Recognition and Distribution of Two North Atlantic Gadiculus Species, G. argenteus and G. thori (Gadidae), Based on Otolith Morphology, Larval Pigmentation, Molecular Evidence, Morphometrics and Meristics
Abstract
1. Introduction
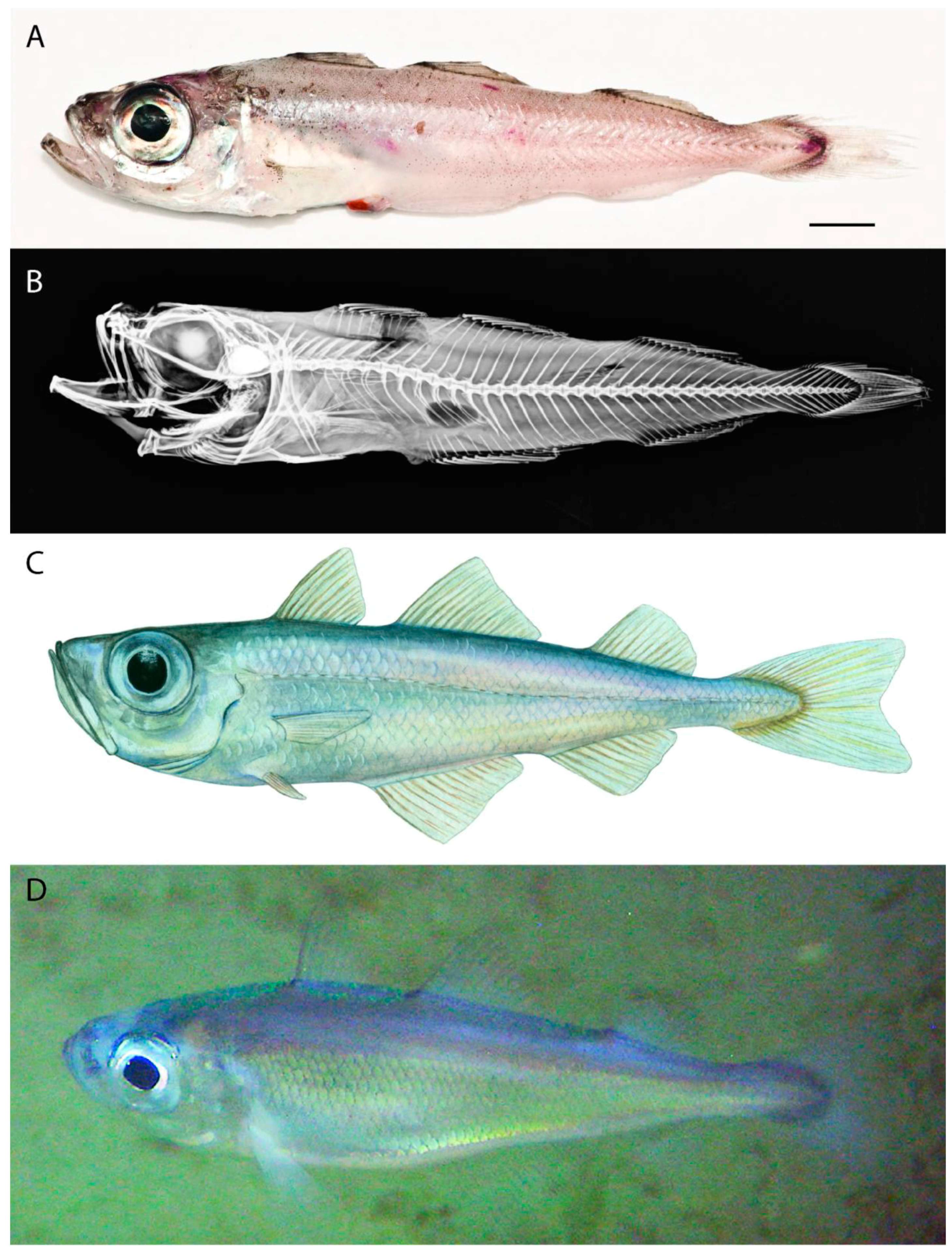
- Different melanophore patterns in post-larvae of the two species. The post-larvae of G. argenteus show three transverse pigmented bars, whereas G. thori only exhibit one (Figure 1).
- At the same stage of development, the post-larvae of G. thori are, in general, larger than those of G. argenteus (Figure 1).
- At the same stage of development, the post-larvae of G. thori are slender compared with G. argenteus, which are stouter and shorter (Figure 1).
- Different number of vertebrae in the two species. Schmidt found that G. thori has 41–43 (usually 42), whereas G. argenteus has 39–41 (usually 40).
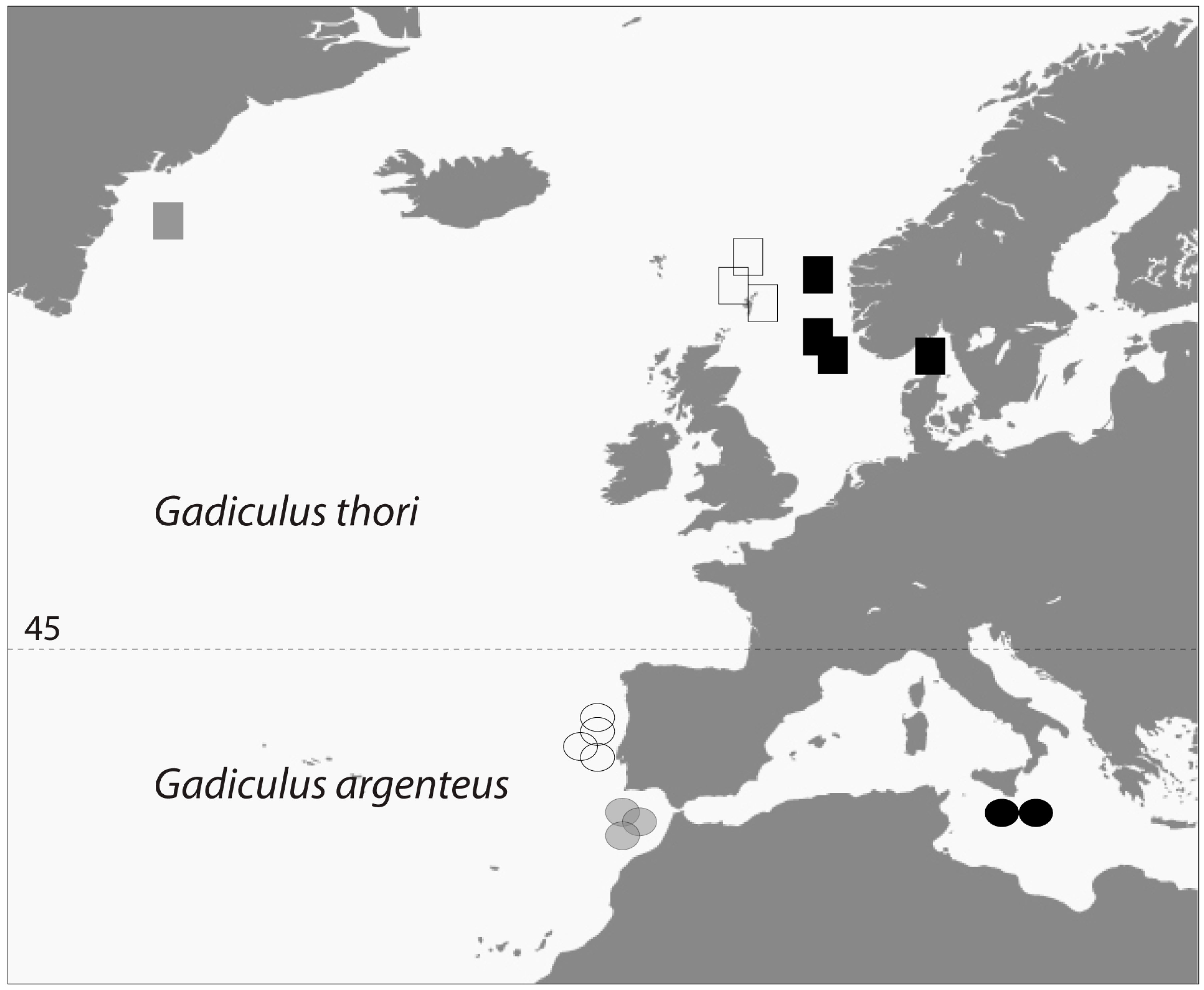
- Geographic distribution. The number of G. thori specimens declines drastically from Ireland in the North to the French Atlantic coast. Conversely, G. argenteus occurs in increasing numbers going south from the mouth of the river Gironde along the Atlantic east coast.
