1. Introduction
Aquaculture in México has great potential, especially of native freshwater species, such as the tropical gar (
Atractosteus tropicus, Gill 1863) in the Southwest. Total fisheries production of this species was ~300 tons per year and it is decreasing every year [
1]. Commercial-scale production of finfish has been limited by several factors including reproductive performance to achieve constant production of high quality eggs and juveniles, zoo-technical management protocols during larval hatching, and nutritional requirements. Therefore, inclusion of specific food components, mainly during the larval stage, could contribute to maximize their growth and survival, and finally increase fry production in mass culture [
2]. Studies related to the use of live prey as well as adaptation to commercial rainbow trout formulated diets (silver cup) show that high rates of cannibalism occur using artificial diets during the first 15 days of larviculture of
A. tropicus [
3]. For this reason, careful handling of larvae is required to decrease attacks among individuals that cause high (up to 95%) mortalities [
2,
4]. Thus, if the main goal is to improve juvenile production, it is essential to develop specific feeds for the larvae by using available information about their digestive capacity and by determining the effects of nutrients on larval growth and survival.
Assimilation of proteins, carbohydrates and lipids offered in different proportions in the diet of fish larvae have been established for particular species depending on their ontogenetic stage [
5]. The use of carbohydrates in marine and freshwater larval fish can depend on the nature and concentration of the ingredient in the diet, digestibility capacities by fish enzymes, food intake level, environmental conditions, and life stage, among other factors [
6]. Starch is a relatively complex polysaccharide; its dietary inclusion is variable, although its use is feasible in finfish diets, especially in species with omnivorous habits [
7], such as common carp (
