Mugilids Display Distinct Trait-Mediated Patterns with a Reinvasion of Para Grass Urochloa mutica in a Tropical Estuary
Abstract
:1. Introduction
2. Results
2.1. Shoreline Physiochemical Characteristics
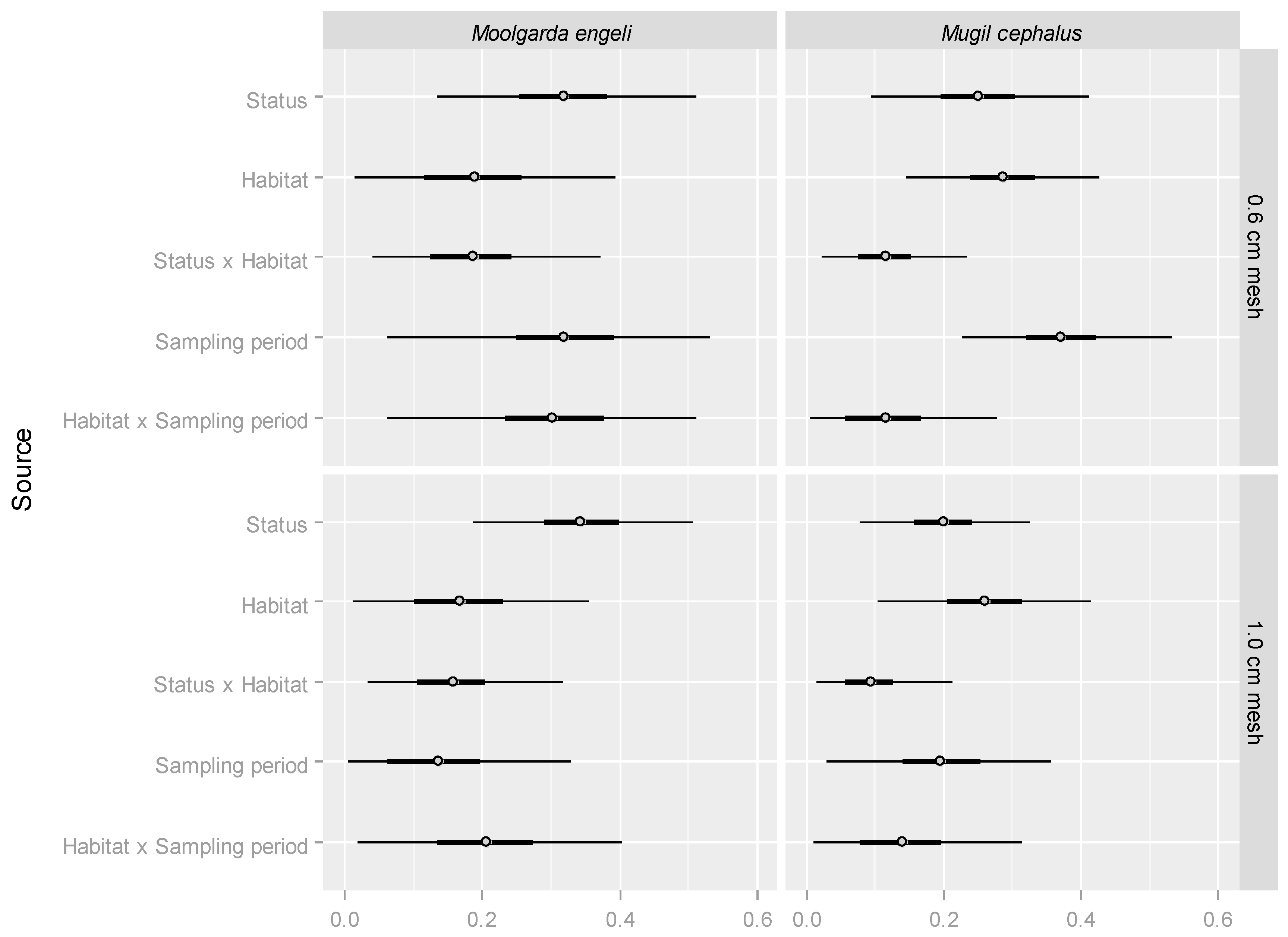
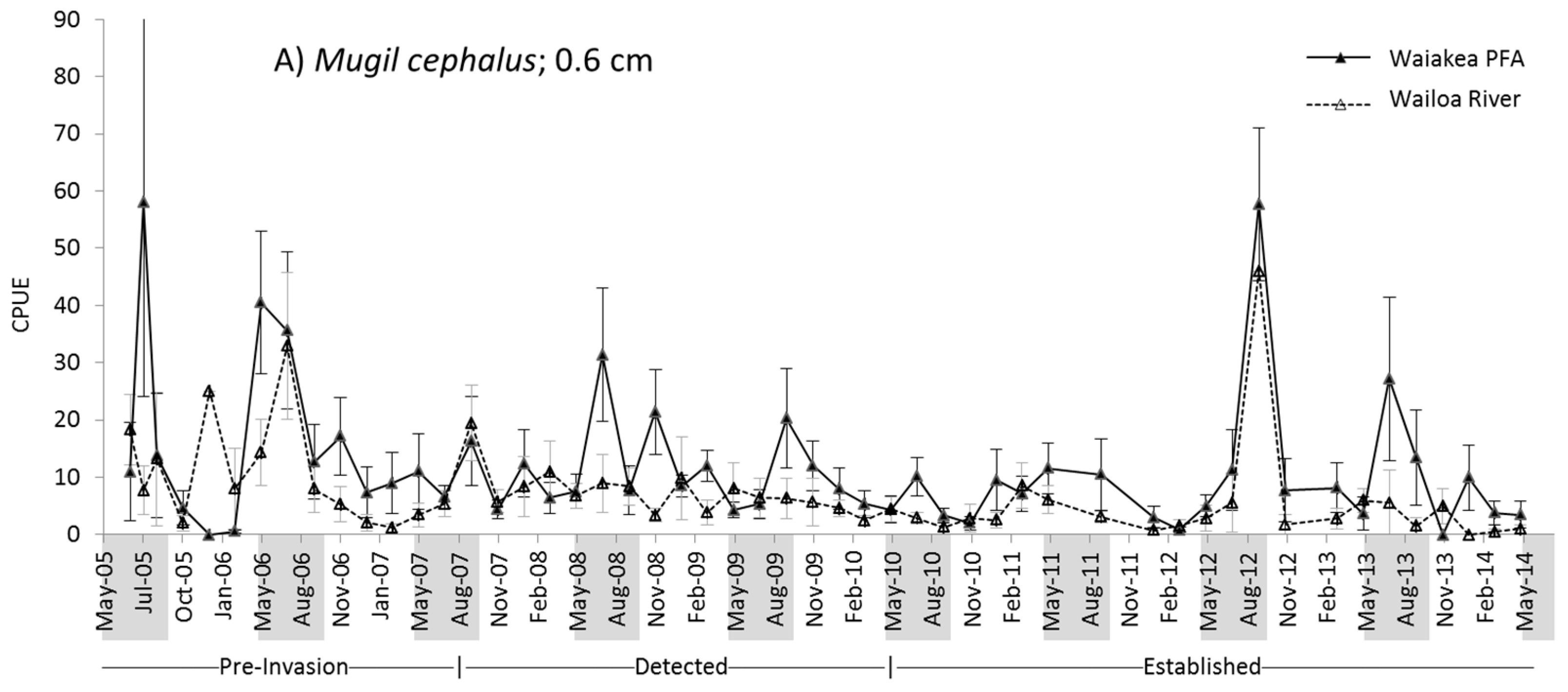
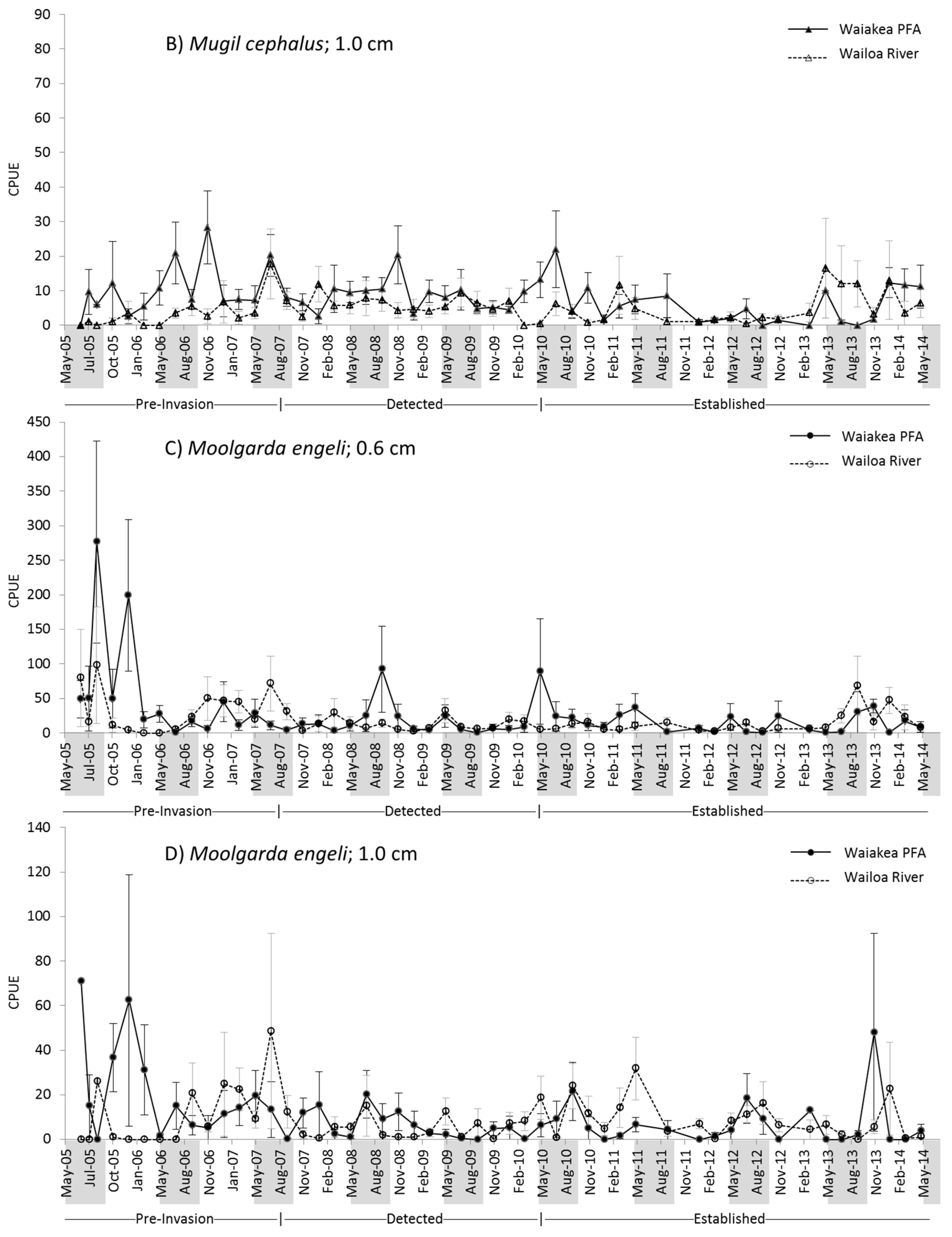
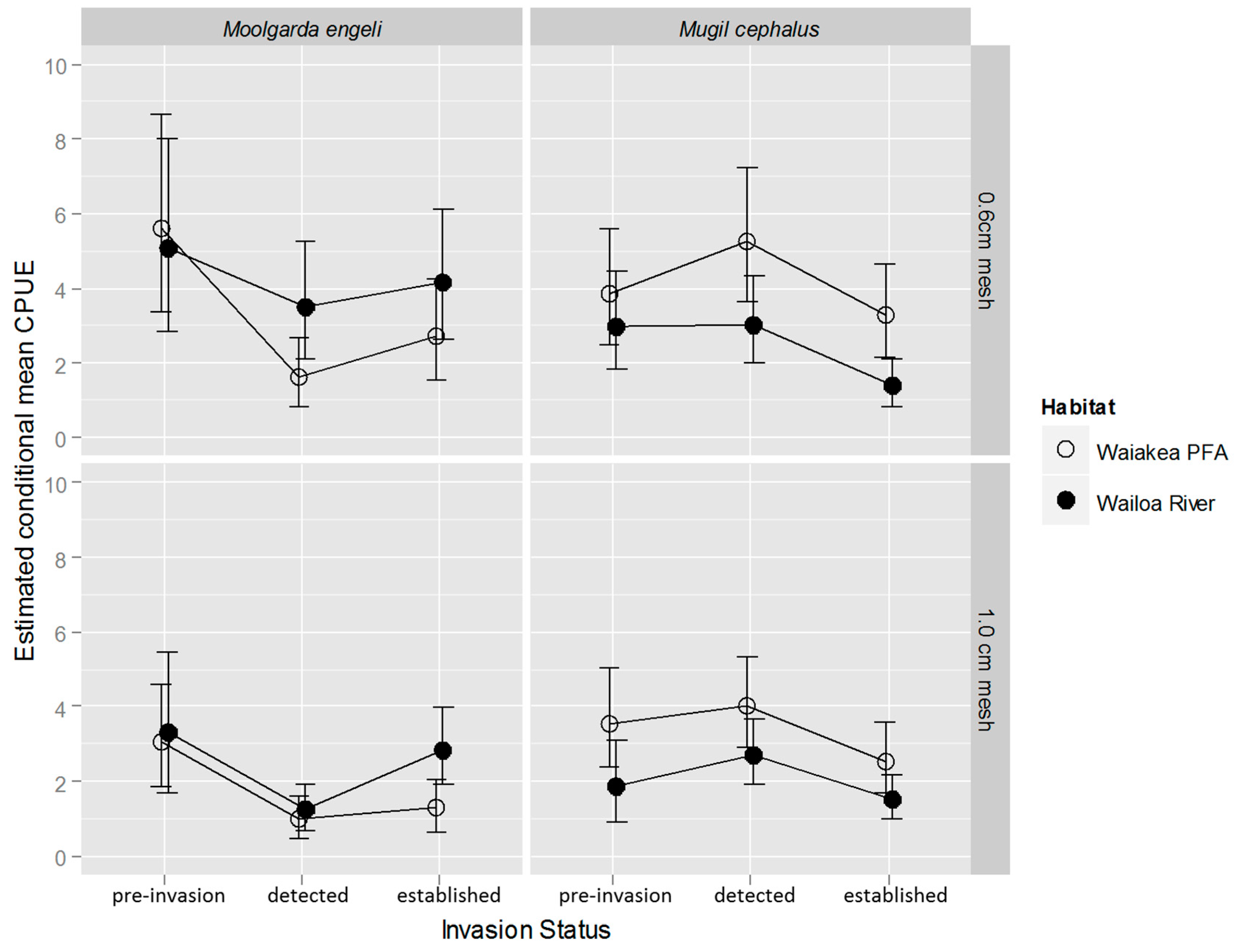
2.2. M. cephalus Catch per Unit Effort (CPUE)
2.3. M. engeli CPUE
3. Discussion
3.1. M. cephalus
3.2. M. engeli
4. Materials and Methods
4.1. Site Description
4.2. U. mutica Expansion Rates and Potential
4.3. Mullet Sampling
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Orth, R.; Heck, K.; van Montfrans, J. Faunal communities in seagrass beds: A review of the influence of plant structure and prey characteristics on predator-prey relationships. Estuaries 1984, 7, 339–350. [Google Scholar] [CrossRef]
- Lubbers, L.; Boynton, W.R.; Kemp, W.M. Variations in structure of estuarine fish communities in relation to abundance of submersed vascular plants. Mar. Ecol. Prog. Ser. 1990, 65, 1–14. [Google Scholar] [CrossRef]
- Randall, R.; Minns, C.; Cairns, V.; Moore, J. The relationship between an index of fish production and submerged macrophytes and other habitat features at three littoral areas in the Great Lakes. Can. J. Fish. Aquat. Sci. 1996, 53, 35–44. [Google Scholar] [CrossRef]
- Reddy, K.; Patrick, W.; Lindau, C. Nitrification-denitrification at the plant root-sediment interface in wetlands. Limnol. Oceanogr. 1989, 34, 1004–1013. [Google Scholar] [CrossRef]
- Brix, H. Do macrophytes play a role in constructed treatment wetlands? Water Sci. Technol. 1997, 35, 11–17. [Google Scholar] [CrossRef]
- Summerfelt, S.T.; Adler, P.R.; Glenn, D.M.; Kretschmann, R.N. Aquaculture sludge removal and stabilization within created wetlands. Aquac. Eng. 1999, 19, 81–92. [Google Scholar] [CrossRef]