2. Materials and Methods
2.1. Otoliths
2.2. New Record of Gadiculus thori off Greenland
2.3. Molecular Analyses
3. Results
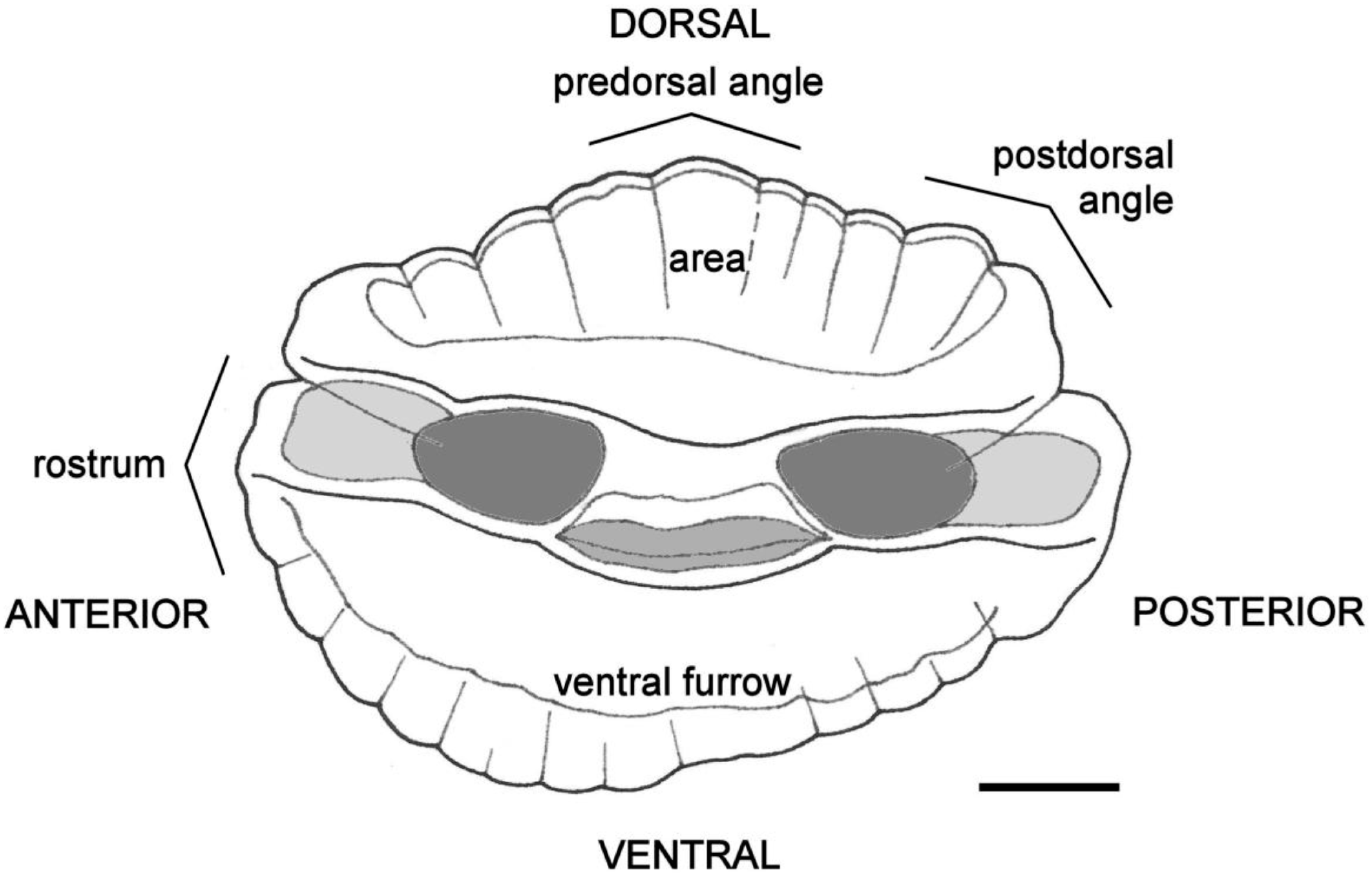
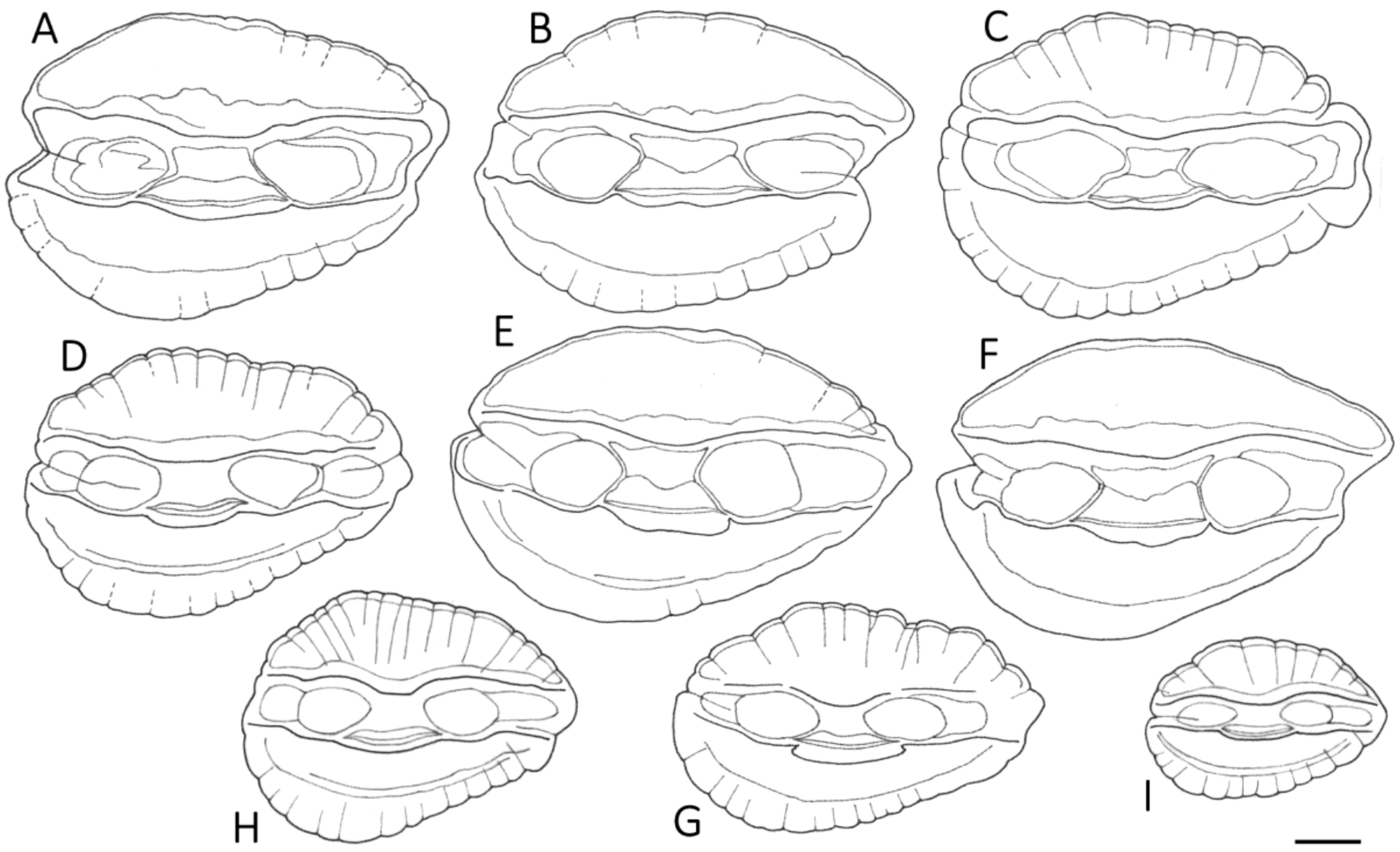
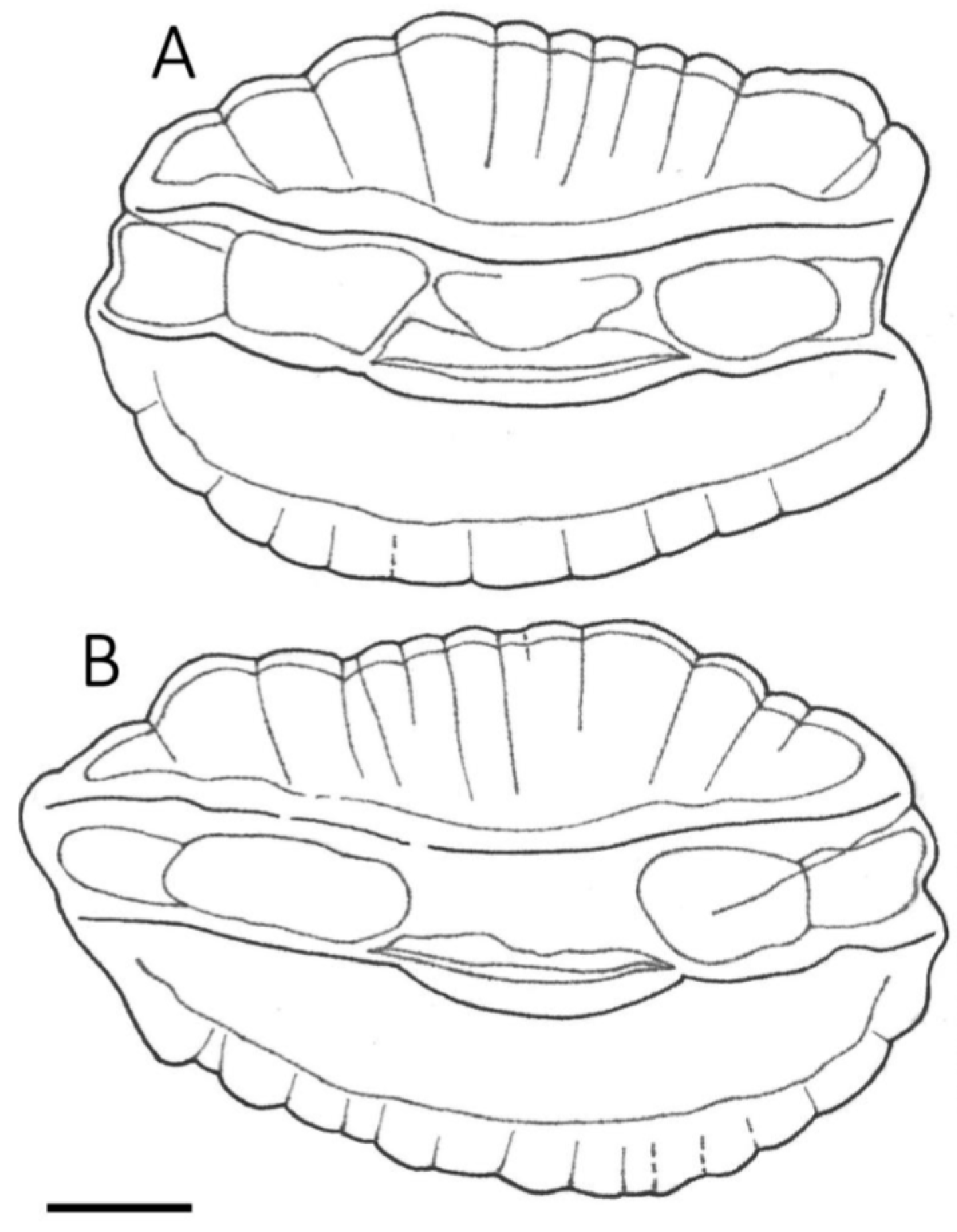
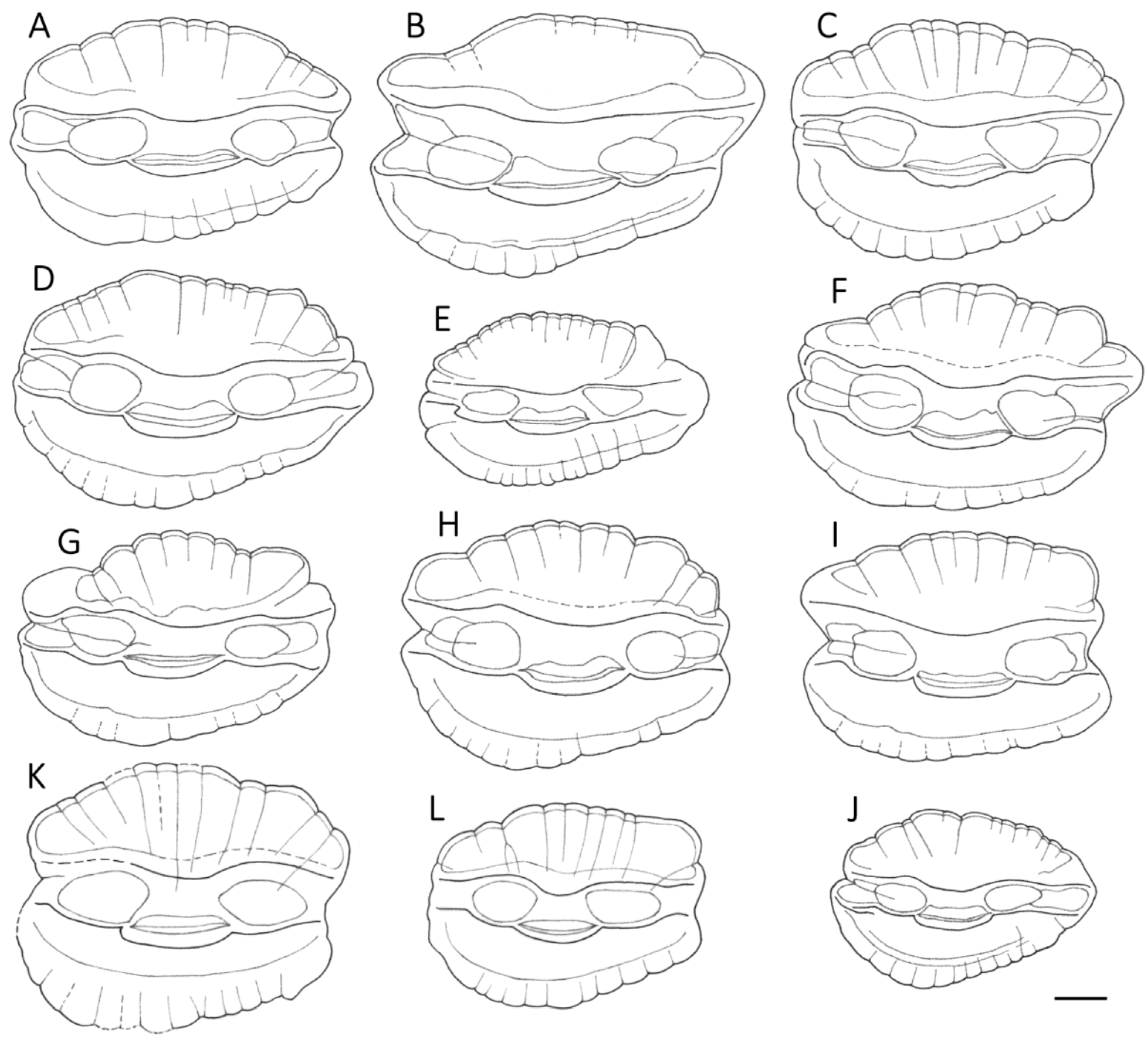
3.1. Otolith Characteristics
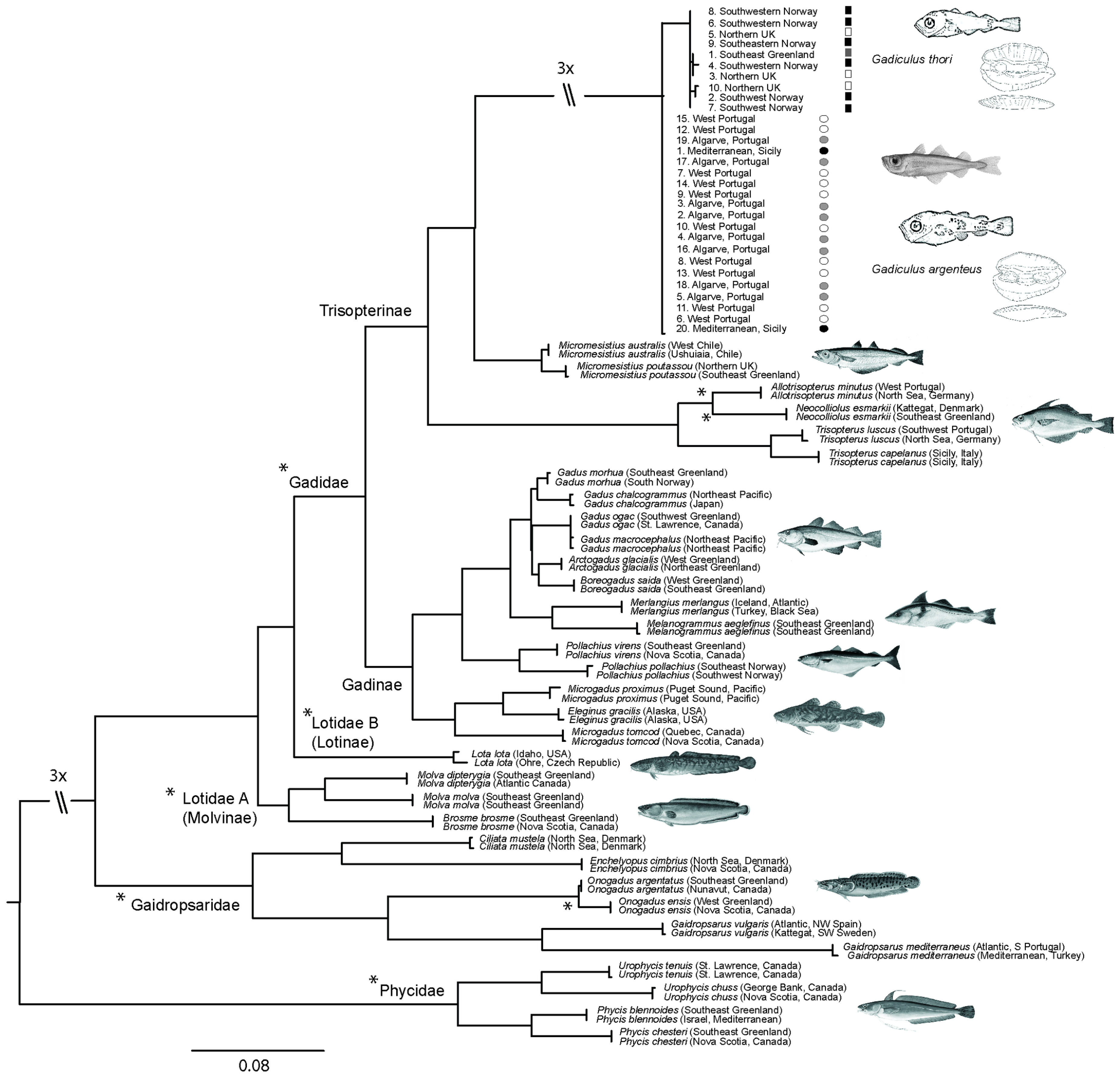
3.2. Molecular Analysis
4. Discussion
4.1. Size and Age
4.2. Morphometric and Meristic Data
- The relatively larger eye in G. argenteus in relation to SL
- The relatively larger head in G. argenteus in relation to SL
- The number of vertebrae in G. thori (39–43)—39 and 40 rarely observed—and G. argenteus (37–41)—37 and 41 rarely observed
- The number of D3 fin rays in G. thori (15–17) and G. argenteus (11–16)
- The number of A1 fin rays in G. thori (15–18)—18 rarely observed—and G. argenteus (11–16)—11 rarely observed
4.3. Post-Larval Pigmentation
4.4. Otolith Morphology
4.5. Molecular Data
4.6. Present Geographic Distributions
4.7. Former Geographic Distributions
4.8. Classification of Codfishes in this Study
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TL | Total fish length in mm |
| TLmax | Largest known total fish length in mm |
| SL | Standard length in mm |
| OL | Otolith length in mm |
| Coll. | Collector |
| ID | Person who originally identified |
| Cox1 | Cytochrome Oxidase 1 |
| K2P | Kimura-2-parameter model |
| T-P spacer | Intergenic non-coding region in all gadiforms between the tRNAs Threonine and Proline |
| ZMUB | Registration number of the Museum of Zoology, University of Bergen, Norway |
References
