes and Ethical Compliance
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Nijhout, H.F.; Emlen, D.J. Competition among body parts in the development and evolution of insect morphology. Proc. Natl. Acad. Sci. USA 1998, 95, 3685–3689. [Google Scholar] [CrossRef] [PubMed]
- Dmitriew, C.M. The evolution of growth trajectories: What limits growth rate? Biol. Rev. 2011, 86, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Nicieza, A.; Wootton, R.J. Compensatory growth in fishes: A response to growth depression. Fish Fish. 2003, 4, 147–190. [Google Scholar] [CrossRef]
- Johnsson, J.I.; Bohlin, T. Compensatory growth for free? A field experiment on brown trout, Salmo trutta. Oikos 2005, 111, 31–38. [Google Scholar] [CrossRef]
- Arendt, J.; Wilson, D.S.; Stark, E. Scale strength as a cost of rapid growth in sunfish. Oikos 2001, 93, 95–100. [Google Scholar] [CrossRef]
- Arendt, J.D.; Wilson, D.S. Population differences in the onset of cranial ossification in pumpkinseed (Lepomis gibbosus), a potential cost of rapid growth. Can. J. Fish. Aquat. Sci. 2000, 57, 351–356. [Google Scholar] [CrossRef]
- Devlin, R.H.; Vandersteen, W.E.; Uh, M.; Stevens, E.D. Genetically modified growth affects allometry of eye and brain in salmonids. Can. J. Zool. 2012, 90, 193–202. [Google Scholar] [CrossRef]
- Roff, D.A. An allocation model of growth and reproduction in fish. Can. J. Fish. Aquat. Sci. 1983, 40, 1395–1404. [Google Scholar] [CrossRef]
- Aiello, L.C.; Wheeler, P. The expensive-tissue hypothesis: The brain and the digestive system in human and primate evolution. Curr. Anthropol. 1995, 36, 199–221. [Google Scholar] [CrossRef]
- Jensen, A.J. The “Gut index”, a new parameter to measure the gross nutritional state of arctic char, Salvelinus alpinus (L.) and brown trout, Salmo trutta L. J. Fish Biol. 1980, 17, 741–747. [Google Scholar] [CrossRef]
- Schmidt-Nielsen, K. Scaling: Why Is Animal Size so Important? Cambridge University Press: Cambridge, UK, 1984; ISBN 978-0521319874. [Google Scholar]
- Rolfe, D.F.; Brown, G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997, 77, 731–758. [Google Scholar] [CrossRef] [PubMed]
- Brijs, J.; Gräns, A.; Hjelmstedt, P.; Sandblom, E.; van Nuland, N.; Berg, C.; Axelsson, M. In vivo aerobic metabolism of the rainbow trout gut and the effects of an acute temperature increase and stress event. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.L.; Iglesias, T.L. No evidence for the “expensive-tissue hypothesis” from an intraspecific study in a highly variable species. J. Evol. Biol. 2012, 25, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Kotrschal, A.; Rogell, B.; Bundsen, A.; Svensson, B.; Zajitschek, S.; Brännström, I.; Immler, S.; Maklakov, A.A.; Kolm, N. Artificial selection on relative brain size in the guppy reveals costs and benefits of evolving a larger brain. Curr. Biol. 2013, 23, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.B.; Lou, S.L.; Zeng, Y.; Kotrschal, A. Large brains, small guts: The expensive tissue hypothesis supported within anurans. Am. Nat. 2016, 188, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, C.Q.; Liao, W.B. Evidence for neither the compensation hypothesis nor the expensive-tissue hypothesis in Carassius auratus. Anim. Biol. 2014, 64, 177–187. [Google Scholar] [CrossRef]