- Casatti, L.; Mendes, H.; Ferreira, K.M. Aquatic macrophytes as feeding site for small fishes in the Rosana Reservoir, Paranapanema River, Southeastern Brazil. Braz. J. Biol. 2003, 63, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Michelan, T.S.; Thomaz, S.; Mormul, R.P.; Carvalho, P. Effects of an exotic invasive macrophyte (tropical signalgrass) on native plant community composition, species richness and functional diversity. Freshw. Biol. 2010, 55, 1315–1326. [Google Scholar] [CrossRef]
- Gordon, D.R. Effects of invasive, non-indigenous plant species on ecosystem processes: Lessons from Florida. Ecol. Appl. 1998, 8, 975–989. [Google Scholar] [CrossRef]
- Schultz, R.; Dibble, E. Effects of invasive macrophytes on freshwater fish and macroinvertebrate communities: The role of invasive plant traits. Hydrobiologia 2012, 684, 1–14. [Google Scholar] [CrossRef]
- Thomaz, S.; Mormul, R.; Michelan, T. Propagule pressure, invasibility of freshwater ecosystems by macrophytes and their ecological impacts: A review of tropical freshwater ecosystems. Hydrobiologia 2015, 746, 39–59. [Google Scholar] [CrossRef]
- Carniatto, N.; Thomaz, S.M.; Cunha, E.R.; Fugi, R.; Ota, R.R. Effects of an invasive alien Poaceae on aquatic macrophytes and fish communities in a Neotropical reservoir. Biotropica 2013, 45, 747–754. [Google Scholar] [CrossRef]
- Casatti, L.; de Paula Ferreira, C.; Carvalho, F.R. Grass-dominated stream sites exhibit low fish species diversity and dominance by guppies: An assessment of two tropical pasture river basins. Hydrobiologia 2009, 632, 273–283. [Google Scholar] [CrossRef]
- Valley, R.D.; Bremigan, M.T. Effects of macrophyte bed architecture on largemouth bass foraging: Implications of exotic macrophyte invasions. Trans. Am. Fish. Soc. 2002, 131, 234–244. [Google Scholar] [CrossRef]
- Theel, H.J.; Dibble, E.D. An experimental simulation of an exotic aquatic macrophyte invasion and its influence on foraging behavior of bluegill. J. Freshw. Ecol. 2008, 23, 79–89. [Google Scholar] [CrossRef]