- Mercader, L.; Vinyoles, D. Révision du statut taxinomique de Gadiculus argenteus thori Schmidt, 1914 (Gadidae). Cybium 2008, 32, 125–130. [Google Scholar]
- Rodríguez-Cabello, C.; Modica, L.; Velasco, F.; Sánchez, F.; Olaso, I. The role of silvery pout (Gadiculus argenteus) as forage prey in the Galician and Cantabrian Sea ecosystem (NE Atlantic) in the last two decades. J. Exp. Mar. Biol. Ecol. 2014, 461, 193–200. [Google Scholar] [CrossRef]
- Mattson, S. The food of Galeus. melastomus, Gadiculus argenteus thori, Trisopterus. esmarkii, Rhinonemus. cimbrius, and Glyptocephalus. cynoglossus (Pisces) caught during the day with shrimp trawl in a West-Norwegian fjord. Sarsia 1981, 66, 109–127. [Google Scholar] [CrossRef]
- Mauchline, J.; Gordon, J.D.M. Feeding and bathymetric distribution of the gadoid and morid fish of the Rockall Trough. J. Mar. Biol. Assoc. UK 1984, 64, 657–665. [Google Scholar] [CrossRef]
- Albert, O.T. Distribution, population structure and diet of silvery pout (Gadiculus argenteus thori J. Schmidt), poor cod (Trisopterus. minutus minutus (L.)), four-bearded rockling (Rhinonemus. cimbrius (L.)), and Vahl’s eelpout (Lycodes. vahlii gracilis Reinhardt) in the Norwegian Deep. Sarsia 1993, 78, 141–154. [Google Scholar]
- Heessen, H.J.L.; Daan, N.; Ellis, J.R. Fish Atlas of the Celtic Sea, North. Sea and Baltic Sea: Based on International Research Vessel Data; Koninklijke Nederlandse Natuurhistorische Vereniging Publishing: Zeist, The Netherlands, 2015; 572p. [Google Scholar]