Cyprinus carpio, Linnaeus 1758). Different authors reported on the use of carbohydrates (starches) with excellent results for growth and survival of freshwater carp [
8]. In this context, it has been suggested that carbohydrates should not exceed 15% of the dietary dry weight for fish, because the excess of these compounds results in stunting and reduced digestibility of other nutrients [
6,
9]. Although it has been possible to include some digestible forms of carbohydrates in larval diets, the amount accepted and its utilization is dependent of the carbohydrate source and fish species [
8]. The aim of this research was thus to assess the effects of increasing dietary levels of carbohydrates (potato starch) and decreasing protein content on growth, survival, cannibalism and digestive enzyme activities
in A. tropicus larvae.
3. Discussion
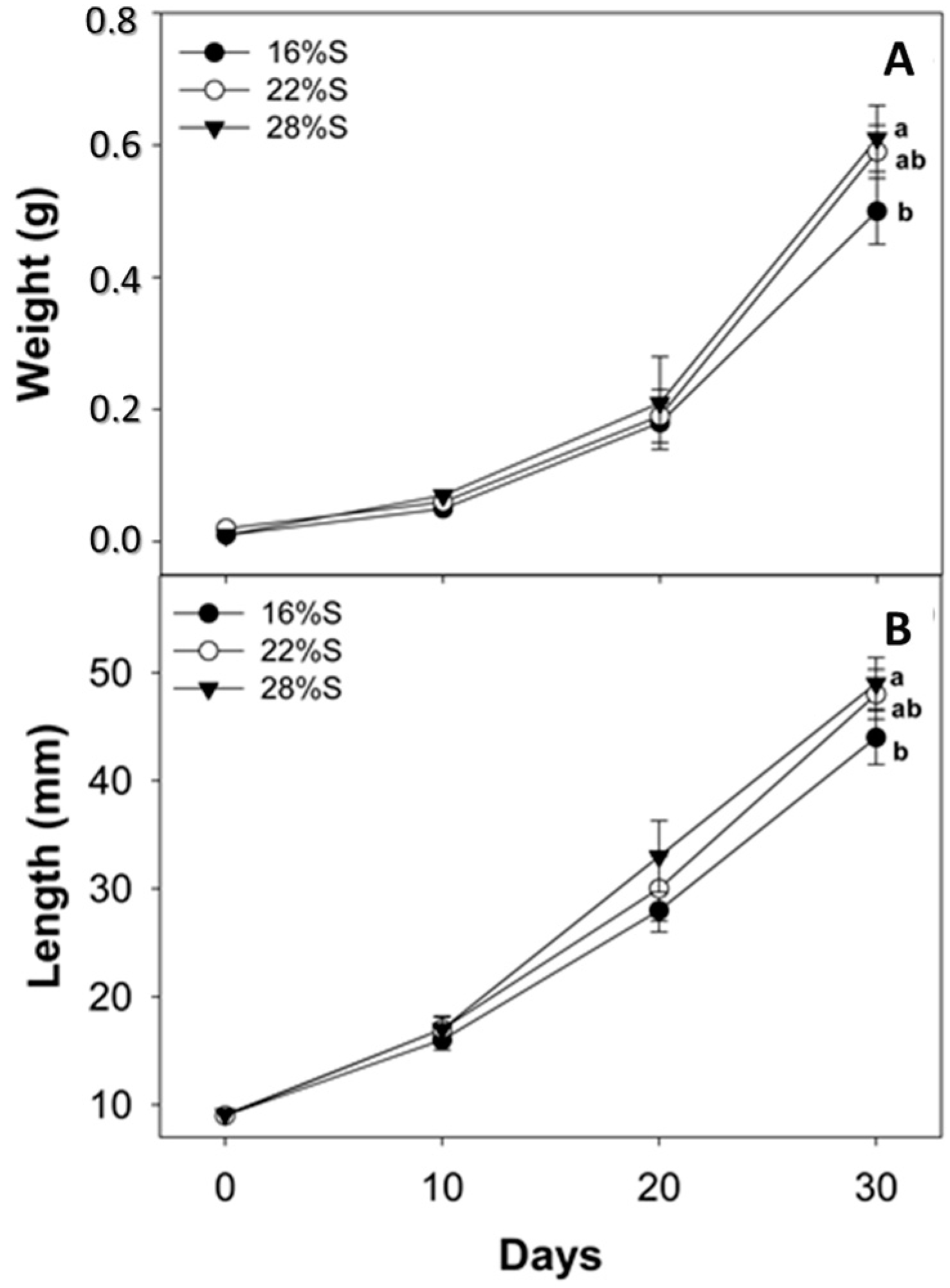
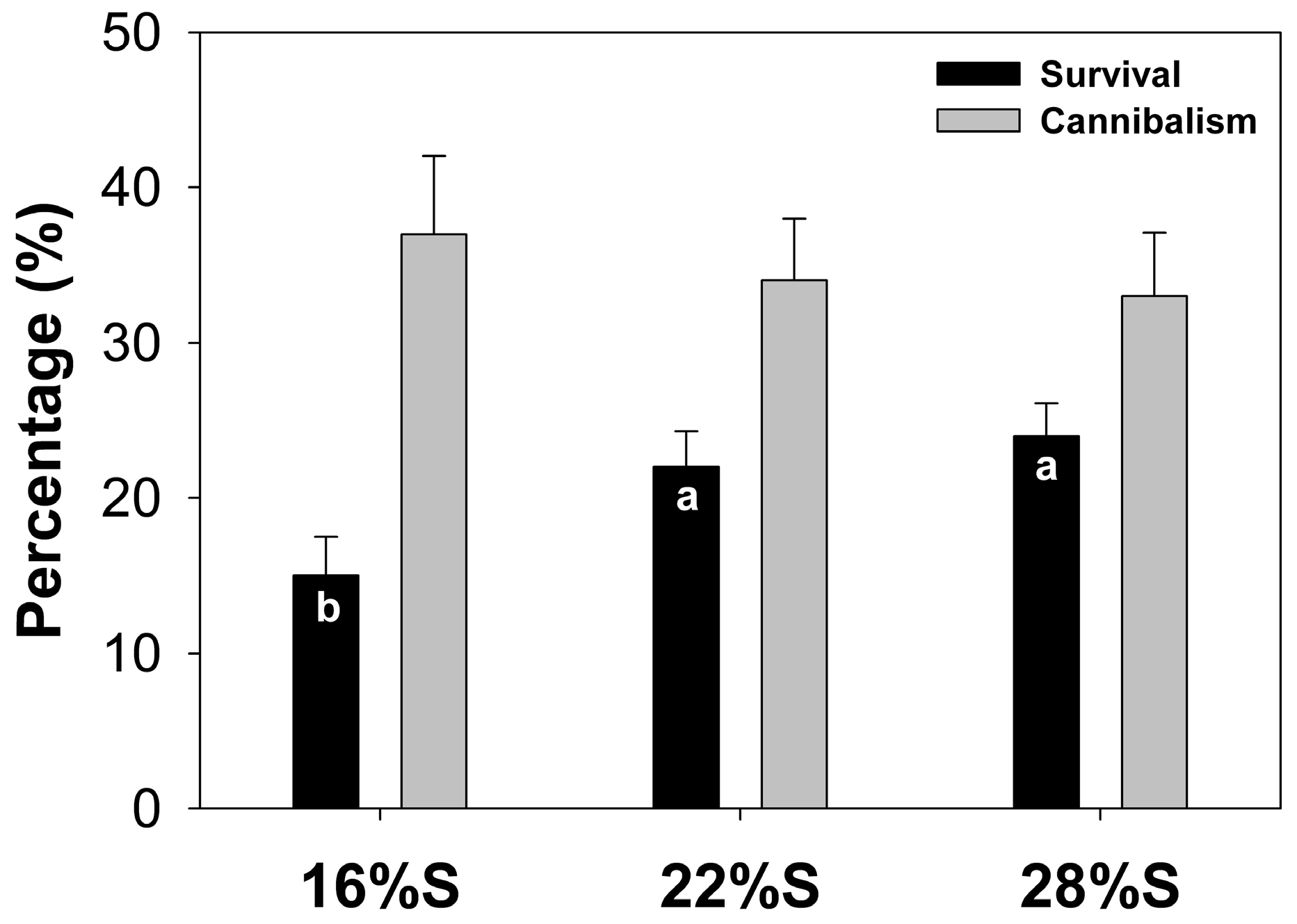
Results of the present study show that larvae fed a diet with 28% potato starch exhibited better growth and survival than those fed diets with a lower starch content. Previous studies indicate that the use of carbohydrates in formulations depends on the type and concentration included, the fish species and growing conditions, which can promote greater digestibility, improving the level of food intake and consequently growth and larval survival [
4,
10]. Prior studies of
A. tropicus larvae using diets based on rendered proteins (poultry byproduct and pork), showed higher growth and survival than results of the present study. However, a combined feeding strategy (
Artemia naupli, live
Artemia and the experimental diets mix) was used until day 14 [
3], whereas experimental feeds in the present study were used as sole feed from the time of first opening of the mouth and anus. Another study on the same species that tested corn starch as a carbohydrates source with two inclusion levels (7.4% and 15.4%), following the same feeding strategy (experimental diets offered from opening of mouth and anus) as the present study, showed comparable growth rates with those reported here, although reduced cannibalism and higher survival were reported [