- Tsuboi, M.; Husby, A.; Kotrschal, A.; Hayward, A.; Büchel, S.; Zidar, J.; Løvlie, H.; Kolm, N. Comparative support for the expensive tissue hypothesis: Big brains are correlated with smaller gut and greater parental investment in Lake Tanganyika cichlids. Evolution 2015, 69, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, M.; Shoji, J.; Sogabe, A.; Ahnesjö, I.; Kolm, N. Within species support for the expensive tissue hypothesis: A negative association between brain size and visceral fat storage in females of the Pacific seaweed pipefish. Ecol. Evol. 2016, 6, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Zera, A.J.; Harshman, L.G. The physiology of life history trade-offs in animals. Annu. Rev. Ecol. Syst. 2001, 32, 95–126. [Google Scholar] [CrossRef]
- Isler, K.; van Schaik, C.P. Metabolic costs of brain size evolution. Biol. Lett. 2006, 2, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, N.W.; Montgomery, J.C. Uncoupling of visual and somatic growth in the rainbow trout Oncorhynchus mykiss. Brain Behav. Evol. 1994, 44, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Rios, F.S.; Kalinin, A.L.; Fernandes, M.N.; Rantin, F.T. Changes in gut gross morphology of traíra, Hoplias malabaricus (Teleostei, Erythrinidae) during long-term starvation and after refeeding. Braz. J. Biol. 2004, 64, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.D.; Devlin, R.H. Gut size in GH-transgenic coho salmon is enhanced by both the GH transgene and increased food intake. J. Fish Biol. 2005, 66, 1633–1648. [Google Scholar] [CrossRef]
- Wang, T.; Hung, C.C.Y.; Randall, D.J. The comparative physiology of food deprivation: From feast to famine. Annu. Rev. Physiol. 2006, 68, 223–251. [Google Scholar] [CrossRef] [PubMed]
- Kotrschal, A.; Szidat, S.; Taborsky, B. Developmental plasticity of growth and digestive efficiency in dependence of early-life food availability. Funct. Ecol. 2014, 28, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Kotrschal, A.; Trombley, S.; Rogell, B.; Brannström, I.; Foconi, E.; Schmitz, M.; Kolm, N. The mating brain: Early maturing sneaker males maintain investment into the brain also under fast body growth in Atlantic salmon (Salmo salar). Evol. Ecol. 2014, 28, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Wiper, M.L.; Britton, S.; Higgs, D.M. Early experience and reproductive morph both affect brain morphology in adult male Chinook salmon (Oncorhynchus tshawytscha). Can. J. Fish. Aquat. Sci. 2014, 71, 1430–1436. [Google Scholar] [CrossRef]
- Walsh, M.R.; Broyles, W.; Beston, S.M.; Munch, S.B. Predator-driven brain size evolution in natural populations of Trinidadian killifish (Rivulus hartii). Proc. R. Soc. B 2016, 283, 20161075. [Google Scholar] [CrossRef] [PubMed]
- Näslund, J.; Larsen, M.H.; Thomassen, S.T.; Aarestrup, K.; Johnsson, J.I. Environment-dependent plasticity and ontogenetic changes in the brain of hatchery-reared Atlantic salmon. J. Zool. 2017, 301, 75–82. [Google Scholar] [CrossRef]
- Näslund, J.; Pauliny, A.; Blomqvist, D.; Johnsson, J.I. Telomere dynamics in wild brown trout: Effects of compensatory growth and early growth investment. Oecologia 2015, 177, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Park, P.J.; Bell, M.A. Variation of telencephalon morphology of the threespine stickleback (Gasterosteus aculeatus) in relation to inferred ecology. J. Evol. Biol. 2010, 23, 1261–1277. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.S.; Andrade, R.; Carneiro, L.A.; Gonçalves, E.J.; Kotrschal, K.; Oliveira, R.F. Sex differences in the dorsolateral telencephalon correlate with home range size in blenniid fish. Brain Behav. Evol. 2011, 77, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Kotrschal, A.; Räsänen, K.; Kristjánsson, B.K.; Senn, M.; Kolm, N. Extreme sexual brain size dimorphism in sticklebacks: A consequence of the cognitive challenges of sex and parenting? PLoS ONE 2012, 7, e30055. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Mirbach, T.; Eifert, C.; Riesch, R.; Farnworth, M.S.; Zimmer, C.; Bierbach, D.; Klaus, S.; Tobler, M.; Streit, B.; Indy, J.R.; et al. Toxic hydrogen sulphide shapes brain anatomy: A comparative study of sulphide-adapted ecotypes in the Poecilia mexicana complex. J. Zool. 2016, 300, 163–176. [Google Scholar] [CrossRef]
- Kolm, N.; Gonzalez-Voyer, A.; Brelin, D.; Winberg, S. Evidence for small scale variation in the vertebrate brain: Mating strategy and sex affect brain size and structure in wild brown trout (Salmo trutta). J. Evol. Biol. 2009, 22, 2524–2531. [Google Scholar] [CrossRef] [PubMed]
- Folmar, L.C.; Dickhoff, W.W. The parr—Smolt transformation (smoltification) and seawater adaptation in salmonids. Aquaculture 1980, 21, 1–37. [Google Scholar] [CrossRef]
- Näslund, J. The Pace of Life of Brown Trout–Inter-and Intra-Individual Variation in Growth and Behaviour. Ph.D. Thesis, University of Gothenburg, Gothenburg, Sweden, 2015. [Google Scholar]
- Dellefors, C.; Faremo, U. Early sexual maturation in males of wild sea trout, Salmo trutta L., inhibits smoltification. J. Fish Biol. 1988, 33, 741–749. [Google Scholar] [CrossRef]
- Bohlin, T.; Dellefors, C.; Faremo, U. Probability of first sexual maturation of male parr in wild sea-run brown trout (Salmo trutta) depends on condition factor 1 yr in advance. Can. J. Fish. Aquat. Sci. 1994, 51, 1920–1926. [Google Scholar] [CrossRef]
- Bohlin, T.; Dellefors, C.; Faremo, U. Date of smolt migration depends on body-size but not age in wild sea-run brown trout. J. Fish Biol. 1996, 49, 157–164. [Google Scholar] [CrossRef]
- Ebbesson, L.O.E.; Braithwaite, V.A. Environmental effects on fish neural plasticity and cognition. J. Fish Biol. 2012, 81, 2151–2174. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, J.C.E. Incidence of Stream Resident Females in Anadromous Brown Trout (Salmo trutta L.) Populations in Small Streams on the Swedish West Coast. Ph.D. Thesis, Göteborg University, Gothenburg, Sweden, 2002. [Google Scholar]
- Jonsson, B.; Jonsson, N. Ecology of Atlantic Salmon and Brown Trout. Habitat as a Template for Life Histories; Springer Science+Business Media, Inc.: Heidelberg, Germany, 2011; ISBN 978-94-007-1189-1. [Google Scholar]
- Nielsen, C.; Aarestrup, K.; Nørum, U.; Madsen, S.S. Pre-migratory differentiation of wild brown trout into migrant and resident individuals. J. Fish Biol. 2003, 63, 1184–1196. [Google Scholar] [CrossRef]
- Fraser, T.W.K.; Fjelldal, P.G.; Skjæraasen, J.E.; Hansen, T.; Mayer, I. Triploidy alters brain morphology in pre-smolt Atlantic salmon Salmo salar: Possible implications for behaviour. J. Fish Biol. 2012, 81, 2199–2212. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.L.; Bryant, M.J. Intestine length in the fishes of a tropical stream: 1. Ontogenetic allometry. Environ. Biol. Fish. 1995, 42, 115–127. [Google Scholar] [CrossRef]