- Pusey, B.J.; Arthington, A.H. Importance of the riparian zone to the conservation and management of freshwater fish: A review. Mar. Freshw. Res. 2003, 54, 1–16. [Google Scholar] [CrossRef]
- Douglas, M.; Bunn, S.; Pidgeon, R.; Davies, P.; Barrow, P.; O’Connor, R.; Winning, M. Weed Management and the Biodiversity and Ecological Processes of Tropical Wetlands; Draft Final Report; National Wetlands R & D Program; Environment Australia & Land and Water Australia: Canberra, Australia, 2002. [Google Scholar]
- Douglas, M.M.; O’Connor, R.A. Effects of the exotic macrophyte, para grass (Urochloa mutica), on benthic and epiphytic macroinvertebrates of a tropical floodplain. Freshw. Biol. 2003, 48, 962–971. [Google Scholar] [CrossRef]
- Fernandes, I.M.; Machado, F.A.; Penha, J. Spatial pattern of a fish assemblage in a seasonal tropical wetland: Effects of habitat, herbaceous plant biomass, water depth, and distance from species sources. Neotropical Ichthyol. 2010, 8, 289–298. [Google Scholar] [CrossRef]
- Cunha, E.R.; Thomaz, S.M.; Evangelista, H.B.A.; Carniato, J.; Souza, C.F.; Fugi, R. Small-sized fish assemblages do not differ between a native and a recently established non-indigenous macrophyte in a Neotropical ecosystem. Nat. Conserv. 2011, 9, 61–66. [Google Scholar] [CrossRef]
- Parsons, J.J. Spread of African pasture grasses to the American tropics. J. Range Manag. 1972, 25, 12–17. [Google Scholar] [CrossRef]
- Humphries, S.E.; Groves, R.H.; Mitchell, D.S. Plant invasions of Australian ecosystems: A status review and management directions. In Kowari; Australian National Parks and Wildlife Service: Sydney, Australia, 1991; Volume 2, pp. 1–134. [Google Scholar]
- Hannan-Jones, M.; Csurhes, S. Invasive Species Risk Assessment: Para Grass (Urochloa mutica); Queensland Department of Agriculture, Fisheries and Forestry: Brisbane, Australia, 2012. [Google Scholar]
- Wagner, W.L.; Herbst, D.R.; Sohmer, S.H. Manual of the Flowering Plants of Hawaii, Volumes 1 and 2; University of Hawaii and Bishop Museum Press: Honolulu, HI, USA, 1999. [Google Scholar]