4]. Differences between these results can be attributed to many factors, including differential utilization of the dietary source of carbohydrates (digestion and metabolism), level included in the diet, differences in the balance of macronutrients, complexity of the carbohydrate source [
11,
12] and even possible differences between egg quality between spawning batches due to the limited information on nutritional requirements of broodstock in the species. Nevertheless, the same findings of acceptance and utilization of corn starch as energy source [
4] and potato starch (present study) make it clear that
A. tropicus larvae show a common pattern of higher growth with increasing starch levels. Hence, differences in survival can be attributed to differences in carbohydrate solubility and limitations of the carbohydrase enzyme battery to digest different starch sources [
13], in conjunction with cannibalism behavior. Other results on apparent digestibility of carbohydrates in species such as olive flounder (
Paralichthys olivaceus) and rockfish (
Sebastes schlegeli) show that different starch sources are differentially digested by the same species, and that these differences are related to the content of indigestible polysaccharides in the food source [
13,
14].
The present study demonstrates acceptance of 28% of dietary potato starch by
A. tropicus larvae. Relatively high levels of carbohydrate in the diet compensate for higher protein retention and lead to higher use of carbohydrates as an energy source, such that the allocation of protein for growth [
15]. In our study, potato starch provides an important source of energy, in this sense, glucose obtained from starch is the principal oxidative substrate for nerve tissue and blood cells, and protein sparing and growth promotion is associated with depression of gluconeogenic activity preferential allocation of amino acids to tissue growth rather than oxidative pathways [
7,
16].