- Biagi, F.; Sartor, P.; Ardizzone, G.D.; Belcari, P.; Belluscio, A.; Serena, F. Analysis of demersal assemblages off the Tuscany and Latium coasts (north-western Mediterranean). Sci. Mar. 2002, 66 (Suppl. 2), 233–242. [Google Scholar] [CrossRef]
- Byrkjedal, I.; Høines, Å. Distribution of demersal fish in the south-western Barents Sea. Polar Res. 2007, 26, 135–151. [Google Scholar] [CrossRef]
- Cohen, D.M.; Inada, T.; Iwamoto, T.; Scialabba, N. FAO Species Catalogue, Volume 10. Gadiform fishes of the world (Order Gadiformes). An annotated and illustrated catalogue of cods, hakes, grenadiers and other gadiform fishes known to date. FAO Fish. Synop. 1990, 125, 1–442. [Google Scholar]
- Jukic-Peladic, S.; Vrgoc, N.; Krstulovic-Sifner, S.; Piccinetti, C.; Piccinetti-Manfrin, G.; Marano, G.; Ungaro, N. Long-term changes in demersal resources of the Adriatic Sea: Comparison between trawl surveys carried out in 1948 and 1998. Fish. Res. 2001, 53, 95–104. [Google Scholar] [CrossRef]
- Labropoulou, M.; Papaconstantinou, C. Community structure of deep-sea demersal fish in the North Aegean Sea (northeastern Mediterranean). Hydrobiology 2000, 440, 281–296. [Google Scholar] [CrossRef]
- Bilecenoglu, M.; Taskavak, E.; Mater, S.; Kaya, M. Checklist of the marine fishes of Turkey. Zootaxa 2002, 113, 1–194. [Google Scholar] [CrossRef]
- Saad, A. Check-list of bony fish collected from the coast of Syria. Turk. J. Fish. Aquat. Sci. 2005, 5, 99–106. [Google Scholar]
- Galil, B.; Goren, M.; Mienis, H. Checklist of marine species in Israel. In Compiled in the Framework of the EU FP7 PESI Project; Available online: http://www.marinespecies.org/aphia.php?p=sourcedetails&id=149096 (accessed on 14 December 2016).