- Space, J.C.; Flynn, T. Observations on Invasive Plant Species in American Samoa; USDA Forest Service, Pacific Southwest Research Station, Institute of Pacific Islands Forestry: Honolulu, HI, USA, 2000. [Google Scholar]
- Space, J.C.; Flynn, T. Report to the Kingdom of Tonga on Invasive Plant Species of Environmental Concern; USDA Forest Service, Pacific Southwest Research Station, Institute of Pacific Islands Forestry: Honolulu, HI, USA, 2001. [Google Scholar]
- Bantilan-Smith, M.; Bruland, G.L.; MacKenzie, R.A.; Henry, A.R.; Ryder, C.R. A comparison of the vegetation and soils of natural, restored, and created coastal lowland wetlands in Hawaii. Wetlands 2009, 29, 1023–1035. [Google Scholar] [CrossRef]
- Bruland, G.L.; MacKenzie, R.A. Nitrogen source tracking with δ15N content of coastal wetland plants in Hawaii. J. Environ. Qual. 2010, 39, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Singh, G. Salinity-related desertification and management strategies: Indian experience. Land Degrad. Dev. 2009, 20, 367–385. [Google Scholar] [CrossRef]
- Boudet, G. The Inventory and Mapping of Rangeland in West Africa, Evaluation and mapping of tropical African rangelands. In Proceedings of the Seminar Held in Bamako, Mali, Bamako, Mali, 3–8 March 1975; pp. 3–8. [Google Scholar]
- Willis, M.; Zerbe, S.; Breitung, W. Habitat survey, mapping, and assessment in the Mai Po Nature Reserve, Hong Kong (China). Arch. Nat. Conserv. Landsc. Res. 2006, 45, 53–70. [Google Scholar]
- Granek, E.F.; Polasky, S.; Kappel, C.V.; Reed, D.J.; Stoms, D.M.; Koch, E.W.; Kennedy, C.J.; Cramer, L.A.; Hacker, S.D.; Barbier, E.B. Ecosystem services as a common language for coastal ecosystem-based management. Conserv. Biol. 2010, 24, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Hadwen, W.L.; Arthington, A.H. Ecology, Threats and Management Options for Small Estuaries and ICOLLS; Sustainable Tourism CRC: Gold Coast, Australia, 2006. [Google Scholar]