Digestive physiology is important to determine the type and amount of nutrients that the body is able to digest and absorb [
10]; thus, digestive and absorptive capabilities play an important role influencing the growth of fish [
17]. The activities of digestive enzymes play an important role in determining the ability of an animal to obtain nutrients from a particular food sourced [
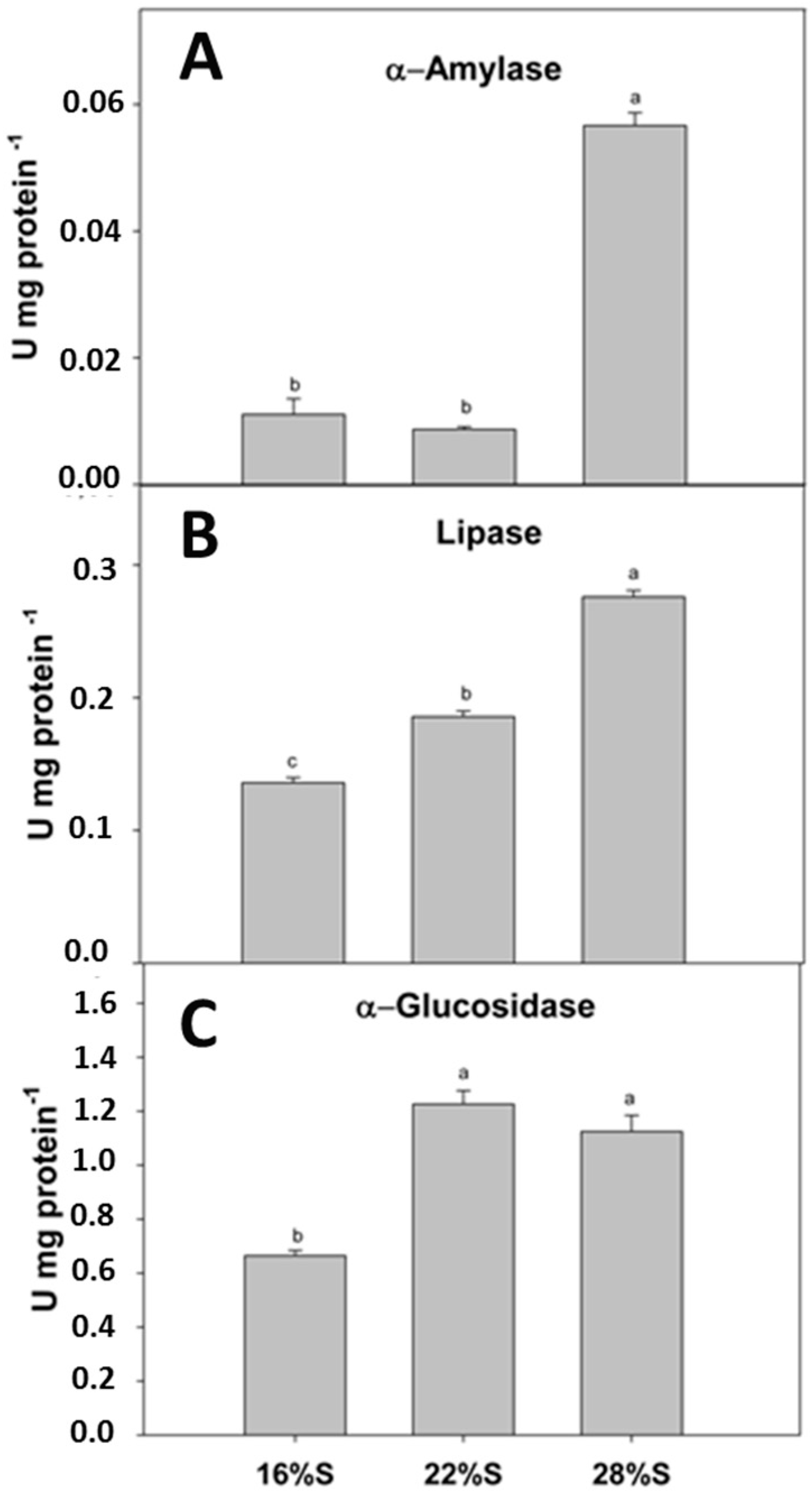
15]. In this study, the inclusion of potato starch in the diet significantly increased the activities of digestive enzymes such as α-amylase, α-glucosidase and lipase. Higher carbohydrase activity was related to the level of potato starch inclusion in the diets. Lipase activity also increased with the potato starch content of the diet. This could be attributed to modification in the lipid source by reduction of the protein source (rendered sources) and consequently balance diets using fish oil, thereby increasing lipid digestibility. There are no studies, however, regarding lipid source digestibility in
A. tropicus. It is known, however, that dietary carbohydrates increase lipid metabolism in other fish species, by stimulation of lipogenic enzymes and an increase in the rate of lipid oxidation that contribute to the energy supply and protein sparing [
17]. In the present study, the higher activities of lipases associated with higher dietary inclusions of potato starch could modify the lipidic metabolism.