- Guichenot, A. Histoire naturelle des reptiles et des poissons. In Exploration Scientifique de l’Algérie Pendant les Années 1840–1842. Sciences Physiques, Zoologie; Oxford University: Oxford, UK, 1850. [Google Scholar]
- Svetovidov, A.N. Gadidae. In Fishes of the North-eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.-L., Hureau, J.-C., Nielsen, J., Tortonese, E., Eds.; UNESCO: France, Paris, 1986; Volume 2, pp. 680–710. [Google Scholar]
- Schmidt, J. Gadiculus Argenteus and Gadiculus Thori. Mindeskrift. I Anledning af Hundredaaret for Japetus. Steenstrups. Fødsel; En kreds af Naturforskere: Copenhagen, Denmark, 1914; Volume 14, pp. 1–10. [Google Scholar]
- Svetovidov, A.N. Fauna SSSR: Ryby, vol. 9, fasc. 4; Treskoobraznye [Gadiformes]; Zoologicheskii Institut Akademii Nauk SSSR: Moscow-Leningrad, Russia, 1948; 294p. [Google Scholar]
- Chaine, J.; Duvergier, J. Recherches sur les otolithes des poisons. Étude descriptive et comparative de la sagitta des téléostéens. Actes Soc. Linn. Bordx. 1934, 86, 1–254. [Google Scholar]
- Schwarzhans, W. Otolith-morphology and its usage for higher systematical units, with special reference to the Myctophiformes s.l. Meded. Werkgr. Tert. Kwart. Geol. 1978, 15, 167–185. [Google Scholar]
- Gaemers, P.A.M. Taxonomic position of the Cichlidae (Pisces, Perciformes) as demonstrated by the morphology of their otoliths. Neth. J. Zool. 1984, 34, 566–595. [Google Scholar] [CrossRef]
- Nolf, D. The Diversity of Fish Otoliths, Past and Present; Royal Belgian Institute of Natural Sciences: Brussels, Belgium, 2013; 222p. [Google Scholar]
- Raitt, D.F.S. A comparison of Gadiculus from Scottish and Mediterranean waters. J. Mar. Biol. Assoc. UK 1964, 44, 693–709. [Google Scholar] [CrossRef]
- Poulsen, J.Y.; Thorkildsen, S.; Arboe, N.H. Identification keys to halosaurs and notacanthids (Notacanthiformes, Elopomorpha) in the subarctic Atlantic Ocean including three new distributional records and multiple molecular OTUs of Notacanthus. cf. chemnitzii. Mar. Biodivers. 2017. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System (www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.P.; Paulay, G. DNA barcoding: Error rates based on comprehensive sampling. PLoS Biol. 2005, 3, e422. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Zhang, G.; Ali, F.; Zamudio, K. The Use of Mean Instead of Smallest Interspecific Distances Exaggerates the Size of the “Barcoding Gap” and Leads to Misidentification. Syst. Biol. 2008, 57, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, J.Y.; Sado, T.; Hahn, C.; Bykjedal, I.; Moku, M.; Miya, M. Preservation Obscures Pelagic Deep-Sea Fish Diversity: Doubling the Number of Sole-Bearing Opisthoproctids and Resurrection of the Genus Monacoa. (Opisthoproctidae, Argentiniformes). PLoS ONE 2016, 11, e0159762. [Google Scholar] [CrossRef] [PubMed]
- McCusker, M.R.; Denti, D.; Van Guelpen, L.; Kenchington, E.; Bentzen, P. Barcoding Atlantic Canada’s commonly encountered marine fishes. Mol. Ecol. Resour. 2012, 13, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Knebelsberger, T.; Landi, M.; Neumann, H.; Kloppmann, M.; Sell, A.F.; Campbell, P.D.; Laakmann, S.; Raupach, M.J.; Carvalho, G.R.; Costa, F.O. A reliable DNA barcode reference library for the identification of the North European shelf fish fauna. Mol. Ecol. Resour. 2014, 14, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.O.; Landi, M.; Martins, R.; Costa, M.H.; Costa, M.E.; Carneiro, M.; Alves, M.J.; Steinke, D.; Carvalho, G.R. A ranking system for reference libraries of DNA barcodes: Application to marine fish species from Portugal. PLoS ONE 2012, 7, e35858. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.; Dimech, M.; Arculeo, M.; Biondo, G.; Martins, R.; Carneiro, M.; Carvalho, G.R.; Brutto, S.L.; Costa, F.O. DNA barcoding for species assignment: The case of Mediterranean marine fishes. PLoS ONE 2014, 9, e106135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.B.; Hanner, R. DNA barcoding is a useful tool for the identification of marine fishes from Japan. Biochem. Syst. Ecol. 2011, 39, 31–42. [Google Scholar] [CrossRef]
- Kochzius, M.; Seidel, C.; Antoniou, A.; Botla, S.K.; Campo, D.; Cariani, A.; Vasquez, E.G.; Hauschild, J.; Hervet, C.; Hjörleifsdottir, S.; et al. Identifying fishes through DNA barcodes and microarrays. PLoS ONE 2010, 5, e12620. [Google Scholar] [CrossRef] [PubMed]
- Hubert, N.; Hanner, R.; Holm, E.; Mandrak, N.E.; Taylor, E.; Burridge, M.; Watkinson, D.; Dumont, P.; Curry, A.; Bentzen, P.; et al. Identifying Canadian freshwater fishes through DNA barcodes. PLoS ONE 2008, 3, e2490. [Google Scholar] [CrossRef] [PubMed]
- Gaemers, P.A.M. New gadiform otoliths from the tertiary of the North Sea basin and a revision of some fossil and recent species. Leidse. Geol. Meded. 1976, 49, 507–537. [Google Scholar]
- Gaemers, P.A.M. New concepts in the evolution of the Gadidae (Vertebrata, Pisces), based on their otoliths. Meded. Werkgr. Tert. Kwart. Geol. 1976, 13, 3–32. [Google Scholar]
- Girone, A.; Nolf, D.; Cappetta, H. Pleistocene fish otoliths from the Mediterranean Basin: A synthesis. Geobios 2006, 39, 651–671. [Google Scholar] [CrossRef]
- Bakke, I.; Shields, G.F.; Johansen, S. Sequence characterization of a unique intergenic spacer in Gadiformes mitochondrial DNA. Mar. Biotechnol. 1999, 1, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Gaemers, P.A.M. Taxonomy, distribution and evolution of trisopterine Gadidae by means of otoliths and other characteristics. Fishes 2017, 1, 18. [Google Scholar] [CrossRef]
- Daan, N.; (formerly Rijksinstituut voor Visserijonderzoek, R.I.V.O., IJmuiden, Netherlands). Personal communication, 2016.