- Beck, M.W.; Heck, K.L., Jr.; Able, K.W.; Childers, D.L.; Eggleston, D.B.; Gillanders, B.M.; Halpern, B.; Hays, C.G.; Hoshino, K.; Minello, T.J.; et al. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates: A better understanding of the habitats that serve as nurseries for marine species and the factors that create site-specific variability in nursery quality will improve conservation and management of these areas. Bioscience 2001, 51, 633–641. [Google Scholar]
- Fitzsimons, J.; McRae, M.; Nishimoto, R. Behavioral ecology of indigenous stream fishes in Hawaii. Biol. Hawaii. Streams Estuaries 2007, 3, 11–21. [Google Scholar]
- Potter, I.C.; Tweedley, J.R.; Elliott, M.; Whitfield, A.K. The ways in which fish use estuaries: A refinement and expansion of the guild approach. Fish Fish. 2013, 16, 230–239. [Google Scholar] [CrossRef]
- McDowall, R.M. Diadromy in Fishes: Migrations between Freshwater and Marine Environments; Timber Press: Portland, OR, USA, 1988. [Google Scholar]
- Cardona, L. Habitat selection by grey mullets (Osteichthyes: Mugilidae) in Mediterranean estuaries: The role of salinity. Sci. Mar. 2006, 70, 443–455. [Google Scholar] [CrossRef]
- Briggs, J.C. Fishes of worldwide (circumtropical) distribution. Copeia 1960, 3, 171–180. [Google Scholar] [CrossRef]
- Thomson, J.M. Synopsis of Biological Data on the Grey Mullet Mugil cephalus, Linnaeus 1758; Commonwealth Scientific and Industrial Research Organization: Melbourne, Australia, 1963. [Google Scholar]
- Nishimoto, R.T.; Shimoda, T.E.; Nishiura, L.K. Mugilids in the muliwai: A tale of two mullets. Biol. Hawaii. Streams Estuaries Bish. Mus. Bull. Cult. Environ. Stud. 2007, 3, 143–156. [Google Scholar]
- Randall, J.E.; Kanayama, R.K. Hawaiian fish immigrants. Sea Front. 1972, 18, 144–153. [Google Scholar]
- Randall, J.E. Introductions of marine fishes to the Hawaiian Islands. Bull. Mar. Sci. 1987, 41, 490–502. [Google Scholar]