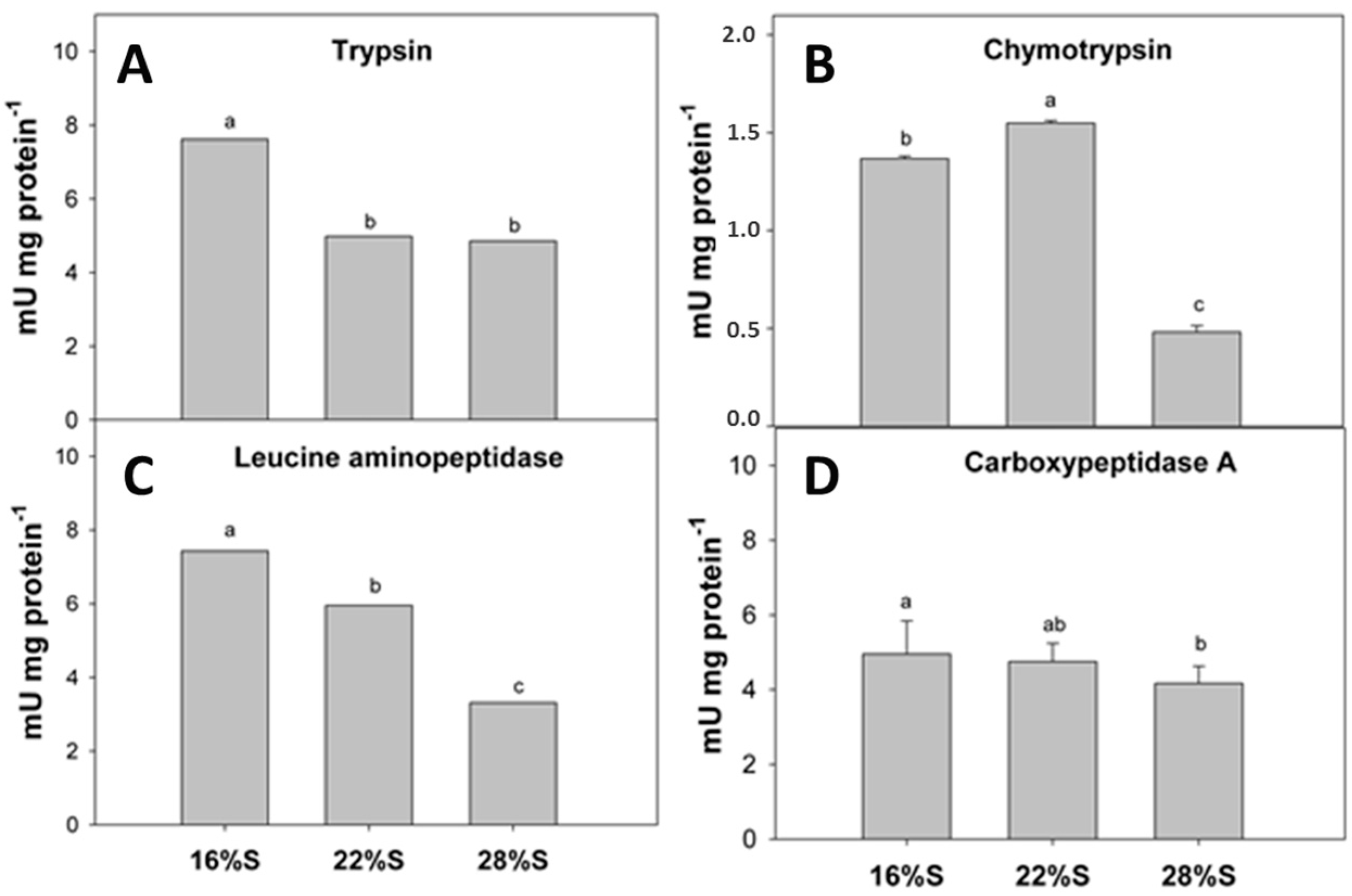
In contrast, the activity of proteases exhibited a more variable response to the percentage of dietary inclusion of potato starch. Previous studies evaluated diets with different levels of corn starch [
4], and found higher digestive enzyme activities (proteases, lipases and carbohydrases) with increasing starch levels in the diet (up to 15%) in
A. tropicus larvae. The present study used higher starch levels (up to 28%), and inclusion of 16% and 22% of potato starch yielded the highest activity values for proteases (alkaline proteases, trypsin, chymotrypsin and leucine aminopeptidases). However, higher protein levels are included in these two diets, which are directly related to the highest protease activity found.