- Stergiou, K.I.; Politou, C.-Y. Biological parameters, body length-weight and length-height relationships for various species in Greek waters. Naga ICLARM Q. 1995, 18, 42–45. [Google Scholar]
- Tuset, V.M.; Lombarte, A.; Assis, C.A. Otolith atlas for the western Mediterranean, north and central eastern Atlantic. Sci. Mar. 2008, 72 (Suppl. 1), 7–198. [Google Scholar] [CrossRef]
- Vassilopoulou, V. Preliminary biological data on silvery pout (Gadiculus argenteus argenteus) in the northern Euboian Gulf (Greece). Rapp. Comm. Int. Mer Médit. 1990, 32, 272. [Google Scholar]
- Bilge, G.; Filiz, H.; Gülşahin, A. Age and growth of Gadiculus argenteus Guichenot, 1850, in the southern Aegean Sea. In Proceedings of the 11th Panhellenic Symposium on Oceanography and Fisheries, Mytilene, Lesvos Island, Greece, 13–17 May 2015; Volume 2015, pp. 61–64. [Google Scholar]
- Giovanardi, O.; Rizzoli, M. Biological data, collected during expeditions Pipeta, on the whiting, Merlangius. merlangius (L.) in the Adriatic Sea. FAO Fish. Rep. 1984, 290, 149–153. [Google Scholar]
- Milić, D.; Kraljević, M. Biometry analysis of the whiting, Merlangius. merlangus (Linnaeus, 1758) from the northern Adriatic Sea. Acta Adriat. 2011, 52, 125–136. [Google Scholar]
- Svetovidov, A.N. Fauna of the U.S.S.R., Fishes, Gadiformes Zoologicheskii Institut, Akademii Nauk SSSR; Israel Program for Scientific Translations: Jerusalem, Israel, 1962; Volume 9, 304p. [Google Scholar]
- Hodges, N.D.C. Relations of temperature to vertebrae among fishes. Science 1891, 18, 104–107. [Google Scholar]
- Wheeler, A.; Jones, A.K.G. Fishes. In Cambridge Manuals in Archaeology; Cambridge University Press: Cambridge, UK, 1989; 210p. [Google Scholar]
- McDowall, R.M. Jordan’s and other ecogeographical rules, and the vertebral number in fishes. J. Biogeogr. 2008, 35, 501–508. [Google Scholar] [CrossRef]
- Halbeisen, H.W.; Schöfer, W. Bestimmungsschlüssel für Fischlarven der Nordsee und angrenzender Gebiete. In Berichte aus dem Institut für Meereskunde an der Christian-Albrechts-Universität Kiel; Christian-Albrechts-Universität Kiel: Kiel, Germany, 1988; Volume 178, 76p. [Google Scholar]
- Izeta, L.M. The larval development of the southern silvery pout Gadiculus argenteus argenteus Guichenot (1850). J. Plankton Res. 1985, 7, 937–946. [Google Scholar] [CrossRef]
- Fahay, M.P. Gadidae, Merlucciidae, Moridae, Bregmacerotidae, Macrouridae. J. Northwest Atl. Fish. Sci. 1983, 4, 168–189. [Google Scholar]
- Fahay, M.P. Early Stages of Fishes in the Western North. Atlantic Ocean. (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras). In Volume 1: Acipenseriformes through Syngnathiformes; Northwest Atlantic Fisheries Organization: Dartmouth, NS, Canada, 2007; 931p. [Google Scholar]
- Gaemers, P.A.M. The definition of the classical Palaeogene-Neogene boundary in the North Sea Basin by means of Gadidae otoliths (Pisces). Tert. Res. 1990, 11, 97–144. [Google Scholar]
- Gaemers, P.A.M. The biostratigraphy of the Nieder Ochtenhausen borehole based on Gadidae otoliths and other fish remains, and the establishment of the Elbian, a new stage for the latest Late Miocene of NW Europe. Geol. Jahrb. A 2001, 152, 301–339. [Google Scholar]
- Schwarzhans, W. Die Fisch-Otolithen aus dem Oberoligozän der Niederrheinischen Bucht. Systematik, Palökologie, Paläobiogeographie, Biostratigraphie und Otolithen-Zonierung. Geol. Jahrb. A 1994, 140. [Google Scholar]
- Schwarzhans, W. The Otoliths from the Miocene of the North. Sea Basin; Backhuys Publishers: Leiden, The Netherlands; Margraf Publishers: Weikersheim, Germany, 2010. [Google Scholar]
- Teletchea, F.; Laudet, V.; Hänni, C. Phylogeny of the Gadidae (sensu Svetovidov, 1948) based on their morphology and two mitochondrial genes. Mol. Phylogenetics Evol. 2006, 38, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.R. The termination of the Gulf Stream and the beginning of the North Atlantic Current. Deep-Sea Res. 1967, 14, 337–359. [Google Scholar] [CrossRef]
- Godfrey, J.S.; Tomczak, M. Regional Oceanography: An Introduction, 2nd ed.; Daya Publishing House: Delhi, India, 2003; 390p. [Google Scholar]
- Bañón, R.; Arronte, J.C.; Vázquez-Dorado, S.; Río, J.L.D.; Carlos, A.D. DNA barcoding of the genus Lepidion. (Gadiformes: Moridae) with recognition of Lepidion. eques as a junior synonym of Lepidion. lepidion. Mol. Ecol. Resour. 2013, 13, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, K.A.T.; Árnason, E.; Smith, P.J.; Mork, J. Mitochondrial DNA differentiation between the antitropical blue whiting species Micromesistius. poutassou and Micromesistius. australis. J. Fish. Biol. 2012, 81, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.R.; Nielsen, J.G.; Knudsen, S.W.; Poulsen, J.Y.; Sünksen, K.; Jørgensen, O.A. A checklist of the fish fauna of Greenland waters. Zootaxa 2010, 2378, 1–84. [Google Scholar]
- Jónsson, G.; Pálsson, J. Islenskir. Fiskar. (Icelandic Fishes), 2nd ed.; Bókabúđ: Reykjavík, Iceland, 2013; 336p. [Google Scholar]
- MacKenzie, B.R.; Payne, M.R.; Boje, J.; Høyer, J.L.; Siegstad, H. A cascade of warming impacts brings bluefin tuna to Greenland waters. Global. Chang. Biol. 2014, 20, 2484–2491. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, J.Y. Fifth confirmed record and North Atlantic range expansion of the rare pelagic bobtail snipe eel genus Neocyema. (Cyematidae, Elopomorpha). Mar. Biodivers. Rec. 2015, 8, 1–5. [Google Scholar] [CrossRef]
- Poulsen, J.Y. A new species of pencil smelt Nansenia. boreacrassicauda (Microstomatidae, Argentiniformes) from the North Atlantic Ocean. Zootaxa 2015, 4020, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Dolgov, A.V. New data on the distribution of rare and new fish species in Russian waters of the Barents Sea. J. Ichthyol. 2006, 46, 139–147. [Google Scholar] [CrossRef]
- Schwarzhans, W. Die tertiäre Teleosteer-Fauna Neuseelands, rekonstruiert anhand von Otolithen. Berl. Geowiss. Abh. A 1980, 26, 1–211. [Google Scholar]
- Endo, H. Phylogeny of the Order Gadiformes (Teleostei, Paracanthopterygii). Mem. Grad. Sch. Fish. Sci. Hokkaido Univ. 2002, 49, 75–149. [Google Scholar]
- Roa-Varón, A.; Ortí, G. Phylogenetic relationships among families of Gadiformes (Teleostei, Paracanthopterygii) based on nuclear and mitochondrial data. Mol. Phylogen Evol. 2009, 52, 688–704. [Google Scholar] [CrossRef] [PubMed]
- Howes, G.J. Anatomy of the Melanonidae (Teleostei: Gadiformes), with Comments on Its Phylogenetic Relationships; Natural History Museum: London, UK, 1993; Volume 59, pp. 11–31. [Google Scholar]
- De Buen, F. Notas sobre los Gaidropsaridae (Peces). Un nuovo género (Onogadus nov. gen.) y una nueva especie (Gaidropsarus barbatus, nov sp.). Bol. Soc. Españ. Hist. Nat. 1934, 34, 499–504. [Google Scholar]