- Maciolek, J. Exotic fishes in Hawaii and other islands of Oceania. In Distribution, Biology, and Management of Exotic Fishes; Johns Hopkins University Press: Baltimore, MD, USA, 1984; pp. 131–161. [Google Scholar]
- Julius, M.L. Why sweat the small stuff: The importance of microalgae in Hawaiian stream ecosystems. Bish. Mus. Bull. Cult. Environ. Stud. 2007, 3, 183–194. [Google Scholar]
- Silva, S.; Silva, E. Biology of young grey mullet, Mugil cephalus L., populatioins in a coastal lagoon in Sri Lanka. J. Fish Biol. 1979, 15, 9–20. [Google Scholar] [CrossRef]
- Blaber, S. Factors affecting recruitment and survival of mugilids in estuaries and coastal waters of southeastern Africa. Am. Fish. Soc. Symp. 1987, 1, 18. [Google Scholar]
- Mwandya, A.; Mgaya, Y.; Öhman, M.; Bryceson, I.; Gullström, M. Distribution patterns of striped mullet Mugil cephalus in mangrove creeks, Zanzibar, Tanzania. Afr. J. Mar. Sci. 2010, 32, 85–93. [Google Scholar] [CrossRef]
- Whitfield, A.K.; Panfili, J.; Durand, J.D. A global review of the cosmopolitan flathead mullet Mugil cephalus Linnaeus 1758 (Teleostei: Mugilidae), with emphasis on the biology, genetics, ecology and fisheries aspects of this apparent species complex. Rev. Fish Biol. Fish. 2012, 22, 641–681. [Google Scholar] [CrossRef]
- Perna, C.; Cappo, M.; Pusey, B.J.; Burrows, D.; Pearson, R. Removal of aquatic weeds greatly enhances fish community richness and diversity: An example from the Burdekin River floodplain, tropical Australia. River Res. Appl. 2012, 28, 1093–1104. [Google Scholar] [CrossRef]
- Munsch, S.H.; Cordell, J.R.; Toft, J.D. Fine-scale habitat use and behavior of a nearshore fish community: Nursery functions, predation avoidance, and spatiotemporal habitat partitioning. Mar. Ecol. Prog. Ser. 2016, 557, 1–15. [Google Scholar] [CrossRef]
- Stone, K.R. Urochloa mutica. Fire Effects Information System. Available online: http://www.webcitation.org/6o71dwj67 (accessed on 1 May 2017).