Higher potato starch inclusion in the diet over the range tested in this study yielded higher α-amylase and α-glucosidase activities. Both enzymes are the main digestive enzymes involved in starch hydrolysis, acting on α(1–4) glycosidic bonds of starch, glycogen and related polysaccharides to produce glucose [
18]. Results of the present study demonstrate that
A. tropicus larvae are capable of digesting relatively high starch levels (28% S). It is important to note, however, that
A. tropicus is a carnivorous fish, in which endogenous enzymes are the principal digestion pathways, contrasting with herbivorous fish, in which exogenous digestion (microbial fermentation) confers them a higher digestion capacity over a wide range of carbohydrate substrates [
9,
19,
20].
Acid proteases in
A. tropicus larvae are present since first feeding [
2], and show high stomach activity compared to alkaline proteases detected in the intestine, as previously reported for this species, a characteristic of monogastric fish [
3,
4,
21]. Results of the present study show a decrease in acid protease activity with increasing starch potato inclusion; larvae feeding on the diet with the lowest starch content (16% S) exhibited higher acid digestion in the stomach and at the same time presented the highest protein content of the three experimental diets. Therefore, the present study aimed to decrease dietary protein content via substitution of potato starch, while previous studies did not change the dietary protein content by substituting a digestible carbohydrate source (corn starch) by an indigestible source (cellulose). It is well established that carbohydrate level and source show effects on growth, feed utilization and metabolism of finfish, and that these are attributable to the interaction between proteins and lipids [
8,
16].
The enzymatic activity of leucine aminopeptidase (LAP) has been used as a further indicator of dietary nutritional quality, indicating maturation of microvilli of enterocytes, but its activity varies in relation to the administered diet as well as the activities of other enzymes [
17]. As an intestinal brush border enzyme, LAP hydrolyzes peptides to amino acids in the final protein digestion phase and is present in the cytosol of intestinal epithelial cells. Therefore, reduced LAP activity with increasing dietary starch levels is closely related to the dietary protein content, as found for other proteolytic enzymes. The inclusion of potato starch in the diet of
A. tropicus larvae could promotes higher activity of amylase and glucosidases, and affects lipase and protease activities. Total digestion capacity of nutrients is required to enhance absorption of products involved in various metabolic processes and morpho-physiological changes during larval development when they experience changes in feeding habits [
22]. Detection of increasing levels of carbohydrases together with growth results indicate the potential of including carbohydrates in diets to contribute to the energy supply during larval development of carnivorous fish such as tropical gar [
7].
4. Materials and Methods
4.1. Larval Culture
Larvae used for this study were obtained from broodstock held at the Laboratorio de Acuicultura Tropical (LAT), División Académica de Ciencias Biológicas (DACBIOL) of the Universidad Juárez Autónoma de Tabasco (UJAT). Females were previously anesthetized with tricaine methasulfonate, MS-222® (Argent Chemical Laboratories™, Redmond, WA, USA) with 200 mg L−1 and then injected with a single dose of luteinizing hormone-releasing analog, LHRHa (35 μg kg−1). They were placed in a 2000 L capacity circular pond in a ratio 1:3 (female:males) (3.5 and 1.5 kg average wet weight of one female and three males, respectively) with synthetic grass, where eggs are fixed by females and fertilized by males. Fertilized eggs were incubated and developed in same tanks. A total of 1350 larvae (5 days post-hatching) were distributed among nine 70 L circular plastic tanks (3 tanks per treatment), connected to a recirculation system driven by a 0.5 HP water pump (Jacuzzi, JWPA5D-230A, Delavan, WI, USA), and a 1500 L reservoir was used for settlement of solids and as a biological filter. Water quality was monitored daily during the 30 days of larval culture: temperature (24.6 ± 2 °C) and dissolved oxygen (5.6 ± 0.3 mg L−1) were measured every day with an oxymeter (YSI 85, Yellow Springs, OH, USA), and pH (7.1 ± 0.2) with a potentiometer (HANNA HI 991001, Nusfalau, Romania). Partial water changes of 50% were made every two days and full changes every 5 days. Food was provided five times a day (at 8:00, 11:00, 13:00, 15:00 and 18:00 h) to apparent satiation. Individuals exhibiting cannibalism were separated in 0.5 L transparent, buoyant containers and isolated in the same experimental tank, where the same food was offered to satiety to allow their adaptation and reintegration within a period of no more than 3 days.