- Howes, G.J. Anatomy, phylogeny and taxonomy of the gadoid fish genus Macruronus. Günther, 1873, with a revised hypothesis of gadoid phylogeny. Bull. Br. Mus. (Nat. Hist.) Zool. 1991, 51, 77–110. [Google Scholar]
- Howes, G.J. Biogeography of gadoid fishes. J. Biogeogr. 1991, 18, 595–622. [Google Scholar] [CrossRef]
- Gaemers, P.A.M. Fish otoliths from the Rupelian of sand-pit Roelants at Heide-Boskant (municipality of Lubbeek, Belgium) and the stratigraphy of the Early Rupelian, 2. Systematic part. Meded. Werkgr. Tert. Kwart. Geol. 1985, 22, 155–172. [Google Scholar]

| Characteristics | Gadiculus thori |
|---|---|
| TL | 126.2 |
| SL | 115.9 (65.0–135.0) |
| % SL | |
| Head length | 30.6 (26.8–35.7) |
| Pre-dorsal dist. | 31.7 (28.0–40.0) |
| Pre-anal dist. | 44.6 |
| Pre-pelvic dist. | 25.5 |
| Pre-orbital dist. | 9.2 |
| Orbit | 11.2 (8.8–12.6) |
| Inter-orbital dist. | 7.5 |
| 1. Dorsal base | 11.2 |
| 1st–2nd Dorsal dist. | 3.3 (0.9–3.7) |
| 2. Dorsal base | 12.1 |
| 2nd–3rd Dorsal dist. | 5.4 (2.2–6.1) |
| 3. Dorsal base | 12.3 |
| 1. Anal base | 14.4 |
| 1st–2nd Anal dist. | 5.0 (2–6.9) |
| 2. Anal base | 15.1 |
| Body depth | 16.1 (13.8–25.8) |
| Caudal depth | 5.5 |
| Premaxillary length | 12.0 |
| 1. Dorsal fin rays | 10 (9–13) |
| 2. Dorsal fin rays | 11 (10–16) |
| 3. Dorsal fin rays | 17 (15–17) |
| 1. Anal fin rays | 17 (15–18) |
| 2. Anal fin rays | 17 (16–17) |
| Vertebrae | 42 (39–43) |
| Specimens | Record ID BOLD | NCBI | Museum | Region, Country and Year of Sampling | Position | Study |
|---|---|---|---|---|---|---|
| Lotidae | ||||||
| Brosme brosme | GLF058 | LC146711 | ZMUB 21890 | SE Greenland 2013 | 64.25° N, 36.51° E | This study |
| Brosme brosme | SCFAC287-06 | KC015253 | ARC 25650 | SE Canada 2006 | 41.93° N, 65.81° E | [29] |
| Lota lota | ANGBF9234 | GU126680 | - | Idaho, USA 2009 | - | Unpubl. |
| Lota lota | IFCZE0693 | HQ961085 | - | Ohre, Czech Republic 2010 | 50.11° N, 12.40° E | Unpubl. |
| Molva dipterygia | GLF056 | LC146709 | ZMUB 21948 | SE Greenland 2013 | 64.18° N, 36.50° E | This study |
| Molva dipterygia | SCFAC413 | KC015694 | ARC 25589 | Unknown, Canada | - | [29] |
| Molva molva | GLF071 | LC146695 | No voucher | SE Greenland 2013 | 66.50° N, 30.28° E | This study |
| Molva molva | GLF176 | LC146701 | ZMUB 22720 | SE Greenland 2014 | 64.27° N, 37.20° E | This study |
| Gaidropsaridae | ||||||
| Ciliata mustela | BNSFI129 | KJ204805 | MT05378 | NW Germany 2010 | 54.14° N, 07.90° E | [30] |
| Ciliata mustela | BNSFI128 | KJ204804 | MT05377 | NW Germany 2010 | 54.14° N, 07.90° E | [30] |
| Enchelyopus cimbrius | SCAFB093 | KC015336 | ARC 24883 | SE Canada 2005 | 44.94° N, 66.09° E | [29] |
| Enchelyopus cimbrius | BNSFI132 | KJ204840 | MT05365 | NW Germany 2010 | 54.14° N, 07.90° E | [30] |
| Gaidropsarus mediterraneus | FCFPS166 | JQ774626 | MB85-005350 | S Portugal | - | [31] |
| Gaidropsarus mediterraneus | GBGCA10850 | KP136735 | J1Bsex-80 | Turkey | - | Unpubl. |
| Gaidropsarus vulgaris | SFM036 | - | AF0036 | NW Spain 2013 | - | Unpubl. |
| Gaidropsarus vulgaris | GBGCA8490 | KJ128491 | NRM46985 | SW Sweden 2001 | 57.88° N, 11.58° E | Unpubl. |
| Onogadus argentatus | GLF114 | LC146708 | ZMUB 21814 | SE Greenland 2013 | 61.57° N, 40.58° E | This study |
| Onogadus argentatus | SCAFB229 | KC015387 | ARC 26385 | E Canada 2006 | 69.83° N, 65.28° E | [29] |
| Onogadus ensis | SCAFB1182 | KC015394 | ARC 28289 | SE Canada 2007 | 44.02° N, 59.01° E | [29] |
| Onogadus ensis | GLF117 | LC146696 | ZMUC P376048 | W Greenland 2013 | 63.31° N, 56.31° E | This study |
| Phycidae | ||||||
| Phycis blennoides | GLF151 | LC146700 | ZMUB 22773 | SE Greenland 2014 | 61.42° N, 41.04° E | This study |
| Phycis blennoides | BIM338 | - | P. 15193 | W Israel 2013 | 32.27° N, 34.36° E | Unpubl. |
| Phycis chesteri | GLF017 | LC146703 | ZMUC P375728 | SE Greenland 2009 | 62.12° N, 40.29° E | This study |
| Phycis chesteri | SCFAC747 | KC015799 | ARC 25896 | SE Canada 2002 | 42.80° N, 63.19° E | [29] |
| Urophycis chuss | SCFAC720 | KC016017 | ARC 25893 | SE Canada 2006 | 43.03° N, 61.61° E | [29] |
| Urophycis chuss | SCFAC714 | KC016018 | ARC 25697 | SE Canada 2006 | 41.39° N, 66.12° E | [29] |
| Urophycis tenuis | SCFAC522 | KC016033 | ARC 25942 | SE Canada | 48.55° N, 63.07° E | [29] |
| Urophycis tenuis | SCFACB855 | KC016030 | ARC 26827 | SE Canada 2007 | 44.36° N, 66.50° E | [29] |
| Gadidae | ||||||
| Eleginus gracilis | WXYZ007 | - | UW150495 | Alaska, USA 2010 | 60.99° N, 167.34° E | Unpubl. |
| Eleginus gracilis | WXYZ005 | - | UW150494 | Alaska, USA 2010 | 60.99° N, 167.34° E | Unpubl. |
| 1. Gadiculus argenteus | CSFOM036 | KJ709531 | CSFOM-044 | Sicily, Italy | - | [32] |
| 2. Gadiculus argenteus | FCFPS164 | JQ774620 | MB85-005348 | S Portugal | - | [31] |
| 3. Gadiculus argenteus | FCFPS133 | JQ774622 | MB85-005315 | S Portugal | - | [31] |
| 4. Gadiculus argenteus | FCFPS130 | JQ774618 | MB85-005317 | S Portugal | - | [31] |
| 5. Gadiculus argenteus | FCFPS154 | JQ774619 | MB85-005338 | S Portugal | - | [31] |
| 6. Gadiculus argenteus | FCFPW097 | JQ775028 | MB85-010501 | W Portugal 2005 | 40.28° N, 09.59° W | [31] |
| 7. Gadiculus argenteus | FCFPW079 | JQ775027 | MB85-010519 | W Portugal 2005 | 40.18° N, 09.59° W | [31] |
| 8. Gadiculus argenteus | FCFPW078 | JQ775024 | FCFOPB064-03 | W Portugal 2005 | 40.18° N, 09.59° W | [31] |
| 9. Gadiculus argenteus | FCFPW076 | JQ775025 | MB85-010520 | W Portugal 2005 | 40.18° N, 09.59° W | [31] |
| 10. Gadiculus argenteus | FCFPW077 | JQ775026 | MB85-010496 | W Portugal 2005 | 40.18° N, 09.59° W | [31] |
| 11. Gadiculus argenteus | FCFP065 | JQ774831 | MB85-004995 | W Portugal 2005 | 39.08° N, 10.00° W | [31] |
| 12. Gadiculus argenteus | FCFP067 | JQ774828 | MB85-004994 | W Portugal 2005 | 39.08° N, 10.00° W | [31] |
| 13. Gadiculus argenteus | FCFP066 | JQ774829 | MB85-004998 | W Portugal 2005 | 39.08° N, 10.00° W | [31] |
| 14. Gadiculus argenteus | FCFP069 | JQ774830 | MB85-004996 | W Portugal 2005 | 39.08° N, 10.00° W | [31] |
| 15. Gadiculus argenteus | FCFP068 | JQ774832 | MB85-004997 | W Portugal 2005 | 39.08° N, 10.00° W | [31] |
| 16. Gadiculus argenteus | FCFPS065 | JQ774623 | MB85-005249 | S Portugal | - | [31] |
| 17. Gadiculus argenteus | FCFPS132 | JQ774624 | MB85-005314 | S Portugal | - | [31] |
| 18. Gadiculus argenteus | FCFPS131 | JQ774621 | MB85-005318 | S Portugal | - | [31] |
| 19. Gadiculus argenteus | FCFPS134 | JQ774625 | MB85-005316 | S Portugal | - | [31] |
| 20. Gadiculus argenteus | CSFOM091 | KJ709532 | CSFOM-117 | Sicily, Italy | - | [32] |
| 1. Gadiculus thori | GLF136 | LC146704 | ZMUB 21452 | SE Greenland 2012 | 64.19° N, 36.45° W | This study |
| 2. Gadiculus thori | NAF001 | LC146706 | ZMUB 21333 | SW Norway 2012 | 62.04° N, 05.02° E | This study |