- Bunn, S.E.; Davies, P.M.; Kellaway, D.M. Contributions of sugar cane and invasive pasture grass to the aquatic food web of a tropical lowland stream. Mar. Freshw. Res. 1997, 48, 173–179. [Google Scholar] [CrossRef]
- Woelfel, J.; Schoknecht, A.; Schaub, I.; Enke, N.; Schumann, R.; Karsten, U. Growth and photosynthesis characteristics of three benthic diatoms from the brackish southern Baltic Sea in relation to varying environmental conditions. Phycologia 2014, 53, 639–651. [Google Scholar] [CrossRef]
- Mariotti, G.; Fagherazzi, S. Modeling the effect of tides and waves on benthic biofilms. J. Geophys. Res. Biogeosci. 2012, 117. [Google Scholar] [CrossRef]
- Laegdsgaard, P.; Johnson, C. Why do juvenile fish utilise mangrove habitats? J. Exp. Mar. Biol. Ecol. 2001, 257, 229–253. [Google Scholar] [CrossRef]
- Lehtiniemi, M. Swim or hide: Predator cues cause species specific reactions in young fish larvae. J. Fish Biol. 2005, 66, 1285–1299. [Google Scholar] [CrossRef]
- Arthington, A.H.; Milton, D.A.; McKay, R.J. Effects of urban development and habitat alterations on the distribution and abundance of native and exotic freshwater fish in the Brisbane region, Queensland. Aust. J. Ecol. 1983, 8, 87–101. [Google Scholar] [CrossRef]
- Low, T. Tropical pasture plants as weeds. Trop. Grassl. 1997, 31, 337–343. [Google Scholar]
- Hyland, S. An Investigation of the Impacts of Ponded Pastures on Barramundi and Other Finfish Populations in Tropical Coastal Wetlands; Department of Primary Industries, Queensland Report QO02005; Fisheries Research and Development Corporation: Brisbane, Australia, 2002. [Google Scholar]
- Wannamaker, C.M.; Rice, J.A. Effects of hypoxia on movements and behavior of selected estuarine organisms from the southeastern United States. J. Exp. Mar. Biol. Ecol. 2000, 249, 145–163. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Harper, D.E.; Turner, R.E. Responses of nekton and demersal and benthic fauna to decreasing oxygen concentrations. In Coastal Hypoxia: Consequences for Living Resources and Ecosystems; American Geophysical Union as Part of the Coastal and Estuarine Studies: Washington, DC, USA, 2001; pp. 115–128. [Google Scholar]
- Vaquer-Sunyer, R.; Duarte, C.M. Thresholds of hypoxia for marine biodiversity. Proc. Natl. Acad. Sci. USA 2008, 105, 15452–15457. [Google Scholar] [CrossRef] [PubMed]
- Paquay, F.S.; Mackenzie, F.T.; Borges, A.V. Carbon dioxide dynamics in rivers and coastal waters of the “big island” of Hawaii, USA, during baseline and heavy rain conditions. Aquat. Geochem. 2007, 13, 1–18. [Google Scholar] [CrossRef]
- Hallacher, L.E.; Kho, E.B.; Bernard, N.D.; Orcutt, A.M.; Dudley, W.C., Jr.; Hammond, T.M. Distribution of arsenic in the sediments and biota of Hilo Bay, Hawaii. Pac. Sci. 1985, 39, 266–273. [Google Scholar]