4.2. Experimental Design
Diets were designed to be isolipidic, with variations in caloric level, by modifying the carbohydrate content (potato starch, S) and decreasing the protein content (P) to evaluate protein sparing in
A. tropicus larvae. Three experimental diets where designed as follows: 16% S (16% S–44% P), 22% S (22% S–40% P) and 28% S (28% S–36% P), which allowed for the reduction of caloric levels of the diets (17.75, 16.68 and 15.57 KJ g
−1, respectively) (
Table 1). Assignments of tanks to each treatment were randomized (
n = 3 tanks per treatment at 150 organisms per tank).
4.3. Formulation and Preparation of Experimental Diets
Diet formulation was performed using MIXITWIN v. 5.0 software (Microsoft windows, Redmond, WA, USA). Experimental diet manufacturing was performed using previously described protocols [
23], according to formulations shown in
Table 1. Macronutrient ingredients were weighed using a 2000 g capacity analytical balance, precision of 0.01 g (Ohaus mod. CS2000, México City, México), and mixed using an industrial mixer (Bathamex, 178716, México City, México) for 15 min. Similarly, micronutrients (vitamin premixes, minerals, vitamin C) were weighed and mixed with the macronutrients for 15 min. The liquid ingredients (fish oil and soybean lecithin) were weighed and mixed in for another 15 min. Finally, water (about 400 mL per kg of diet) was added and final mixing was performed for another 15 min. The mixture obtained was placed in a 1 HP beef mill (Torrey, M-22RI, Monterrey, México) to obtain pellets that were oven dried at 60 °C for 24 h (Coriat, HC-35-D, México City, México). Grinding and sieving of the diets was performed to obtain specific particle sizes that met larvae requirements over ontogeny (250–500 μm). The experimental diets were maintained at −20 °C until use.
4.4. Proximal Composition
Moisture, protein, lipid and ash levels in the diets were determined using standard methods [
24]. Samples were homogenized and dried at 105 °C for 24 h prior to chemical analyses. The level of crude protein was analyzed using the micro-Kjeldahl method by the Labcocnco System (Labconco, Kansas City, MO, USA). The lipid content was obtained after extraction with petroleum ether, using a micro Foss Soxtec Avanti 2050 Automatic System (Foss Soxtec, Hoganäs, Sweden). The ash content was determined by combustion of the samples in a muffle furnace at 550 °C for 24 h (Fisher Scientific International, Inc., Pittsburgh, PA, USA). Fiber was determined by an enzymatic-gravimetric method, using three enzymes (heat stable α-amylase, protease, and amyloglucosidase), in which the sum of soluble and insoluble polysaccharides and lignin were measured and considered as total dietary fiber (TDF). The gross energy content of the diets was measured by combustion in a Parr bomb semi micro-calorimeter 6725 (Parr, Instrument Company, Moline, IL, USA) using benzoic acid as standard.
4.5. Assessment of Growth, Survival and Cannibalism
The experiment lasted 30 days from the start of exogenous feeding immediately after yolk absorption and opening of mouth/anus (5 days after hatching, DAH). Biometric measurements were performed every 10 days, recording individual weight and total length of the entire live test population. For weight measurement (g) a digital balance (Ohaus A5200, México City, México) was used and total length (cm) was obtained with Vernier calipers (to 0.01 cm). At the end of the experiment, percent survival was calculated using the following formula: S = (Nf/Ni) × 100, where Ni = initial number of live individuals, and Nf = final number of live individuals. Mortalities due to cannibalism were calculated as the difference between the theoretical survival rate (SR) that was obtained by subtracting from the initial number of fish, the running sums of dead fish (both intact and truncated) collected each day and the actual survival rate (SO; counts of survivors until day 30, considering only the truncated fish) following the next formula: C = ((SO − SR)/SO) × 100 [
25].
4.6. Enzymatic Analysis
At the end of the experiment, samples of the digestive tract were obtained to perform enzyme activity assays. Extracts were obtained by maceration of viscera from pooled larvae, using 50 larvae per tank (150 per treatment,
n = 3 pooled samples per treatment); heads and tails were cut off and left on ice, to prepare multienzymatic extracts. Each sample was homogenized at 4 °C in Tris-HCl 50 mmol L
−1, pH 7.5 (15 mg mL
−1) and centrifuged at 16,000×
g for 15 min at 4 °C. The supernatant was collected and stored at −20 °C before biochemical analysis. The concentration of soluble protein in samples was measured [
26], using bovine serum albumin as standard (1 mg mL
−1). All enzyme assays were performed in triplicate.