| 3. Gadiculus thori | BNSFI055 | KJ204873 | MT04119 | N United Kingdom 2012 | 59.71° N, 00.56° W | [30] |
| 4. Gadiculus thori | BNSFI030 | KJ204872 | MT04118 | SW Norway 2012 | 58.22° N, 04.38° E | [30] |
| 5. Gadiculus thori | BNSFI056 | KJ204867 | MT04120 | N United Kingdom 2012 | 59.71° N, 00.56° W | [30] |
| 6. Gadiculus thori | BNSFI029 | KJ204865 | MT04117 | SW Norway 2012 | 58.22° N, 04.38° E | [30] |
| 7. Gadiculus thori | BNSFI028 | KJ204864 | MT04116 | SW Norway 2012 | 58.22° N, 04.38° E | [30] |
| 8. Gadiculus thori | BNSF269 | KJ204869 | MT02313 | SW Norway 2012 | 59.14° N, 03.13° E | [30] |
| 9. Gadiculus thori | GBGCA6718 | KJ128488 | NRM476 | SE Norway 2000 | 58.07° N, 10.02° E | Unpubl. |
| 10. Gadiculus thori | BNSFI057 | KJ204871 | MT04121 | N United Kingdom 2012 | 59.71° N, 00.56° W | [30] |
| Arctogadus glacialis | GLF145 | LC146697 | ZMUB 22974 | W Greenland 2014 | 68.36° N, 55.10° W | This study |
| Arctogadus glacialis | DSFNG010 | - | ZMUB 21027 | NE Greenland 2010 | 72.00° N, 21.02° W | Unpubl. |
| Boreogadus saida | GLF148 | LC146698 | ZMUB 22936 | W Greenland 2014 | 69.31° N, 51.53° W | This study |
| Boreogadus saida | GLF105 | LC146694 | ZMUB 21932 | SE Greenland 2013 | 65.38° N, 30.19° W | This study |
| Gadus ogac | GLF065 | LC146707 | ZMUB 21811 | SW Greenland 2013 | 60.43° N, 46.02° W | This study |
| Gadus ogac | SCAFB565 | KC015369 | ARC 26244 | SE Canada 2006 | 50.05° N, 57.88° W | [29] |
| Gadus macrocephalus | FMV221 | JQ354100 | UW110223 | NE Pacific 2004 | - | Unpubl. |
| Gadus macrocephalus | UKFBI444 | KF929903 | KU 28473 | NE Pacific 1999 | 55.16° N, 133.99° W | Unpubl. |
| Gadus morhua | GLF052 | LC146693 | No voucher | SE Greenland 2013 | 65.28° N, 33.45° W | This study |
| Gadus morhua | NOFIS088 | - | NHMO-f-541 | S Norway 2009 | 58.11° N, 08.13° E | Unpubl. |
| Gadus chalcogrammus | FMV536 | JQ354517 | UW150214 | NE Pacific 2008 | 33.87° N, 118.43° W | Unpubl. |
| Gadus chalcogrammus | ABFJ129 | JF952737 | - | NE Japan 2005 | - | [33] |
| Merlangius merlangus | ANGBF9794 | FN689176 | - | Iceland 2003 | - | [34] |
| Merlangius merlangus | ANGBF9862 | FN689040 | - | Black Sea, Turkey 2003 | - | [30] |
| Melanogrammus aeglefinus | GLF171 | LC146702 | ZMUB 22913 | SE Greenland 2014 | 66.35° N, 29.15° W | This study |
| Melanogrammus aeglefinus | GLF057 | LC146710 | ZMUB 21891 | SE Greenland 2014 | 64.25° N, 36.51° W | This study |
| Microgadus proximus | FMV009 | JQ354228 | UW047300 | NW USA 2003 | - | Unpubl. |
| Microgadus proximus | WXYZ011 | - | UW 150512 | NW USA 2010 | 47.13° S, 122.69° W | Unpubl. |
| Microgadus tomcod | BCF621 | EU524129 | ROM-T03570 | SE Canada 2006 | 47.06° S, 70.42° W | [35] |
| Microgadus tomcod | SCAFB629 | KC015691 | ARC26844 | SE Canada | 44.26° S, 64.36° W | [29] |
| Micromesistius australis | FCHIL259 | - | - | S Chile | 56.50° S, 68.62° W | Unpubl. |
| Micromesistius australis | FCHIL239 | - | - | W Chile | 47.13° S, 75.58° W | Unpubl. |
| Micromesistius poutassou | GLF149 | LC146699 | ZMUB 22716 | SE Greenland 2014 | 61.10° N, 41.40° W | This study |
| Micromesistius poutassou | BNSFI089 | KJ205044 | MT04159 | N United Kingdom 2012 | 57.85° N, 01.17° E | [30] |
| Pollachius pollachius | BNSFI033 | KJ205137 | MT04178 | SW Norway 2012 | 58.22° N, 04.38° E | [30] |
| Pollachius pollachius | NOFIS084 | - | NHMO-f-537 | SE Norway 2009 | 58.11° S, 08.13° E | Unpubl. |
| Pollachius virens | GLF053 | LC146692 | No voucher | SE Greenland 2013 | 65.28° N, 33.45° E | This study |
| Pollachius virens | SCAFB100 | KC015818 | ARC 24890 | SE Canada 2005 | 42.91° N, 63.53° E | [29] |
| Trisopterus capelanus | CSFOM166 | KJ709669 | CSFOM-246 | Sicily, Italy | - | [32] |
| Trisopterus capelanus | CSFOM165 | KJ709671 | CSFOM-245 | Sicily, Italy | - | [32] |
| Neocolliolus esmarkii | GLF012 | LC146705 | ZMUB 21421 | SE Greenland 2012 | 65.53° N, 32.36° W | This study |
| Neocolliolus esmarkii | GBGCA7771 | KJ128652 | NRM5415 | SW Sweden 2007 | 57.31° N, 11.47° E | Unpubl. |
| Trisopterus luscus | FCFP125 | JQ774953 | MB85-004867 | SW Portugal 2005 | 38.22° N, 08.83° W | [31] |
| Trisopterus luscus | BNSFI090 | KJ205243 | MT04227 | NW Germany 2011 | 53.76° N, 06.45° E | [30] |
| Allotrisopterus minutus | BNSFI133 | KJ205252 | MT05367 | N Germany 2010 | 54.14° N, 07.90° E | [30] |
| Allotrisopterus minutus | FCFPW193 | JQ775159 | FCFOPB086-05 | W Portugal 2005 | 41.62° N, 08.99° W | [31] |
| Species | Gadiculus argenteus | Gadiculus thori |
|---|---|---|
| Maximum size | ca. 15 cm | ca. 20 cm |
| Head size | Relatively large | Relatively small |
| Proportion of the eye | Relatively large | Relatively small |
| OL/OT ratio | Relatively large | Relatively small |
| Shape of adult otoliths | Drop-shaped Moderately variable Posterior end usually not truncated, but if so, truncation is oblique to long axis | Oval (irregular) Highly variable Posterior end usually truncated; truncation perpendicular to long axis |
| Postlarvae at same stage of development | Three transverse pigmented bars Relatively small size Relatively stout | One transverse pigmented bar Relatively large size Relatively slender |
| Number of vertebrae | 37–41, usually 40 (usual range 38–40; 37 and 41 rare) | 39–43, usually 42 (usual range 41–43; 39 and 40 rare) |
| Number of D3 fin rays | 11–16 | 15–17 |
| Number of A1 fin rays | 11–16 (11 rare) | 15–18 (18 rare) |
| Cox1 barcodes | Little intraspecific variation | Little intraspecific variation |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaemers, P.A.M.; Poulsen, J.Y. Recognition and Distribution of Two North Atlantic Gadiculus Species, G. argenteus and G. thori (Gadidae), Based on Otolith Morphology, Larval Pigmentation, Molecular Evidence, Morphometrics and Meristics. Fishes 2017, 2, 15. https://doi.org/10.3390/fishes2030015
Gaemers PAM, Poulsen JY. Recognition and Distribution of Two North Atlantic Gadiculus Species, G. argenteus and G. thori (Gadidae), Based on Otolith Morphology, Larval Pigmentation, Molecular Evidence, Morphometrics and Meristics. Fishes. 2017; 2(3):15. https://doi.org/10.3390/fishes2030015
Chicago/Turabian StyleGaemers, Pieter A. M., and Jan Y. Poulsen. 2017. "Recognition and Distribution of Two North Atlantic Gadiculus Species, G. argenteus and G. thori (Gadidae), Based on Otolith Morphology, Larval Pigmentation, Molecular Evidence, Morphometrics and Meristics" Fishes 2, no. 3: 15. https://doi.org/10.3390/fishes2030015
APA StyleGaemers, P. A. M., & Poulsen, J. Y. (2017). Recognition and Distribution of Two North Atlantic Gadiculus Species, G. argenteus and G. thori (Gadidae), Based on Otolith Morphology, Larval Pigmentation, Molecular Evidence, Morphometrics and Meristics. Fishes, 2(3), 15. https://doi.org/10.3390/fishes2030015