- Wiegner, T.N.; Mead, L. Water Quality in Hilo Bay, Hawaii, USA, under Baseflow and Storm Conditions; Professional Report to Hawaii County’s Department of Public Works; Engineering Division: Hilo, HI, USA, 2009. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 12 May 2017).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest: Tests in Linear Mixed Effects Models. Cran, R Package, Version 2. 2015. Available online: http://CRAN.R-project.org/package=lmerTest (accessed on 12 May 2017).
- Gelman, A. Analysis of variance—Why it is more important than ever. Ann. Stat. 2005, 33, 1–53. [Google Scholar] [CrossRef]
- Plummer, M. Jags: Just Another Gibbs Sampler; Version 3.4.0. Available online: http://mcmc-jags.sourceforge.net (accessed on 1 October 2015).
- Smith, T.; Marshall, L.; Sharma, A. Predicting hydrologic response through a hierarchical catchment knowledgebase: A Bayes empirical Bayes approach. Water Resour. Res. 2014, 50, 1189–1204. [Google Scholar] [CrossRef]
- Brooks, S.; Gelman, A. General methods for monitoring convergence of iterative simulations. J. Comput. Graph. Stat. 1998, 7, 434–455. [Google Scholar] [CrossRef]

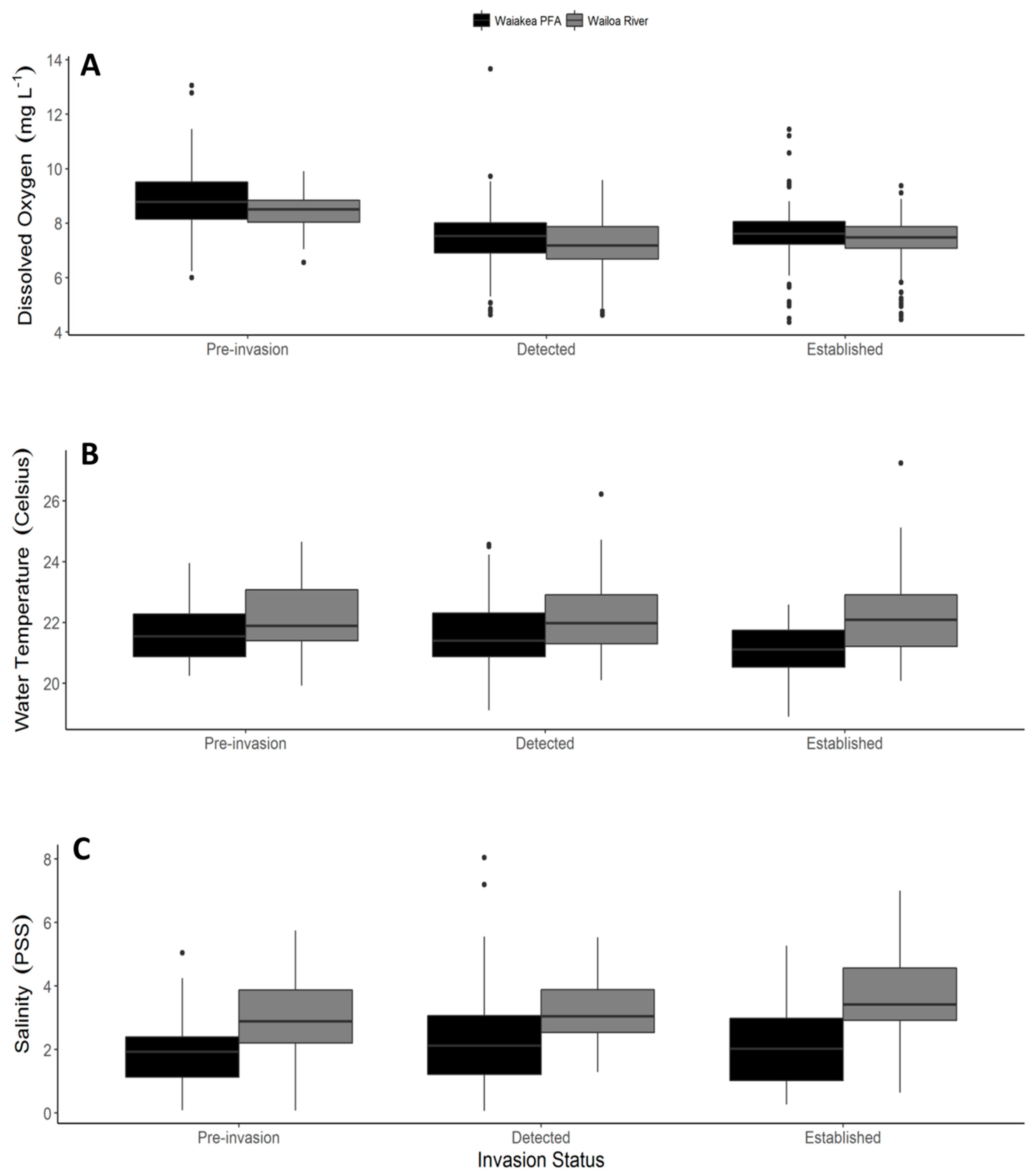
| Dissolved Oxygen (mg L−1) | ||||||
|---|---|---|---|---|---|---|
| Source | 1SS | 2MS | 3numDF | 4denDF | F | 5p |
| Habitat | 0.074 | 0.074 | 1 | 41.98 | 8.735 | 0.0051 |
| Invasion status | 0.092 | 0.046 | 2 | 43.07 | 5.469 | 0.0076 |
| Habitat: Invasion status | 0.0005 | 0.0003 | 2 | 42.17 | 0.0298 | 0.97 |
| Water Temperature (°C) | ||||||
| Source | 1SS | 2MS | 3numDF | 4denDF | F | 5p |
| Habitat | 0.079 | 0.079 | 1 | 40.76 | 44.76 | <0.001 |
| Invasion status | 0.0031 | 0.0016 | 2 | 41.58 | 0.89 | 0.42 |
| Habitat: Invasion status | 0.016 | 0.0079 | 2 | 41.06 | 4.46 | 0.018 |
| Salinity (PSS) | ||||||
| Source | 1SS | 2MS | 3numDF | 4denDF | F | 5p |
| Habitat | 16.68 | 16.68 | 1 | 434.08 | 126.94 | <0.001 |
| Invasion status | 0.31 | 0.16 | 2 | 42.12 | 1.20 | 0.31 |
| Habitat: Invasion status | 0.70 | 0.35 | 2 | 434.13 | 2.67 | 0.07 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakihara, T.S.; Fukunaga, A.; Peyton, K.A. Mugilids Display Distinct Trait-Mediated Patterns with a Reinvasion of Para Grass Urochloa mutica in a Tropical Estuary. Fishes 2017, 2, 7. https://doi.org/10.3390/fishes2020007
Sakihara TS, Fukunaga A, Peyton KA. Mugilids Display Distinct Trait-Mediated Patterns with a Reinvasion of Para Grass Urochloa mutica in a Tropical Estuary. Fishes. 2017; 2(2):7. https://doi.org/10.3390/fishes2020007
Chicago/Turabian StyleSakihara, Troy S., Atsuko Fukunaga, and Kimberly A. Peyton. 2017. "Mugilids Display Distinct Trait-Mediated Patterns with a Reinvasion of Para Grass Urochloa mutica in a Tropical Estuary" Fishes 2, no. 2: 7. https://doi.org/10.3390/fishes2020007
APA StyleSakihara, T. S., Fukunaga, A., & Peyton, K. A. (2017). Mugilids Display Distinct Trait-Mediated Patterns with a Reinvasion of Para Grass Urochloa mutica in a Tropical Estuary. Fishes, 2(2), 7. https://doi.org/10.3390/fishes2020007