Alkaline protease activity was measured using casein (0.5%) as substrate in Tris-HCl 50 mmol L
−1, pH 9 [
27]. Acid protease (pepsin activity) was measured using hemoglobin (0.5%) as substrate in glycine-HCl 100 mmol L
−1, pH 2 [
28]. The mixtures were incubated at 37 °C, the reaction was stopped by adding 0.5 mL 20 % TCA, and the absorbance of the reaction products was measured at 280 nm. The unit of enzymatic activity was defined as 1 µg of tyrosine liberated per minute, based on a molar extinction coefficient (MEC) of 0.005 at 280 nm. Trypsin activity was measured at 25 °C using BAPNA (
N-a-benzoyl-
dl-arginine
p-nitroanilide) as substrate in Tris-HCl 50 mmol L
−1, pH 8.2 and CaCl
2 10 mmol L
−1 [
29]. Chymotrypsin activity was measured at 25 °C, using SAAPNA (
N-succinyl-ala-ala-pro-phe
p-nitroanilide) as substrate in dimethyl sulfoxide, DMSO, 10 mmol L
−1 and Tris-HCl 100 mmol L
−1, pH 7.8 and CaCl
2 10 mmol L
−1 [
30]. Leucine aminopeptidase activity was measured with leucine
p-nitroanilide in DMSO (0.1 mL) and 50 mmol L
−1 sodium phosphate, pH 7.2 at 25 °C [
31]. Carboxypeptidase A activity was measured using hippuryl-
l-phenyl-alanine as substrate in 25 mmol L
−1 Tris-HCl and NaCl (500 mmol L
−1) at pH 7.5 [
32]. The reactions in all the above-described techniques were stopped with 30% acetic acid. Enzymatic activity was defined as 1 µg of nitroanilide released per minute, using an MEC of 0.0088 for trypsin, and 8.2 for chymotrypsin and leucine aminopeptidase at 410 nm. The α-amylase activity was measured using starch (2%) as substrate in phosphate-citrate 100 mmol L
−1, NaCl 50 mmol L
−1, pH 7.5, at 600 nm [
33]. Lipase activity was quantified using 2-naphthyl caprylate (200 mmol L
−1) as substrate in Tris-HCl 50 mmol L
−1, pH 7.2 and sodium taurocholate (100 mmol L
−1), incubated for 30 min, and the reaction was stopped with TCA (0.72 N); fast blue (100 mmol L
−1) was added and ethanol/ethyl acetate (1:1
v/
v) was added to clarify [
34]. The activity unit was defined as 1 µg of naphthol released per minute at 540 nm, with an MEC of 0.02. The activity of α-glucosidase was determined using α-
p-nitrophenol glucoside (10 mmol L
−1) as substrate, dissolved in buffer solution (potassium phosphate 67 mmol L
−1 at pH 6.8) and incubated for 60 min with enzyme extract and stopped with potassium carbonate (100 mmol/L). Absorbance was measured at 420 nm using a standard calibration curve for
p-nitrophenol α-glucoside (Sigma, St. Louis, MO, USA) [
35]. The α-glucosidase activity was expressed as a unit of enzyme, which liberates 1 mol of
d-glucose
p-nitrophenyl α-
d-glucoside per minute.
All assays were performed in triplicate. For the calculation of the specific, activity of extracts, the following equations were used: (1) units mL−1 = [ΔAbs × final reaction volume (mL)]/[MEC × time (min) × volume of extract (mL)]; and (2) units mg protein−1 = [Units per mL]/ [mg of soluble protein], where ΔAbs is the increase in absorbance at a given wavelength and MEC is the molar extinction coefficient for the reaction product (mL µg/cm−1).
4.7. Statistical Analysis
For evaluation of larval growth in weight and length, data from the experiment were tested for normality (Kolmogorov–Smirnov test) and homoscedasticity (Levene test), and analysis of variance (ANOVA) was applied to detect differences among treatments followed by a posteriori Tukey tests. Survival, cannibalism and enzyme activity data were analyzed using a non-parametric Kruskal–Wallis test and Nemenyi posteriori tests. All tests were conducted using a 0.05 significance value. STATISTICA v 7.0 statistical software (Statsoft, Tulsa, OK, USA) was used to perform all statistical analyses.