- Aldvén, D.; Davidsen, J.G. Marine migrations of sea trout (Salmo trutta). In Sea Trout: Science & Management. Proceedings of the 2nd International Sea Trout Symposium; Harris, G., Ed.; Troubador Publishing Ltd./Matador: Leicester, UK, 2017; pp. 267–276. ISBN 9781788035354. [Google Scholar]
- Johnsson, J.I.; Näslund, J. Studying behavioural variation in salmonids from an ecological perspective: Observations questions methodological considerations. Rev. Fish Biol. Fish. 2018, in press. [Google Scholar] [CrossRef]
- Olsson, R. Kordatzoologi; Almqvist & Wiksell: Stockholm, Sweden, 1971; ISBN 91-20-04499-2. [Google Scholar]
- Meek, J.; Nieuwenhuys, R. Holosteans and teleosts. In The Central Nervous System of Vertebrates; Nieuwenhuys, R., ten Donkelaar, H.J., Nicholson, C., Eds.; Springer Verlag: Heidelberg, Germany, 1998; Volume 2, pp. 759–937. [Google Scholar]
- Burnstock, G. The morphology of the gut of the brown trout (Salmo trutta). Q. J. Microsc. Sci. 1959, 100, 183–198. [Google Scholar]
- Näslund, J.; Sundström, L.F.; Johnsson, J.I. Autumn food restriction reduces smoltification rate, but not over-winter survival, in juvenile brown trout Salmo trutta. Ecol. Freshw. Fish 2017, 26, 205–216. [Google Scholar] [CrossRef]
- Rosengren, M.; Kvingedal, E.; Näslund, J.; Johnsson, J.I.; Sundell, K. Born to be wild: Effects of rearing density and environmental enrichment on stress, welfare, and smolt migration in hatchery-reared Atlantic salmon. Can. J. Fish. Aquat. Sci. 2017, 74, 396–405. [Google Scholar] [CrossRef]
| Estimate | SE | t-Value | p-Value | |
|---|---|---|---|---|
| Intercept | −5.4702 | 0.1023 | −53.46 | <0.0001 |
| Length | 0.0090 | 0.0008 | 11.25 | <0.0001 |
| Sex (Male) | −0.3300 | 0.1368 | −2.41 | 0.018 |
| Treatment (Starved) | 0.0194 | 0.0133 | 1.45 | 0.149 |
| Length × Sex (Male) | 0.0027 | 0.0011 | 2.52 | 0.014 |
| Estimate | SE | t-Value | p-Value | |
|---|---|---|---|---|
| Intercept | −6.6471 | 0.4087 | −16.27 | <0.0001 |
| Length | 0.0265 | 0.0032 | 8.27 | <0.0001 |
| Sex (Male) | 1.3720 | 0.5550 | 2.47 | 0.015 |
| Treatment (Starved) | 0.0474 | 0.0541 | 0.88 | 0.384 |
| Length × Sex (Male) | −0.0113 | 0.0044 | −2.57 | 0.012 |
| Estimate | SE | t-Value | p-Value | |
|---|---|---|---|---|
| Intercept | 1.1841 | 0.1712 | 6.92 | <0.0001 |
| Length | −0.0064 | 0.0013 | −4.81 | <0.0001 |
| Sex (Male) | −0.5864 | 0.2288 | −2.56 | 0.012 |
| Treatment (Starved) | −0.0080 | 0.0222 | −0.36 | 0.719 |
| Length × Sex (Male) | 0.0048 | 0.0018 | 2.66 | 0.009 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Näslund, J. Relative Mass of Brain- and Intestinal Tissue in Juvenile Brown Trout: No Long-Term Effects of Compensatory Growth; with Additional Notes on Emerging Sex-Differences. Fishes 2018, 3, 38. https://doi.org/10.3390/fishes3040038
Näslund J. Relative Mass of Brain- and Intestinal Tissue in Juvenile Brown Trout: No Long-Term Effects of Compensatory Growth; with Additional Notes on Emerging Sex-Differences. Fishes. 2018; 3(4):38. https://doi.org/10.3390/fishes3040038
Chicago/Turabian StyleNäslund, Joacim. 2018. "Relative Mass of Brain- and Intestinal Tissue in Juvenile Brown Trout: No Long-Term Effects of Compensatory Growth; with Additional Notes on Emerging Sex-Differences" Fishes 3, no. 4: 38. https://doi.org/10.3390/fishes3040038
APA StyleNäslund, J. (2018). Relative Mass of Brain- and Intestinal Tissue in Juvenile Brown Trout: No Long-Term Effects of Compensatory Growth; with Additional Notes on Emerging Sex-Differences. Fishes, 3(4), 38. https://doi.org/10.3390/fishes3040038





